9233ce7c3ab60f281b941d16c688ecf8.ppt
- Количество слайдов: 51
 Optimizing Treatment for Her 2 Positive Early Stage Breast Cancer Patients Sunil Verma MD, MSEd, FRCPC Medical Oncologist Chair, Breast Medical Oncology Sunnybrook Odette Cancer Centre Associate Professor, University of Toronto
Optimizing Treatment for Her 2 Positive Early Stage Breast Cancer Patients Sunil Verma MD, MSEd, FRCPC Medical Oncologist Chair, Breast Medical Oncology Sunnybrook Odette Cancer Centre Associate Professor, University of Toronto
 Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
 Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
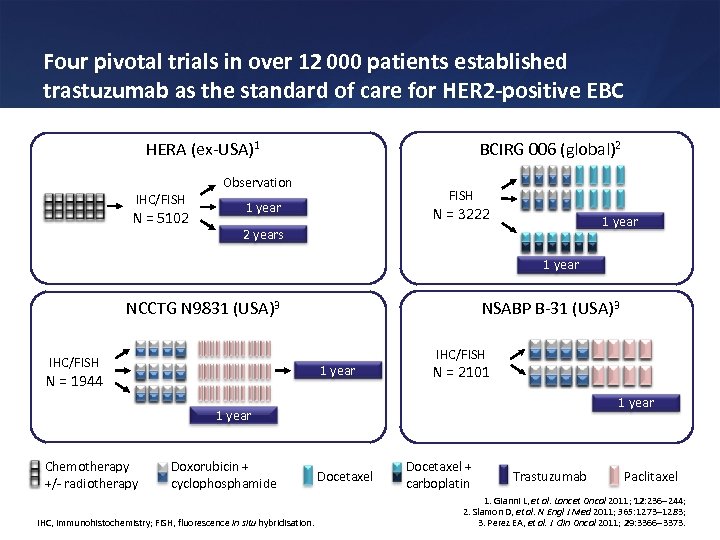 Four pivotal trials in over 12 000 patients established trastuzumab as the standard of care for HER 2 -positive EBC HERA (ex-USA)1 IHC/FISH N = 5102 BCIRG 006 (global)2 Observation FISH 1 year N = 3222 1 year 2 years 1 year NCCTG N 9831 (USA)3 IHC/FISH NSABP B-31 (USA)3 1 year N = 1944 IHC/FISH N = 2101 1 year Chemotherapy +/- radiotherapy Doxorubicin + cyclophosphamide IHC, immunohistochemistry; FISH, fluorescence in situ hybridisation. Docetaxel + carboplatin Trastuzumab Paclitaxel 1. Gianni L, et al. Lancet Oncol 2011; 12: 236 244; 2. Slamon D, et al. N Engl J Med 2011; 365: 1273 1283; 3. Perez EA, et al. J Clin Oncol 2011; 29: 3366 3373.
Four pivotal trials in over 12 000 patients established trastuzumab as the standard of care for HER 2 -positive EBC HERA (ex-USA)1 IHC/FISH N = 5102 BCIRG 006 (global)2 Observation FISH 1 year N = 3222 1 year 2 years 1 year NCCTG N 9831 (USA)3 IHC/FISH NSABP B-31 (USA)3 1 year N = 1944 IHC/FISH N = 2101 1 year Chemotherapy +/- radiotherapy Doxorubicin + cyclophosphamide IHC, immunohistochemistry; FISH, fluorescence in situ hybridisation. Docetaxel + carboplatin Trastuzumab Paclitaxel 1. Gianni L, et al. Lancet Oncol 2011; 12: 236 244; 2. Slamon D, et al. N Engl J Med 2011; 365: 1273 1283; 3. Perez EA, et al. J Clin Oncol 2011; 29: 3366 3373.
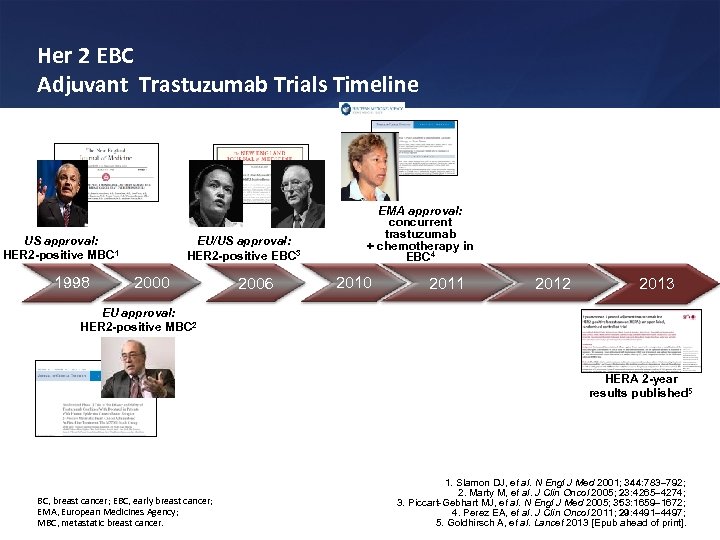 Her 2 EBC Adjuvant Trastuzumab Trials Timeline US approval: HER 2 -positive MBC 1 1998 EU/US approval: HER 2 -positive EBC 3 2000 2006 EMA approval: concurrent trastuzumab + chemotherapy in EBC 4 2010 2011 2012 2013 EU approval: HER 2 -positive MBC 2 HERA 2 -year results published 5 BC, breast cancer; EBC, early breast cancer; EMA, European Medicines Agency; MBC, metastatic breast cancer. 1. Slamon DJ, et al. N Engl J Med 2001; 344: 783 792; 2. Marty M, et al. J Clin Oncol 2005; 23: 4265 4274; 3. Piccart-Gebhart MJ, et al. N Engl J Med 2005; 353: 1659 1672; 4. Perez EA, et al. J Clin Oncol 2011; 29: 4491 4497; 5. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print].
Her 2 EBC Adjuvant Trastuzumab Trials Timeline US approval: HER 2 -positive MBC 1 1998 EU/US approval: HER 2 -positive EBC 3 2000 2006 EMA approval: concurrent trastuzumab + chemotherapy in EBC 4 2010 2011 2012 2013 EU approval: HER 2 -positive MBC 2 HERA 2 -year results published 5 BC, breast cancer; EBC, early breast cancer; EMA, European Medicines Agency; MBC, metastatic breast cancer. 1. Slamon DJ, et al. N Engl J Med 2001; 344: 783 792; 2. Marty M, et al. J Clin Oncol 2005; 23: 4265 4274; 3. Piccart-Gebhart MJ, et al. N Engl J Med 2005; 353: 1659 1672; 4. Perez EA, et al. J Clin Oncol 2011; 29: 4491 4497; 5. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print].
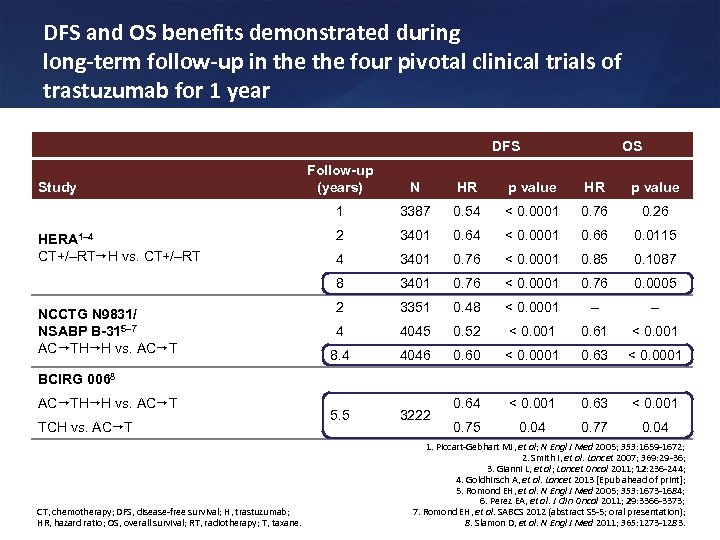 DFS and OS benefits demonstrated during long-term follow-up in the four pivotal clinical trials of trastuzumab for 1 year DFS OS NCCTG N 9831/ NSABP B-315– 7 AC TH H vs. AC T HR p value 3387 0. 54 < 0. 0001 0. 76 0. 26 2 3401 0. 64 < 0. 0001 0. 66 0. 0115 4 3401 0. 76 < 0. 0001 0. 85 0. 1087 8 HERA 1– 4 CT+/–RT H vs. CT+/–RT N 1 Study Follow-up (years) 3401 0. 76 < 0. 0001 0. 76 0. 0005 2 3351 0. 48 < 0. 0001 – – 4 4045 0. 52 < 0. 001 0. 61 < 0. 001 8. 4 4046 0. 60 < 0. 0001 0. 63 < 0. 0001 5. 5 3222 0. 64 < 0. 001 0. 63 < 0. 001 0. 75 0. 04 0. 77 0. 04 BCIRG 0068 AC TH H vs. AC T TCH vs. AC T CT, chemotherapy; DFS, disease-free survival; H, trastuzumab; HR, hazard ratio; OS, overall survival; RT, radiotherapy; T, taxane. 1. Piccart-Gebhart MJ, et al; N Engl J Med 2005; 353: 1659 -1672; 2. Smith I, et al. Lancet 2007; 369: 29 -36; 3. Gianni L, et al; Lancet Oncol 2011; 12: 236 -244; 4. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 5. Romond EH, et al. N Engl J Med 2005; 353: 1673 -1684; 6. Perez EA, et al. J Clin Oncol 2011; 29: 3366 -3373; 7. Romond EH, et al. SABCS 2012 (abstract S 5 -5; oral presentation); 8. Slamon D, et al. N Engl J Med 2011; 365: 1273 -1283.
