a0e63cff83632ba8afb5d5cbce41d578.ppt
- Количество слайдов: 20

Orchid Chemicals & Pharmaceuticals Ltd. Creating Sustainable Value BSE: 524372 NSE: ORCHIDCHEM Bloomberg: OCP@IN Reuters: ORCD. BO April 2011 www. orchidpharma. com 1

Disclaimer n This presentation includes forward-looking statements / projections, which are based on current expectations and forecasts about future events. Such statements involve known / unknown risks, uncertainties and other factors and may cause and defer the actual results materially. Such factors include, but are not limited to, changes in local and global economic conditions, Orchid Chemicals & Pharmaceuticals Ltd. (‘Orchid’ or the ‘Company’) ability to successfully implement its strategies, the market acceptance and demand of the Company’s products and services, the Company’s growth rates, expansion, technological change and the Company’s exposure to market risks. By this nature, these indications and projections are only estimates and actual results could differ from these in the future. n These materials are not an offer for sale of securities in the United States or in any other jurisdiction. No securities of the Company may be offered or sold in the United States absent registration or an exemption from registration under the U. S. Securities Act of 1933, as amended. The Company does not intend to register any of its securities for offer or sale in the United States or elsewhere, or to conduct a public offering of securities in the United States or elsewhere. No money or other consideration is being solicited pursuant to these materials. www. orchidpharma. com 2
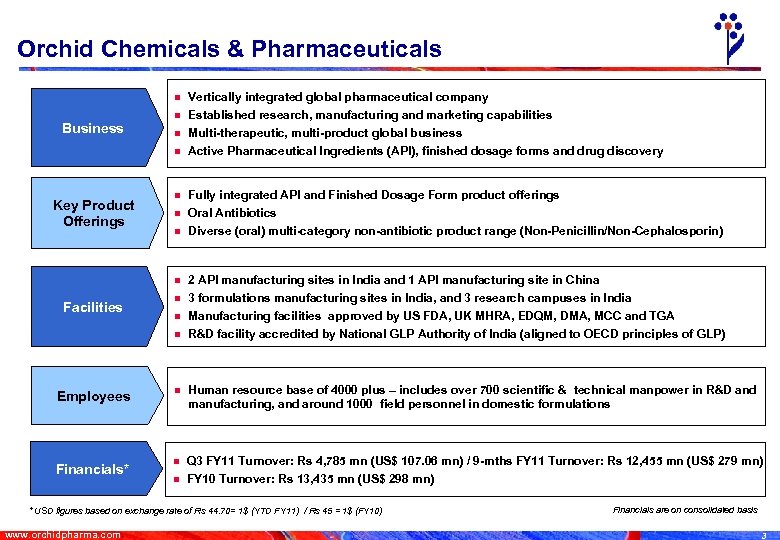
Orchid Chemicals & Pharmaceuticals n Business n n n Key Product Offerings n n Facilities n n n Vertically integrated global pharmaceutical company Established research, manufacturing and marketing capabilities Multi-therapeutic, multi-product global business Active Pharmaceutical Ingredients (API), finished dosage forms and drug discovery Fully integrated API and Finished Dosage Form product offerings Oral Antibiotics Diverse (oral) multi-category non-antibiotic product range (Non-Penicillin/Non-Cephalosporin) 2 API manufacturing sites in India and 1 API manufacturing site in