7b275f0658bed8afe7367e29617fc420.ppt
- Количество слайдов: 46

Orbitrap Mass Analyser - Overview and Applications in Proteomics Alexander Makarov, Michaela Scigelova Thermo Electron Corporation
Outline • Orbitrap mass analyser • Linking orbitrap to linear ion trap • Flexibility of use of LTQ Orbitrap • Focus on: – High resolution – Sensitivity – Speed – Dynamic range • Conclusion 2
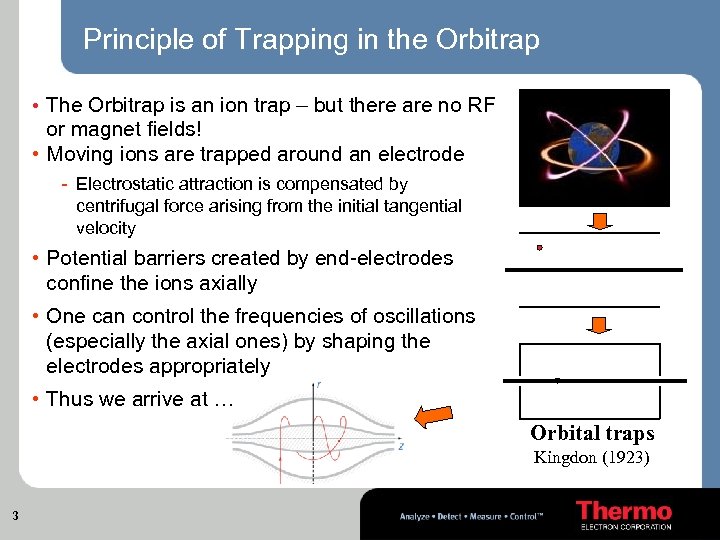
Principle of Trapping in the Orbitrap • The Orbitrap is an ion trap – but there are no RF or magnet fields! • Moving ions are trapped around an electrode - Electrostatic attraction is compensated by centrifugal force arising from the initial tangential velocity • Potential barriers created by end-electrodes confine the ions axially • One can control the frequencies of oscillations (especially the axial ones) by shaping the electrodes appropriately • Thus we arrive at … Orbital traps Kingdon (1923) 3
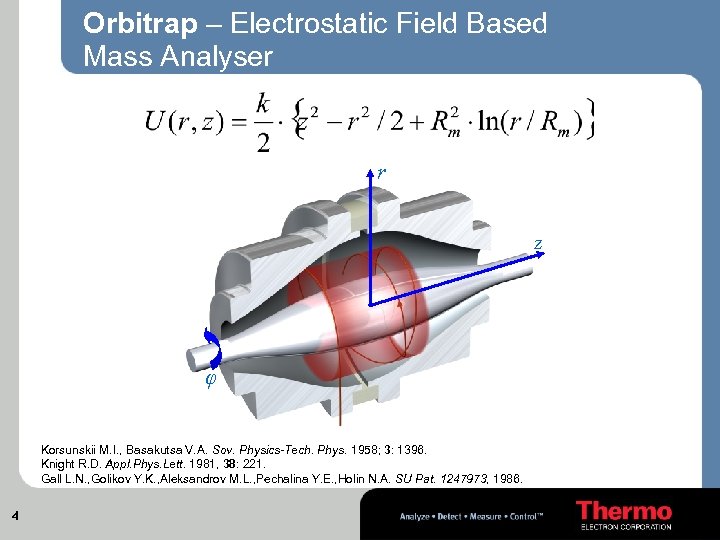
Orbitrap – Electrostatic Field Based Mass Analyser r z φ Korsunskii M. I. , Basakutsa V. A. Sov. Physics-Tech. Phys. 1958; 3: 1396. Knight R. D. Appl. Phys. Lett. 1981, 38: 221. Gall L. N. , Golikov Y. K. , Aleksandrov M. L. , Pechalina Y. E. , Holin N. A. SU Pat. 1247973, 1986. 4
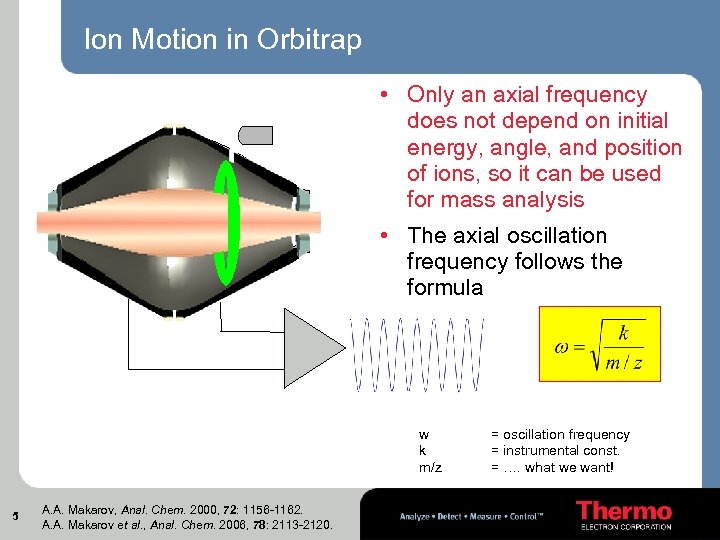
Ion Motion in Orbitrap • Only an axial frequency does not depend on initial energy, angle, and position of ions, so it can be used for mass analysis • The axial oscillation frequency follows the formula w k m/z 5 A. A. Makarov, Anal. Chem. 2000, 72: 1156 -1162. A. A. Makarov et al. , Anal. Chem. 2006, 78: 2113 -2120. = oscillation frequency = instrumental const. = …. what we want!