DFS and OS benefits demonstrated during long-term follow-up in the four pivotal clinical trials of trastuzumab for 1 year DFS OS NCCTG N 9831/ NSABP B-315– 7 AC TH H vs. AC T HR p value 3387 0. 54 < 0. 0001 0. 76 0. 26 2 3401 0. 64 < 0. 0001 0. 66 0. 0115 4 3401 0. 76 < 0. 0001 0. 85 0. 1087 8 HERA 1– 4 CT+/–RT H vs. CT+/–RT N 1 Study Follow-up (years) 3401 0. 76 < 0. 0001 0. 76 0. 0005 2 3351 0. 48 < 0. 0001 – – 4 4045 0. 52 < 0. 001 0. 61 < 0. 001 8. 4 4046 0. 60 < 0. 0001 0. 63 < 0. 0001 5. 5 3222 0. 64 < 0. 001 0. 63 < 0. 001 0. 75 0. 04 0. 77 0. 04 BCIRG 0068 AC TH H vs. AC T TCH vs. AC T CT, chemotherapy; DFS, disease-free survival; H, trastuzumab; HR, hazard ratio; OS, overall survival; RT, radiotherapy; T, taxane. 1. Piccart-Gebhart MJ, et al; N Engl J Med 2005; 353: 1659 -1672; 2. Smith I, et al. Lancet 2007; 369: 29 -36; 3. Gianni L, et al; Lancet Oncol 2011; 12: 236 -244; 4. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 5. Romond EH, et al. N Engl J Med 2005; 353: 1673 -1684; 6. Perez EA, et al. J Clin Oncol 2011; 29: 3366 -3373; 7. Romond EH, et al. SABCS 2012 (abstract S 5 -5; oral presentation); 8. Slamon D, et al. N Engl J Med 2011; 365: 1273 -1283.
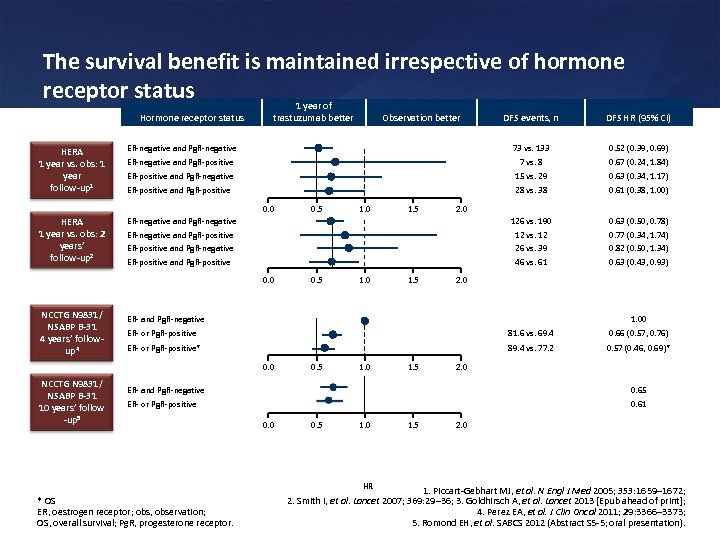 The survival benefit is maintained irrespective of hormone receptor status 1 year of Hormone receptor status DFS events, n DFS HR (95% CI) 73 vs. 133 0. 52 (0. 39, 0. 69) ER-negative and Pg. R-positive 7 vs. 8 0. 67 (0. 24, 1. 84) ER-positive and Pg. R-negative 15 vs. 29 0. 63 (0. 34, 1. 17) ER-positive and Pg. R-positive 28 vs. 38 0. 61 (0. 38, 1. 00) 126 vs. 190 12 vs. 12 26 vs. 39 46 vs. 61 0. 63 (0. 50, 0. 78) 0. 77 (0. 34, 1. 74) 0. 82 (0. 50, 1. 34) 0. 63 (0. 43, 0. 93) ER- and Pg. R-negative ER- or Pg. R-positive 81. 6 vs. 69. 4 1. 00 0. 66 (0. 57, 0. 76) ER- or Pg. R-positive* 89. 4 vs. 77. 2 0. 57 (0. 46, 0. 69)* HERA 1 year vs. obs: 1 year follow-up 1 Observation better ER-negative and Pg. R-negative HERA 1 year vs. obs: 2 years’ follow-up 2 trastuzumab better ER-negative and Pg. R-negative ER-negative and Pg. R-positive ER-positive and Pg. R-negative ER-positive and Pg. R-positive 0. 0 NCCTG N 9831/ NSABP B-31 4 years’ followup 4 0. 0 NCCTG N 9831/ NSABP B-31 10 years’ follow -up 5 0. 5 1. 0 1. 5 2. 0 ER- and Pg. R-negative ER- or Pg. R-positive 0. 65 0. 61 0. 0 0. 5 1. 0 HR * OS ER, oestrogen receptor; obs, observation; OS, overall survival; Pg. R, progesterone receptor. 1. 5 2. 0 1. Piccart-Gebhart MJ, et al. N Engl J Med 2005; 353: 1659 1672; 2. Smith I, et al. Lancet 2007; 369: 29 36; 3. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 4. Perez EA, et al. J Clin Oncol 2011; 29: 3366 3373; 5. Romond EH, et al. SABCS 2012 (Abstract S 5 -5; oral presentation).
The survival benefit is maintained irrespective of hormone receptor status 1 year of Hormone receptor status DFS events, n DFS HR (95% CI) 73 vs. 133 0. 52 (0. 39, 0. 69) ER-negative and Pg. R-positive 7 vs. 8 0. 67 (0. 24, 1. 84) ER-positive and Pg. R-negative 15 vs. 29 0. 63 (0. 34, 1. 17) ER-positive and Pg. R-positive 28 vs. 38 0. 61 (0. 38, 1. 00) 126 vs. 190 12 vs. 12 26 vs. 39 46 vs. 61 0. 63 (0. 50, 0. 78) 0. 77 (0. 34, 1. 74) 0. 82 (0. 50, 1. 34) 0. 63 (0. 43, 0. 93) ER- and Pg. R-negative ER- or Pg. R-positive 81. 6 vs. 69. 4 1. 00 0. 66 (0. 57, 0. 76) ER- or Pg. R-positive* 89. 4 vs. 77. 2 0. 57 (0. 46, 0. 69)* HERA 1 year vs. obs: 1 year follow-up 1 Observation better ER-negative and Pg. R-negative HERA 1 year vs. obs: 2 years’ follow-up 2 trastuzumab better ER-negative and Pg. R-negative ER-negative and Pg. R-positive ER-positive and Pg. R-negative ER-positive and Pg. R-positive 0. 0 NCCTG N 9831/ NSABP B-31 4 years’ followup 4 0. 0 NCCTG N 9831/ NSABP B-31 10 years’ follow -up 5 0. 5 1. 0 1. 5 2. 0 ER- and Pg. R-negative ER- or Pg. R-positive 0. 65 0. 61 0. 0 0. 5 1. 0 HR * OS ER, oestrogen receptor; obs, observation; OS, overall survival; Pg. R, progesterone receptor. 1. 5 2. 0 1. Piccart-Gebhart MJ, et al. N Engl J Med 2005; 353: 1659 1672; 2. Smith I, et al. Lancet 2007; 369: 29 36; 3. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 4. Perez EA, et al. J Clin Oncol 2011; 29: 3366 3373; 5. Romond EH, et al. SABCS 2012 (Abstract S 5 -5; oral presentation).
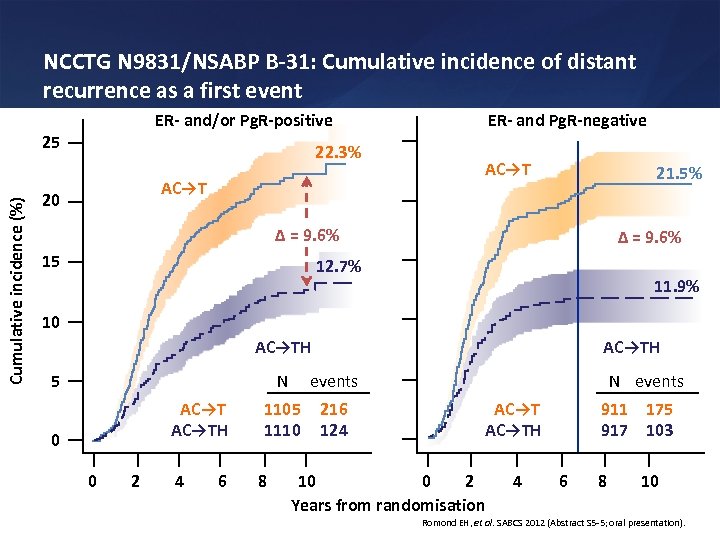 NCCTG N 9831/NSABP B-31: Cumulative incidence of distant recurrence as a first event 25 Cumulative incidence (%) ER- and Pg. R-negative ER- and/or Pg. R-positive 22. 3% AC→T 20 AC→T 21. 5% Δ = 9. 6% 15 Δ = 9. 6% 12. 7% 11. 9% 10 AC→TH N events 1105 1110 216 124 5 AC→TH 0 0 2 4 6 8 N events AC→TH 10 0 2 Years from randomisation 4 911 175 917 103 6 8 10 Romond EH, et al. SABCS 2012 (Abstract S 5 -5; oral presentation).
