4fee071e8c03e32ac3217785e432454b.ppt
- Количество слайдов: 59
 Options for the Control of Influenza VI June 17 -23, 2007 Toronto, Ontario, Canada Conference Summary
Options for the Control of Influenza VI June 17 -23, 2007 Toronto, Ontario, Canada Conference Summary
 Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
 Global surveillance efforts u Varies widely, resources an issue l Africa: emerging programs l Asia: some seasonal surveillance, focus on avian l Oceania: little information presented l Europe: significant national and EU efforts l Latin America: increasing number of programs l US & Canada: significant government, military (US) programs l International: WHO, collaborative groups
Global surveillance efforts u Varies widely, resources an issue l Africa: emerging programs l Asia: some seasonal surveillance, focus on avian l Oceania: little information presented l Europe: significant national and EU efforts l Latin America: increasing number of programs l US & Canada: significant government, military (US) programs l International: WHO, collaborative groups
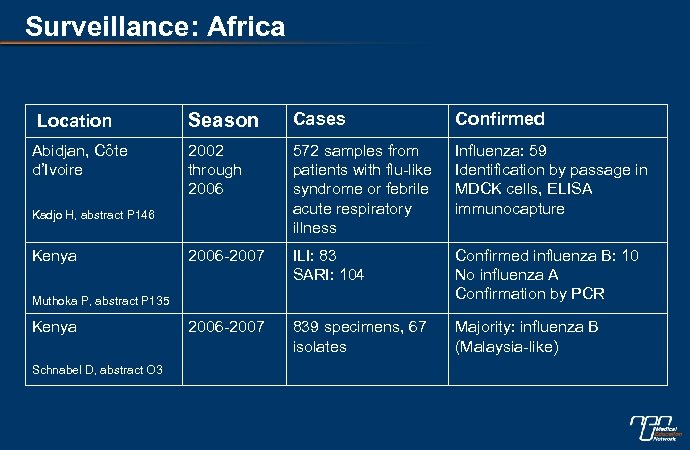 Surveillance: Africa Location Abidjan, Côte d’Ivoire Season Cases Confirmed 2002 through 2006 572 samples from patients with flu-like syndrome or febrile acute respiratory illness Influenza: 59 Identification by passage in MDCK cells, ELISA immunocapture 2006 -2007 ILI: 83 SARI: 104 Confirmed influenza B: 10 No influenza A Confirmation by PCR 2006 -2007 839 specimens, 67 isolates Majority: influenza B (Malaysia-like) Kadjo H, abstract P 146 Kenya Muthoka P, abstract P 135 Kenya Schnabel D, abstract O 3
Surveillance: Africa Location Abidjan, Côte d’Ivoire Season Cases Confirmed 2002 through 2006 572 samples from patients with flu-like syndrome or febrile acute respiratory illness Influenza: 59 Identification by passage in MDCK cells, ELISA immunocapture 2006 -2007 ILI: 83 SARI: 104 Confirmed influenza B: 10 No influenza A Confirmation by PCR 2006 -2007 839 specimens, 67 isolates Majority: influenza B (Malaysia-like) Kadjo H, abstract P 146 Kenya Muthoka P, abstract P 135 Kenya Schnabel D, abstract O 3
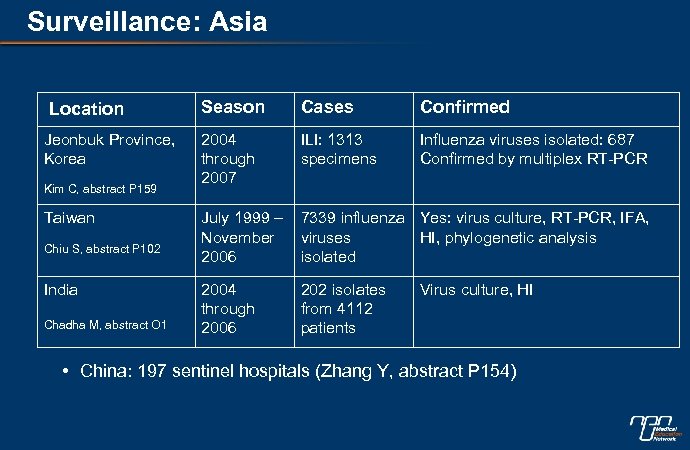 Surveillance: Asia Location Jeonbuk Province, Korea Kim C, abstract P 159 Taiwan Chiu S, abstract P 102 India Chadha M, abstract O 1 Season Cases Confirmed 2004 through 2007 ILI: 1313 specimens Influenza viruses isolated: 687 Confirmed by multiplex RT-PCR July 1999 – November 2006 7339 influenza Yes: virus culture, RT-PCR, IFA, viruses HI, phylogenetic analysis isolated 2004 through 2006 202 isolates from 4112 patients Virus culture, HI • China: 197 sentinel hospitals (Zhang Y, abstract P 154)
Surveillance: Asia Location Jeonbuk Province, Korea Kim C, abstract P 159 Taiwan Chiu S, abstract P 102 India Chadha M, abstract O 1 Season Cases Confirmed 2004 through 2007 ILI: 1313 specimens Influenza viruses isolated: 687 Confirmed by multiplex RT-PCR July 1999 – November 2006 7339 influenza Yes: virus culture, RT-PCR, IFA, viruses HI, phylogenetic analysis isolated 2004 through 2006 202 isolates from 4112 patients Virus culture, HI • China: 197 sentinel hospitals (Zhang Y, abstract P 154)
 Surveillance: Europe Location England Hayward A, abstract P 168 Poland Romanowska M, abstract P 107 Season Cases Confirmed October ILI: 253 nasal 2006 -March swabs 2007 3 of 11 analyzed (PCR) swabs were positive for influenza Remainder still to be processed 2004 -2005 -2006 -2007 Influenza: 63 (21%) Influenza: 47 (5%) All respiratory infections: 27 (analysis in progress) ILI cases: 399 ILI cases: 949 ILI cases: 650 • Established sentinel networks in Sweden (Andersson E, abstract P 136), Portugal (Gonçalves P, abstract P 147), France (Mosnier A, abstract P 166) • Evidence of west-to-east spread through season (W Paget, abstract O 4) • Peaks: no link to prior cold weather (Mangtani P, abstract P 111)
Surveillance: Europe Location England Hayward A, abstract P 168 Poland Romanowska M, abstract P 107 Season Cases Confirmed October ILI: 253 nasal 2006 -March swabs 2007 3 of 11 analyzed (PCR) swabs were positive for influenza Remainder still to be processed 2004 -2005 -2006 -2007 Influenza: 63 (21%) Influenza: 47 (5%) All respiratory infections: 27 (analysis in progress) ILI cases: 399 ILI cases: 949 ILI cases: 650 • Established sentinel networks in Sweden (Andersson E, abstract P 136), Portugal (Gonçalves P, abstract P 147), France (Mosnier A, abstract P 166) • Evidence of west-to-east spread through season (W Paget, abstract O 4) • Peaks: no link to prior cold weather (Mangtani P, abstract P 111)
 Surveillance: Latin America u Argentina: National Influenza Centre Network 1 l Important peak in winter everywhere; additional summer/autumn peaks @ extreme latitudes u Brazil: l National Influenza Surveillance Network: 958 samples collected in 2006 season, virus strain surveillance 2 l Single-centre ILI cases 2006 -2007: 115913 l Climate analysis: spread associated with rainfall in equatorial areas, low temperatures in other areas 4 u Cuba: l Laboratory surveillance of circulating strains 5 1. Savy V. abstract P 117, Options VI, 2007. 2. Paiva T. abstract P 119, Options VI, 2007. 3. Cintra O. abstract P 169, Options VI, 2007. 4. Alonso W. abstract P 171, Options VI, 2007. 5. Acosta B. abstract P 153, Options VI, 2007.
Surveillance: Latin America u Argentina: National Influenza Centre Network 1 l Important peak in winter everywhere; additional summer/autumn peaks @ extreme latitudes u Brazil: l National Influenza Surveillance Network: 958 samples collected in 2006 season, virus strain surveillance 2 l Single-centre ILI cases 2006 -2007: 115913 l Climate analysis: spread associated with rainfall in equatorial areas, low temperatures in other areas 4 u Cuba: l Laboratory surveillance of circulating strains 5 1. Savy V. abstract P 117, Options VI, 2007. 2. Paiva T. abstract P 119, Options VI, 2007. 3. Cintra O. abstract P 169, Options VI, 2007. 4. Alonso W. abstract P 171, Options VI, 2007. 5. Acosta B. abstract P 153, Options VI, 2007.
 Surveillance: US u CDC: 122 -City Mortality Reporting System 1 l Provides early data on influenza mortality l Reported area of jurisdiction covers ~69 million people (23. 2% of US population) u CDC: Sentinel Provider Surveillance Network 2 l ~2500 participating physicians, weekly reports of ILI cases l High correlation between ILI reports and WHO lab isolates l Regional differences currently being addressed 1. Blanton L. abstract P 118, Options VI, 2007. 2. Johnson A. abstract P 132, Options VI, 2007.
Surveillance: US u CDC: 122 -City Mortality Reporting System 1 l Provides early data on influenza mortality l Reported area of jurisdiction covers ~69 million people (23. 2% of US population) u CDC: Sentinel Provider Surveillance Network 2 l ~2500 participating physicians, weekly reports of ILI cases l High correlation between ILI reports and WHO lab isolates l Regional differences currently being addressed 1. Blanton L. abstract P 118, Options VI, 2007. 2. Johnson A. abstract P 132, Options VI, 2007.
 Surveillance: Canada u Flu. Watch programme: analysis of 11 years of data 1 l Comprehensive system for timely surveillance, in line with other international efforts l Lacks real-time severity indicators of adult hospitalizations, mortality u Web-based model for real-time electronic reporting 2 l Currently being piloted in Atlantic Canada u IMPACT paediatric surveillance programme: 3 Season Influenza-related admissions 2003 -2004 505 2004 -2005 391 2005 -2006 374 2006 -2007 140 (to Feb 24)
Surveillance: Canada u Flu. Watch programme: analysis of 11 years of data 1 l Comprehensive system for timely surveillance, in line with other international efforts l Lacks real-time severity indicators of adult hospitalizations, mortality u Web-based model for real-time electronic reporting 2 l Currently being piloted in Atlantic Canada u IMPACT paediatric surveillance programme: 3 Season Influenza-related admissions 2003 -2004 505 2004 -2005 391 2005 -2006 374 2006 -2007 140 (to Feb 24)
 Surveillance: international efforts u u US Naval Medical Research Unit 3: eastern Mediterranean, Africa, eastern Europe, central Asia 1 WHO global network: determination of vaccine strains 2 l Expansion of network in collaboration w/ CDC u CDC: general guidelines for seasonal surveillance & early pandemic detection 3 l Fills gaps in WHO approach re: pandemic detection u Asian group: surveillance of online news sources 4 l Development of downloadable, searchable “intelligent” Webbased surveillance system 1. Soliman A. abstract P 122, Options VI, 2007. 2. Daves S. abstract P 145, Options VI, 2007. 3. Ortiz J. abstract P 128, Options VI, 2007. 4. Collier N. abstract P 157, Options VI, 2007.