China 3 formulations manufacturing sites in India, and 3 research campuses in India Manufacturing facilities approved by US FDA, UK MHRA, EDQM, DMA, MCC and TGA R&D facility accredited by National GLP Authority of India (aligned to OECD principles of GLP) Employees n Human resource base of 4000 plus – includes over 700 scientific & technical manpower in R&D and manufacturing, and around 1000 field personnel in domestic formulations Financials* n Q 3 FY 11 Turnover: Rs 4, 785 mn (US$ 107. 06 mn) / 9 -mths FY 11 Turnover: Rs 12, 455 mn (US$ 279 mn) FY 10 Turnover: Rs 13, 435 mn (US$ 298 mn) n * USD figures based on exchange rate of Rs 44. 70= 1$ (YTD FY 11) / Rs 45 = 1$ (FY 10) www. orchidpharma. com Financials are on consolidated basis 3
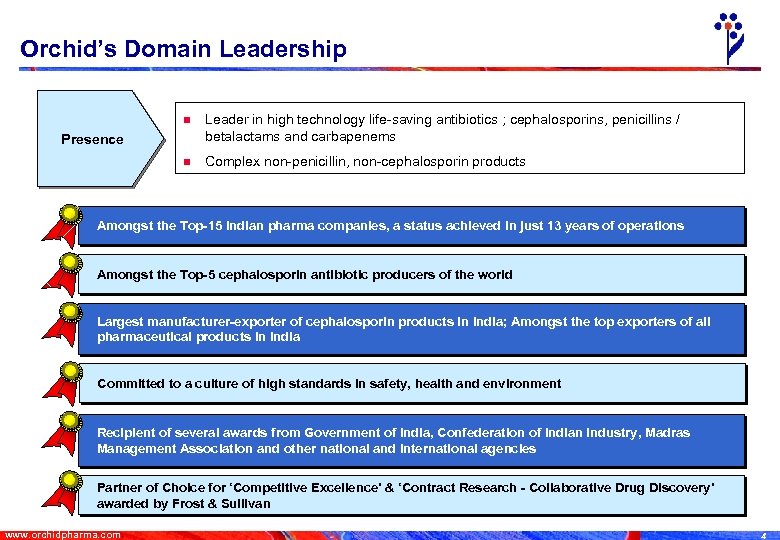
Orchid’s Domain Leadership n Leader in high technology life-saving antibiotics ; cephalosporins, penicillins / betalactams and carbapenems n Complex non-penicillin, non-cephalosporin products Presence Amongst the Top-15 Indian pharma companies, a status achieved in just 13 years of operations Amongst the Top-5 cephalosporin antibiotic producers of the world Largest manufacturer-exporter of cephalosporin products in India; Amongst the top exporters of all pharmaceutical products in India Committed to a culture of high standards in safety, health and environment Recipient of several awards from Government of India, Confederation of Indian Industry, Madras Management Association and other national and international agencies Partner of Choice for ‘Competitive Excellence’ & ‘Contract Research - Collaborative Drug Discovery’ awarded by Frost & Sullivan www. orchidpharma. com 4
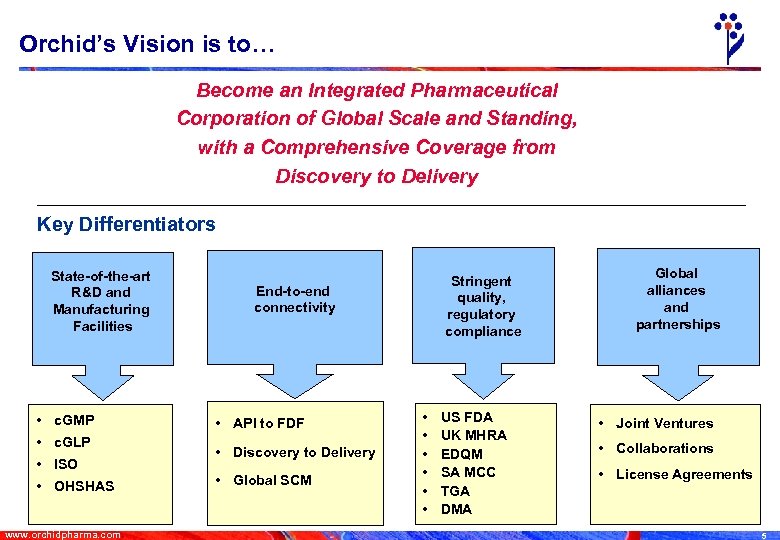
Orchid’s Vision is to… Become an Integrated Pharmaceutical Corporation of Global Scale and Standing, with a Comprehensive Coverage from Discovery to Delivery Key Differentiators State-of-the-art R&D and Manufacturing Facilities • c. GMP • c. GLP • ISO • OHSHAS www. orchidpharma. com Stringent quality, regulatory compliance End-to-end connectivity • API to FDF • Discovery to Delivery • Global SCM • • • US FDA UK MHRA EDQM SA MCC TGA DMA Global alliances and partnerships • Joint Ventures • Collaborations • License Agreements 5