NCCTG N 9831/NSABP B-31: Cumulative incidence of distant recurrence as a first event 25 Cumulative incidence (%) ER- and Pg. R-negative ER- and/or Pg. R-positive 22. 3% AC→T 20 AC→T 21. 5% Δ = 9. 6% 15 Δ = 9. 6% 12. 7% 11. 9% 10 AC→TH N events 1105 1110 216 124 5 AC→TH 0 0 2 4 6 8 N events AC→TH 10 0 2 Years from randomisation 4 911 175 917 103 6 8 10 Romond EH, et al. SABCS 2012 (Abstract S 5 -5; oral presentation).
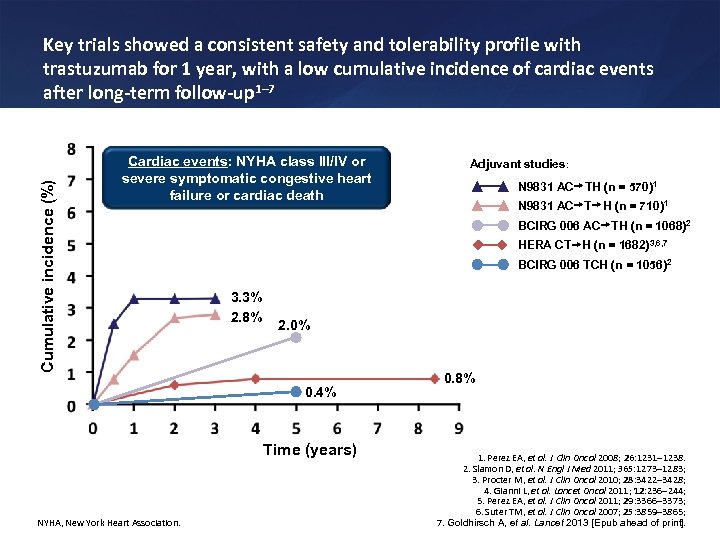 Cumulative incidence (%) Key trials showed a consistent safety and tolerability profile with trastuzumab for 1 year, with a low cumulative incidence of cardiac events after long-term follow-up 1– 7 Cardiac events: NYHA class III/IV or severe symptomatic congestive heart failure or cardiac death N 9831 AC TH (n = 570)1 N 9831 AC T H (n = 710)1 BCIRG 006 AC TH (n = 1068)2 HERA CT H (n = 1682)3, 6, 7 BCIRG 006 TCH (n = 1056)2 3. 3% 2. 8% 2. 0% 0. 4% Time (years) NYHA, New York Heart Association. Adjuvant studies: 0. 8% 1. Perez EA, et al. J Clin Oncol 2008; 26: 1231 1238. 2. Slamon D, et al. N Engl J Med 2011; 365: 1273 1283; 3. Procter M, et al. J Clin Oncol 2010; 28: 3422 3428; 4. Gianni L, et al. Lancet Oncol 2011; 12: 236 244; 5. Perez EA, et al. J Clin Oncol 2011; 29: 3366 3373; 6. Suter TM, et al. J Clin Oncol 2007; 25: 3859 3865; 7. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print].
Cumulative incidence (%) Key trials showed a consistent safety and tolerability profile with trastuzumab for 1 year, with a low cumulative incidence of cardiac events after long-term follow-up 1– 7 Cardiac events: NYHA class III/IV or severe symptomatic congestive heart failure or cardiac death N 9831 AC TH (n = 570)1 N 9831 AC T H (n = 710)1 BCIRG 006 AC TH (n = 1068)2 HERA CT H (n = 1682)3, 6, 7 BCIRG 006 TCH (n = 1056)2 3. 3% 2. 8% 2. 0% 0. 4% Time (years) NYHA, New York Heart Association. Adjuvant studies: 0. 8% 1. Perez EA, et al. J Clin Oncol 2008; 26: 1231 1238. 2. Slamon D, et al. N Engl J Med 2011; 365: 1273 1283; 3. Procter M, et al. J Clin Oncol 2010; 28: 3422 3428; 4. Gianni L, et al. Lancet Oncol 2011; 12: 236 244; 5. Perez EA, et al. J Clin Oncol 2011; 29: 3366 3373; 6. Suter TM, et al. J Clin Oncol 2007; 25: 3859 3865; 7. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print].
 Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Trastuzumab Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Trastuzumab Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
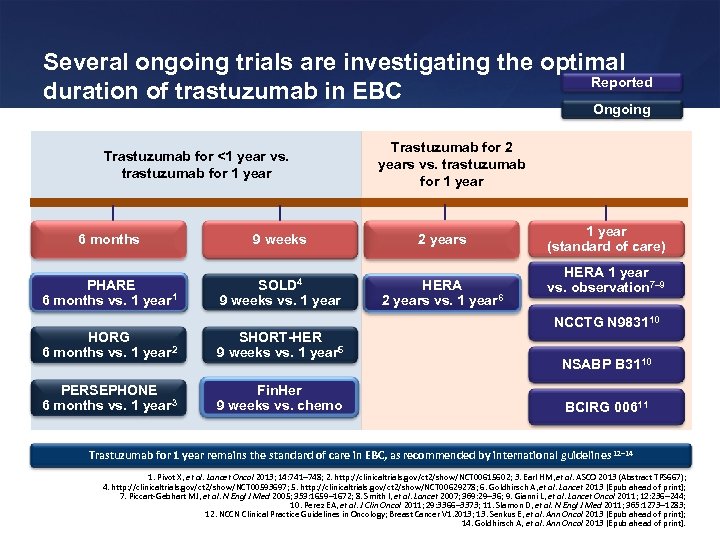 Several ongoing trials are investigating the optimal Reported duration of trastuzumab in EBC Ongoing Trastuzumab for <1 year vs. trastuzumab for 1 year Trastuzumab for 2 years vs. trastuzumab for 1 year 6 months 9 weeks 2 years PHARE 6 months vs. 1 year 1 SOLD 4 9 weeks vs. 1 year HERA 2 years vs. 1 year 6 HORG 6 months vs. 1 year 2 SHORT-HER 9 weeks vs. 1 year 5 PERSEPHONE 6 months vs. 1 year 3 Fin. Her 9 weeks vs. chemo 1 year (standard of care) HERA 1 year vs. observation 7– 9 NCCTG N 983110 NSABP B 3110 BCIRG 00611 Trastuzumab for 1 year remains the standard of care in EBC, as recommended by international guidelines 12– 14 1. Pivot X, et al. Lancet Oncol 2013; 14: 741– 748; 2. http: //clinicaltrials. gov/ct 2/show/NCT 00615602; 3. Earl HM, et al. ASCO 2013 (Abstract TPS 667); 4. http: //clinicaltrials. gov/ct 2/show/NCT 00593697; 5. http: //clinicaltrials. gov/ct 2/show/NCT 00629278; 6. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 7. Piccart-Gebhart MJ, et al. N Engl J Med 2005; 353: 1659– 1672; 8. Smith I, et al. Lancet 2007; 369: 29 36; 9. Gianni L, et al. Lancet Oncol 2011; 12: 236 244; 10. Perez EA, et al. J Clin Oncol 2011; 29: 3366– 3373; 11. Slamon D, et al. N Engl J Med 2011; 365: 1273 1283; 12. NCCN Clinical Practice Guidelines in Oncology; Breast Cancer V 1. 2013; 13. Senkus E, et al. Ann Oncol 2013 [Epub ahead of print]; 14. Goldhirsch A, et al. Ann Oncol 2013 [Epub ahead of print].