Surveillance: international efforts u u US Naval Medical Research Unit 3: eastern Mediterranean, Africa, eastern Europe, central Asia 1 WHO global network: determination of vaccine strains 2 l Expansion of network in collaboration w/ CDC u CDC: general guidelines for seasonal surveillance & early pandemic detection 3 l Fills gaps in WHO approach re: pandemic detection u Asian group: surveillance of online news sources 4 l Development of downloadable, searchable “intelligent” Webbased surveillance system 1. Soliman A. abstract P 122, Options VI, 2007. 2. Daves S. abstract P 145, Options VI, 2007. 3. Ortiz J. abstract P 128, Options VI, 2007. 4. Collier N. abstract P 157, Options VI, 2007.
 Surveillance: children & families Location Data England & Wales 40 years of incidence u. No consistent time lags between ILI data on children as peaks in children & others drivers of community u. Bronchitis always peaked in children ILI & bronchitis spread before elderly Elliot A, abstract P 124 Leicester, UK Democratis J, abstract P 123 Japan Hirotsu N, abstract O 5 Questionnaire: families of 35 children with confirmed influenza Results u 35% of adult, 63% of child household contacts experienced ~2 days of ILI u 35% of households: parental time off work to care for ill child (mean 1. 7 days) Analysis of 1609 u. Most common index cases: age 0 -6 influenza patients from u. Influenza A: commonly passed from 1234 families children (any age) to mother, younger sib u. Influenza B: transmitted from children 04, to wider spread of age ranges
Surveillance: children & families Location Data England & Wales 40 years of incidence u. No consistent time lags between ILI data on children as peaks in children & others drivers of community u. Bronchitis always peaked in children ILI & bronchitis spread before elderly Elliot A, abstract P 124 Leicester, UK Democratis J, abstract P 123 Japan Hirotsu N, abstract O 5 Questionnaire: families of 35 children with confirmed influenza Results u 35% of adult, 63% of child household contacts experienced ~2 days of ILI u 35% of households: parental time off work to care for ill child (mean 1. 7 days) Analysis of 1609 u. Most common index cases: age 0 -6 influenza patients from u. Influenza A: commonly passed from 1234 families children (any age) to mother, younger sib u. Influenza B: transmitted from children 04, to wider spread of age ranges
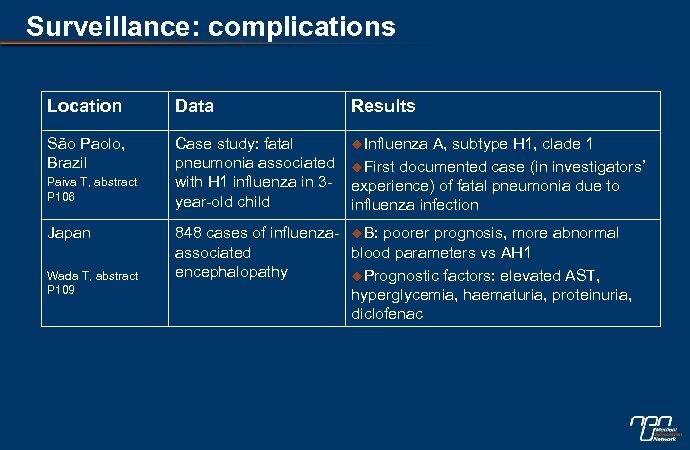 Surveillance: complications Location Data Results São Paolo, Brazil Case study: fatal pneumonia associated with H 1 influenza in 3 year-old child u. Influenza Paiva T, abstract P 106 Japan Wada T, abstract P 109 A, subtype H 1, clade 1 u. First documented case (in investigators’ experience) of fatal pneumonia due to influenza infection 848 cases of influenza- u. B: poorer prognosis, more abnormal associated blood parameters vs AH 1 encephalopathy u. Prognostic factors: elevated AST, hyperglycemia, haematuria, proteinuria, diclofenac
Surveillance: complications Location Data Results São Paolo, Brazil Case study: fatal pneumonia associated with H 1 influenza in 3 year-old child u. Influenza Paiva T, abstract P 106 Japan Wada T, abstract P 109 A, subtype H 1, clade 1 u. First documented case (in investigators’ experience) of fatal pneumonia due to influenza infection 848 cases of influenza- u. B: poorer prognosis, more abnormal associated blood parameters vs AH 1 encephalopathy u. Prognostic factors: elevated AST, hyperglycemia, haematuria, proteinuria, diclofenac
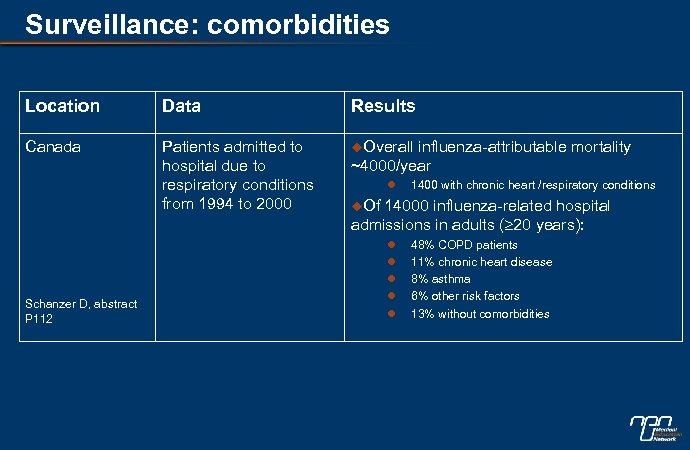 Surveillance: comorbidities Location Data Results Canada Patients admitted to hospital due to respiratory conditions from 1994 to 2000 u. Overall Schanzer D, abstract P 112 influenza-attributable mortality ~4000/year l 1400 with chronic heart /respiratory conditions u. Of 14000 influenza-related hospital admissions in adults ( 20 years): l l l 48% COPD patients 11% chronic heart disease 8% asthma 6% other risk factors 13% without comorbidities
Surveillance: comorbidities Location Data Results Canada Patients admitted to hospital due to respiratory conditions from 1994 to 2000 u. Overall Schanzer D, abstract P 112 influenza-attributable mortality ~4000/year l 1400 with chronic heart /respiratory conditions u. Of 14000 influenza-related hospital admissions in adults ( 20 years): l l l 48% COPD patients 11% chronic heart disease 8% asthma 6% other risk factors 13% without comorbidities
 Mathematical models: influenza spread and intervention u u D Smith (Cambridge): models must be questioned & tested 1 D Shay (CDC, USA): comparison of excess mortality modeling methods 2 l All yielded similar (22000 to 34900 deaths) estimates, risk-difference w/ summer baseline higher l Estimates varied with age group, viral type/subtype u u L Denoeud (France): validation of morbidity as mortality predictor 3 N Ferguson (UK): current models of pandemic control 4 l l l Containment: requires early detection, antiviral stockpiles Treatment: must be fast (12 -24 h) to reduce transmission Travel restrictions: would only buy time, not contain Social distancing: difficult to measure or enforce Combination of strategies: could reduce attack rate by 75% 1. Smith D. TS 5, Options VI, 2007. 2. Shay D. abstract O 7, Options VI, 2007. 3. Denoeud L. abstract P 115, Options VI, 2007 4. Ferguson N. TS 5, Options VI, 2007.
Mathematical models: influenza spread and intervention u u D Smith (Cambridge): models must be questioned & tested 1 D Shay (CDC, USA): comparison of excess mortality modeling methods 2 l All yielded similar (22000 to 34900 deaths) estimates, risk-difference w/ summer baseline higher l Estimates varied with age group, viral type/subtype u u L Denoeud (France): validation of morbidity as mortality predictor 3 N Ferguson (UK): current models of pandemic control 4 l l l Containment: requires early detection, antiviral stockpiles Treatment: must be fast (12 -24 h) to reduce transmission Travel restrictions: would only buy time, not contain Social distancing: difficult to measure or enforce Combination of strategies: could reduce attack rate by 75% 1. Smith D. TS 5, Options VI, 2007. 2. Shay D. abstract O 7, Options VI, 2007. 3. Denoeud L. abstract P 115, Options VI, 2007 4. Ferguson N. TS 5, Options VI, 2007.
 Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
 Pathogenesis: seasonal influenza u Seasonal factors affecting transmission (guinea pigs)1 l varies with humidity (highest at 20 -35%) and temperature (highest at 5 C) u PB 1 -F 2 protein and pneumonia (mice)2 l “ 1918” version of protein associated with: u increased virulence u heightened immunopathology u priming for secondary bacterial pneumonia 1. Lowen A. abstract O 91, Options VI, 2007. 2. Mc. Auley J. abstract O 89, Options VI, 2007.
Pathogenesis: seasonal influenza u Seasonal factors affecting transmission (guinea pigs)1 l varies with humidity (highest at 20 -35%) and temperature (highest at 5 C) u PB 1 -F 2 protein and pneumonia (mice)2 l “ 1918” version of protein associated with: u increased virulence u heightened immunopathology u priming for secondary bacterial pneumonia 1. Lowen A. abstract O 91, Options VI, 2007. 2. Mc. Auley J. abstract O 89, Options VI, 2007.
 Pathogenesis: pandemic-potential influenza u Viral polymerase impact on virulence (mice, ferrets)1 l swapping polymerase gene from non-lethal CH 58 into VN 1203 attenuated virulence in ferrets and mice l inhibition of polymerase by Mx 1 may protect vs death u NS 1 protein C-terminus and virulence (mice)2 l 4 -aa truncation abolishes plaque formation l “avian-like” sequences most virulent u HPAI H 5 N 1 and interferon response (cell culture)3 l HP virus associated with reduced & delayed IFN induction, decreased expression of IFN-stimulated genes 1. Salomon R. abstract O 92, Options VI, 2007. 2. Jackson D. abstract O 90, Options VI, 2007. 3. Zeng H, abstract O 93, Options VI, 2007.
Pathogenesis: pandemic-potential influenza u Viral polymerase impact on virulence (mice, ferrets)1 l swapping polymerase gene from non-lethal CH 58 into VN 1203 attenuated virulence in ferrets and mice l inhibition of polymerase by Mx 1 may protect vs death u NS 1 protein C-terminus and virulence (mice)2 l 4 -aa truncation abolishes plaque formation l “avian-like” sequences most virulent u HPAI H 5 N 1 and interferon response (cell culture)3 l HP virus associated with reduced & delayed IFN induction, decreased expression of IFN-stimulated genes 1. Salomon R. abstract O 92, Options VI, 2007. 2. Jackson D. abstract O 90, Options VI, 2007. 3. Zeng H, abstract O 93, Options VI, 2007.
 Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
 Seasonal influenza vaccines: pre-clinical evaluation u Solvay: new cell-derived products l Qualification of MDCK cells as safe vaccine production system 1 u u u Risk assessment Elimination of residual cellular DNA (DNAse treatment) MDCK cells are as safe as other cell lines l Pre-clinical validation of Grippol TC adjuvanted cell-derived (MDCK) vaccine 2 u u u Novel adjuvant: polyoxidonium Sterile cell-derived antigens, immunogenic at 3 -fold lower levels than split or subunit vaccines Pre-clinical validation complete, clinical trials to begin soon 1. Kersten A. abstract P 1404, Options VI, 2007. 2. Nekrasov A. abstract P 1433, Options VI, 2007.
Seasonal influenza vaccines: pre-clinical evaluation u Solvay: new cell-derived products l Qualification of MDCK cells as safe vaccine production system 1 u u u Risk assessment Elimination of residual cellular DNA (DNAse treatment) MDCK cells are as safe as other cell lines l Pre-clinical validation of Grippol TC adjuvanted cell-derived (MDCK) vaccine 2 u u u Novel adjuvant: polyoxidonium Sterile cell-derived antigens, immunogenic at 3 -fold lower levels than split or subunit vaccines Pre-clinical validation complete, clinical trials to begin soon 1. Kersten A. abstract P 1404, Options VI, 2007. 2. Nekrasov A. abstract P 1433, Options VI, 2007.
 Seasonal influenza vaccines: pre-clinical evaluation u Dynavax: “universal influenza vaccine” approach 1 l Conserved viral antigen (NP) with immunostimulatory DNA l Promising results in mice; NP-ISS plus Fluzone enhanced antibody response, viral neutralization in baboons u NIBSC: DNA vaccine with truncated HA 2 l Spontaneous insertion of bacterial DNA into HA construct l Induces 10 -fold increase in HA antibody titre vs normal HA l Antibodies to truncated HA can recognize intact virus u Del. Site: dry powder (Gel. Vac) delivery system 3 l Ionic polysaccharide (nasal delivery or reconstitution for injection); whole virion or split antigens l Safe & tolerable through nasal route; immunogenic when injected 1. Higgins D. abstract P 1419, Options VI, 2007. 2. Robertson J. abstract P 1421, Options VI, 2007. 3. Ni Y. abstract P 1431, Options VI, 2007.
Seasonal influenza vaccines: pre-clinical evaluation u Dynavax: “universal influenza vaccine” approach 1 l Conserved viral antigen (NP) with immunostimulatory DNA l Promising results in mice; NP-ISS plus Fluzone enhanced antibody response, viral neutralization in baboons u NIBSC: DNA vaccine with truncated HA 2 l Spontaneous insertion of bacterial DNA into HA construct l Induces 10 -fold increase in HA antibody titre vs normal HA l Antibodies to truncated HA can recognize intact virus u Del. Site: dry powder (Gel. Vac) delivery system 3 l Ionic polysaccharide (nasal delivery or reconstitution for injection); whole virion or split antigens l Safe & tolerable through nasal route; immunogenic when injected 1. Higgins D. abstract P 1419, Options VI, 2007. 2. Robertson J. abstract P 1421, Options VI, 2007. 3. Ni Y. abstract P 1431, Options VI, 2007.
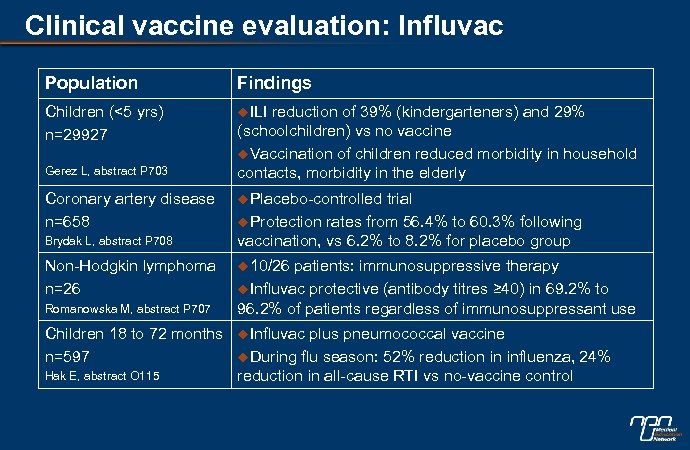 Clinical vaccine evaluation: Influvac Population Findings Children (<5 yrs) n=29927 u. ILI Gerez L, abstract P 703 Coronary artery disease n=658 Brydak L, abstract P 708 Non-Hodgkin lymphoma n=26 Romanowska M, abstract P 707 Children 18 to 72 months n=597 Hak E, abstract O 115 reduction of 39% (kindergarteners) and 29% (schoolchildren) vs no vaccine u. Vaccination of children reduced morbidity in household contacts, morbidity in the elderly u. Placebo-controlled trial u. Protection rates from 56. 4% to 60. 3% following vaccination, vs 6. 2% to 8. 2% for placebo group u 10/26 patients: immunosuppressive therapy u. Influvac protective (antibody titres ≥ 40) in 69. 2% to 96. 2% of patients regardless of immunosuppressant use u. Influvac plus pneumococcal vaccine u. During flu season: 52% reduction in influenza, 24% reduction in all-cause RTI vs no-vaccine control
Clinical vaccine evaluation: Influvac Population Findings Children (<5 yrs) n=29927 u. ILI Gerez L, abstract P 703 Coronary artery disease n=658 Brydak L, abstract P 708 Non-Hodgkin lymphoma n=26 Romanowska M, abstract P 707 Children 18 to 72 months n=597 Hak E, abstract O 115 reduction of 39% (kindergarteners) and 29% (schoolchildren) vs no vaccine u. Vaccination of children reduced morbidity in household contacts, morbidity in the elderly u. Placebo-controlled trial u. Protection rates from 56. 4% to 60. 3% following vaccination, vs 6. 2% to 8. 2% for placebo group u 10/26 patients: immunosuppressive therapy u. Influvac protective (antibody titres ≥ 40) in 69. 2% to 96. 2% of patients regardless of immunosuppressant use u. Influvac plus pneumococcal vaccine u. During flu season: 52% reduction in influenza, 24% reduction in all-cause RTI vs no-vaccine control
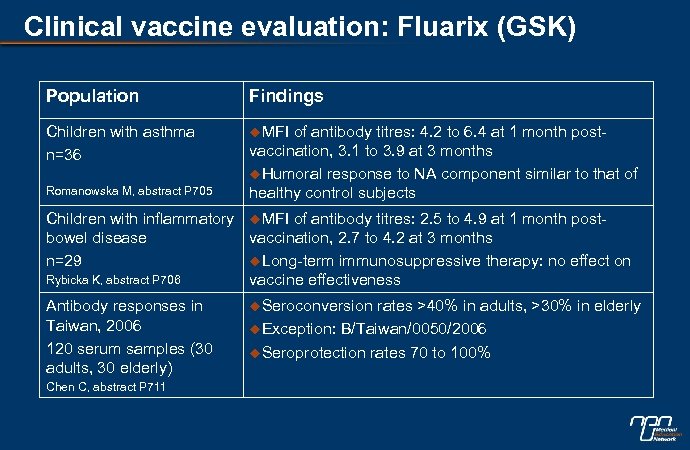 Clinical vaccine evaluation: Fluarix (GSK) Population Findings Children with asthma n=36 u. MFI Romanowska M, abstract P 705 of antibody titres: 4. 2 to 6. 4 at 1 month postvaccination, 3. 1 to 3. 9 at 3 months u. Humoral response to NA component similar to that of healthy control subjects Children with inflammatory u. MFI of antibody titres: 2. 5 to 4. 9 at 1 month postbowel disease vaccination, 2. 7 to 4. 2 at 3 months n=29 u. Long-term immunosuppressive therapy: no effect on Rybicka K, abstract P 706 vaccine effectiveness Antibody responses in Taiwan, 2006 120 serum samples (30 adults, 30 elderly) Chen C, abstract P 711 u. Seroconversion rates >40% in adults, >30% in elderly u. Exception: B/Taiwan/0050/2006 u. Seroprotection rates 70 to 100%
Clinical vaccine evaluation: Fluarix (GSK) Population Findings Children with asthma n=36 u. MFI Romanowska M, abstract P 705 of antibody titres: 4. 2 to 6. 4 at 1 month postvaccination, 3. 1 to 3. 9 at 3 months u. Humoral response to NA component similar to that of healthy control subjects Children with inflammatory u. MFI of antibody titres: 2. 5 to 4. 9 at 1 month postbowel disease vaccination, 2. 7 to 4. 2 at 3 months n=29 u. Long-term immunosuppressive therapy: no effect on Rybicka K, abstract P 706 vaccine effectiveness Antibody responses in Taiwan, 2006 120 serum samples (30 adults, 30 elderly) Chen C, abstract P 711 u. Seroconversion rates >40% in adults, >30% in elderly u. Exception: B/Taiwan/0050/2006 u. Seroprotection rates 70 to 100%
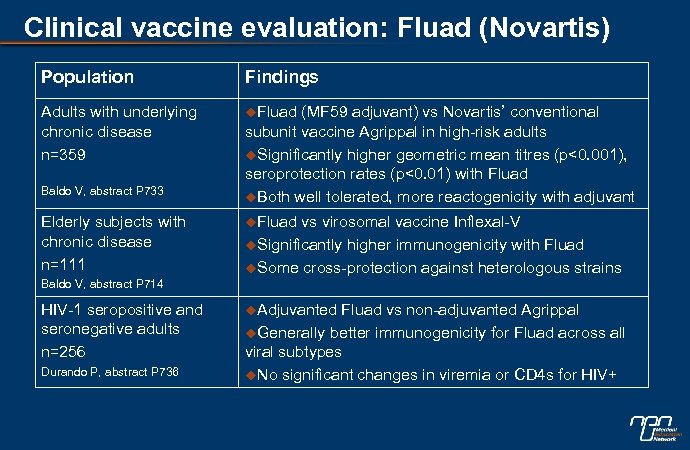 Clinical vaccine evaluation: Fluad (Novartis) Population Findings Adults with underlying chronic disease n=359 u. Fluad Baldo V, abstract P 733 Elderly subjects with chronic disease n=111 (MF 59 adjuvant) vs Novartis’ conventional subunit vaccine Agrippal in high-risk adults u. Significantly higher geometric mean titres (p<0. 001), seroprotection rates (p<0. 01) with Fluad u. Both well tolerated, more reactogenicity with adjuvant u. Fluad vs virosomal vaccine Inflexal-V u. Significantly higher immunogenicity with Fluad u. Some cross-protection against heterologous strains Baldo V, abstract P 714 HIV-1 seropositive and seronegative adults n=256 Durando P, abstract P 736 u. Adjuvanted Fluad vs non-adjuvanted Agrippal u. Generally better immunogenicity for Fluad across all viral subtypes u. No significant changes in viremia or CD 4 s for HIV+
