349bab4ed39676967e040ca536b6bc83.ppt
- Количество слайдов: 22

Orbital energies
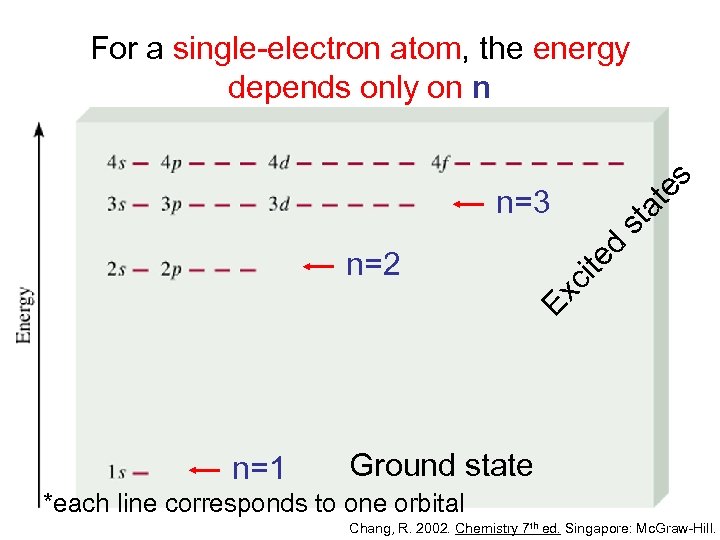
es For a single-electron atom, the energy depends only on n d st at n=3 Ex ci te n=2 n=1 Ground state *each line corresponds to one orbital Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
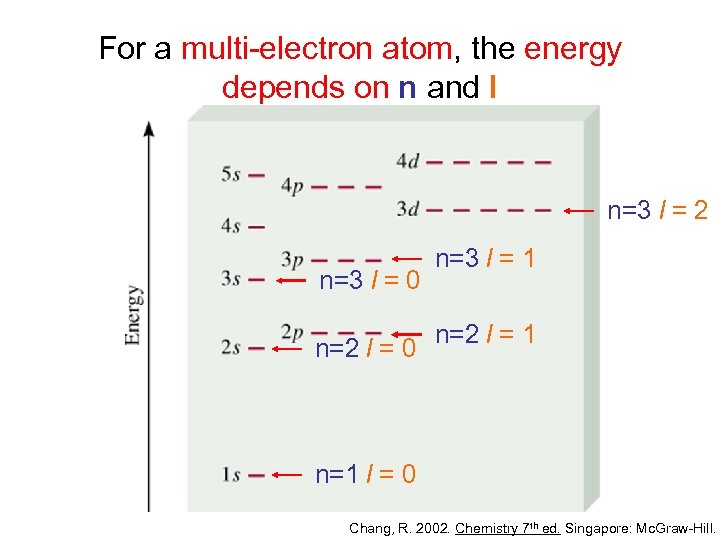
For a multi-electron atom, the energy depends on n and l n=3 l = 2 n=3 l = 0 n=2 l = 0 n=3 l = 1 n=2 l = 1 n=1 l = 0 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Electron configuration
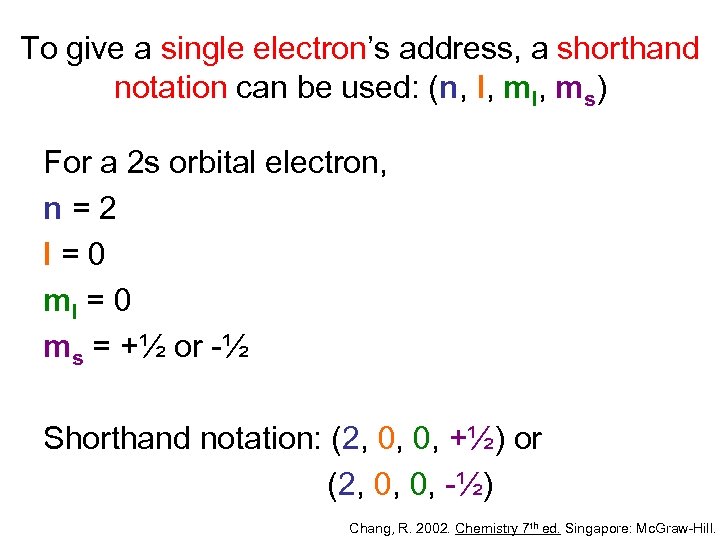
To give a single electron’s address, a shorthand notation can be used: (n, l, ms) For a 2 s orbital electron, n=2 l=0 ml = 0 ms = +½ or -½ Shorthand notation: (2, 0, 0, +½) or (2, 0, 0, -½) Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
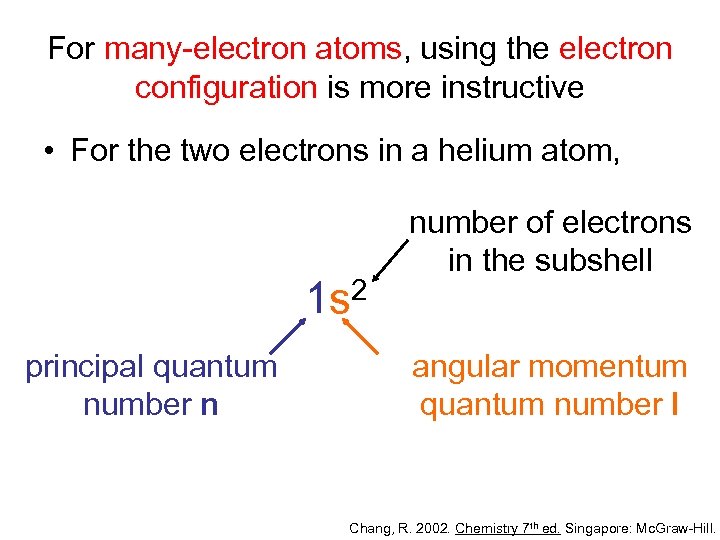