Several ongoing trials are investigating the optimal Reported duration of trastuzumab in EBC Ongoing Trastuzumab for <1 year vs. trastuzumab for 1 year Trastuzumab for 2 years vs. trastuzumab for 1 year 6 months 9 weeks 2 years PHARE 6 months vs. 1 year 1 SOLD 4 9 weeks vs. 1 year HERA 2 years vs. 1 year 6 HORG 6 months vs. 1 year 2 SHORT-HER 9 weeks vs. 1 year 5 PERSEPHONE 6 months vs. 1 year 3 Fin. Her 9 weeks vs. chemo 1 year (standard of care) HERA 1 year vs. observation 7– 9 NCCTG N 983110 NSABP B 3110 BCIRG 00611 Trastuzumab for 1 year remains the standard of care in EBC, as recommended by international guidelines 12– 14 1. Pivot X, et al. Lancet Oncol 2013; 14: 741– 748; 2. http: //clinicaltrials. gov/ct 2/show/NCT 00615602; 3. Earl HM, et al. ASCO 2013 (Abstract TPS 667); 4. http: //clinicaltrials. gov/ct 2/show/NCT 00593697; 5. http: //clinicaltrials. gov/ct 2/show/NCT 00629278; 6. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 7. Piccart-Gebhart MJ, et al. N Engl J Med 2005; 353: 1659– 1672; 8. Smith I, et al. Lancet 2007; 369: 29 36; 9. Gianni L, et al. Lancet Oncol 2011; 12: 236 244; 10. Perez EA, et al. J Clin Oncol 2011; 29: 3366– 3373; 11. Slamon D, et al. N Engl J Med 2011; 365: 1273 1283; 12. NCCN Clinical Practice Guidelines in Oncology; Breast Cancer V 1. 2013; 13. Senkus E, et al. Ann Oncol 2013 [Epub ahead of print]; 14. Goldhirsch A, et al. Ann Oncol 2013 [Epub ahead of print].
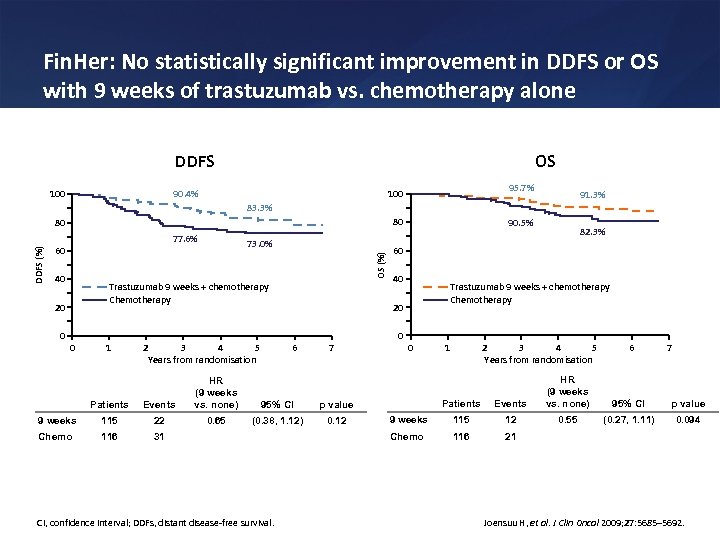 Fin. Her: No statistically significant improvement in DDFS or OS with 9 weeks of trastuzumab vs. chemotherapy alone DDFS OS 90. 4% 100 95. 7% 100 91. 3% 83. 3% 80 80 73. 0% 60 OS (%) DDFS (%) 77. 6% 40 Trastuzumab 9 weeks + chemotherapy Chemotherapy 20 0 1 2 3 4 5 Years from randomisation 40 Patients Events HR (9 weeks vs. none) 9 weeks 115 22 0. 65 Chemo 116 31 Trastuzumab 9 weeks + chemotherapy Chemotherapy 20 6 7 95% CI 0. 12 0 CI, confidence interval; DDFs, distant disease-free survival. 1 2 3 4 5 Years from randomisation Patients Events HR (9 weeks vs. none) 9 weeks 115 12 0. 55 Chemo 116 6 7 21 p value (0. 38, 1. 12) 82. 3% 60 0 0 90. 5% 95% CI p value (0. 27, 1. 11) 0. 094 Joensuu H, et al. J Clin Oncol 2009; 27: 5685– 5692.
Fin. Her: No statistically significant improvement in DDFS or OS with 9 weeks of trastuzumab vs. chemotherapy alone DDFS OS 90. 4% 100 95. 7% 100 91. 3% 83. 3% 80 80 73. 0% 60 OS (%) DDFS (%) 77. 6% 40 Trastuzumab 9 weeks + chemotherapy Chemotherapy 20 0 1 2 3 4 5 Years from randomisation 40 Patients Events HR (9 weeks vs. none) 9 weeks 115 22 0. 65 Chemo 116 31 Trastuzumab 9 weeks + chemotherapy Chemotherapy 20 6 7 95% CI 0. 12 0 CI, confidence interval; DDFs, distant disease-free survival. 1 2 3 4 5 Years from randomisation Patients Events HR (9 weeks vs. none) 9 weeks 115 12 0. 55 Chemo 116 6 7 21 p value (0. 38, 1. 12) 82. 3% 60 0 0 90. 5% 95% CI p value (0. 27, 1. 11) 0. 094 Joensuu H, et al. J Clin Oncol 2009; 27: 5685– 5692.
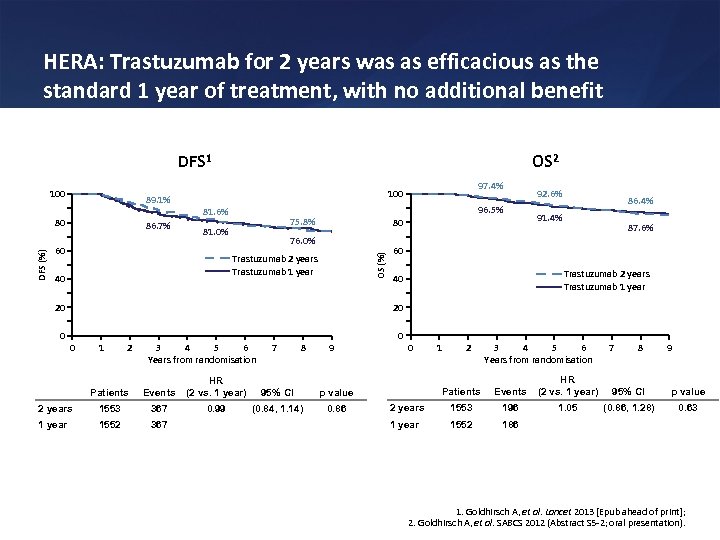 HERA: Trastuzumab for 2 years was as efficacious as the standard 1 year of treatment, with no additional benefit DFS 1 89. 1% DFS (%) 80 86. 7% 81. 6% 96. 5% 75. 8% 81. 0% 60 80 76. 0% Trastuzumab 2 years Trastuzumab 1 year 40 92. 6% 86. 4% 91. 4% 87. 6% 60 Trastuzumab 2 years Trastuzumab 1 year 40 20 20 0 97. 4% 100 OS (%) 100 OS 2 0 0 1 2 3 4 5 6 Years from randomisation 7 8 9 Patients Events HR (2 vs. 1 year) 95% CI 1553 367 0. 99 (0. 84, 1. 14) 0. 86 1 year 1552 367 1 2 3 4 5 6 Years from randomisation 7 8 9 Patients Events HR (2 vs. 1 year) 95% CI p value 2 years 1553 196 1. 05 (0. 86, 1. 28) 0. 63 1 year 1552 186 p value 2 years 0 1. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 2. Goldhirsch A, et al. SABCS 2012 (Abstract S 5 -2; oral presentation).
HERA: Trastuzumab for 2 years was as efficacious as the standard 1 year of treatment, with no additional benefit DFS 1 89. 1% DFS (%) 80 86. 7% 81. 6% 96. 5% 75. 8% 81. 0% 60 80 76. 0% Trastuzumab 2 years Trastuzumab 1 year 40 92. 6% 86. 4% 91. 4% 87. 6% 60 Trastuzumab 2 years Trastuzumab 1 year 40 20 20 0 97. 4% 100 OS (%) 100 OS 2 0 0 1 2 3 4 5 6 Years from randomisation 7 8 9 Patients Events HR (2 vs. 1 year) 95% CI 1553 367 0. 99 (0. 84, 1. 14) 0. 86 1 year 1552 367 1 2 3 4 5 6 Years from randomisation 7 8 9 Patients Events HR (2 vs. 1 year) 95% CI p value 2 years 1553 196 1. 05 (0. 86, 1. 28) 0. 63 1 year 1552 186 p value 2 years 0 1. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 2. Goldhirsch A, et al. SABCS 2012 (Abstract S 5 -2; oral presentation).