Clinical vaccine evaluation: Fluad (Novartis) Population Findings Adults with underlying chronic disease n=359 u. Fluad Baldo V, abstract P 733 Elderly subjects with chronic disease n=111 (MF 59 adjuvant) vs Novartis’ conventional subunit vaccine Agrippal in high-risk adults u. Significantly higher geometric mean titres (p<0. 001), seroprotection rates (p<0. 01) with Fluad u. Both well tolerated, more reactogenicity with adjuvant u. Fluad vs virosomal vaccine Inflexal-V u. Significantly higher immunogenicity with Fluad u. Some cross-protection against heterologous strains Baldo V, abstract P 714 HIV-1 seropositive and seronegative adults n=256 Durando P, abstract P 736 u. Adjuvanted Fluad vs non-adjuvanted Agrippal u. Generally better immunogenicity for Fluad across all viral subtypes u. No significant changes in viremia or CD 4 s for HIV+
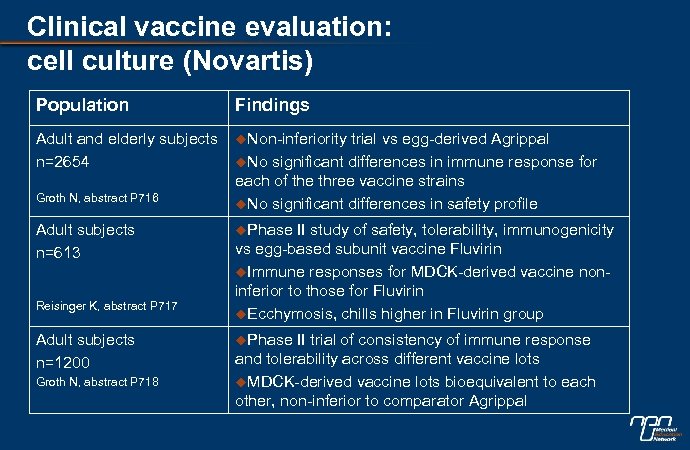 Clinical vaccine evaluation: cell culture (Novartis) Population Findings Adult and elderly subjects n=2654 u. Non-inferiority Groth N, abstract P 716 Adult subjects n=613 Reisinger K, abstract P 717 Adult subjects n=1200 Groth N, abstract P 718 trial vs egg-derived Agrippal u. No significant differences in immune response for each of the three vaccine strains u. No significant differences in safety profile u. Phase II study of safety, tolerability, immunogenicity vs egg-based subunit vaccine Fluvirin u. Immune responses for MDCK-derived vaccine noninferior to those for Fluvirin u. Ecchymosis, chills higher in Fluvirin group u. Phase II trial of consistency of immune response and tolerability across different vaccine lots u. MDCK-derived vaccine lots bioequivalent to each other, non-inferior to comparator Agrippal
Clinical vaccine evaluation: cell culture (Novartis) Population Findings Adult and elderly subjects n=2654 u. Non-inferiority Groth N, abstract P 716 Adult subjects n=613 Reisinger K, abstract P 717 Adult subjects n=1200 Groth N, abstract P 718 trial vs egg-derived Agrippal u. No significant differences in immune response for each of the three vaccine strains u. No significant differences in safety profile u. Phase II study of safety, tolerability, immunogenicity vs egg-based subunit vaccine Fluvirin u. Immune responses for MDCK-derived vaccine noninferior to those for Fluvirin u. Ecchymosis, chills higher in Fluvirin group u. Phase II trial of consistency of immune response and tolerability across different vaccine lots u. MDCK-derived vaccine lots bioequivalent to each other, non-inferior to comparator Agrippal
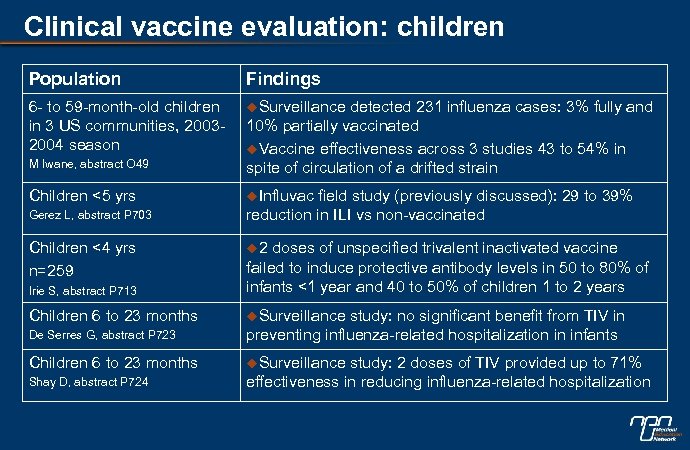 Clinical vaccine evaluation: children Population Findings 6 - to 59 -month-old children in 3 US communities, 20032004 season u. Surveillance M Iwane, abstract O 49 detected 231 influenza cases: 3% fully and 10% partially vaccinated u. Vaccine effectiveness across 3 studies 43 to 54% in spite of circulation of a drifted strain Children <5 yrs u. Influvac Gerez L, abstract P 703 Children <4 yrs n=259 field study (previously discussed): 29 to 39% reduction in ILI vs non-vaccinated u 2 Irie S, abstract P 713 doses of unspecified trivalent inactivated vaccine failed to induce protective antibody levels in 50 to 80% of infants <1 year and 40 to 50% of children 1 to 2 years Children 6 to 23 months u. Surveillance De Serres G, abstract P 723 study: no significant benefit from TIV in preventing influenza-related hospitalization in infants Children 6 to 23 months u. Surveillance Shay D, abstract P 724 study: 2 doses of TIV provided up to 71% effectiveness in reducing influenza-related hospitalization
Clinical vaccine evaluation: children Population Findings 6 - to 59 -month-old children in 3 US communities, 20032004 season u. Surveillance M Iwane, abstract O 49 detected 231 influenza cases: 3% fully and 10% partially vaccinated u. Vaccine effectiveness across 3 studies 43 to 54% in spite of circulation of a drifted strain Children <5 yrs u. Influvac Gerez L, abstract P 703 Children <4 yrs n=259 field study (previously discussed): 29 to 39% reduction in ILI vs non-vaccinated u 2 Irie S, abstract P 713 doses of unspecified trivalent inactivated vaccine failed to induce protective antibody levels in 50 to 80% of infants <1 year and 40 to 50% of children 1 to 2 years Children 6 to 23 months u. Surveillance De Serres G, abstract P 723 study: no significant benefit from TIV in preventing influenza-related hospitalization in infants Children 6 to 23 months u. Surveillance Shay D, abstract P 724 study: 2 doses of TIV provided up to 71% effectiveness in reducing influenza-related hospitalization
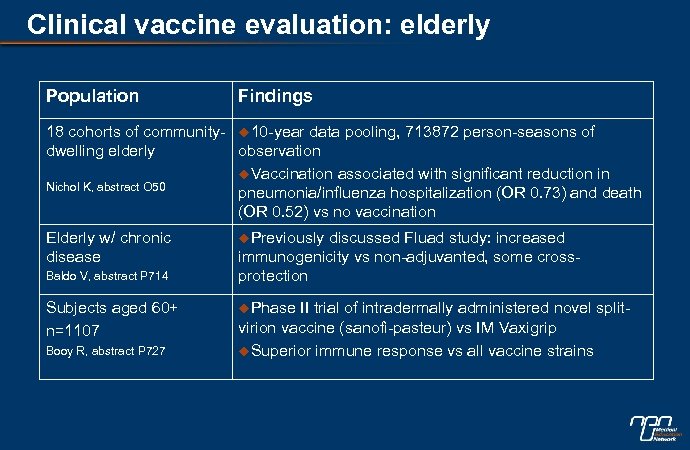 Clinical vaccine evaluation: elderly Population Findings 18 cohorts of community- u 10 -year data pooling, 713872 person-seasons of dwelling elderly observation u. Vaccination associated with significant reduction in Nichol K, abstract O 50 pneumonia/influenza hospitalization (OR 0. 73) and death (OR 0. 52) vs no vaccination Elderly w/ chronic disease Baldo V, abstract P 714 Subjects aged 60+ n=1107 Booy R, abstract P 727 u. Previously discussed Fluad study: increased immunogenicity vs non-adjuvanted, some crossprotection u. Phase II trial of intradermally administered novel splitvirion vaccine (sanofi-pasteur) vs IM Vaxigrip u. Superior immune response vs all vaccine strains
Clinical vaccine evaluation: elderly Population Findings 18 cohorts of community- u 10 -year data pooling, 713872 person-seasons of dwelling elderly observation u. Vaccination associated with significant reduction in Nichol K, abstract O 50 pneumonia/influenza hospitalization (OR 0. 73) and death (OR 0. 52) vs no vaccination Elderly w/ chronic disease Baldo V, abstract P 714 Subjects aged 60+ n=1107 Booy R, abstract P 727 u. Previously discussed Fluad study: increased immunogenicity vs non-adjuvanted, some crossprotection u. Phase II trial of intradermally administered novel splitvirion vaccine (sanofi-pasteur) vs IM Vaxigrip u. Superior immune response vs all vaccine strains
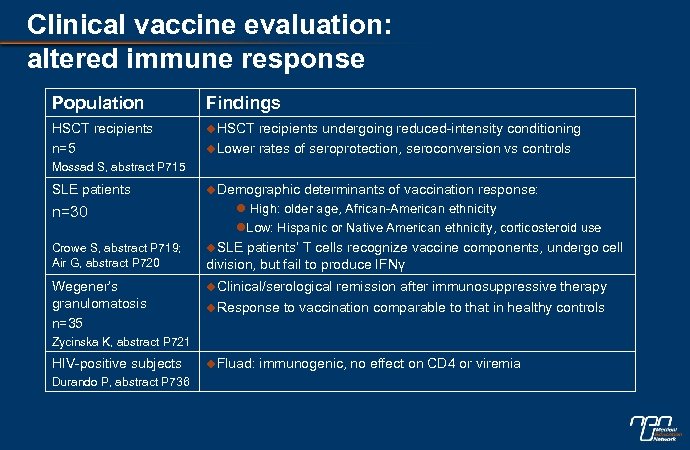 Clinical vaccine evaluation: altered immune response Population Findings HSCT recipients n=5 u. HSCT recipients undergoing reduced-intensity conditioning u. Lower rates of seroprotection, seroconversion vs controls Mossad S, abstract P 715 SLE patients u. Demographic n=30 l High: older age, African-American ethnicity l. Low: Hispanic or Native American ethnicity, corticosteroid use u. SLE patients’ T cells recognize vaccine components, undergo cell division, but fail to produce IFNγ Crowe S, abstract P 719; Air G, abstract P 720 Wegener’s granulomatosis n=35 determinants of vaccination response: u. Clinical/serological remission after immunosuppressive therapy u. Response to vaccination comparable to that in healthy controls Zycinska K, abstract P 721 HIV-positive subjects Durando P, abstract P 736 u. Fluad: immunogenic, no effect on CD 4 or viremia
Clinical vaccine evaluation: altered immune response Population Findings HSCT recipients n=5 u. HSCT recipients undergoing reduced-intensity conditioning u. Lower rates of seroprotection, seroconversion vs controls Mossad S, abstract P 715 SLE patients u. Demographic n=30 l High: older age, African-American ethnicity l. Low: Hispanic or Native American ethnicity, corticosteroid use u. SLE patients’ T cells recognize vaccine components, undergo cell division, but fail to produce IFNγ Crowe S, abstract P 719; Air G, abstract P 720 Wegener’s granulomatosis n=35 determinants of vaccination response: u. Clinical/serological remission after immunosuppressive therapy u. Response to vaccination comparable to that in healthy controls Zycinska K, abstract P 721 HIV-positive subjects Durando P, abstract P 736 u. Fluad: immunogenic, no effect on CD 4 or viremia