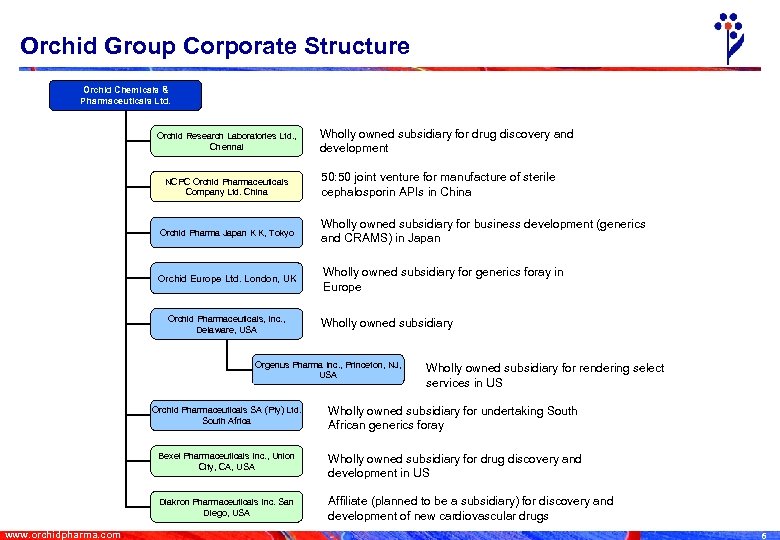
Orchid Group Corporate Structure Orchid Chemicals & Pharmaceuticals Ltd. Orchid Research Laboratories Ltd. , Chennai NCPC Orchid Pharmaceuticals Company Ltd. China Wholly owned subsidiary for drug discovery and development 50: 50 joint venture for manufacture of sterile cephalosporin APIs in China Orchid Pharma Japan K K, Tokyo Wholly owned subsidiary for business development (generics and CRAMS) in Japan Orchid Europe Ltd. London, UK Wholly owned subsidiary for generics foray in Europe Orchid Pharmaceuticals, Inc. , Delaware, USA Wholly owned subsidiary Orgenus Pharma Inc. , Princeton, NJ, USA Wholly owned subsidiary for rendering select services in US Orchid Pharmaceuticals SA (Pty) Ltd. South Africa Bexel Pharmaceuticals Inc. , Union City, CA, USA Wholly owned subsidiary for drug discovery and development in US Diakron Pharmaceuticals Inc. San Diego, USA www. orchidpharma. com Wholly owned subsidiary for undertaking South African generics foray Affiliate (planned to be a subsidiary) for discovery and development of new cardiovascular drugs 6

API facilities (Alathur, Chennai) n GMP - Approved (WHO) n US-FDA – Approved (Nov’ 02, Sep’ 03 & Dec’ 06) Approved by US FDA, UK MHRA and other international regulatory agencies n EDQM – Approved (Sep’ 03) n MHRA – Approved (Jan’ 06) n Hamburg Health Authority – Approved (Jan’ 06) Australian-TGA - Approved (Feb’ 03) n ISO 9001: 2000 -certified Quality Management System (Re-certified in May’ 08) n ISO 14001 -certified Environment Management System (Re-certified in May’ 08) n Danish Medicines Agency (EU-GMP) – Approval (Jan’ 09) n n Largest cephalosporin API manufacturing complex n n Pharmaceuticals & Medical Devices Agency (PMDA, Japan) – Oct’ 09) n Variety of non-sterile and sterile (crystalline & lyophilised) APIs n High levels of throughput and manufacturing integration (980 MT of capacity per annum) n State-of-the-art infrastructure designed for highest levels of process efficiencies and environmental friendliness www. orchidpharma. com 7