For many-electron atoms, using the electron configuration is more instructive • For the two electrons in a helium atom, 2 1 s principal quantum number n number of electrons in the subshell angular momentum quantum number l Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
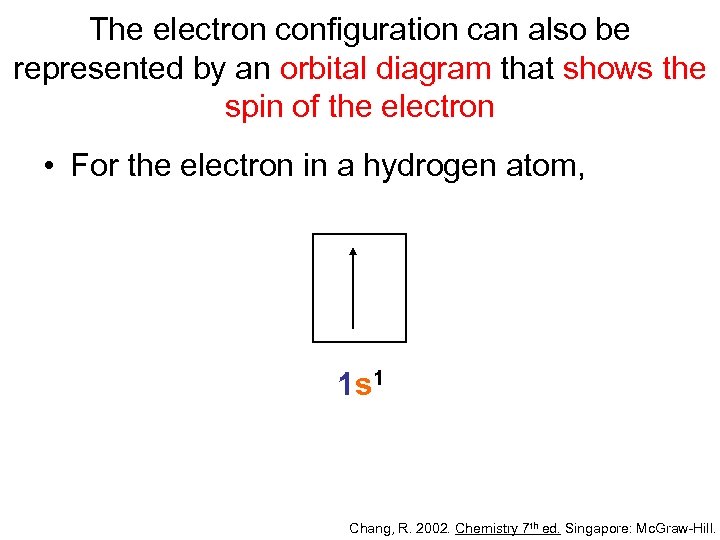
The electron configuration can also be represented by an orbital diagram that shows the spin of the electron • For the electron in a hydrogen atom, 1 s 1 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
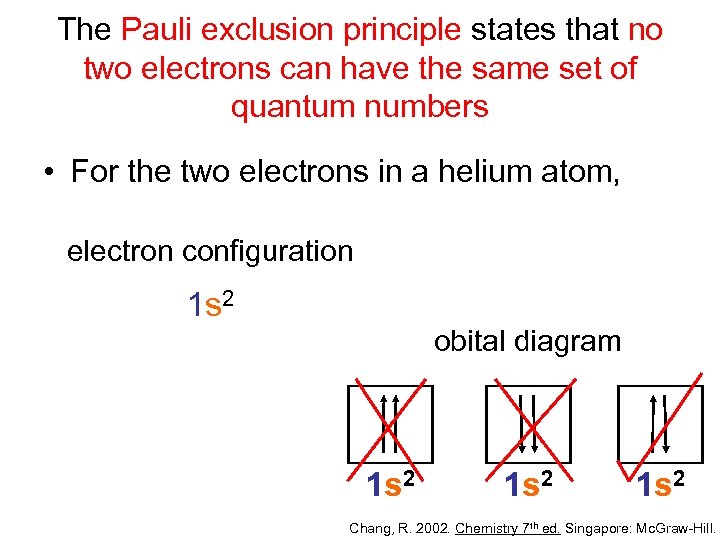
The Pauli exclusion principle states that no two electrons can have the same set of quantum numbers • For the two electrons in a helium atom, electron configuration 1 s 2 obital diagram 1 s 2 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
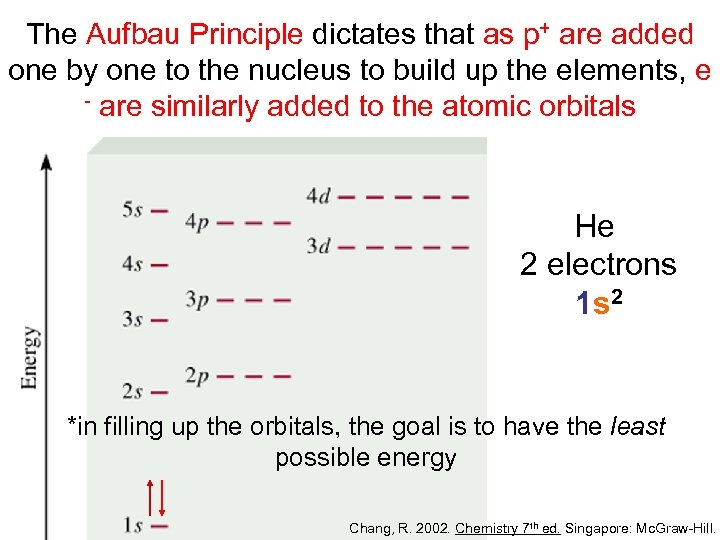