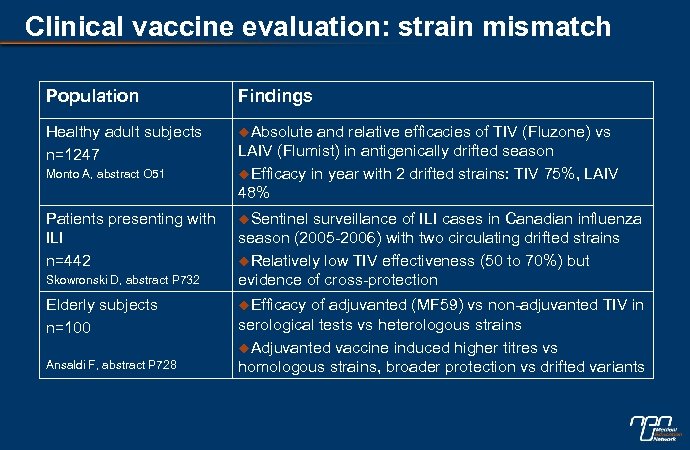 Clinical vaccine evaluation: strain mismatch Population Findings Healthy adult subjects n=1247 u. Absolute Monto A, abstract O 51 Patients presenting with ILI n=442 Skowronski D, abstract P 732 Elderly subjects n=100 Ansaldi F, abstract P 728 and relative efficacies of TIV (Fluzone) vs LAIV (Flumist) in antigenically drifted season u. Efficacy in year with 2 drifted strains: TIV 75%, LAIV 48% u. Sentinel surveillance of ILI cases in Canadian influenza season (2005 -2006) with two circulating drifted strains u. Relatively low TIV effectiveness (50 to 70%) but evidence of cross-protection u. Efficacy of adjuvanted (MF 59) vs non-adjuvanted TIV in serological tests vs heterologous strains u. Adjuvanted vaccine induced higher titres vs homologous strains, broader protection vs drifted variants
Clinical vaccine evaluation: strain mismatch Population Findings Healthy adult subjects n=1247 u. Absolute Monto A, abstract O 51 Patients presenting with ILI n=442 Skowronski D, abstract P 732 Elderly subjects n=100 Ansaldi F, abstract P 728 and relative efficacies of TIV (Fluzone) vs LAIV (Flumist) in antigenically drifted season u. Efficacy in year with 2 drifted strains: TIV 75%, LAIV 48% u. Sentinel surveillance of ILI cases in Canadian influenza season (2005 -2006) with two circulating drifted strains u. Relatively low TIV effectiveness (50 to 70%) but evidence of cross-protection u. Efficacy of adjuvanted (MF 59) vs non-adjuvanted TIV in serological tests vs heterologous strains u. Adjuvanted vaccine induced higher titres vs homologous strains, broader protection vs drifted variants
 Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
 Avian influenza: general considerations u Veterinary aspects of avian influenza 1 l For every human infected, 1 million infected animals l Spread to wild birds: unprecedented ecological & epidemiological situation l Should be seen as disease of animals, not just birds u Study of pathogenesis in ducks 2 l Virus present 1 day post infection in nasal cavity, lungs, spleen l Better understanding of targets for surveillance sampling u State of bird vaccines 3 l Potentially useful for eradication, management, prevention l Issues: administration & coverage, new variants l Future directions: replaceable “cassette”, improved vectors 1. Capua I. PS 3, Options VI, 2007. 2. Banks J. abstract O 95, Options VI, 2007. 3. Swayne D. PS 3, Options VI, 2007.
Avian influenza: general considerations u Veterinary aspects of avian influenza 1 l For every human infected, 1 million infected animals l Spread to wild birds: unprecedented ecological & epidemiological situation l Should be seen as disease of animals, not just birds u Study of pathogenesis in ducks 2 l Virus present 1 day post infection in nasal cavity, lungs, spleen l Better understanding of targets for surveillance sampling u State of bird vaccines 3 l Potentially useful for eradication, management, prevention l Issues: administration & coverage, new variants l Future directions: replaceable “cassette”, improved vectors 1. Capua I. PS 3, Options VI, 2007. 2. Banks J. abstract O 95, Options VI, 2007. 3. Swayne D. PS 3, Options VI, 2007.
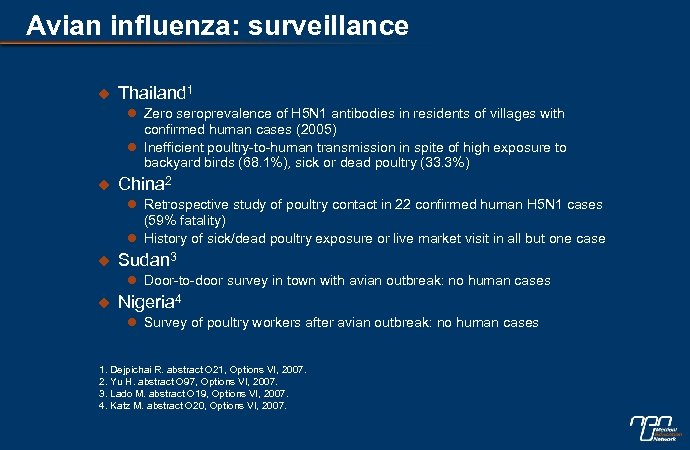 Avian influenza: surveillance u Thailand 1 l Zero seroprevalence of H 5 N 1 antibodies in residents of villages with confirmed human cases (2005) l Inefficient poultry-to-human transmission in spite of high exposure to backyard birds (68. 1%), sick or dead poultry (33. 3%) u China 2 l Retrospective study of poultry contact in 22 confirmed human H 5 N 1 cases (59% fatality) l History of sick/dead poultry exposure or live market visit in all but one case u Sudan 3 l Door-to-door survey in town with avian outbreak: no human cases u Nigeria 4 l Survey of poultry workers after avian outbreak: no human cases 1. Dejpichai R. abstract O 21, Options VI, 2007. 2. Yu H. abstract O 97, Options VI, 2007. 3. Lado M. abstract O 19, Options VI, 2007. 4. Katz M. abstract O 20, Options VI, 2007.
Avian influenza: surveillance u Thailand 1 l Zero seroprevalence of H 5 N 1 antibodies in residents of villages with confirmed human cases (2005) l Inefficient poultry-to-human transmission in spite of high exposure to backyard birds (68. 1%), sick or dead poultry (33. 3%) u China 2 l Retrospective study of poultry contact in 22 confirmed human H 5 N 1 cases (59% fatality) l History of sick/dead poultry exposure or live market visit in all but one case u Sudan 3 l Door-to-door survey in town with avian outbreak: no human cases u Nigeria 4 l Survey of poultry workers after avian outbreak: no human cases 1. Dejpichai R. abstract O 21, Options VI, 2007. 2. Yu H. abstract O 97, Options VI, 2007. 3. Lado M. abstract O 19, Options VI, 2007. 4. Katz M. abstract O 20, Options VI, 2007.
 Avian influenza: spread & control u Control in birds in SE Asia 1 l Effective surveillance, rapid eradication, proper disposal, enhanced biosecurity, vaccination l Will need to be sensitive to regional issues/practices u Control of spread into Europe/Africa 2 l No new wild cases in Europe since June 2006 l Need to enhance systems for early detection l Establishment of protection/surveillance zones l Increase/improve vaccination, biosecurity 1. Kalpravidh W. PS 3, Options VI, 2007. 2. Brown I. abstract PS 3, Options VI, 2007.
Avian influenza: spread & control u Control in birds in SE Asia 1 l Effective surveillance, rapid eradication, proper disposal, enhanced biosecurity, vaccination l Will need to be sensitive to regional issues/practices u Control of spread into Europe/Africa 2 l No new wild cases in Europe since June 2006 l Need to enhance systems for early detection l Establishment of protection/surveillance zones l Increase/improve vaccination, biosecurity 1. Kalpravidh W. PS 3, Options VI, 2007. 2. Brown I. abstract PS 3, Options VI, 2007.
 Avian influenza: spread & control u Control of avian/human clusters in UK: l Feb 2007 - H 5 N 1 outbreak on Suffolk poultry farm 1 u All 160000 birds culled u Oseltamivir prophylaxis and seasonal influenza vaccination for exposed people u No human H 5 N 1 cases detected l H 7 N 2 outbreak in north Wales 2 u Several infected premises, all traced to same vendor u Flu-like symptoms in exposed people; 4 H 7 N 2 cases (2 serious) u Prophylaxis offered to all exposed 1. Van Tam J. abstract O 18, Options VI, 2007. 2. Van Tam J. no abstract available, Options VI, 2007.
Avian influenza: spread & control u Control of avian/human clusters in UK: l Feb 2007 - H 5 N 1 outbreak on Suffolk poultry farm 1 u All 160000 birds culled u Oseltamivir prophylaxis and seasonal influenza vaccination for exposed people u No human H 5 N 1 cases detected l H 7 N 2 outbreak in north Wales 2 u Several infected premises, all traced to same vendor u Flu-like symptoms in exposed people; 4 H 7 N 2 cases (2 serious) u Prophylaxis offered to all exposed 1. Van Tam J. abstract O 18, Options VI, 2007. 2. Van Tam J. no abstract available, Options VI, 2007.