API facilities (Aurangabad) n Multi-site API complex with dedicated facilities for ä Penicillins ä Sterile Carbapenems ä Non-penicillin, Non-cephalosporins (NPNC) n Manufacturing capacities : 48 MT for Penicillins, 12 MT for Sterile Carbapenems and 120 MT for NPNC n ISPE, INTERPHEX and Pharmaceutical Processing Facility Awards 2009 Facilities designed for automated production n Sterile Carbapenem Facility Approvals: ä UK MHRA (Approved - Jan ’ 07) ä US FDA (Approved - Feb ’ 07) ä OHSAS 18001: 1999 ä Danish Medicines Agency [EU-GMP] (Approved - Jan ’ 09) ä WHO GMP Audit (Approved - Apr ‘ 10) www. orchidpharma. com 8

Formulation Facilities – Advanced Markets (Irungattukottai, Chennai) n Large state-of-the-art oral dosage form complex with multi-therapeutic facilities n World-class facilities with US FDA & UK MHRA and other international regulatory approvals n High throughput Oral cephalosporin formulations facility n Pilot and commercial scale NPNC (non-antibiotic) dosage forms plant n Global scale capacities www. orchidpharma. com 9

Highlights of Orchid’s Generics Contracts – US, EU model § Orchid has long term partnerships with leading global generic players in US and Europe to distribute its products in the respective markets. § Orchid licenses the generic products in return for development cum licensing fees received from the partners. § Orchid manufactures and supplies the products from its US FDA, UK MHRA approved facilities based on pre-agreed costs of goods and also receives share of revenues or net profits as per agreements. § The arrangements incorporate mutual performance parameters in terms of market and supply performance. § The typical initial term ranges upwards of five years from product launch, with the agreements providing for renewal. § The partnerships have been expanded in terms of product range and extended in terms of geographic coverage reflecting Orchid’s performance and partnership equity Orchid is currently working on creating front-end marketing organisations of its own in the advanced markets www. orchidpharma. com 10

Novel Drug Delivery Systems (NDDS) n A new initiative to support product life cycle management of established drugs n Undertakes development of new dosage forms and strengths to enhance the efficacy and safety of existing drugs n Develops novel combinations of existing drugs for better compliance and / or synergistic effect n Platform technologies deployed to undertake NDDS