The Aufbau Principle dictates that as p+ are added one by one to the nucleus to build up the elements, e - are similarly added to the atomic orbitals He 2 electrons 1 s 2 *in filling up the orbitals, the goal is to have the least possible energy Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
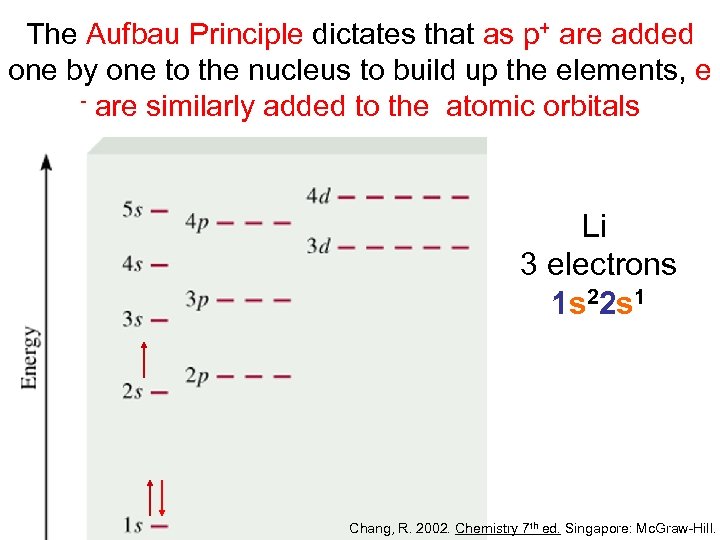
The Aufbau Principle dictates that as p+ are added one by one to the nucleus to build up the elements, e - are similarly added to the atomic orbitals Li 3 electrons 1 s 22 s 1 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
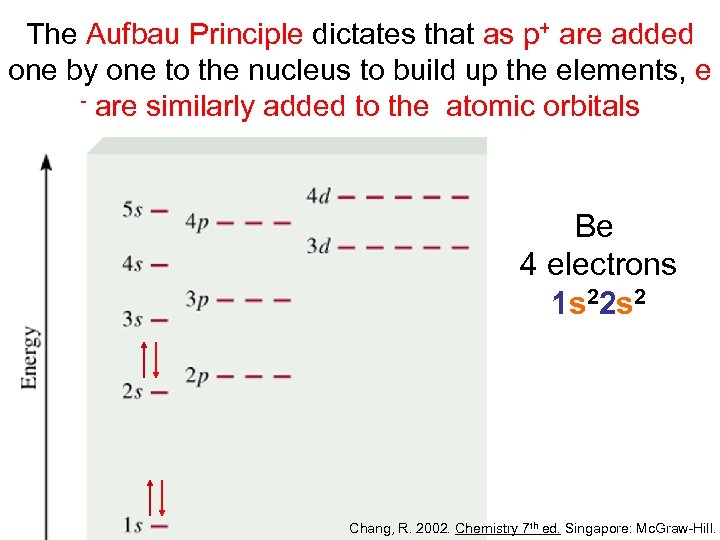
The Aufbau Principle dictates that as p+ are added one by one to the nucleus to build up the elements, e - are similarly added to the atomic orbitals Be 4 electrons 1 s 22 s 2 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
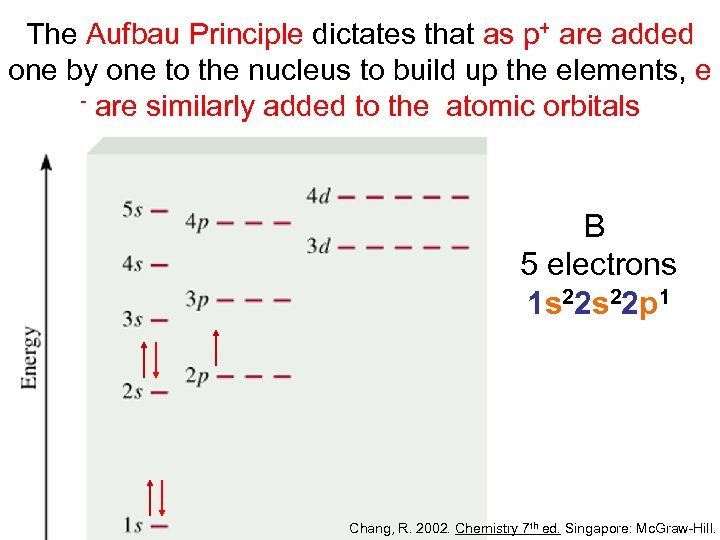
