3f1cae44add48902ac2d02a43626100a.ppt
- Количество слайдов: 17

Optimizing the global pharmaceutical value chain for improved health and development

PRESENTATION OUTLINE Background and milestones 5 principles underpinning the AU model for promoting and developing Africa’s pharmaceutical manufacturing industry Overview of the pharmaceutical manufacturing system situation in Africa Key achievements, challenges and opportunities Recommendations on way forward

Introduction and milestones PMPA: Borne out of the recognition by African Heads of state of the tremendous challenges facing African healthcare systems Medicines regulatory systems strengthening: borne out of need to ensure quality, safety, and efficacy of products Original decision to develop a PMPA – Abuja 2005 q Initial Plan endorsed by Heads of State – Accra 2007 q Endorsement of Business plan – 2012 q q AMRH Programme developed -2009 q AU model law on medical product regulations-2013 q Endorsement of the concept and milestones for establishment of AMA- 2015

5 principles underpinning the promotion of Africa’s Pharmaceutical Manufacturing agenda(1/2) 1. 2. 3. Quality and affordability of medical products is essential for promoting public health and for viability and sustainability of the local industry ; Expansion of the local industry will trigger strengthening of regulatory systems to promote safety, efficacy and curtail counterfeit products; Competitiveness driven by enhancing skills, innovation & technology base will bring the industry closer to the consumer market;

5 principles underpinning the promotion of Africa’s Pharmaceutical Manufacturing agenda 4. Self reliance will emanate from optimizing the global pharmaceutical value chains as opposed to dependence on imported finished products; 5. Development and enforcement of coherent policies across supporting sectors will create enabling environment for sustainability of robust healthcare delivery systems.
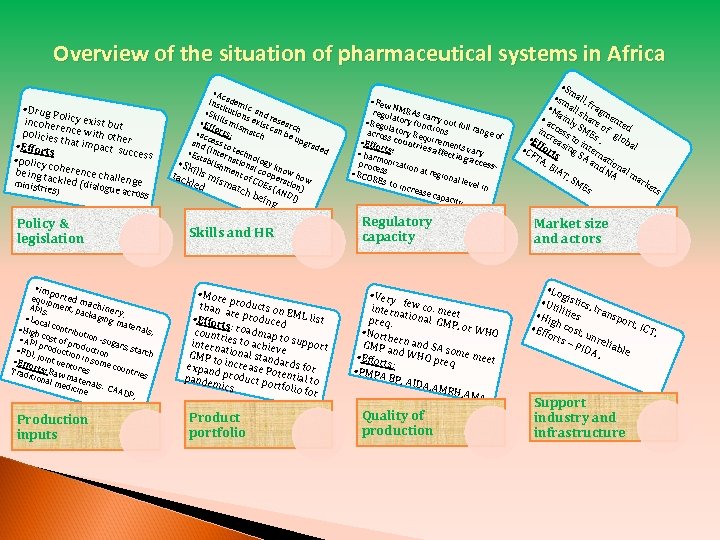
Overview of the situation of pharmaceutical systems in Africa • Ac • Drug P o incoher licy exist but policies ence with oth er t • Effort hat impact su ccess s • policy c being t oherence chal ackled minist (dialo lenge ries) gue acr o ss Policy & legislation • imp equi orted m p APIs ment, p achiner acka. ging y, • Loc mate al co rials n • Hig , h cos tribution t of p -suga • API rodu prod rs, st ction uctio arch • FDI , j n • Effo oint vent in some coun Trad rts: Raw ures tries ition al me material s: CA dicin ADP e , Production inputs inst adem i i • Sk tution c and ills s ex rese m • Ef for isma ist can arch ts: tch • ac be u pgr and cess to ade (int tec d • Es hno ern t • Sk ablishm ational logy kn o i c tack lls mis ent of C ooperaw how OEs tion ma led ) ( tch bei ANDI) n g Skills and HR • More p than a roducts on E r • Effor e produced ML list t count s: roadmap ries to to sup intern port ationa achieve GMP t l stand o expan increase P ards for d prod otenti pande a mics uct portfoli l to o for Product portfolio • Few N regu MRAs ca lator y fun rry out fu • Reg ct u ll ran ge of acros latory Re ions quire s cou • Effo ment ntrie r s affe s vary • har ts: cting moni acce z proc ssess ation at r egion • RCO al lev REs to inc el in rease capa city Regulatory capacity • Very intern few co. mee t a preq. tional GMP , or W HO • North ern an GMP a d SA s n o • Effor d WHO preq me meet ts: • PMPA BP, AI DA, AM RH, AM A, Quality of production • Sm • sm all, • M all s fragm • a ainly hare ent e c inc cess SME of g d rea to s, lob • E i al • C ffort sing nter FT s SA na A, an tion BIA d N al T, A ma SM rke Es ts Market size and actors • Lo g • Ut istics, iliti tran e • Hi spo gh c s rt, I • Eff ost, CT, orts unr el – PI DA iable , Support industry and infrastructure