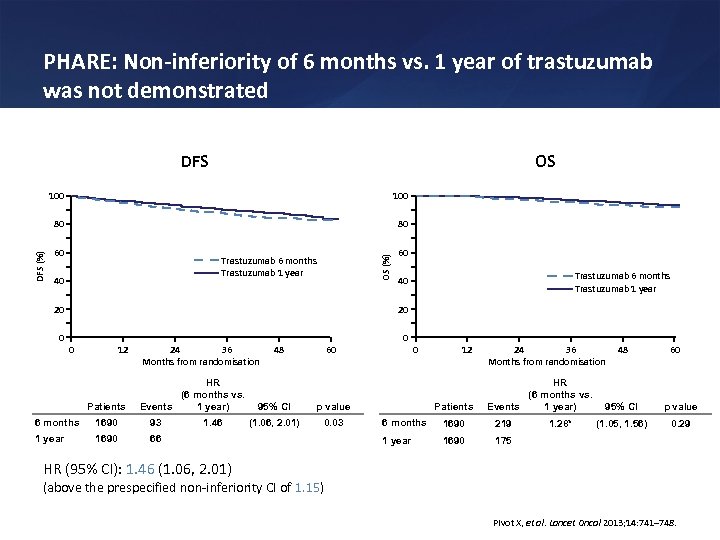 PHARE: Non-inferiority of 6 months vs. 1 year of trastuzumab was not demonstrated DFS OS 80 60 OS (%) 100 80 DFS (%) 100 Trastuzumab 6 months Trastuzumab 1 year 40 20 20 0 60 0 12 Patients 24 36 Months from randomisation HR (6 months vs. Events 1 year) 6 months 1690 93 1 year 1690 48 60 95% CI (1. 06, 2. 01) 0. 03 0 p value 66 1. 46 0 12 Patients 24 36 Months from randomisation HR (6 months vs. Events 1 year) 6 months 1690 219 1 year 1690 48 60 95% CI p value (1. 05, 1. 56) 0. 29 175 1. 28* HR (95% CI): 1. 46 (1. 06, 2. 01) (above the prespecified non-inferiority CI of 1. 15) Pivot X, et al. Lancet Oncol 2013; 14: 741– 748.
PHARE: Non-inferiority of 6 months vs. 1 year of trastuzumab was not demonstrated DFS OS 80 60 OS (%) 100 80 DFS (%) 100 Trastuzumab 6 months Trastuzumab 1 year 40 20 20 0 60 0 12 Patients 24 36 Months from randomisation HR (6 months vs. Events 1 year) 6 months 1690 93 1 year 1690 48 60 95% CI (1. 06, 2. 01) 0. 03 0 p value 66 1. 46 0 12 Patients 24 36 Months from randomisation HR (6 months vs. Events 1 year) 6 months 1690 219 1 year 1690 48 60 95% CI p value (1. 05, 1. 56) 0. 29 175 1. 28* HR (95% CI): 1. 46 (1. 06, 2. 01) (above the prespecified non-inferiority CI of 1. 15) Pivot X, et al. Lancet Oncol 2013; 14: 741– 748.
 Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
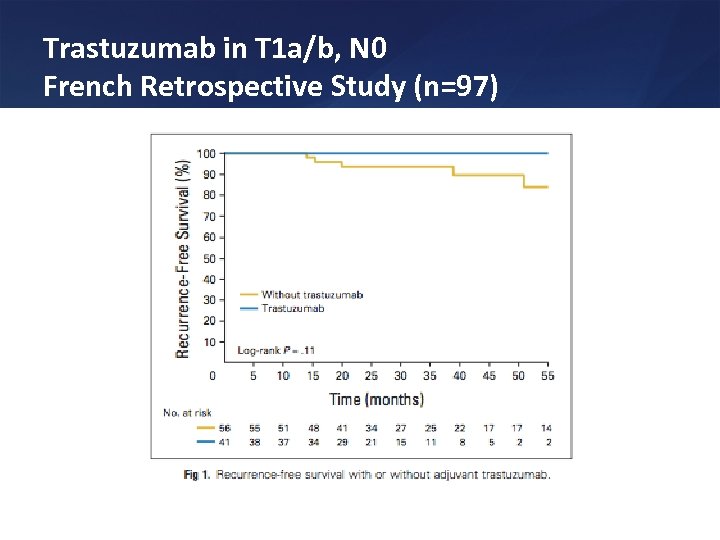 Trastuzumab in T 1 a/b, N 0 French Retrospective Study (n=97)
Trastuzumab in T 1 a/b, N 0 French Retrospective Study (n=97)
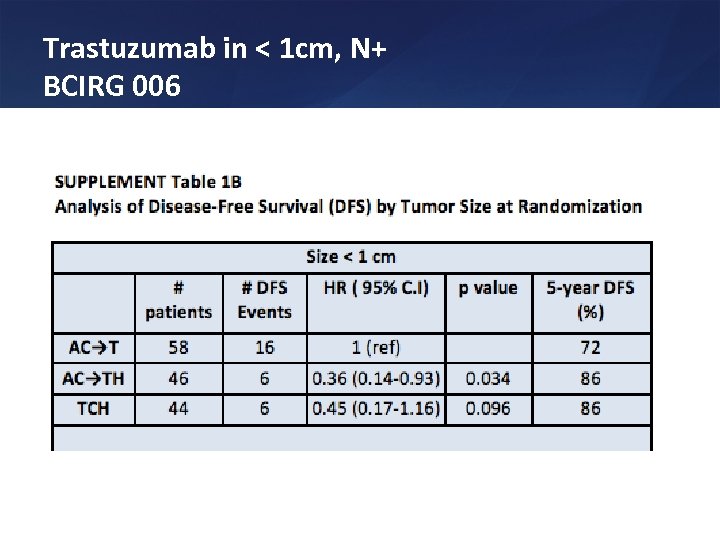 Trastuzumab in < 1 cm, N+ BCIRG 006
Trastuzumab in < 1 cm, N+ BCIRG 006

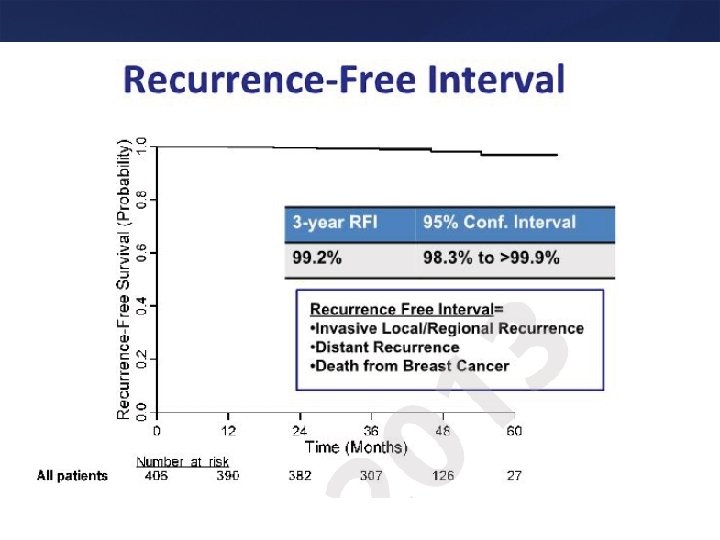
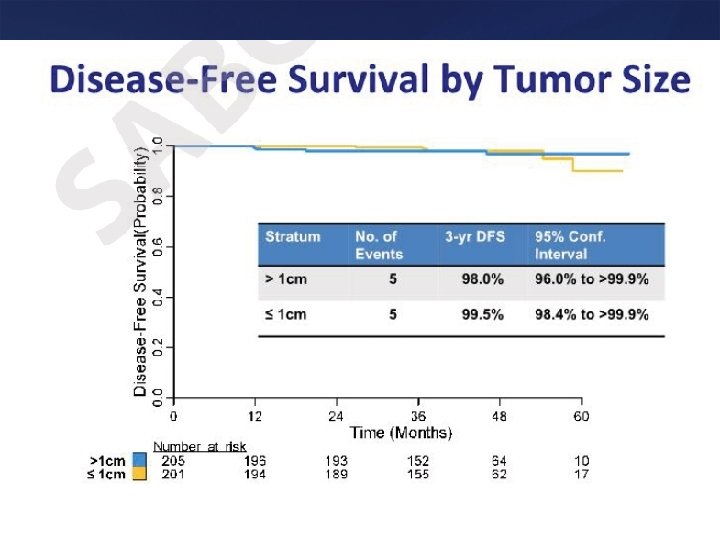
 Summary • < 1 cm node negative patients should be considered for trastuzumab – One has to weigh potential toxicity and absolute benefit, especially for T 1 a tumors – Paclitaxel + Trastuzumab may be a very effective and well tolerated approach for T 1 N 0 Her 2 positive tumors or for patients not deemed to be suitable for anthracyclines and docetaxel based regimens
Summary • < 1 cm node negative patients should be considered for trastuzumab – One has to weigh potential toxicity and absolute benefit, especially for T 1 a tumors – Paclitaxel + Trastuzumab may be a very effective and well tolerated approach for T 1 N 0 Her 2 positive tumors or for patients not deemed to be suitable for anthracyclines and docetaxel based regimens
 Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
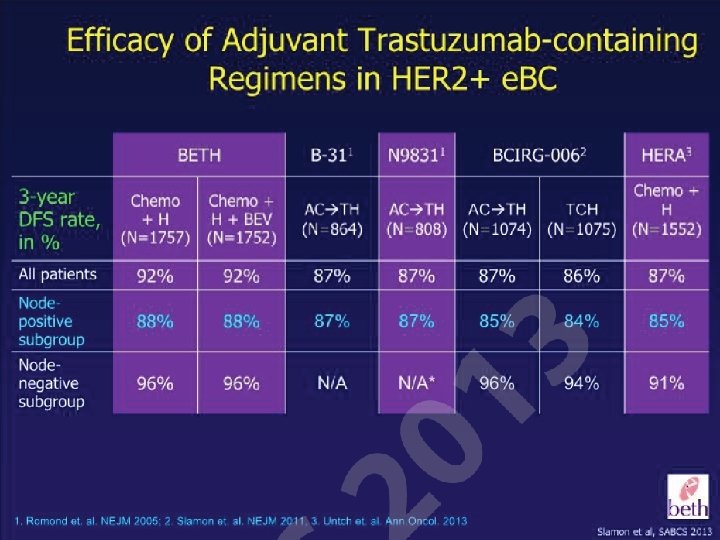