 Pre-pandemic planning: initiatives u EU assessment 1 l All member states: good start on planning surveillance, outbreak control, non-pharma strategies, public education l More support needed on: integration across agencies, seasonal flu control, research u Chinese assessment 2 l Good coverage of alert phase, pandemic phase responses l Needs: detailed implementation plan; strategies for risk communication, stockpiling, essential service continuity u Importance of stockpiling l Antivirals, pre-pandemic vaccines once available 1. Kreidl P. abstract O 23, Options VI, 2007. 2. Peng Z. abstract P 324, Options VI, 2007.
Pre-pandemic planning: initiatives u EU assessment 1 l All member states: good start on planning surveillance, outbreak control, non-pharma strategies, public education l More support needed on: integration across agencies, seasonal flu control, research u Chinese assessment 2 l Good coverage of alert phase, pandemic phase responses l Needs: detailed implementation plan; strategies for risk communication, stockpiling, essential service continuity u Importance of stockpiling l Antivirals, pre-pandemic vaccines once available 1. Kreidl P. abstract O 23, Options VI, 2007. 2. Peng Z. abstract P 324, Options VI, 2007.
 Pre-pandemic planning: challenges u u u Alignment of pandemic plans with rapidly evolving knowledge & technology (especially developing countries) Allocation of adequate resources & facilities to underserved countries (Africa, Asia) Communication & collaboration among different nations & agencies Modification of human attitudes and risk behaviours Retaining adequate capacity for response to other emergencies Ensuring prompt & equitable distribution of available antivirals & vaccines PS 1, Options VI, 2007
Pre-pandemic planning: challenges u u u Alignment of pandemic plans with rapidly evolving knowledge & technology (especially developing countries) Allocation of adequate resources & facilities to underserved countries (Africa, Asia) Communication & collaboration among different nations & agencies Modification of human attitudes and risk behaviours Retaining adequate capacity for response to other emergencies Ensuring prompt & equitable distribution of available antivirals & vaccines PS 1, Options VI, 2007
 Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
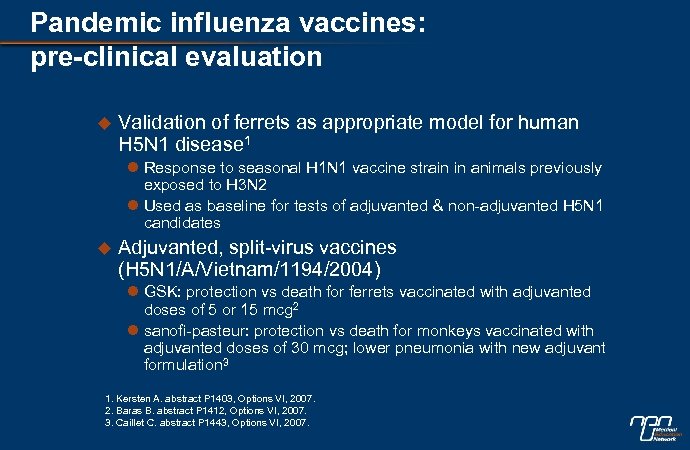 Pandemic influenza vaccines: pre-clinical evaluation u Validation of ferrets as appropriate model for human H 5 N 1 disease 1 l Response to seasonal H 1 N 1 vaccine strain in animals previously exposed to H 3 N 2 l Used as baseline for tests of adjuvanted & non-adjuvanted H 5 N 1 candidates u Adjuvanted, split-virus vaccines (H 5 N 1/A/Vietnam/1194/2004) l GSK: protection vs death for ferrets vaccinated with adjuvanted doses of 5 or 15 mcg 2 l sanofi-pasteur: protection vs death for monkeys vaccinated with adjuvanted doses of 30 mcg; lower pneumonia with new adjuvant formulation 3 1. Kersten A. abstract P 1403, Options VI, 2007. 2. Baras B. abstract P 1412, Options VI, 2007. 3. Caillet C. abstract P 1443, Options VI, 2007.
Pandemic influenza vaccines: pre-clinical evaluation u Validation of ferrets as appropriate model for human H 5 N 1 disease 1 l Response to seasonal H 1 N 1 vaccine strain in animals previously exposed to H 3 N 2 l Used as baseline for tests of adjuvanted & non-adjuvanted H 5 N 1 candidates u Adjuvanted, split-virus vaccines (H 5 N 1/A/Vietnam/1194/2004) l GSK: protection vs death for ferrets vaccinated with adjuvanted doses of 5 or 15 mcg 2 l sanofi-pasteur: protection vs death for monkeys vaccinated with adjuvanted doses of 30 mcg; lower pneumonia with new adjuvant formulation 3 1. Kersten A. abstract P 1403, Options VI, 2007. 2. Baras B. abstract P 1412, Options VI, 2007. 3. Caillet C. abstract P 1443, Options VI, 2007.
 Pandemic influenza vaccines: pre-clinical evaluation u Live attenuated vaccines (Med. Immune) l Reverse genetics – HA and NA genes from A/HK/213/2003(H 5 N 1) in cold-adapted donor strain: protects ferrets vs homologous challenge after 1 dose, cross-protects with 2 doses 1 l Same technique, A/VN/1203/2004(H 5 N 1) strain: homologous and heterologous protection in ferrets after 1 dose 2 1. Suguitan A. abstract P 1430, Options VI, 2007. 2. Jin H. abstract P 1436, Options VI, 2007.
Pandemic influenza vaccines: pre-clinical evaluation u Live attenuated vaccines (Med. Immune) l Reverse genetics – HA and NA genes from A/HK/213/2003(H 5 N 1) in cold-adapted donor strain: protects ferrets vs homologous challenge after 1 dose, cross-protects with 2 doses 1 l Same technique, A/VN/1203/2004(H 5 N 1) strain: homologous and heterologous protection in ferrets after 1 dose 2 1. Suguitan A. abstract P 1430, Options VI, 2007. 2. Jin H. abstract P 1436, Options VI, 2007.
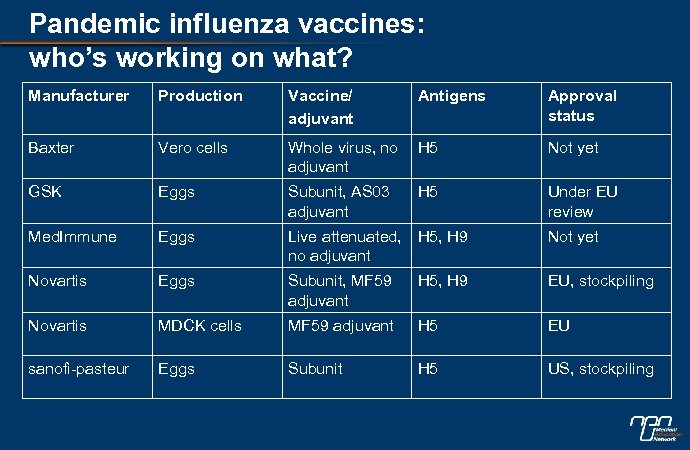 Pandemic influenza vaccines: who’s working on what? Manufacturer Production Vaccine/ adjuvant Antigens Approval status Baxter Vero cells Whole virus, no adjuvant H 5 Not yet GSK Eggs Subunit, AS 03 adjuvant H 5 Under EU review Med. Immune Eggs Live attenuated, no adjuvant H 5, H 9 Not yet Novartis Eggs Subunit, MF 59 adjuvant H 5, H 9 EU, stockpiling Novartis MDCK cells MF 59 adjuvant H 5 EU sanofi-pasteur Eggs Subunit H 5 US, stockpiling
Pandemic influenza vaccines: who’s working on what? Manufacturer Production Vaccine/ adjuvant Antigens Approval status Baxter Vero cells Whole virus, no adjuvant H 5 Not yet GSK Eggs Subunit, AS 03 adjuvant H 5 Under EU review Med. Immune Eggs Live attenuated, no adjuvant H 5, H 9 Not yet Novartis Eggs Subunit, MF 59 adjuvant H 5, H 9 EU, stockpiling Novartis MDCK cells MF 59 adjuvant H 5 EU sanofi-pasteur Eggs Subunit H 5 US, stockpiling
 Pandemic influenza vaccines: clinical evaluation u CSL Limited: aluminium-adjuvanted inactivated split-virion A/Vietnam/1194/2004 NIBRG(H 5 N 1) vaccine l Phase I, II in healthy adults: adequately immunogenic (MN ≥ 1: 20 for 73% of subjects at 30 or 45 mcg), generally safe/well tolerated 1 l Serological analysis: clade 1 vaccine gives some limited cross-protection against clade 2 viruses 2 1. Nolan T. abstract P 7266, Options VI, 2007. 2. Hoschler K. abstract P 729, Options VI, 2007.
Pandemic influenza vaccines: clinical evaluation u CSL Limited: aluminium-adjuvanted inactivated split-virion A/Vietnam/1194/2004 NIBRG(H 5 N 1) vaccine l Phase I, II in healthy adults: adequately immunogenic (MN ≥ 1: 20 for 73% of subjects at 30 or 45 mcg), generally safe/well tolerated 1 l Serological analysis: clade 1 vaccine gives some limited cross-protection against clade 2 viruses 2 1. Nolan T. abstract P 7266, Options VI, 2007. 2. Hoschler K. abstract P 729, Options VI, 2007.
 Pandemic influenza vaccines: clinical evaluation u sanofi-pasteur: inactivated subvirion rg. A/VN/1203/2004(H 5 N 1), aluminium adjuvant l Dose-ranging in healthy adults: dose relationship observed but antigenicity low after 2 doses at all dose levels (3. 75 to 45 mcg); little to no effect from adjuvant 1 l 2 similar studies in elderly: still limited antigenicity after 2 doses (35 -37% of subjects achieving HAI titers ≥ 40)2, 3 l Use of a 3 rd (unadjuvanted) dose 6 months later induces higher antibody levels that persist after a further 6 months; support for “prime-boost” strategy 4 1. Keitel W. abstract P 722, Options VI, 2007. 2. Brady R. abstract P 739, Options VI, 2007. 3. Treanor J. abstract P 731, Options VI, 2007. 4. Zangwill K, abstract P 737, Options VI, 2007.