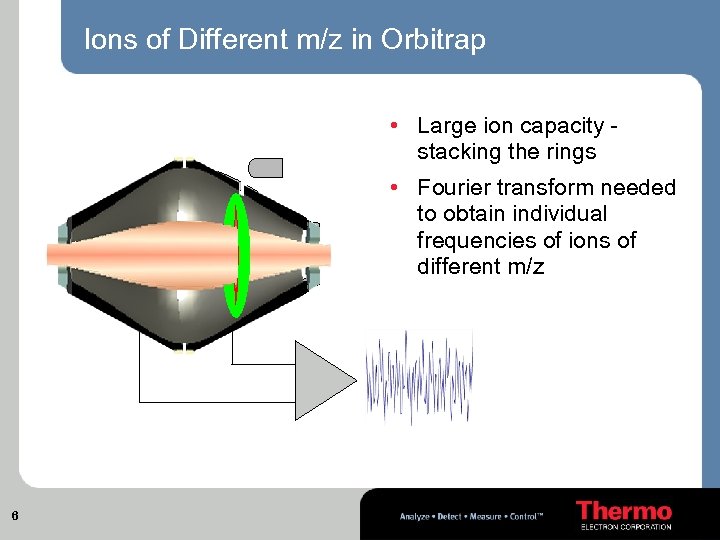
Ions of Different m/z in Orbitrap • Large ion capacity stacking the rings • Fourier transform needed to obtain individual frequencies of ions of different m/z 6

How Big Is Orbitrap? 7
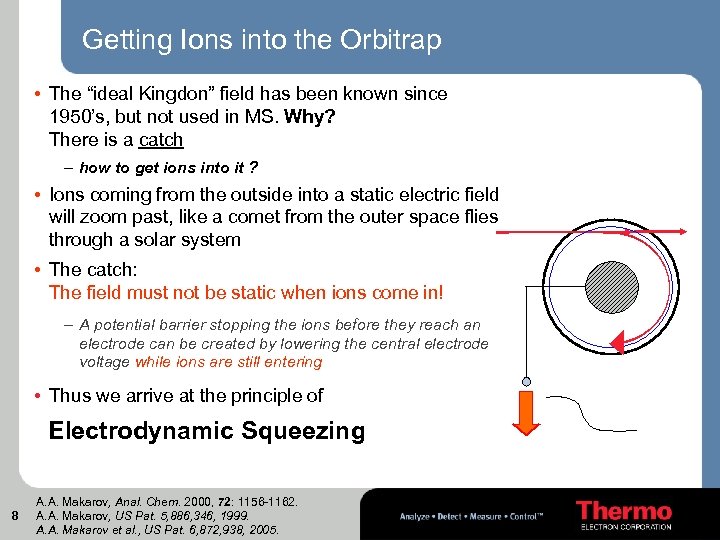
Getting Ions into the Orbitrap • The “ideal Kingdon” field has been known since 1950’s, but not used in MS. Why? There is a catch – how to get ions into it ? • Ions coming from the outside into a static electric field will zoom past, like a comet from the outer space flies through a solar system • The catch: The field must not be static when ions come in! – A potential barrier stopping the ions before they reach an electrode can be created by lowering the central electrode voltage while ions are still entering • Thus we arrive at the principle of Electrodynamic Squeezing 8 A. A. Makarov, Anal. Chem. 2000, 72: 1156 -1162. A. A. Makarov, US Pat. 5, 886, 346, 1999. A. A. Makarov et al. , US Pat. 6, 872, 938, 2005.
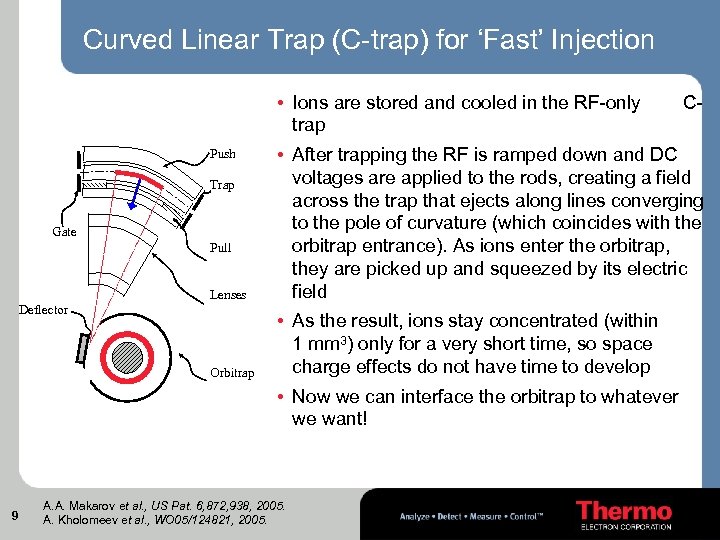
Curved Linear Trap (C-trap) for ‘Fast’ Injection • Ions are stored and cooled in the RF-only trap C- Lenses • After trapping the RF is ramped down and DC voltages are applied to the rods, creating a field across the trap that ejects along lines converging to the pole of curvature (which coincides with the orbitrap entrance). As ions enter the orbitrap, they are picked up and squeezed by its electric field Orbitrap • As the result, ions stay concentrated (within 1 mm 3) only for a very short time, so space charge effects do not have time to develop Push Trap Gate Pull Deflector • Now we can interface the orbitrap to whatever we want! 9 A. A. Makarov et al. , US Pat. 6, 872, 938, 2005. A. Kholomeev et al. , WO 05/124821, 2005.
Outline • Orbitrap mass analyser • Linking orbitrap to linear ion trap • Flexibility of use of LTQ Orbitrap • Focus on: – High resolution – Sensitivity – Speed – Dynamic range • Conclusion 10