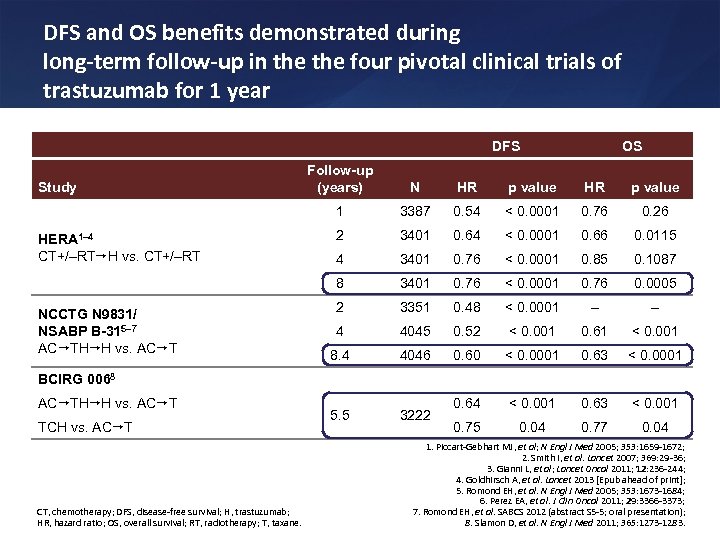 DFS and OS benefits demonstrated during long-term follow-up in the four pivotal clinical trials of trastuzumab for 1 year DFS OS NCCTG N 9831/ NSABP B-315– 7 AC TH H vs. AC T HR p value 3387 0. 54 < 0. 0001 0. 76 0. 26 2 3401 0. 64 < 0. 0001 0. 66 0. 0115 4 3401 0. 76 < 0. 0001 0. 85 0. 1087 8 HERA 1– 4 CT+/–RT H vs. CT+/–RT N 1 Study Follow-up (years) 3401 0. 76 < 0. 0001 0. 76 0. 0005 2 3351 0. 48 < 0. 0001 – – 4 4045 0. 52 < 0. 001 0. 61 < 0. 001 8. 4 4046 0. 60 < 0. 0001 0. 63 < 0. 0001 5. 5 3222 0. 64 < 0. 001 0. 63 < 0. 001 0. 75 0. 04 0. 77 0. 04 BCIRG 0068 AC TH H vs. AC T TCH vs. AC T CT, chemotherapy; DFS, disease-free survival; H, trastuzumab; HR, hazard ratio; OS, overall survival; RT, radiotherapy; T, taxane. 1. Piccart-Gebhart MJ, et al; N Engl J Med 2005; 353: 1659 -1672; 2. Smith I, et al. Lancet 2007; 369: 29 -36; 3. Gianni L, et al; Lancet Oncol 2011; 12: 236 -244; 4. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 5. Romond EH, et al. N Engl J Med 2005; 353: 1673 -1684; 6. Perez EA, et al. J Clin Oncol 2011; 29: 3366 -3373; 7. Romond EH, et al. SABCS 2012 (abstract S 5 -5; oral presentation); 8. Slamon D, et al. N Engl J Med 2011; 365: 1273 -1283.
DFS and OS benefits demonstrated during long-term follow-up in the four pivotal clinical trials of trastuzumab for 1 year DFS OS NCCTG N 9831/ NSABP B-315– 7 AC TH H vs. AC T HR p value 3387 0. 54 < 0. 0001 0. 76 0. 26 2 3401 0. 64 < 0. 0001 0. 66 0. 0115 4 3401 0. 76 < 0. 0001 0. 85 0. 1087 8 HERA 1– 4 CT+/–RT H vs. CT+/–RT N 1 Study Follow-up (years) 3401 0. 76 < 0. 0001 0. 76 0. 0005 2 3351 0. 48 < 0. 0001 – – 4 4045 0. 52 < 0. 001 0. 61 < 0. 001 8. 4 4046 0. 60 < 0. 0001 0. 63 < 0. 0001 5. 5 3222 0. 64 < 0. 001 0. 63 < 0. 001 0. 75 0. 04 0. 77 0. 04 BCIRG 0068 AC TH H vs. AC T TCH vs. AC T CT, chemotherapy; DFS, disease-free survival; H, trastuzumab; HR, hazard ratio; OS, overall survival; RT, radiotherapy; T, taxane. 1. Piccart-Gebhart MJ, et al; N Engl J Med 2005; 353: 1659 -1672; 2. Smith I, et al. Lancet 2007; 369: 29 -36; 3. Gianni L, et al; Lancet Oncol 2011; 12: 236 -244; 4. Goldhirsch A, et al. Lancet 2013 [Epub ahead of print]; 5. Romond EH, et al. N Engl J Med 2005; 353: 1673 -1684; 6. Perez EA, et al. J Clin Oncol 2011; 29: 3366 -3373; 7. Romond EH, et al. SABCS 2012 (abstract S 5 -5; oral presentation); 8. Slamon D, et al. N Engl J Med 2011; 365: 1273 -1283.
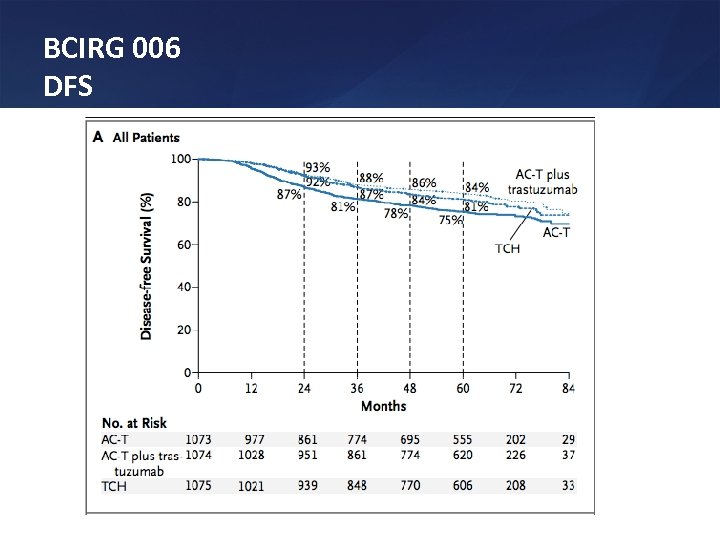 BCIRG 006 DFS
BCIRG 006 DFS
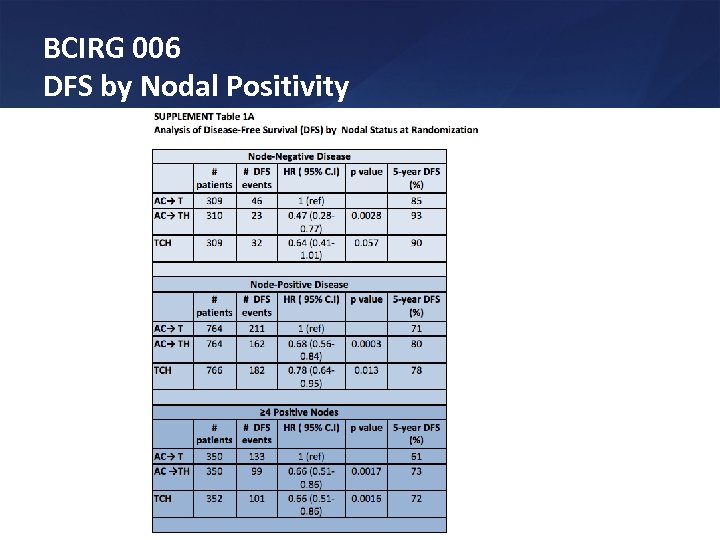 BCIRG 006 DFS by Nodal Positivity
BCIRG 006 DFS by Nodal Positivity


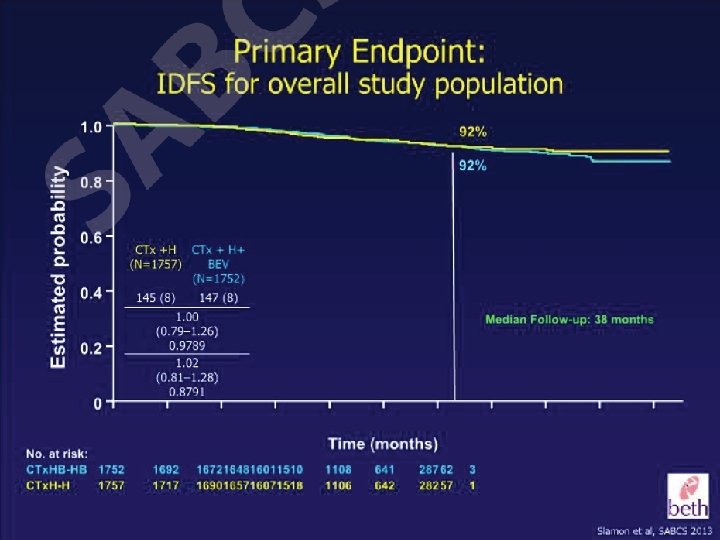
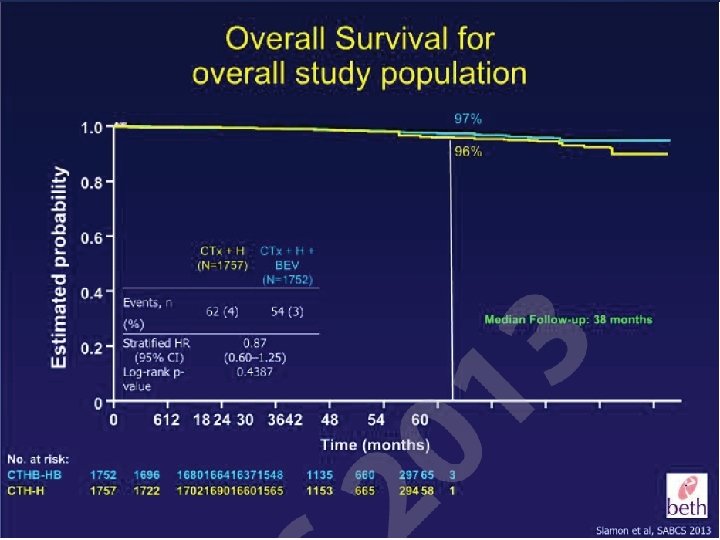
 Which patients should we considered for a nonanthracycline based anti Her 2 alternative option? • Patients with cardiac risk factors or underlying cardiac disease • Patients where the absolute benefit of adjuvant therapy may be low – T 1 a, T 1 b tumors • Older patients (? >70 years of age)
Which patients should we considered for a nonanthracycline based anti Her 2 alternative option? • Patients with cardiac risk factors or underlying cardiac disease • Patients where the absolute benefit of adjuvant therapy may be low – T 1 a, T 1 b tumors • Older patients (? >70 years of age)
 Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies
 First results from the phase III ALTTO trial (BIG 02 -06; NCCTG 063 D) comparing one year of anti-HER 2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T L) or their combination (L + T) in the adjuvant treatment of HER 2 -positive early breast cancer (EBC) Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
First results from the phase III ALTTO trial (BIG 02 -06; NCCTG 063 D) comparing one year of anti-HER 2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T L) or their combination (L + T) in the adjuvant treatment of HER 2 -positive early breast cancer (EBC) Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
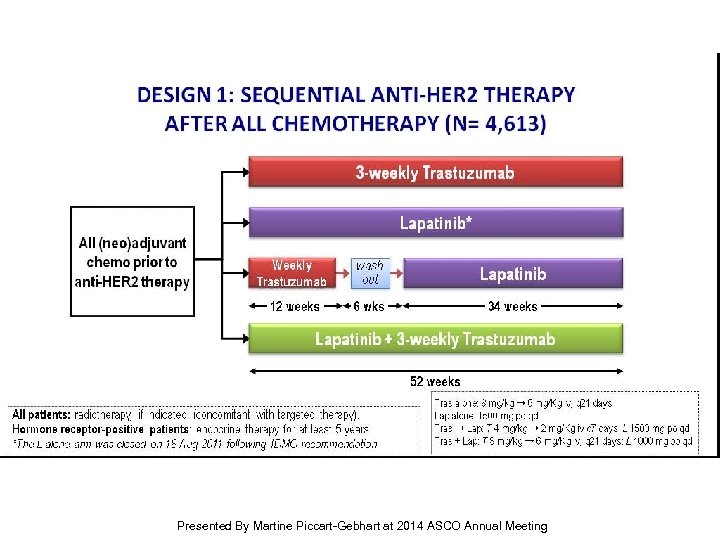 Slide 5 Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
Slide 5 Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
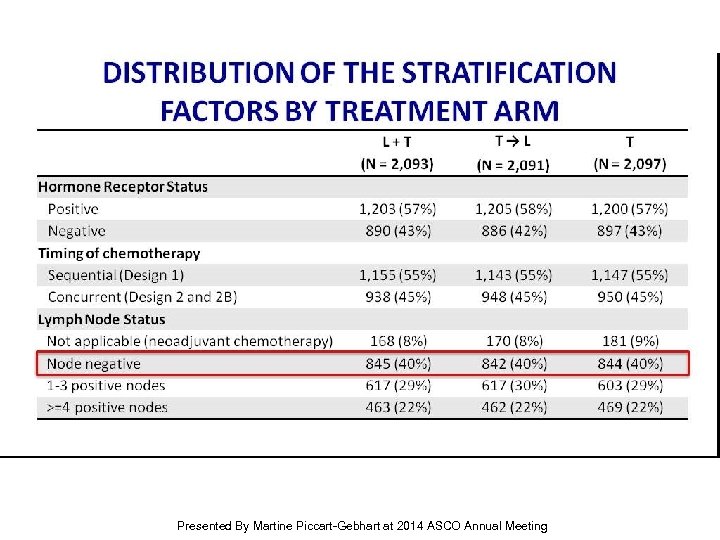 Distribution of the Stratification Factors by Treatment Arm Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
Distribution of the Stratification Factors by Treatment Arm Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
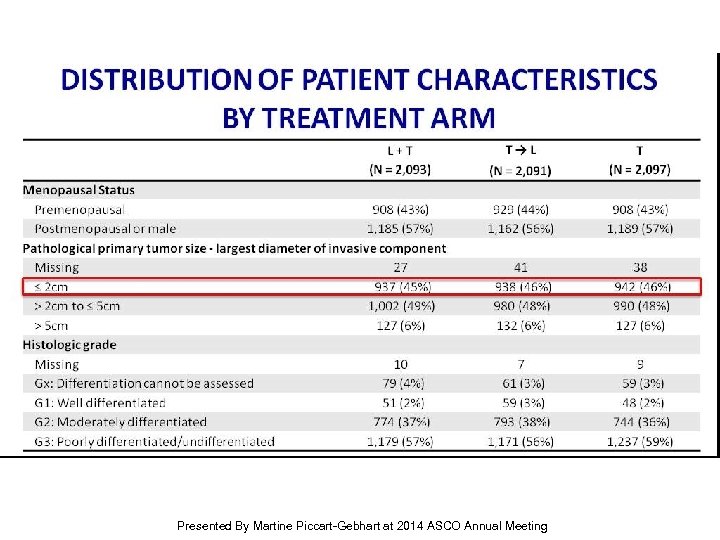 Distribution of patient characteristics by Treatment Arm Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
Distribution of patient characteristics by Treatment Arm Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
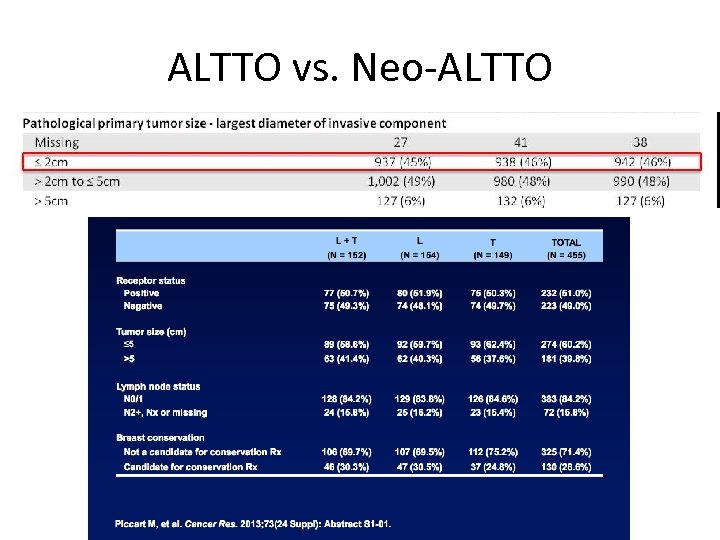 ALTTO vs. Neo-ALTTO
ALTTO vs. Neo-ALTTO
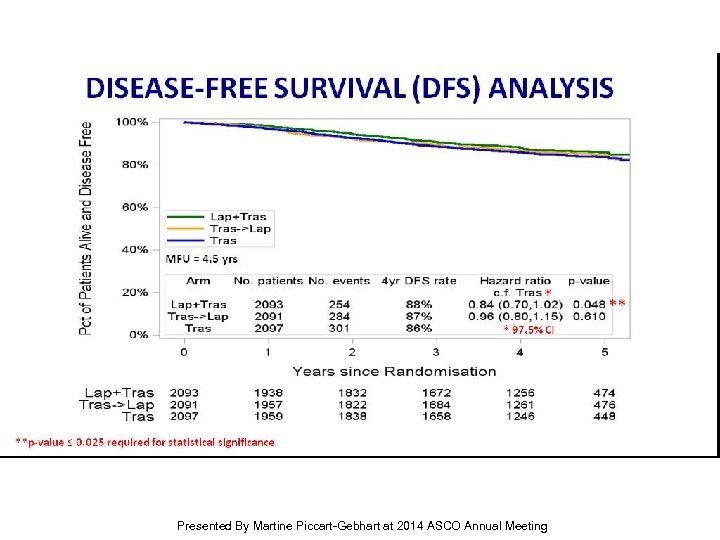 DISEASE-FREE SURVIVAL (DFS) ANALYSIS Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
DISEASE-FREE SURVIVAL (DFS) ANALYSIS Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
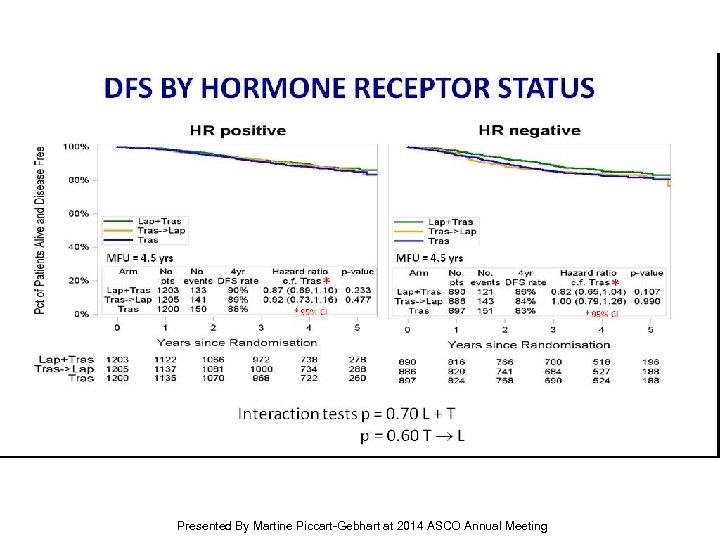 DFS BY Hormone Receptor Status Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
DFS BY Hormone Receptor Status Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
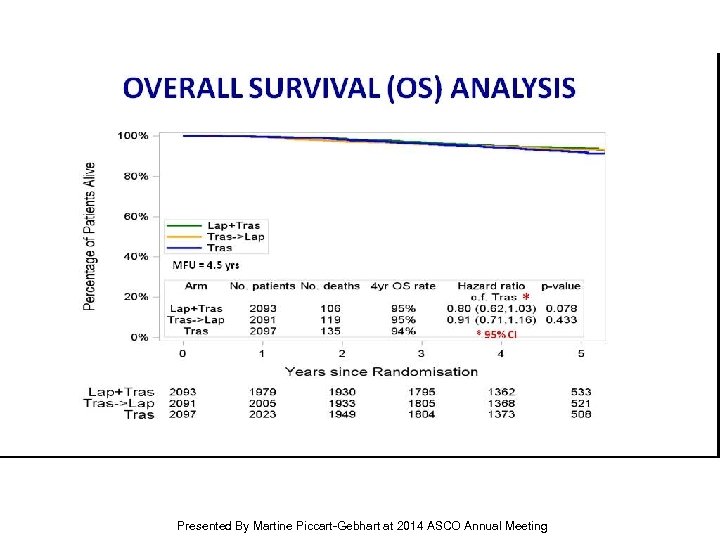 OVERALL SURVIVAL (OS) ANALYSIS Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
OVERALL SURVIVAL (OS) ANALYSIS Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
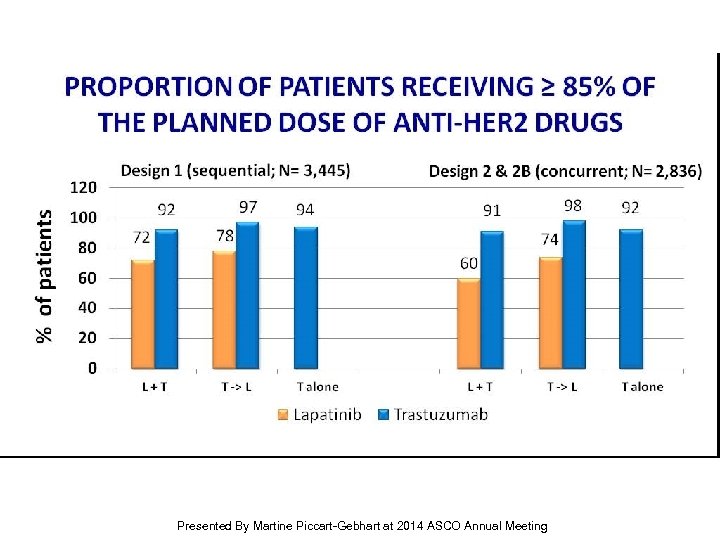 PROPORTION OF PATIENTS RECEIVING ≥ 85% OF THE PLANNED DOSE OF ANTI-HER 2 DRUGS Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
PROPORTION OF PATIENTS RECEIVING ≥ 85% OF THE PLANNED DOSE OF ANTI-HER 2 DRUGS Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
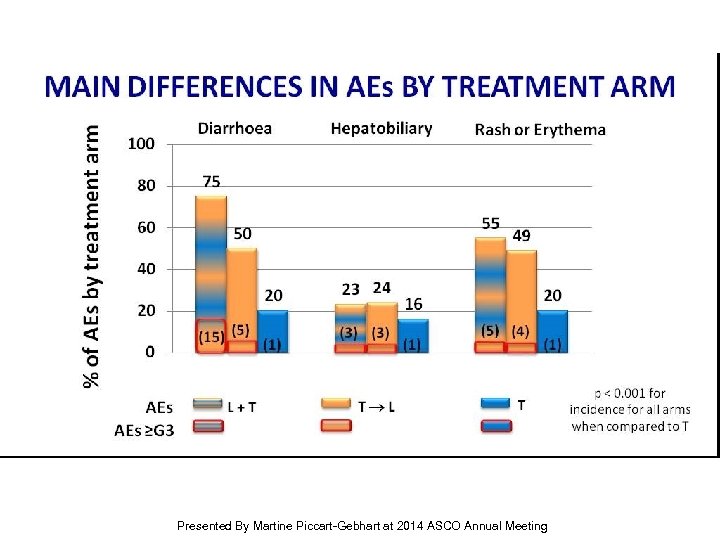 MAIN DIFFERENCES IN AEs BY TREATMENT ARM Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
MAIN DIFFERENCES IN AEs BY TREATMENT ARM Presented By Martine Piccart-Gebhart at 2014 ASCO Annual Meeting
 Bottom Line • There is no role for Lapatinib in the adjuvant setting • Why is ALTTO negative? – The ‘Ceiling Effect’ • OS > 95% in the control arm • low risk patient population • very effective and well-tolerated control arm – Dose Delivery in the experimental arm – Is it related to MOA for TKI