Achievements(1/3) Maintaining sector on continental and global agenda while leveraging support through international multilateral and bilateral cooperation China, EU, USA, India. . ; Creating strategic partnerships, collaboration with local and global public and private sector actors; Facilitating country and regional level adoption/adaption /implementation of continentally agreed policy, legislative and regulatory frameworks/strategies/initiatives e. g AU Roadmap on SRGS, AMRH, AMA, AU model law on medical product regulation;

Achievements(2/3) Supporting enhancement of national and regional institutional manufacturing and regulatory capacities and capabilities(sector strategies for some MS and RECs e. g. EAC, ECOWAS, SADC; Anchor/leverage other continental socio economic development efforts (industry(AIDA), trade(CFTA), infrastructure(PIDA), agriculture(CAADP), Science and Technology and Innovation(STISA) , CAP etc. )”.
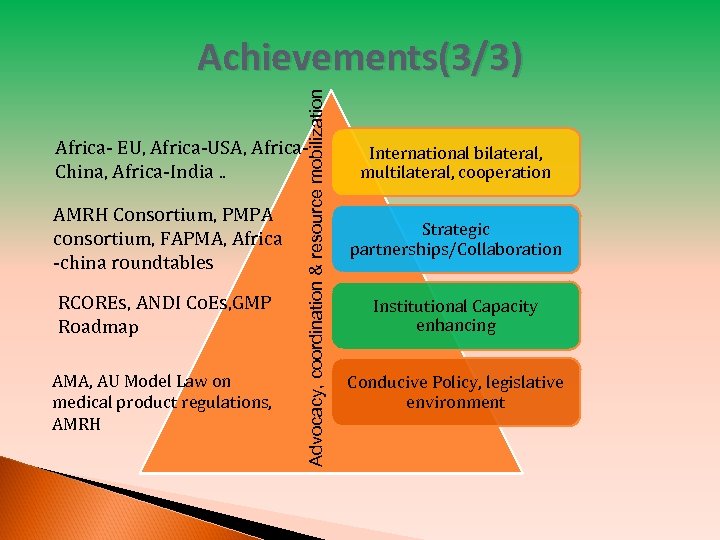
Advocacy, coordination & resource mobilization Achievements(3/3) Africa- EU, Africa-USA, Africa. China, Africa-India. . AMRH Consortium, PMPA consortium, FAPMA, Africa -china roundtables RCOREs, ANDI Co. Es, GMP Roadmap AMA, AU Model Law on medical product regulations, AMRH International bilateral, multilateral, cooperation Strategic partnerships/Collaboration Institutional Capacity enhancing Conducive Policy, legislative environment
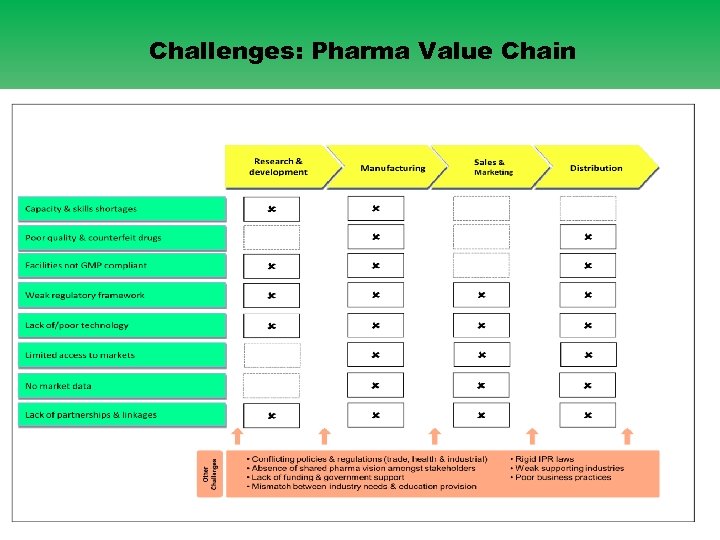
Challenges: Pharma Value Chain 10