Pandemic influenza vaccines: clinical evaluation u sanofi-pasteur: inactivated subvirion rg. A/VN/1203/2004(H 5 N 1), aluminium adjuvant l Dose-ranging in healthy adults: dose relationship observed but antigenicity low after 2 doses at all dose levels (3. 75 to 45 mcg); little to no effect from adjuvant 1 l 2 similar studies in elderly: still limited antigenicity after 2 doses (35 -37% of subjects achieving HAI titers ≥ 40)2, 3 l Use of a 3 rd (unadjuvanted) dose 6 months later induces higher antibody levels that persist after a further 6 months; support for “prime-boost” strategy 4 1. Keitel W. abstract P 722, Options VI, 2007. 2. Brady R. abstract P 739, Options VI, 2007. 3. Treanor J. abstract P 731, Options VI, 2007. 4. Zangwill K, abstract P 737, Options VI, 2007.
 Pandemic influenza vaccines: clinical evaluation u sanofi-pasteur: PER. C 6 -derived H 7 N 1 inactivated split reverse genetics vaccine 1 l HPAI A/Chicken/Italy/13474/99(H 7 N 1) in PR 8 carrier l Antibody responses in 21 of 54 participants; best responses in high-dose (24 mcg HA) aluminium-adjuvanted group l Priming may be necessary given weak immunogenicity u Value of pre-pandemic priming 2 l Single dose of A/VN 1203/2004(H 5 N 1) vaccine given to individuals with 2 previous doses of A/HK/156/1997(H 5 N 1) vaccine l Robust increases in H 5 HA-specific B-cell response 1. Cox R. abstract O 56, Options VI, 2007. 2. Topham D. abstract O 55, Options VI, 2007.
Pandemic influenza vaccines: clinical evaluation u sanofi-pasteur: PER. C 6 -derived H 7 N 1 inactivated split reverse genetics vaccine 1 l HPAI A/Chicken/Italy/13474/99(H 7 N 1) in PR 8 carrier l Antibody responses in 21 of 54 participants; best responses in high-dose (24 mcg HA) aluminium-adjuvanted group l Priming may be necessary given weak immunogenicity u Value of pre-pandemic priming 2 l Single dose of A/VN 1203/2004(H 5 N 1) vaccine given to individuals with 2 previous doses of A/HK/156/1997(H 5 N 1) vaccine l Robust increases in H 5 HA-specific B-cell response 1. Cox R. abstract O 56, Options VI, 2007. 2. Topham D. abstract O 55, Options VI, 2007.
 Pandemic influenza vaccines: clinical evaluation u GSK: split-virus H 5 N 1 candidate vaccine with novel oil-in-water adjuvant system (AS 03) l 3. 8 mcg dose established as effective, chosen for further development l Phase III trial in 5071 subjects: safety profile of 15 mcg dose vs seasonal vaccine Fluarix l Significantly higher levels of solicited AEs with H 5 N 1 vaccine; medically acceptable reactogenicity Ballou W. abstract O 54, Options VI, 2007.
Pandemic influenza vaccines: clinical evaluation u GSK: split-virus H 5 N 1 candidate vaccine with novel oil-in-water adjuvant system (AS 03) l 3. 8 mcg dose established as effective, chosen for further development l Phase III trial in 5071 subjects: safety profile of 15 mcg dose vs seasonal vaccine Fluarix l Significantly higher levels of solicited AEs with H 5 N 1 vaccine; medically acceptable reactogenicity Ballou W. abstract O 54, Options VI, 2007.
 Pandemic influenza vaccines: clinical evaluation u Antigen-sparing strategies l Berna: intradermal administration of virosomal adjuvanted seasonal vaccine 1 u Highly immunogenic, well tolerated at a five-fold reduced dose compared to IM administration l Novartis: non-inferiority of low-dose MF 59 -adjuvanted H 5 N 1 vaccine 2 u 2 doses of 7. 5 (low) vs 15 mcg (standard) of A/Vietnam/1194/2004 like(H 5 N 1) antigen with MF 59 u Low-dose: non-inferior, may be a valid dose-sparing candidate, pre-priming agent 1. Kunzi V. abstract P 704, Options VI, 2007. 2. Banzhoff A. abstract P 734, Options VI, 2007.
Pandemic influenza vaccines: clinical evaluation u Antigen-sparing strategies l Berna: intradermal administration of virosomal adjuvanted seasonal vaccine 1 u Highly immunogenic, well tolerated at a five-fold reduced dose compared to IM administration l Novartis: non-inferiority of low-dose MF 59 -adjuvanted H 5 N 1 vaccine 2 u 2 doses of 7. 5 (low) vs 15 mcg (standard) of A/Vietnam/1194/2004 like(H 5 N 1) antigen with MF 59 u Low-dose: non-inferior, may be a valid dose-sparing candidate, pre-priming agent 1. Kunzi V. abstract P 704, Options VI, 2007. 2. Banzhoff A. abstract P 734, Options VI, 2007.
 Pandemic influenza vaccines: future directions u No chickens = no eggs = no vaccines l Development of cell culture systems u Rapidly expandable u Enhanced immunogenicity? u Reverse genetics u Use of adjuvants l Antigen sparing l Increased immunogenicity in elderly l Cross-protection?
Pandemic influenza vaccines: future directions u No chickens = no eggs = no vaccines l Development of cell culture systems u Rapidly expandable u Enhanced immunogenicity? u Reverse genetics u Use of adjuvants l Antigen sparing l Increased immunogenicity in elderly l Cross-protection?
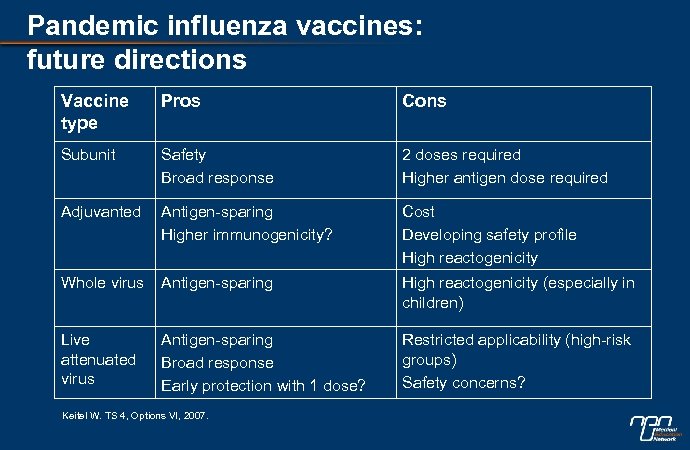 Pandemic influenza vaccines: future directions Vaccine type Pros Cons Subunit Safety Broad response 2 doses required Higher antigen dose required Adjuvanted Antigen-sparing Higher immunogenicity? Cost Developing safety profile High reactogenicity Whole virus Antigen-sparing High reactogenicity (especially in children) Live attenuated virus Antigen-sparing Broad response Early protection with 1 dose? Restricted applicability (high-risk groups) Safety concerns? Keitel W. TS 4, Options VI, 2007.
Pandemic influenza vaccines: future directions Vaccine type Pros Cons Subunit Safety Broad response 2 doses required Higher antigen dose required Adjuvanted Antigen-sparing Higher immunogenicity? Cost Developing safety profile High reactogenicity Whole virus Antigen-sparing High reactogenicity (especially in children) Live attenuated virus Antigen-sparing Broad response Early protection with 1 dose? Restricted applicability (high-risk groups) Safety concerns? Keitel W. TS 4, Options VI, 2007.
 Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
 Antivirals and seasonal influenza u L Gubareva (CDC, USA): antiviral resistance of >1500 isolates from last 3 seasons l 133 A(H 1 N 1), 186 A(H 3 N 2), 118 B l All susceptible to oseltamivir and zanamivir, one exception: B virus, R 371 K active site substitution l Another B mutant (H 274 Y) detected: susceptible to oseltamivir & zanamivir, peramivir-resistant u A Hurt (WHO, Australia): mutations in N 1 l 4 A(H 1 N 1) strains detected with reduced NAI sensitivity l 3 novel mutations in NA gene: Q 136 K, K 150 T, K 143 R l All affect the recently identified ‘ 150 -cavity’ 1. Gubareva L. abstract O 66, Options VI, 2007. 2. Hurt A. abstract O 67, Options VI, 2007.
Antivirals and seasonal influenza u L Gubareva (CDC, USA): antiviral resistance of >1500 isolates from last 3 seasons l 133 A(H 1 N 1), 186 A(H 3 N 2), 118 B l All susceptible to oseltamivir and zanamivir, one exception: B virus, R 371 K active site substitution l Another B mutant (H 274 Y) detected: susceptible to oseltamivir & zanamivir, peramivir-resistant u A Hurt (WHO, Australia): mutations in N 1 l 4 A(H 1 N 1) strains detected with reduced NAI sensitivity l 3 novel mutations in NA gene: Q 136 K, K 150 T, K 143 R l All affect the recently identified ‘ 150 -cavity’ 1. Gubareva L. abstract O 66, Options VI, 2007. 2. Hurt A. abstract O 67, Options VI, 2007.
 Antivirals and seasonal influenza: children u N Sugaya (Japan): effectiveness of oseltamivir vs zanamivir in children with influenza A l H 1 N 1: u total febrile period, duration of fever after treatment initiation: no difference l H 3 N 2: u u total febrile period: oseltamivir 1. 7 days, zanamivir 2. 3 days (p = 0. 01) duration of fever after treatment initiation: no difference survival of virus in throat @ day 5: zanamivir 8%, oseltamivir 47% R Dutkowski (Roche, Switzerland): earlier (<24 h) vs later (≥ 24 h) initiation of oseltamivir in children l time to freedom from illness: 78. 8% absolute improvement l duration of fever: 25. 4% absolute improvement l resistance: 5. 5% overall; fewer cases in early initiation group; no impact on illness duration 1. Sugaya N. abstract O 65, Options VI, 2007. 2. Dutkowski R. abstract O 98, Options VI, 2007.
Antivirals and seasonal influenza: children u N Sugaya (Japan): effectiveness of oseltamivir vs zanamivir in children with influenza A l H 1 N 1: u total febrile period, duration of fever after treatment initiation: no difference l H 3 N 2: u u total febrile period: oseltamivir 1. 7 days, zanamivir 2. 3 days (p = 0. 01) duration of fever after treatment initiation: no difference survival of virus in throat @ day 5: zanamivir 8%, oseltamivir 47% R Dutkowski (Roche, Switzerland): earlier (<24 h) vs later (≥ 24 h) initiation of oseltamivir in children l time to freedom from illness: 78. 8% absolute improvement l duration of fever: 25. 4% absolute improvement l resistance: 5. 5% overall; fewer cases in early initiation group; no impact on illness duration 1. Sugaya N. abstract O 65, Options VI, 2007. 2. Dutkowski R. abstract O 98, Options VI, 2007.