Linking Linear Trap with Orbitrap • Combining the features of the Finnigan LTQ… – ESI, nanospray, APCI, APPI ionsation methods – outstanding sensitivity – MSn operation – Ruggedness and ease of use It adds capabilities for the most demanding analyses • …with excellent performance of orbitrap – High resolution – Accurate mass determination It is fast - even with high resolution/accurate mass detection 11
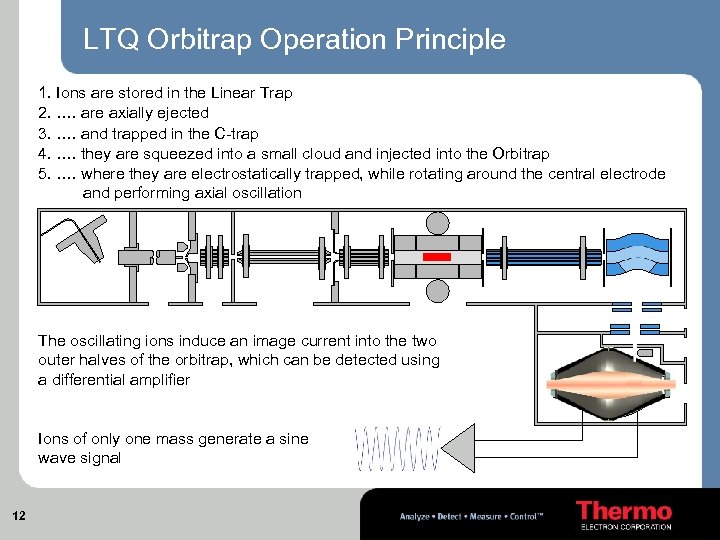
LTQ Orbitrap Operation Principle 1. Ions are stored in the Linear Trap 2. …. are axially ejected 3. …. and trapped in the C-trap 4. …. they are squeezed into a small cloud and injected into the Orbitrap 5. …. where they are electrostatically trapped, while rotating around the central electrode and performing axial oscillation The oscillating ions induce an image current into the two outer halves of the orbitrap, which can be detected using a differential amplifier Ions of only one mass generate a sine wave signal 12

How Big Is LTQ Orbitrap? 13
What LTQ Orbitrap Delivers • Mass resolution > 60, 000 at m/z 400 at 1 sec cycle • Max. resolution over 100, 000 (FWHM) • Mass accuracy < 5 ppm external calibration • Mass accuracy < 2 ppm internal calibration • Mass range 50 – 2, 000; 200 – 4, 000 • Sensitivity sub-femtomole on column • Throughput 4 scans per second (1 high-resolution scan in the orbitrap + 3 MS/MS scans in the LTQ) 14
Outline • Orbitrap mass analyser • Linking orbitrap to linear ion trap • Flexible method design for LTQ Orbitrap • Focus on: – High resolution – Sensitivity – Speed – Dynamic range • Conclusion 15
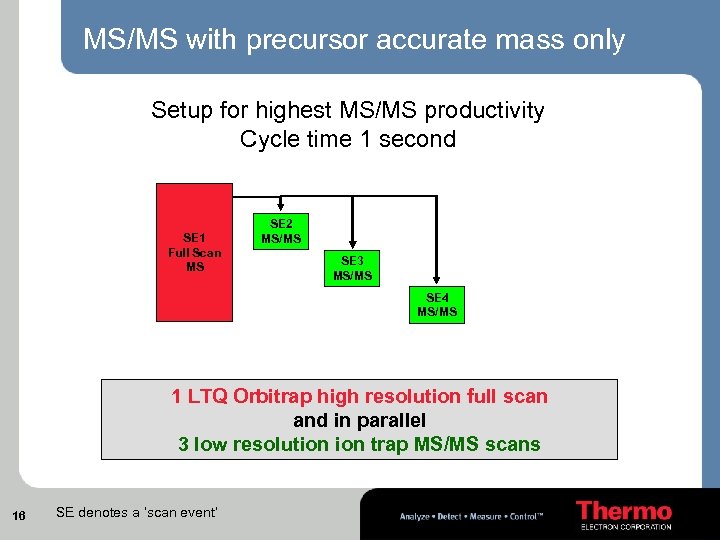
MS/MS with precursor accurate mass only Setup for highest MS/MS productivity Cycle time 1 second SE 1 Full Scan MS SE 2 MS/MS SE 3 MS/MS SE 4 MS/MS 1 LTQ Orbitrap high resolution full scan and in parallel 3 low resolution trap MS/MS scans 16 SE denotes a ‘scan event’