The Aufbau Principle dictates that as p+ are added one by one to the nucleus to build up the elements, e - are similarly added to the atomic orbitals B 5 electrons 1 s 22 p 1 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
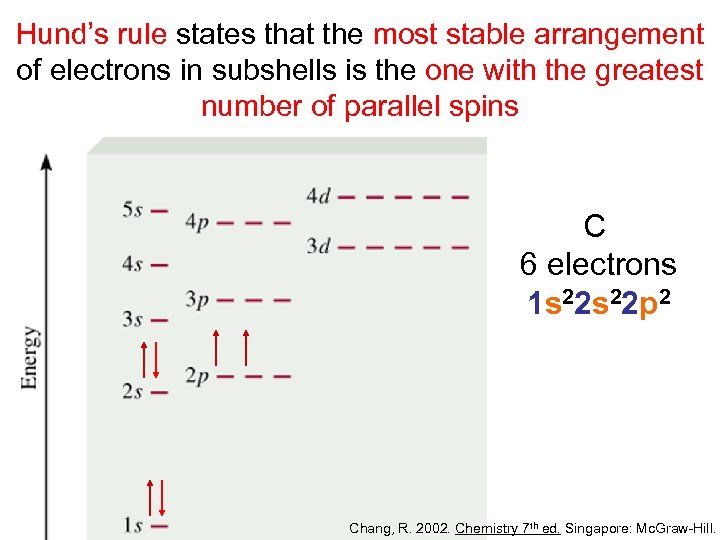
Hund’s rule states that the most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins C 6 electrons 1 s 22 p 2 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
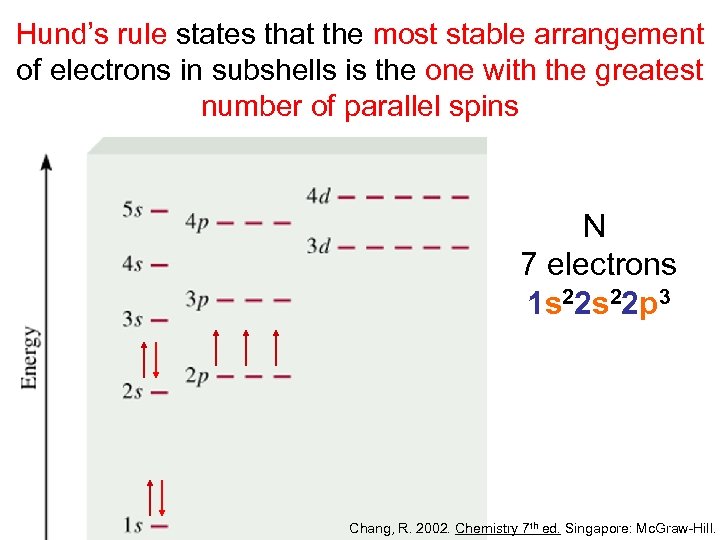
Hund’s rule states that the most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins N 7 electrons 1 s 22 p 3 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
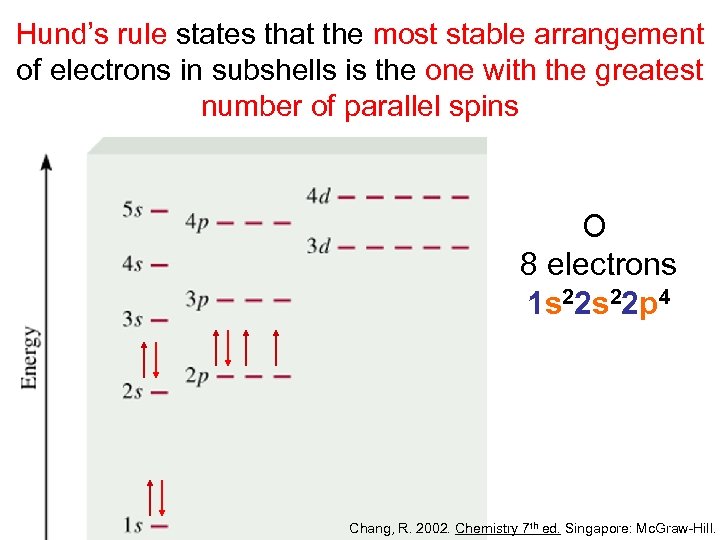
Hund’s rule states that the most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins O 8 electrons 1 s 22 p 4 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
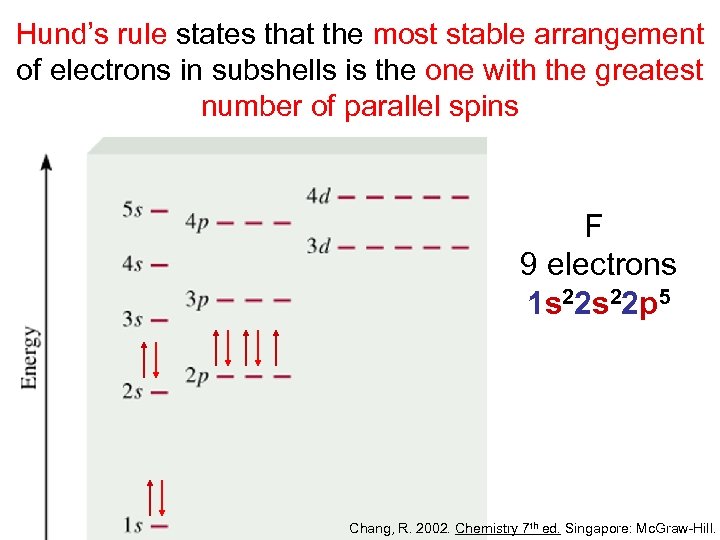