 Antivirals and H 5 N 1 u J Belser (CDC, USA): novel sialidase (DAS 181, Fludase) vs H 5 N 1 in mice l removes sialic acids from respiratory epithelium l 70% prevention of infection, 100% prevention of death l Phase I imminent (Nex. Bio) u E Gorovkova (USA): effectiveness of oseltamivir vs H 5 N 1 in ferrets l 5 mg/kg/day within 4 hours of infection: protection vs death from VM/1203 l Treatment delay to 24 hours: 25 mg/kg/day required l All animals protected on homologous re-challenge (21 days) 1. Belser J, abstract O 71, Options VI, 2007. 2. Gorovkova E. abstract O 72, Options VI, 2007.
Antivirals and H 5 N 1 u J Belser (CDC, USA): novel sialidase (DAS 181, Fludase) vs H 5 N 1 in mice l removes sialic acids from respiratory epithelium l 70% prevention of infection, 100% prevention of death l Phase I imminent (Nex. Bio) u E Gorovkova (USA): effectiveness of oseltamivir vs H 5 N 1 in ferrets l 5 mg/kg/day within 4 hours of infection: protection vs death from VM/1203 l Treatment delay to 24 hours: 25 mg/kg/day required l All animals protected on homologous re-challenge (21 days) 1. Belser J, abstract O 71, Options VI, 2007. 2. Gorovkova E. abstract O 72, Options VI, 2007.
 Antivirals and H 5 N 1 u Decreased oseltamivir sensitivity among Indonesian H 5 N 1 isolates 1 l clade 2: 25 - to 30 -fold decrease in sensitivity compared to clade 1 l zanamivir should be incorporated into all stockpiles u Clinical experience in Thailand 2 l oseltamivir used in 16/25 human H 5 N 1 cases u 5 of 8 surviving patients had received it u earlier treatment associated with higher survival probability? 1. Mc. Kimm-Breschkin J, abstract O 69, Options VI, 2007. 2. Chotpitayasunondh T. TS 3, Options VI, 2007.
Antivirals and H 5 N 1 u Decreased oseltamivir sensitivity among Indonesian H 5 N 1 isolates 1 l clade 2: 25 - to 30 -fold decrease in sensitivity compared to clade 1 l zanamivir should be incorporated into all stockpiles u Clinical experience in Thailand 2 l oseltamivir used in 16/25 human H 5 N 1 cases u 5 of 8 surviving patients had received it u earlier treatment associated with higher survival probability? 1. Mc. Kimm-Breschkin J, abstract O 69, Options VI, 2007. 2. Chotpitayasunondh T. TS 3, Options VI, 2007.
 Overcoming antiviral resistance: new directions u New methods of targeting NA 1 l potential for design of conformation-specific drugs (group 1: 150 cavity, group 2: closed) l further studies of existing agents: combinations, multimers u HA as antiviral target 1 l block receptor binding, inhibit membrane fusion u NS 1 as antiviral target 2 l RNA-binding domain: required for replication l effector domain: interferes with IFN m. RNA processing u M 1 and PB 1 gene silencing by catalytic nucleic acids 3 l DNAzymes and ribozymes both effective; different cleavage sites = more effective when combined 1. Hay A. TS 2, Options VI, 2007. 2. Krug R. TS 2, Options VI, 2007. 3. Khanna M. abstract O 70, Options VI, 2007.
Overcoming antiviral resistance: new directions u New methods of targeting NA 1 l potential for design of conformation-specific drugs (group 1: 150 cavity, group 2: closed) l further studies of existing agents: combinations, multimers u HA as antiviral target 1 l block receptor binding, inhibit membrane fusion u NS 1 as antiviral target 2 l RNA-binding domain: required for replication l effector domain: interferes with IFN m. RNA processing u M 1 and PB 1 gene silencing by catalytic nucleic acids 3 l DNAzymes and ribozymes both effective; different cleavage sites = more effective when combined 1. Hay A. TS 2, Options VI, 2007. 2. Krug R. TS 2, Options VI, 2007. 3. Khanna M. abstract O 70, Options VI, 2007.
 Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
Overview u Disease surveillance and modeling u Virus-host interactions and pathogenesis u Seasonal influenza: vaccine evaluation u Pandemic influenza: outbreak and pre-pandemic response u Pandemic influenza: vaccine evaluation u Antivirals u Clinical guidance and policies
 Clinical guidance and policies: recurring themes u Need for a universal seasonal vaccination recommendation : l current age-based and disease-based recommendations can be confusing/inconsistent u Need to increase seasonal vaccine use: l for its own sake (reduced morbidity/mortality from seasonal influenza) l as a means of increasing production capacity, to be converted to pandemic vaccine production when required l as a catalyst for developing more effective/antigen-sparing vaccines u Need to ensure reliable and rapid communication during a pandemic: l within an affected areas l across different jurisdictions
Clinical guidance and policies: recurring themes u Need for a universal seasonal vaccination recommendation : l current age-based and disease-based recommendations can be confusing/inconsistent u Need to increase seasonal vaccine use: l for its own sake (reduced morbidity/mortality from seasonal influenza) l as a means of increasing production capacity, to be converted to pandemic vaccine production when required l as a catalyst for developing more effective/antigen-sparing vaccines u Need to ensure reliable and rapid communication during a pandemic: l within an affected areas l across different jurisdictions
 Seasonal vaccination coverage rates Germany, UK, Italy: Szucs et al, abstracts P 1321, P 1323, P 1324, Options VI, 2007. Korean studies: Kee et al. , abstracts P 1301, P 1302, Options VI, 2007.
Seasonal vaccination coverage rates Germany, UK, Italy: Szucs et al, abstracts P 1321, P 1323, P 1324, Options VI, 2007. Korean studies: Kee et al. , abstracts P 1301, P 1302, Options VI, 2007.
 Factors influencing vaccine uptake in health care providers u Survey of determinants/deterrents of choice to receive vaccination 1 l 103 health care workers in BC, Canada l 77% vaccinated l Vaccination seen as “personal” choice u u perceived risks/benefits to self & family workplace policy (where overlap with personal considerations) access to in-depth, personalized education Survey of vaccination uptake by vaccination providers 2 l 335 nurses, 343 physicians in BC, Canada l 89% intended to receive vaccine, 78% received it ≥ 75% of the time l Drivers for vaccine use: positive direct attitudes to vaccination (2. 5 times more likely), direct social norms (3. 2 times more likely) 1. Masaro C. abstract P 1303, Options VI, 2007. 2. Buxton J. abstract P 1304, Options VI, 2007.
Factors influencing vaccine uptake in health care providers u Survey of determinants/deterrents of choice to receive vaccination 1 l 103 health care workers in BC, Canada l 77% vaccinated l Vaccination seen as “personal” choice u u perceived risks/benefits to self & family workplace policy (where overlap with personal considerations) access to in-depth, personalized education Survey of vaccination uptake by vaccination providers 2 l 335 nurses, 343 physicians in BC, Canada l 89% intended to receive vaccine, 78% received it ≥ 75% of the time l Drivers for vaccine use: positive direct attitudes to vaccination (2. 5 times more likely), direct social norms (3. 2 times more likely) 1. Masaro C. abstract P 1303, Options VI, 2007. 2. Buxton J. abstract P 1304, Options VI, 2007.
 Vaccination issues in the elderly u Timing of vaccination in elderly patients in France 1 l Median time of vaccination: weeks 43 to 44 (end of October), consistent across years u Controversy over mortality benefits of influenza vaccination 2 l Weaknesses of some analyses: u frailty selection bias u non-specific endpoints u insufficient adjustment approaches 1. Mosnier A. abstract P 1315, Options VI, 2007. 2. Simonsen L. abstract P 1317, Options VI, 2007.
Vaccination issues in the elderly u Timing of vaccination in elderly patients in France 1 l Median time of vaccination: weeks 43 to 44 (end of October), consistent across years u Controversy over mortality benefits of influenza vaccination 2 l Weaknesses of some analyses: u frailty selection bias u non-specific endpoints u insufficient adjustment approaches 1. Mosnier A. abstract P 1315, Options VI, 2007. 2. Simonsen L. abstract P 1317, Options VI, 2007.
 Cost-effectiveness of seasonal influenza vaccination u Systematic review of vaccine cost-effectiveness in 50 - to 64 -year-olds 1 l Few age-based recommendations include 50 - to 64 -year-olds on basis of age alone l Across 4 studies , favourable QALY ratio for vaccination of 50 - to 64 -year-olds regardless of risk factors u Incremental cost-effectiveness of adjuvanted vaccine in France 2 l adjuvanted vaccines: more effective in case of drift l the greater the likelihood of drift, the more cost-effective MF 59 adjuvanted vaccine is l becomes cost-saving at drift rate of 0. 5 (1 mismatch/2 years) 1. Nichol K. abstract O 101, Options VI, 2007. 2. Piercy J. abstract O 102, Options VI, 2007.
Cost-effectiveness of seasonal influenza vaccination u Systematic review of vaccine cost-effectiveness in 50 - to 64 -year-olds 1 l Few age-based recommendations include 50 - to 64 -year-olds on basis of age alone l Across 4 studies , favourable QALY ratio for vaccination of 50 - to 64 -year-olds regardless of risk factors u Incremental cost-effectiveness of adjuvanted vaccine in France 2 l adjuvanted vaccines: more effective in case of drift l the greater the likelihood of drift, the more cost-effective MF 59 adjuvanted vaccine is l becomes cost-saving at drift rate of 0. 5 (1 mismatch/2 years) 1. Nichol K. abstract O 101, Options VI, 2007. 2. Piercy J. abstract O 102, Options VI, 2007.
 Trends and future issues u T Tam (Canada): macroepidemiology of vaccination in 70 countries 1 l Despite H 5 N 1 concern, little change in global vaccine use between 2002 and 2005 l 9 major vaccine-producing countries: 12% of world’s population, ~60% of total vaccine use – political & public health implications u M Miller (NIH, USA): prioritization of pandemic vaccines 2 l YLL models: largest impact in younger age groups with 1918 -like outbreak, older groups with 1957 - or 1968 -like l Outcomes depend on prior exposure/immunity 1. Tam T. abstract O 103, Options VI, 2007. 2. Miller M. abstract O 104, Options VI, 2007.
Trends and future issues u T Tam (Canada): macroepidemiology of vaccination in 70 countries 1 l Despite H 5 N 1 concern, little change in global vaccine use between 2002 and 2005 l 9 major vaccine-producing countries: 12% of world’s population, ~60% of total vaccine use – political & public health implications u M Miller (NIH, USA): prioritization of pandemic vaccines 2 l YLL models: largest impact in younger age groups with 1918 -like outbreak, older groups with 1957 - or 1968 -like l Outcomes depend on prior exposure/immunity 1. Tam T. abstract O 103, Options VI, 2007. 2. Miller M. abstract O 104, Options VI, 2007.