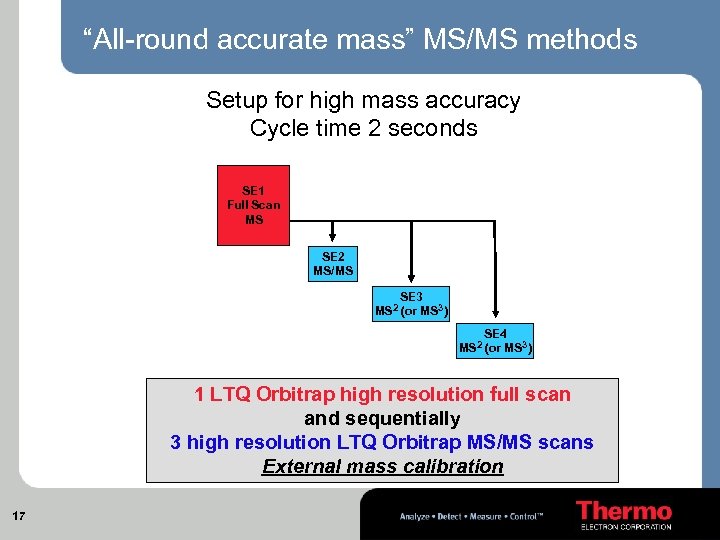
“All-round accurate mass” MS/MS methods Setup for high mass accuracy Cycle time 2 seconds SE 1 Full Scan MS SE 2 MS/MS SE 3 MS 2 (or MS 3) MS 2 SE 4 (or MS 3) 1 LTQ Orbitrap high resolution full scan and sequentially 3 high resolution LTQ Orbitrap MS/MS scans External mass calibration 17
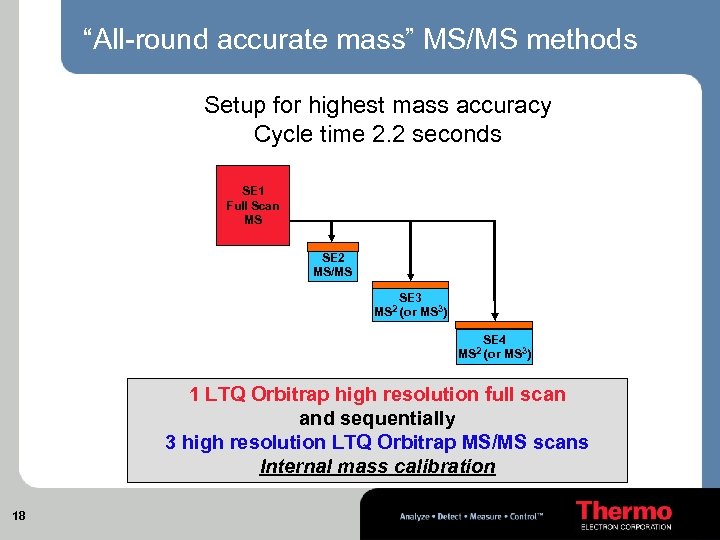
“All-round accurate mass” MS/MS methods Setup for highest mass accuracy Cycle time 2. 2 seconds SE 1 Full Scan MS SE 2 MS/MS MS 2 SE 3 (or MS 3) MS 2 SE 4 (or MS 3) 1 LTQ Orbitrap high resolution full scan and sequentially 3 high resolution LTQ Orbitrap MS/MS scans Internal mass calibration 18
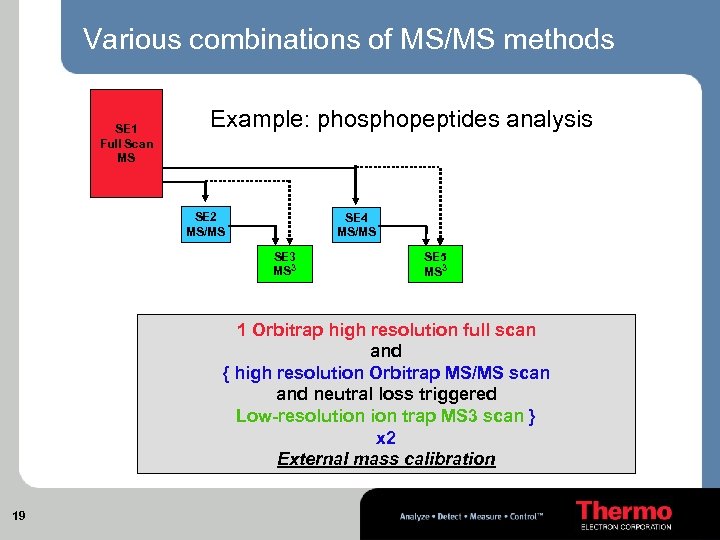
Various combinations of MS/MS methods SE 1 Full Scan MS Example: phosphopeptides analysis SE 2 MS/MS SE 4 MS/MS SE 3 MS 3 SE 5 MS 3 1 Orbitrap high resolution full scan and { high resolution Orbitrap MS/MS scan and neutral loss triggered Low-resolution trap MS 3 scan } x 2 External mass calibration 19
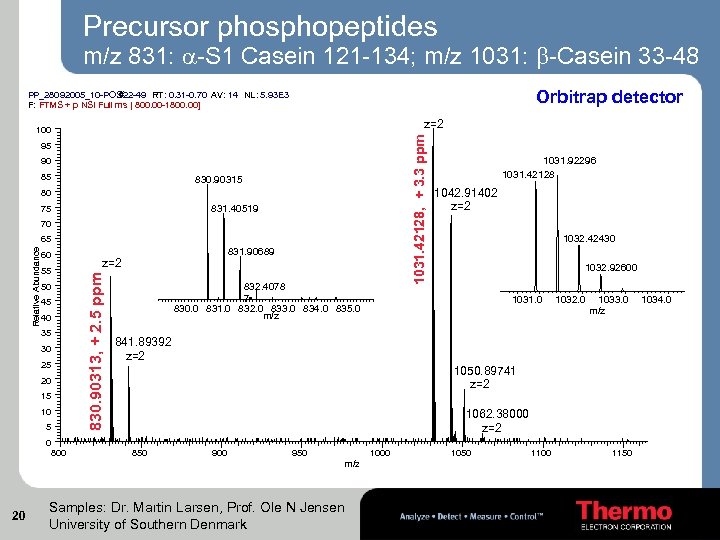
Precursor phosphopeptides m/z 831: -S 1 Casein 121 -134; m/z 1031: -Casein 33 -48 Orbitrap detector PP_28092005_10 -POS #22 -49 RT: 0. 31 -0. 70 AV: 14 NL: 5. 93 E 3 F: FTMS + p NSI Full ms [ 800. 00 -1800. 00] z=2 1031. 42128, + 3. 3 ppm 100 95 90 85 830. 90315 80 831. 40519 75 70 60 55 50 45 40 35 30 25 20 15 10 5 0 800 831. 90689 z=2 830. 90313, + 2. 5 ppm Relative Abundance 65 832. 4078 7 830. 0 831. 0 832. 0 833. 0 834. 0 835. 0 m/z 1042. 91402 z=2 1032. 42430 1032. 92600 1031. 0 1032. 0 1033. 0 m/z 841. 89392 z=2 1050. 89741 z=2 1062. 38000 z=2 850 900 950 1000 m/z 20 1031. 92296 1031. 42128 Samples: Dr. Martin Larsen, Prof. Ole N Jensen University of Southern Denmark 1050 1100 1150 1034. 0
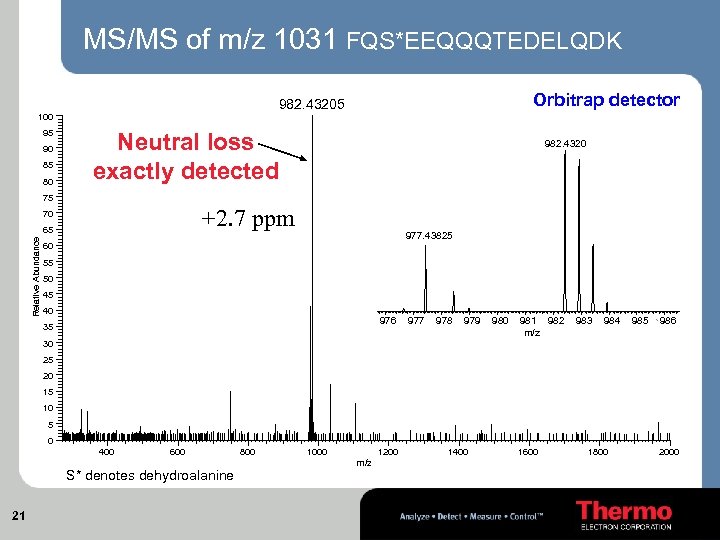