Hund’s rule states that the most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins F 9 electrons 1 s 22 p 5 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
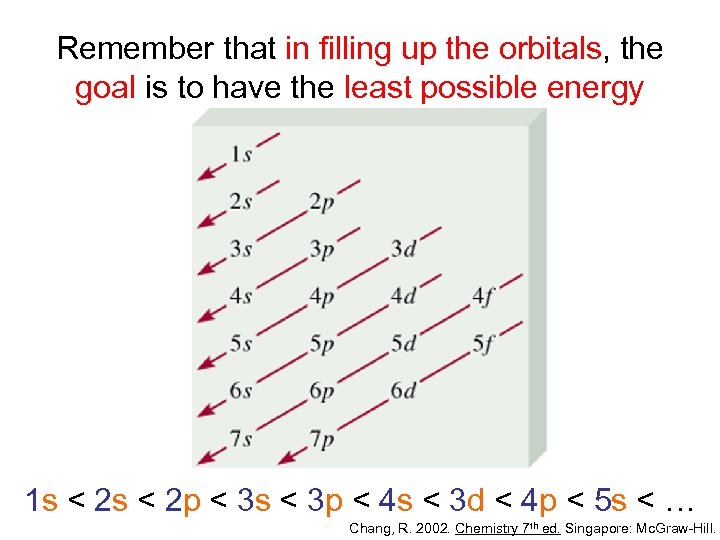
Remember that in filling up the orbitals, the goal is to have the least possible energy 1 s < 2 p < 3 s < 3 p < 4 s < 3 d < 4 p < 5 s < … Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
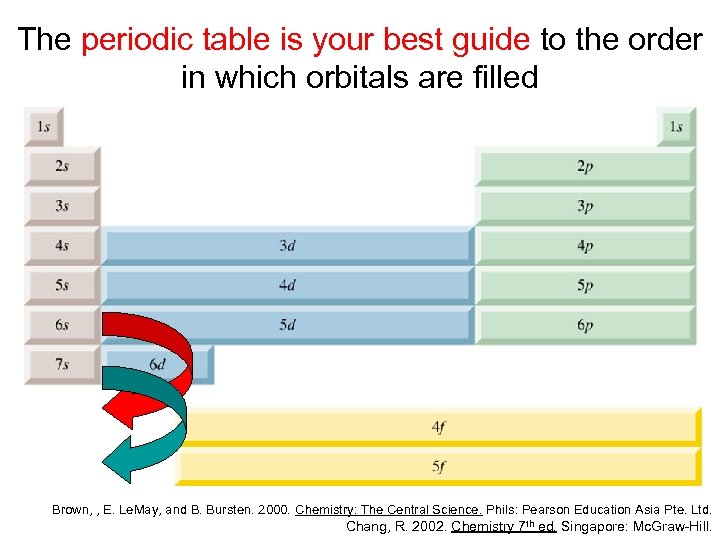
The periodic table is your best guide to the order in which orbitals are filled Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.

Draw the orbital diagram and write the electron configuration for the following • Ne • Na
![The abbreviated electron configuration: [noble gas that nearly precedes the element]outer e- Noble gases The abbreviated electron configuration: [noble gas that nearly precedes the element]outer e- Noble gases](https://present5.com/presentation/349bab4ed39676967e040ca536b6bc83/image-20.jpg)