developments n Strategy focuses on developing NDDS on bench scale and establishing limited proof-of- concept n Business strategy envisages collaborative approaches with MNCs and specialist delivery companies for further studies and eventual commercialization n Also provides support to development of conventional and novel formulations for New Chemical Entities www. orchidpharma. com 11

Orchid Research Laboratories Limited (ORLL) n Business n n Objectives n n A wholly owned subsidiary of Orchid Chemicals & Pharmaceuticals for undertaking drug discovery and development An integrated research company with end-to-end capabilities in drug discovery and development Among the top ranked pharmaceutical discovery entities in India, with global standing Discovery and development of New Chemical Entities (NCEs) Custom Research and Manufacturing Services (CRAMS) n Drug Discovery Centre, Biology Centre, Animal House cum Pre-clinical Facility, CMC Facility, Analytical Centre, Formulation Facility in Chennai, India R&D Facilities GLP accredited by National GLP Authority of India, aligned with OECD Principles n n Scientific base of 130 plus with several doctoral and post-doctoral scientists Access to 200 plus scientists in other R&D and technical domains of the Parent organization n Partner of choice in ‘Contract Research – Collaborative Drug Discovery’ awarded by Frost & Sullivan n Facilities Employees Recognition www. orchidpharma. com 12
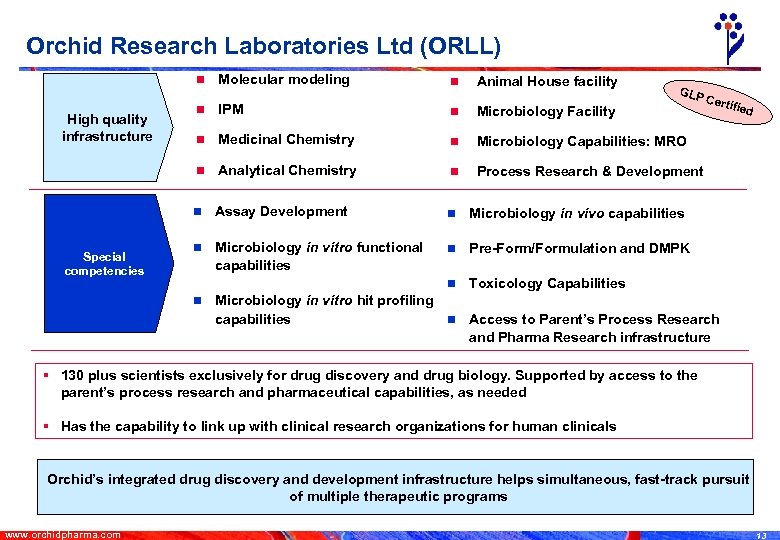
Orchid Research Laboratories Ltd (ORLL) n Animal House facility n IPM n Microbiology Facility n Medicinal Chemistry n Microbiology Capabilities: MRO Analytical Chemistry n Process Research & Development n Assay Development n Microbiology in vivo capabilities n Microbiology in vitro functional capabilities n Pre-Form/Formulation and DMPK n Special competencies n n High quality infrastructure Molecular modeling Toxicology Capabilities n GLP Cert ified Microbiology in vitro hit profiling n Access to Parent’s Process Research capabilities and Pharma Research infrastructure § 130 plus scientists exclusively for drug discovery and drug biology. Supported by access to the parent’s process research and pharmaceutical capabilities, as needed § Has the capability to link up with clinical research organizations for human clinicals Orchid’s integrated drug discovery and development infrastructure helps simultaneous, fast-track pursuit of multiple therapeutic programs www. orchidpharma. com 13