MS/MS of m/z 1031 FQS*EEQQQTEDELQDK Orbitrap detector 982. 43205 100 95 90 85 80 Neutral loss exactly detected 982. 4320 75 +2. 7 ppm 70 Relative Abundance 65 977. 43825 60 55 50 45 40 976 35 977 978 979 980 981 982 m/z 983 984 985 986 30 25 20 15 10 5 0 400 600 S* denotes dehydroalanine 21 800 1000 1200 m/z 1400 1600 1800 2000
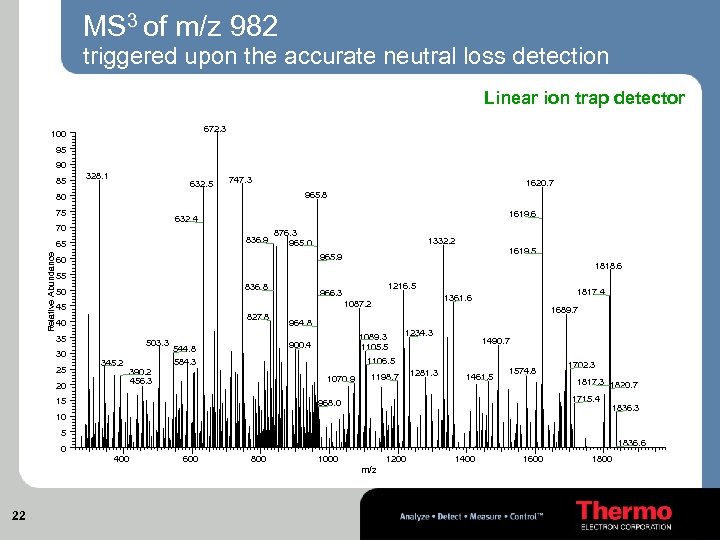
MS 3 of m/z 982 triggered upon the accurate neutral loss detection Linear ion trap detector 672. 3 100 95 90 85 328. 1 632. 5 747. 3 80 75 1619. 6 632. 4 70 836. 9 65 Relative Abundance 1620. 7 965. 8 876. 3 965. 0 1332. 2 1619. 5 965. 9 60 1818. 6 55 836. 8 50 827. 8 40 35 25 20 503. 3 345. 2 390. 2 456. 3 1689. 7 964. 8 1089. 3 1105. 5 900. 4 544. 8 584. 3 1817. 4 1361. 6 1087. 2 45 30 1216. 5 966. 3 1234. 3 1490. 7 1106. 5 1070. 9 15 1198. 7 1281. 3 1461. 5 1574. 8 1702. 3 1817. 3 1820. 7 1715. 4 968. 0 10 1836. 3 5 1836. 6 0 400 600 800 1000 1200 m/z 22 1400 1600 1800
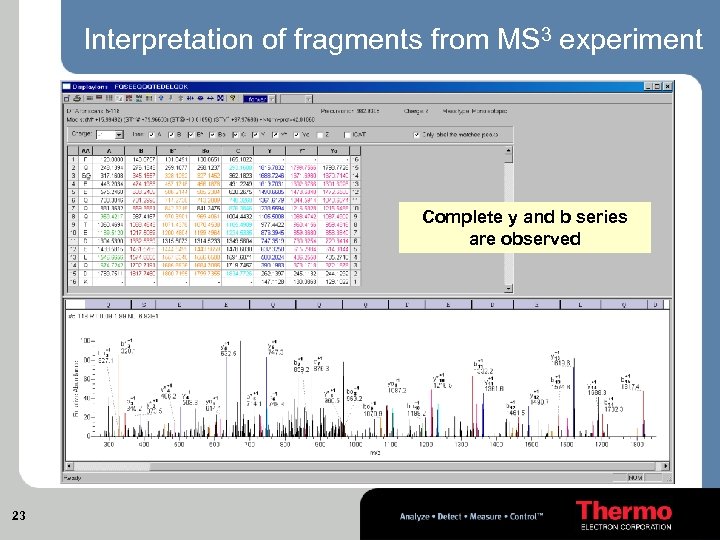
Interpretation of fragments from MS 3 experiment Complete y and b series are observed 23
Outline • Orbitrap mass analyser • Linking orbitrap to linear ion trap • Flexibility of use of LTQ Orbitrap • Focus on: – High resolution and mass accuracy – Sensitivity – Speed – Dynamic range • Conclusion 24
High Resolution & Accurate Mass. . confident ID, PTMs, de novo sequencing, top-down 25
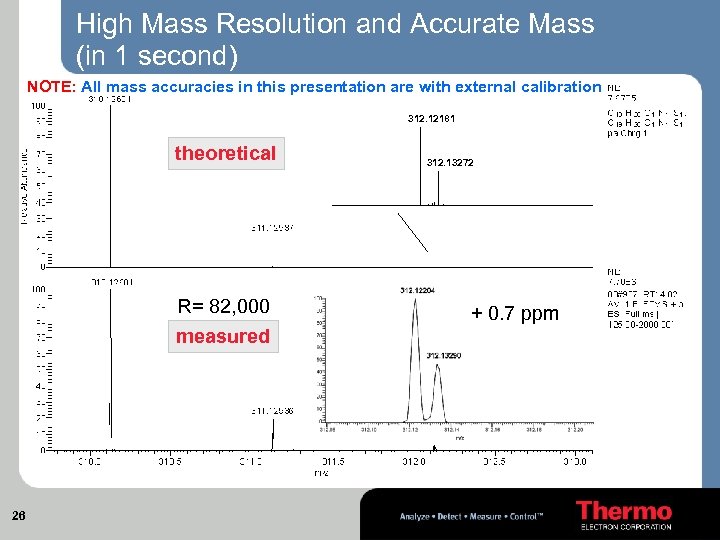
High Mass Resolution and Accurate Mass (in 1 second) NOTE: All mass accuracies in this presentation are with external calibration 312. 12181 theoretical R= 82, 000 measured 26 312. 13272 + 0. 7 ppm
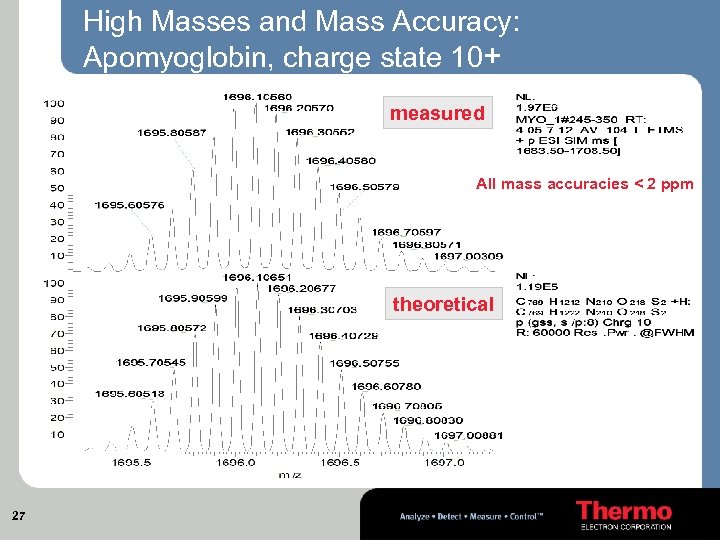
High Masses and Mass Accuracy: Apomyoglobin, charge state 10+ measured All mass accuracies < 2 ppm theoretical 27
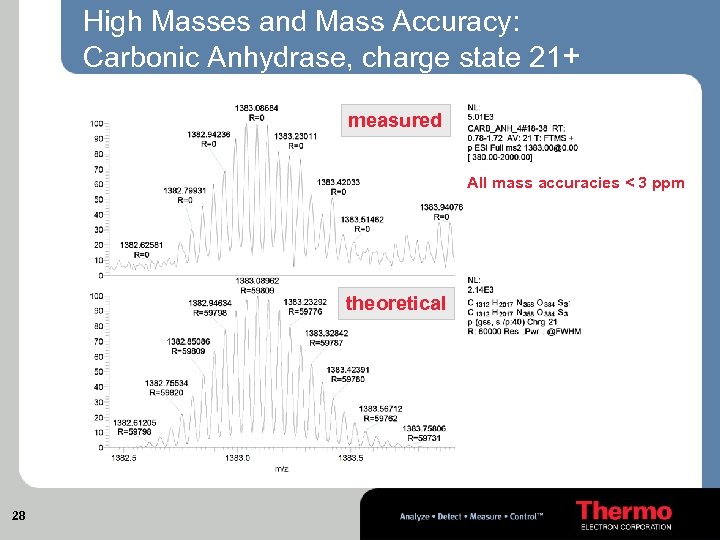
High Masses and Mass Accuracy: Carbonic Anhydrase, charge state 21+ measured All mass accuracies < 3 ppm theoretical 28
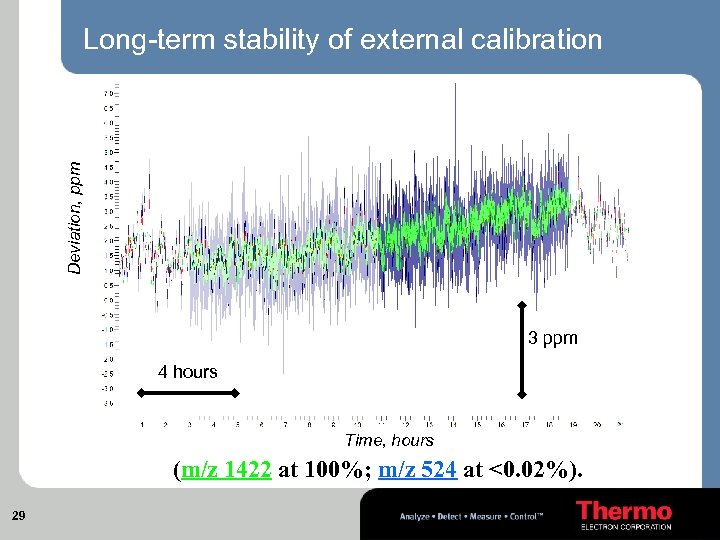