challenges –public health Disease burden Infrastructure • 25% of the global disease burden • High infectious disease burden • Growing non communicable disease • Software – Shortage of HR ~ 3% of the world’s healthcare workers deployed in Africa – inequitably distributed • Hard – clinics, hospitals, labs, inadequate • Supply chain • 1% of global healthcare expenditure Funding • Limited National budgets (<10 countries have met Abuja’s target of 15% allocation) • Dependence external aid 11

Opportunities for growth Economic Public health Support industry • 1. 3 billion population by 2020 • Combined GDP of 2. 9 trn • Healthcare expenditure of 200 billion • Pharma market of USD 23 billion • Household income >20 USD Investments in healthcare reforms • Patent expiries and TRIPS flexibilities • Changing disease patterns towards chronic • Towards universal health coverage • Infrastructure boom- transport, communication, energy • Education –innovation, R&D, skills matching market requirement • Agriculture – Raw materials • Diversification -shift commodity driven to optimization of global value chains

Conclusion Access to quality healthcare is a fundamental Human Right q The promotion of industrial development and the safeguarding and protection of public health are not mutually exclusive priorities q The production of quality medicines and the development of an international GMP compliant industry in Africa are possible, desirable and eminently doable q Local production has huge potential to not only contribute to improved healthcare provision, but also to stimulate economic growth, self reliance and develop skills and increase the knowledge base. q

Key recommendations for sustaining achievements(1/3) Support and facilitate: Increased dialogue at national, regional, continental and international fora to maintain awareness and recognition by policy and decision makers of the potential contribution of the pharma sector to both industrial agenda and the healthcare system of the continent; Implementation of continental strategies that define Africa's trajectory for promoting and developing the sector that have been elaborated and endorsed by high level policy makers and attracted the interest of a broad range of stakeholders from both public and private sectors and stimulated action at national and regional level;

Key recommendations for sustaining achievements (2/3) Support and facilitate: strategic partnerships and platforms for international cooperation fostered by AU leadership to leverage support for growth of the local manufacturing industry across the its value chain; Continental level efforts to promote the creation of enabling policy and legislative environment for the growth of the sector to sustain action at national and regional level and trigger investments/support from public and private sector, local and international actors

Key recommendations for sustaining achievements (3/3) Agree to support and adopt the following as key instruments and mechanisms for sustaining national and regional level achievements in promoting and developing the pharmaceutical sector for improved health and development : ◦ PMPA business plan ◦ AU model law on medical product regulations ◦ Task team to facilitate the establishment African Medicines Agency and take note its progress report ◦ Biennial African regulators forum

sa. au. int/en/sites/default/files/pmpa%20 bp%20 ebook. pdf Contributing to The Africa We Want THANK YOU FOR YOUR KIND ATTENTION !
3f1cae44add48902ac2d02a43626100a.ppt