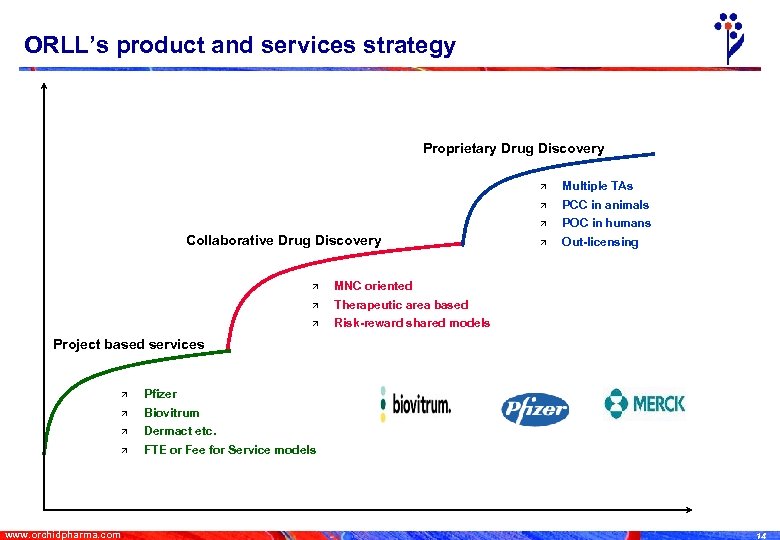
ORLL’s product and services strategy Proprietary Drug Discovery ä ä Out-licensing Therapeutic area based ä POC in humans MNC oriented ä PCC in animals ä Collaborative Drug Discovery Multiple TAs Risk-reward shared models Project based services ä ä Biovitrum ä Dermact etc. ä www. orchidpharma. com Pfizer FTE or Fee for Service models 14
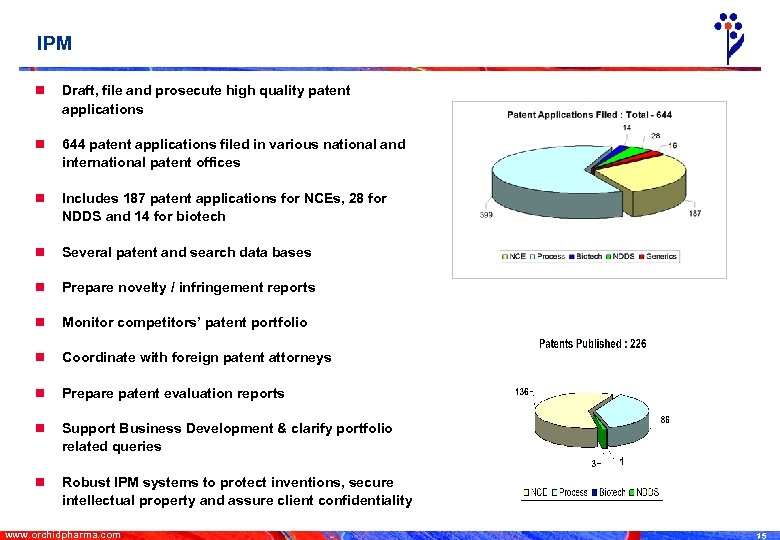
IPM n Draft, file and prosecute high quality patent applications n 644 patent applications filed in various national and international patent offices n Includes 187 patent applications for NCEs, 28 for NDDS and 14 for biotech n Several patent and search data bases n Prepare novelty / infringement reports n Monitor competitors’ patent portfolio n Coordinate with foreign patent attorneys n Prepare patent evaluation reports n Support Business Development & clarify portfolio related queries n Robust IPM systems to protect inventions, secure intellectual property and assure client confidentiality www. orchidpharma. com 15
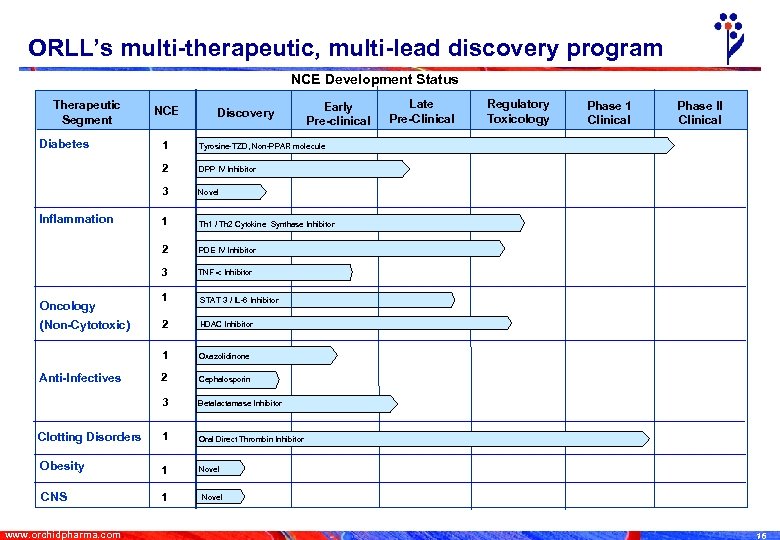
ORLL’s multi-therapeutic, multi-lead discovery program NCE Development Status Therapeutic Segment Diabetes NCE Discovery Early Pre-clinical 1 Th 1 / Th 2 Cytokine Synthase Inhibitor 2 PDE IV Inhibitor 3 TNF Inhibitor 1 STAT 3 / IL-6 Inhibitor 2 HDAC Inhibitor 1 Oxazolidinone 2 Cephalosporin 3 Betalactamase Inhibitor Clotting Disorders 1 Oral Direct Thrombin Inhibitor Obesity 1 Novel CNS 1 Phase II Clinical Novel 1 Phase 1 Clinical DPP IV Inhibitor 3 Regulatory Toxicology Tyrosine-TZD, Non-PPAR molecule 2 Late Pre-Clinical Inflammation Oncology (Non-Cytotoxic) Anti-Infectives www. orchidpharma. com Novel 16
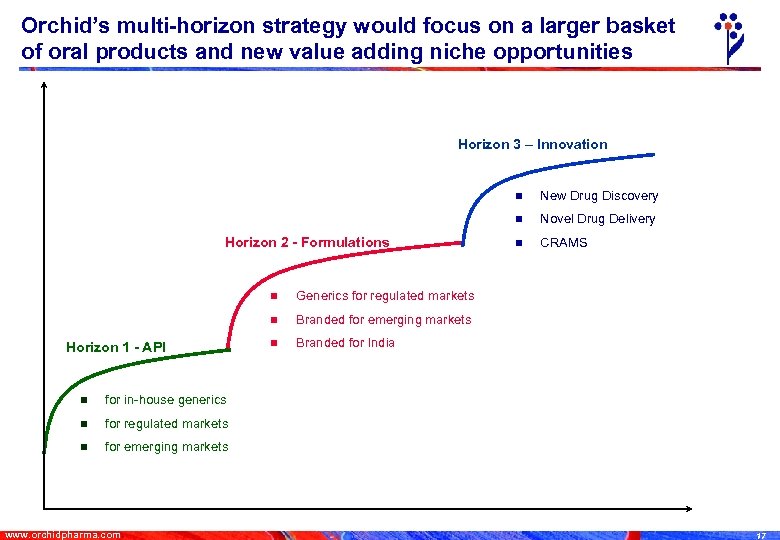
Orchid’s multi-horizon strategy would focus on a larger basket of oral products and new value adding niche opportunities Horizon 3 – Innovation n n Horizon 2 - Formulations n Horizon 1 - API n Branded for India for regulated markets n CRAMS for in-house generics n n Branded for emerging markets n Novel Drug Delivery Generics for regulated markets n New Drug Discovery for emerging markets www. orchidpharma. com 17