Deviation, ppm Long-term stability of external calibration 3 ppm 4 hours Time, hours (m/z 1422 at 100%; m/z 524 at <0. 02%). 29
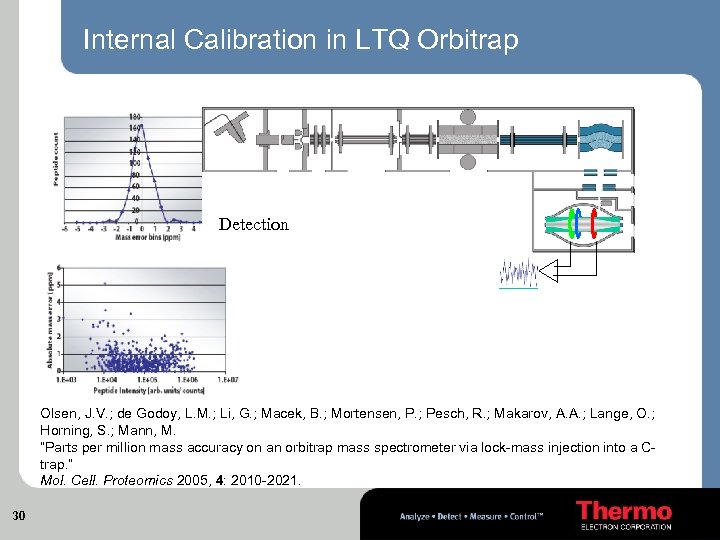
Internal Calibration in LTQ Orbitrap Mixing of ion populations Injection of analyte Injection of the calibrant and ejection Detection Olsen, J. V. ; de Godoy, L. M. ; Li, G. ; Macek, B. ; Mortensen, P. ; Pesch, R. ; Makarov, A. A. ; Lange, O. ; Horning, S. ; Mann, M. “Parts per million mass accuracy on an orbitrap mass spectrometer via lock-mass injection into a Ctrap. ” Mol. Cell. Proteomics 2005, 4: 2010 -2021. 30

Speed. . while delivering accurate mass in MS, MS/MS and MSn 31

Complex Protein Digests: ‘Big 5’ Experiment Digging deep into the baseline for low abundant co-eluting peptides Total time 2. 4 seconds SE 1 Full Scan MS SE 2 MS/MS SE 3 MS/MS SE 4 MS/MS SE 5 MS/MS SE 6 MS/MS 1 LTQ Orbitrap high resolution full scan and 5 fast ion trap MS/MS scans 32 SE denotes a ‘scan event’
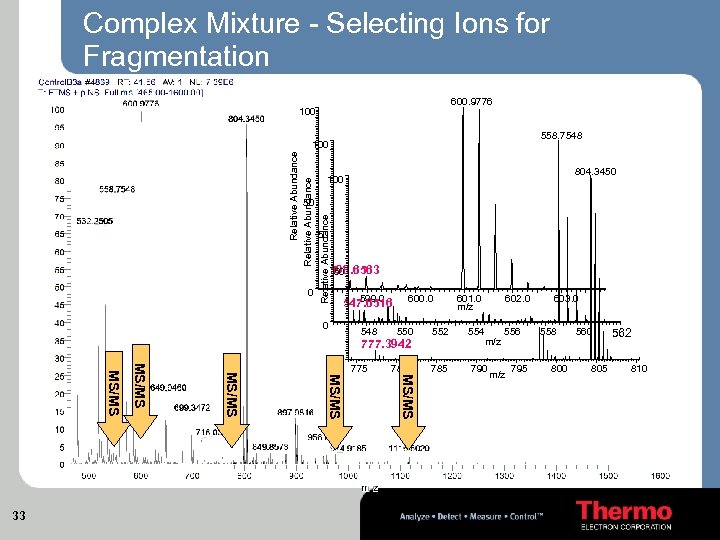
Complex Mixture - Selecting Ions for Fragmentation 600. 9776 100 558. 7548 Relative Abundance 100 804. 3450 100 0 Relative Abundance 50 50 598. 6563 50 599. 0 547. 6516 0 548 600. 0 550 777. 3942 775 780 MS/MS MS/MS 33 0 601. 0 m/z 602. 0 552 554 556 m/z 785 790 m/z 795 603. 0 558 800 560 805 562 810
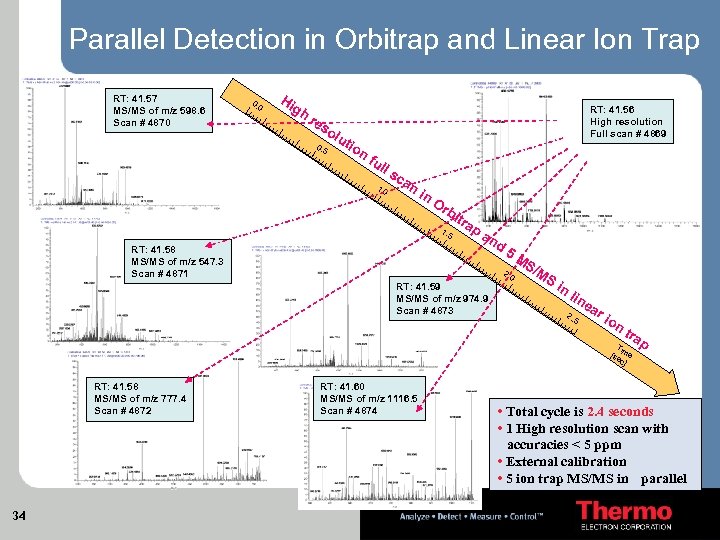
Parallel Detection in Orbitrap and Linear Ion Trap RT: 41. 57 MS/MS of m/z 598. 6 Scan # 4870 0. 0 Hi gh re so 0. 5 lut ion RT: 41. 56 High resolution Full scan # 4869 fu ll s 1. 0 ca n in Or b 1. 5 RT: 41. 58 MS/MS of m/z 547. 3 Scan # 4871 itr ap a nd 5 M 2. 0 RT: 41. 59 MS/MS of m/z 974. 9 Scan # 4873 S/ MS in l ine 2. . 5 ar ion tra Tim [se e c] RT: 41. 58 MS/MS of m/z 777. 4 Scan # 4872 34 RT: 41. 60 MS/MS of m/z 1116. 5 Scan # 4874 p • Total cycle is 2. 4 seconds • 1 High resolution scan with accuracies < 5 ppm • External calibration • 5 ion trap MS/MS in parallel
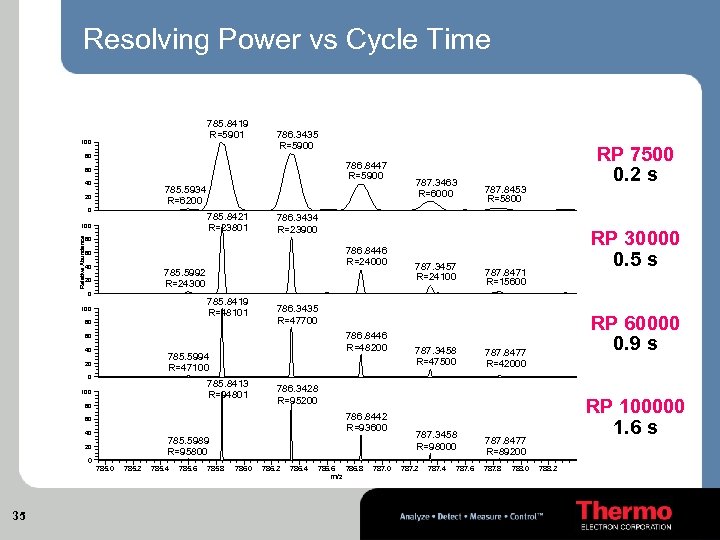