The abbreviated electron configuration: [noble gas that nearly precedes the element]outer e- Noble gases
![The abbreviated electron configuration: [noble gas that nearly precedes the element]outer e- [Ne]3 s The abbreviated electron configuration: [noble gas that nearly precedes the element]outer e- [Ne]3 s](https://present5.com/presentation/349bab4ed39676967e040ca536b6bc83/image-21.jpg)
The abbreviated electron configuration: [noble gas that nearly precedes the element]outer e- [Ne]3 s 23 p 3
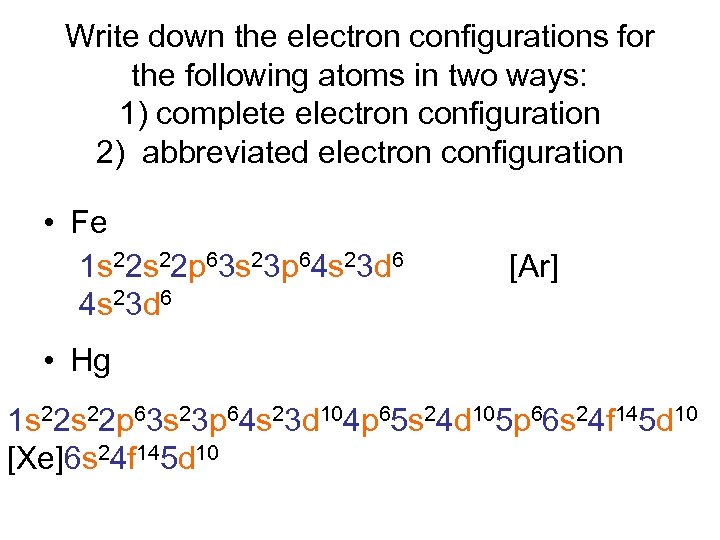
Write down the electron configurations for the following atoms in two ways: 1) complete electron configuration 2) abbreviated electron configuration • Fe 1 s 22 p 63 s 23 p 64 s 23 d 6 [Ar] • Hg 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 10 [Xe]6 s 24 f 145 d 10
349bab4ed39676967e040ca536b6bc83.ppt