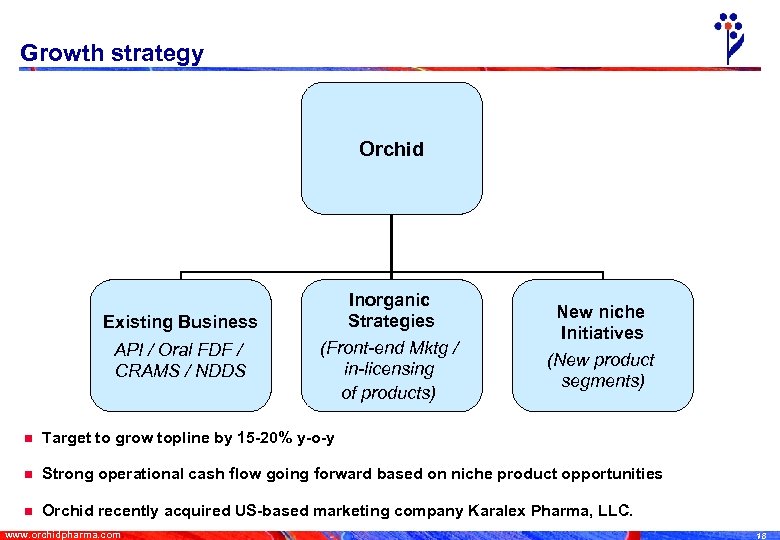
Growth strategy Orchid Existing Business API / Oral FDF / CRAMS / NDDS Inorganic Strategies (Front-end Mktg / in-licensing of products) New niche Initiatives (New product segments) n Target to grow topline by 15 -20% y-o-y n Strong operational cash flow going forward based on niche product opportunities n Orchid recently acquired US-based marketing company Karalex Pharma, LLC. www. orchidpharma. com 18
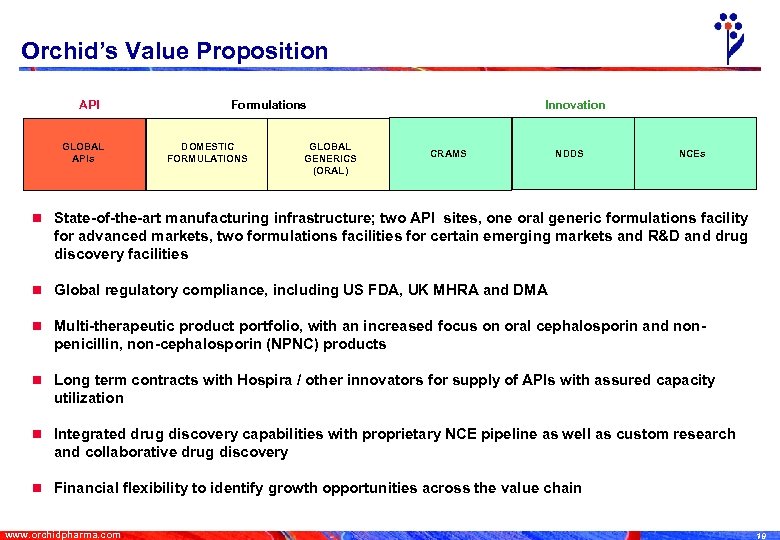
Orchid’s Value Proposition API GLOBAL APIs Formulations DOMESTIC FORMULATIONS GLOBAL GENERICS (ORAL) Innovation CRAMS NDDS NCEs n State-of-the-art manufacturing infrastructure; two API sites, one oral generic formulations facility for advanced markets, two formulations facilities for certain emerging markets and R&D and drug discovery facilities n Global regulatory compliance, including US FDA, UK MHRA and DMA n Multi-therapeutic product portfolio, with an increased focus on oral cephalosporin and non- penicillin, non-cephalosporin (NPNC) products n Long term contracts with Hospira / other innovators for supply of APIs with assured capacity utilization n Integrated drug discovery capabilities with proprietary NCE pipeline as well as custom research and collaborative drug discovery n Financial flexibility to identify growth opportunities across the value chain www. orchidpharma. com 19

THANK YOU For any information or clarifications, please visit www. orchidpharma. com or call Ch Ram, Head - Communications & Investor Relations on +91 98400 11626 / ram@orchidpharma. com © 2011 Orchid Chemicals & Pharmaceuticals Ltd. , All Rights Reserved. This material was used during an oral presentation; it is not a complete record of the discussion. This work may not be used, sold, transferred, adapted, abridged, copied or reproduced in whole on or in part in any manner or form or in any media without the prior written consent. All product names and company names and logos mentioned herein are the trademarks or registered trademarks of their respective owners. www. orchidpharma. com 20
a0e63cff83632ba8afb5d5cbce41d578.ppt