Resolving Power vs Cycle Time 785. 8419 R=5901 100 786. 3435 R=5900 RP 7500 0. 2 s 80 786. 8447 R=5900 60 40 785. 5934 R=6200 20 0 785. 8421 R=23801 Relative Abundance 100 787. 3463 R=6000 787. 8453 R=5800 786. 3434 R=23900 RP 30000 0. 5 s 80 786. 8446 R=24000 60 40 785. 5992 R=24300 20 0 785. 8419 R=48101 100 80 786. 8446 R=48200 785. 5994 R=47100 20 0 785. 8413 R=94801 100 80 786. 8442 R=93600 785. 5989 R=95800 20 0 785. 0 35 785. 2 785. 4 785. 6 785. 8 786. 0 787. 3458 R=47500 RP 60000 0. 9 s 787. 8477 R=42000 786. 3428 R=95200 60 40 787. 8471 R=15600 786. 3435 R=47700 60 40 787. 3457 R=24100 786. 2 786. 4 786. 6 786. 8 m/z 787. 0 787. 3458 R=98000 787. 2 787. 4 787. 6 RP 100000 1. 6 s 787. 8477 R=89200 787. 8 788. 0 788. 2

Sensitivity 36
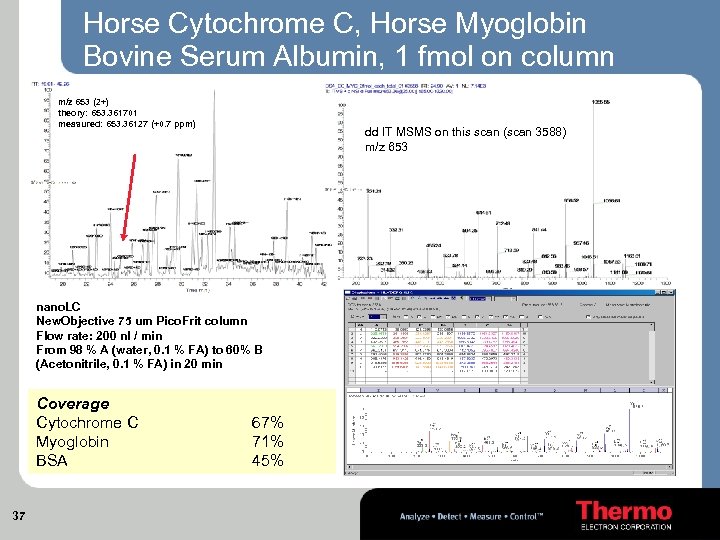
Horse Cytochrome C, Horse Myoglobin Bovine Serum Albumin, 1 fmol on column m/z 653 (2+) theory: 653. 361701 measured: 653. 36127 (+0. 7 ppm) dd IT MSMS on this scan (scan 3588) m/z 653 nano. LC New. Objective 75 um Pico. Frit column Flow rate: 200 nl / min From 98 % A (water, 0. 1 % FA) to 60% B (Acetonitrile, 0. 1 % FA) in 20 min Coverage Cytochrome C Myoglobin BSA 37 67% 71% 45%
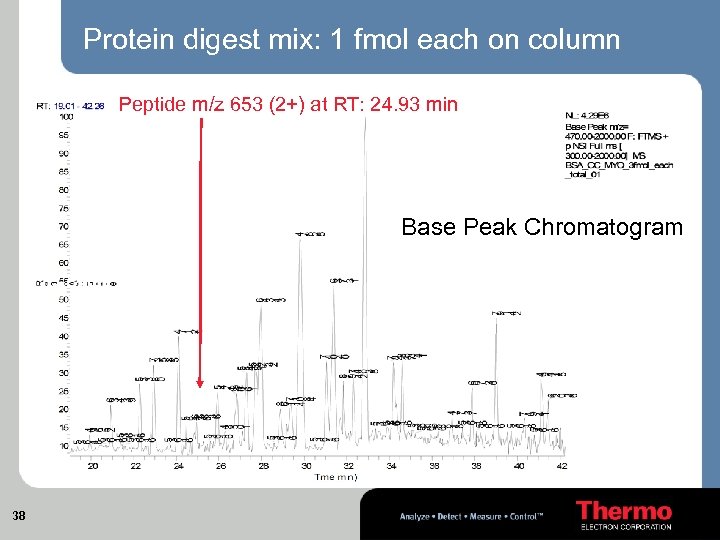
Protein digest mix: 1 fmol each on column Peptide m/z 653 (2+) at RT: 24. 93 min Base Peak Chromatogram 38
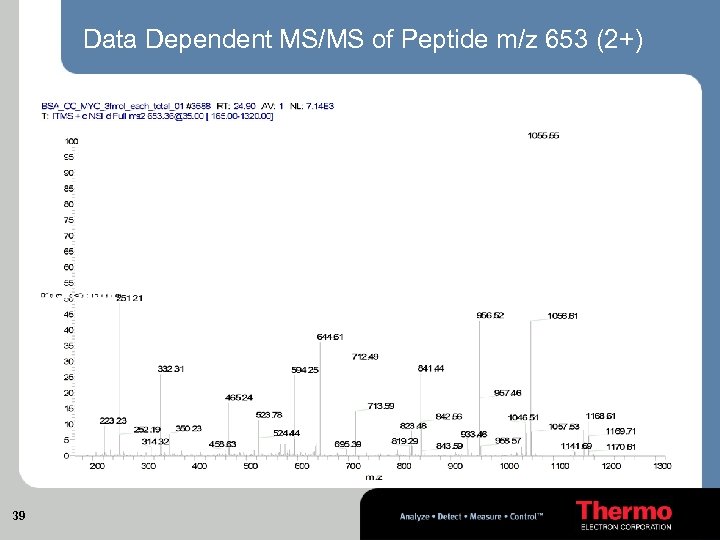
Data Dependent MS/MS of Peptide m/z 653 (2+) 39
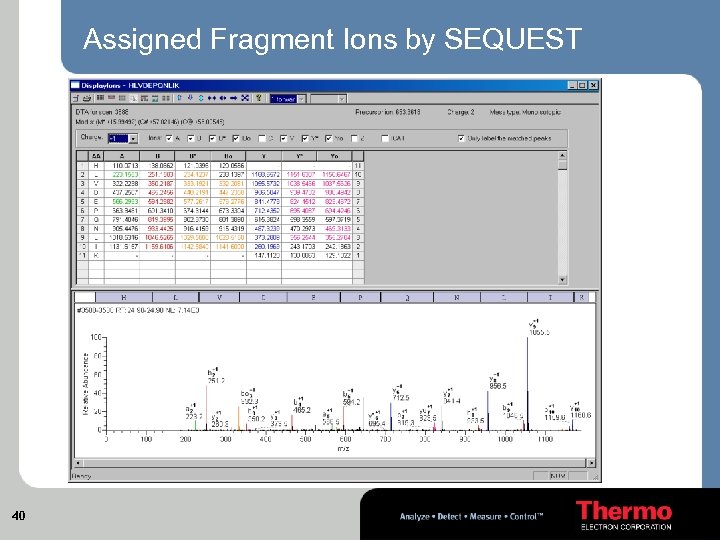
Assigned Fragment Ions by SEQUEST 40
Dynamic Range. . detecting minor components in complex mixtures 41
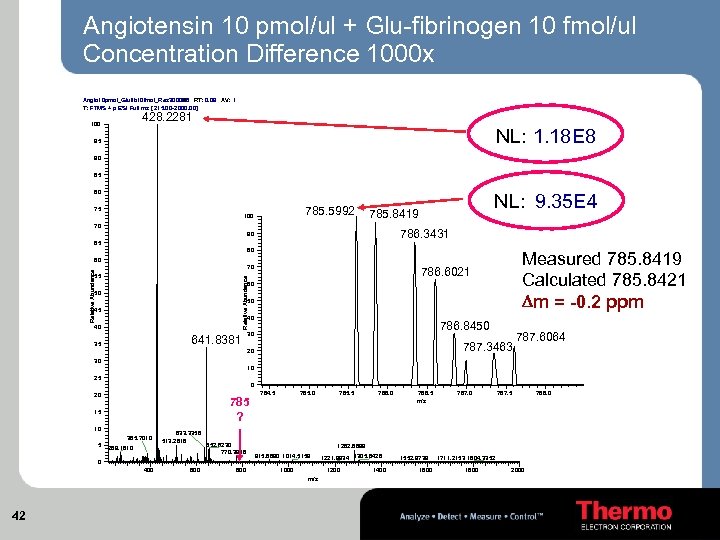
Angiotensin 10 pmol/ul + Glu-fibrinogen 10 fmol/ul Concentration Difference 1000 x Angio 10 pmol_Glufib 10 fmol_Res 30000 RT: 0. 09 AV: 1 #6 T: FTMS + p ESI Full ms [ 215. 00 -2000. 00] 428. 2281 100 NL: 1. 18 E 8 95 90 85 80 785. 5992 75 100 NL: 9. 35 E 4 785. 8419 70 786. 3431 90 65 80 Measured 785. 8419 Calculated 785. 