Bottom Line • There is no role for Lapatinib in the adjuvant setting • Why is ALTTO negative? – The ‘Ceiling Effect’ • OS > 95% in the control arm • low risk patient population • very effective and well-tolerated control arm – Dose Delivery in the experimental arm – Is it related to MOA for TKI
 Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies Future Directions and Concluding Remarks
Outline • • • Overview of Adjuvant EBC Her 2 Positive Trials Duration of Therapy < 1 cm Node Negative Role of Non-Anthracycline based chemotherapy Incorporation of other Anti Her 2 Therapies Future Directions and Concluding Remarks
 Future Questions in EBC • Which is the best combination of targeted agents? – Lapatinib and Trastuzumab – Pertuzumab and Trastuzumab – ? T-DM 1 and Pertuzumab • Do we need to offer patients one year of Herceptin or one year of combined blockade? • ? Role of anti-HER 2 agents in HER 2 1+ or 2+ patients 49
Future Questions in EBC • Which is the best combination of targeted agents? – Lapatinib and Trastuzumab – Pertuzumab and Trastuzumab – ? T-DM 1 and Pertuzumab • Do we need to offer patients one year of Herceptin or one year of combined blockade? • ? Role of anti-HER 2 agents in HER 2 1+ or 2+ patients 49
 Improving the outcome of EBC patients A Cost-Effective Approach • Which patients are ‘cured’ with surgery alone? • Which patients are cured with surgery and traditional chemotherapy? • Which patients require trastuzumab, only for a short duration i. e. 9 weeks or 6 months? • Which patients don’t benefit from chemotherapy and trastuzumab?
Improving the outcome of EBC patients A Cost-Effective Approach • Which patients are ‘cured’ with surgery alone? • Which patients are cured with surgery and traditional chemotherapy? • Which patients require trastuzumab, only for a short duration i. e. 9 weeks or 6 months? • Which patients don’t benefit from chemotherapy and trastuzumab?
 Conclusion • There is continued and maintained benefit with adjuvant trastuzumab • Duration of Trastuzumab remains to be fully defined – At present one year is standard of treatment • Identifying the patients who are at a greater risk – Address those who remain at high risk • The focus needs to be on how we can gain better outcomes with less toxicity – Integration of novel therapies vs. conventional traditional chemotherapy
Conclusion • There is continued and maintained benefit with adjuvant trastuzumab • Duration of Trastuzumab remains to be fully defined – At present one year is standard of treatment • Identifying the patients who are at a greater risk – Address those who remain at high risk • The focus needs to be on how we can gain better outcomes with less toxicity – Integration of novel therapies vs. conventional traditional chemotherapy