8421 Dm = -0. 2 ppm 60 Relative Abundance 70 786. 6021 Relative Abundance 55 60 50 50 45 40 40 641. 8381 35 786. 8450 30 787. 3463 20 787. 6064 30 10 25 0 20 785 ? 15 10 385. 7010 5 633. 3358 513. 2818 269. 1610 652. 8230 770. 3946 0 400 600 800 784. 5 785. 0 786. 5 m/z 787. 0 787. 5 1282. 6699 915. 6690 1014. 5159 1000 1221. 9934 1305. 6428 1200 m/z 42 785. 5 1400 1552. 9739 1600 1711. 2153 1804. 3352 1800 2000 788. 0
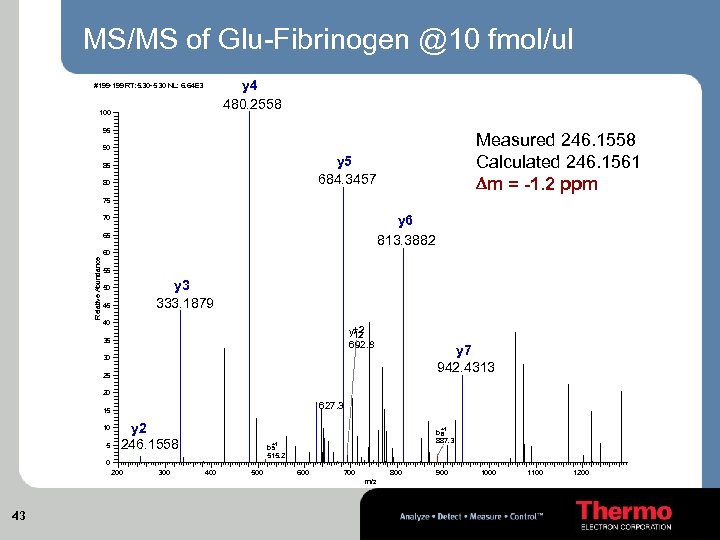
MS/MS of Glu-Fibrinogen @10 fmol/ul y 4 480. 2558 #199 -199 RT: 5. 30 -5. 30 NL: 6. 64 E 3 100 95 Measured 246. 1558 Calculated 246. 1561 Dm = -1. 2 ppm 90 y 5 684. 3457 85 80 75 y 6 813. 3882 70 65 Relative Abundance 60 55 y 3 333. 1879 50 45 40 y+2 12 692. 8 35 y 7 942. 4313 30 25 20 627. 3 15 10 5 y 2 246. 1558 +1 +1 b 5 515. 2 0 200 300 b 8 887. 3 400 500 600 700 800 m/z 43 900 1000 1100 1200
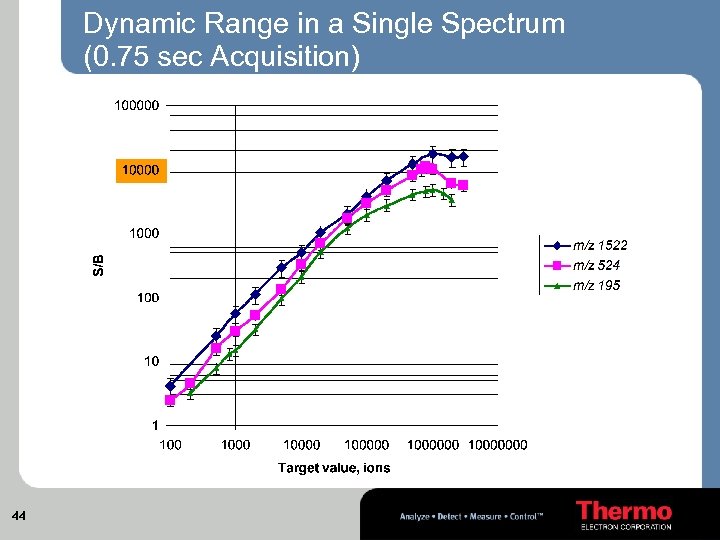
Dynamic Range in a Single Spectrum (0. 75 sec Acquisition) 44
Conclusion • The orbitrap mass analyzer is first fundamentally new mass analyzer introduced commercially in over 20 years – The last novel mass spectrometer introduction was the RF Ion Trap (Finnigan MAT) in the early 1980’s • The main advantages of the orbitrap mass analyzer are: – Unsurpassed dynamic range of mass accuracy – High resolution – High sensitivity – High stability – Compact package – Maintenance-free • The LTQ Orbitrap is the first implementation of the orbitrap analyzer in a hybrid instrument – Isolation, fragmentation and MSn is provided mainly by the linear trap – The C-trap supports multiple ion fills, CID and future expansion – The orbitrap is and will be used as a detector 45

About the Authors Dr. Alexander Makarov The inventor of orbitrap mass analyser Research Manager at Thermo Electron in Bremen Dr. Michaela Scigelova LC/MS application expert at Thermo Electron in UK 46
7b275f0658bed8afe7367e29617fc420.ppt