6af1360bf716a1d17ecc8db9f3907bf6.ppt
- Количество слайдов: 44
 Optics in Confocal Microscopy
Optics in Confocal Microscopy
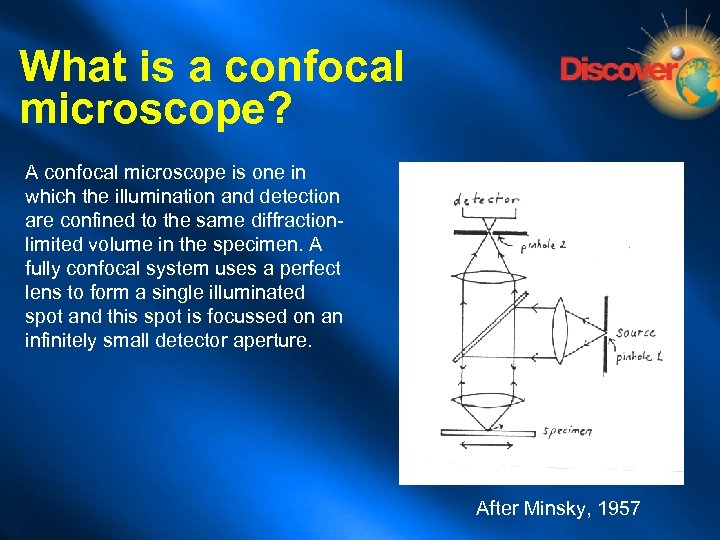 What is a confocal microscope? A confocal microscope is one in which the illumination and detection are confined to the same diffractionlimited volume in the specimen. A fully confocal system uses a perfect lens to form a single illuminated spot and this spot is focussed on an infinitely small detector aperture. After Minsky, 1957
What is a confocal microscope? A confocal microscope is one in which the illumination and detection are confined to the same diffractionlimited volume in the specimen. A fully confocal system uses a perfect lens to form a single illuminated spot and this spot is focussed on an infinitely small detector aperture. After Minsky, 1957
 What is the correct aperture size in a real confocal microscope? Short answer: It should be not more than the Airy disk diameter. Real answer: To form an image, the detector aperture cannot be infinitely small. It has to be opened up until the signal strength (after averaging, if necessary) is high enough to see the required detail.
What is the correct aperture size in a real confocal microscope? Short answer: It should be not more than the Airy disk diameter. Real answer: To form an image, the detector aperture cannot be infinitely small. It has to be opened up until the signal strength (after averaging, if necessary) is high enough to see the required detail.
 So what is an Airy disc? The image of a single point in the specimen has a characteristic pattern at the level of the aperture: a bright central spot with bright rings around it. The central part of this pattern is called the Airy disk.
So what is an Airy disc? The image of a single point in the specimen has a characteristic pattern at the level of the aperture: a bright central spot with bright rings around it. The central part of this pattern is called the Airy disk.
 How big is this Airy disc then? The size of the Airy disk is determined by the wavelength of the light, the numerical aperture of the objective lens and the magnification of the image at the level of the aperture. Optical experts use so-called ‘optical units’ when discussing the size of an Airy disk. The radius of an Airy disk (measuring to the darkest part of the surrounding ring) is 3. 833 optical units. One expert (H. T. M. van der Voort) has proved theoretically that the aperture radius should be 2 units or less for near-ideal confocal resolution, but that a radius of 4 units gives the best compromise between resolution and signal strength for point objects.
How big is this Airy disc then? The size of the Airy disk is determined by the wavelength of the light, the numerical aperture of the objective lens and the magnification of the image at the level of the aperture. Optical experts use so-called ‘optical units’ when discussing the size of an Airy disk. The radius of an Airy disk (measuring to the darkest part of the surrounding ring) is 3. 833 optical units. One expert (H. T. M. van der Voort) has proved theoretically that the aperture radius should be 2 units or less for near-ideal confocal resolution, but that a radius of 4 units gives the best compromise between resolution and signal strength for point objects.
 A practical approach. . . The aperture diameter should be about the same as the Airy disk diameter under practical conditions, but it should be possible to close it a little further to get better axial resolution if the signal strength is high enough. When the object is not a point, but has vertical extent, it is useful to be able to open up the detector aperture much more. • Variable apertures are important • Its better to set it too big than too small • Often, the fluorescence signal determines the ‘correct’ size
A practical approach. . . The aperture diameter should be about the same as the Airy disk diameter under practical conditions, but it should be possible to close it a little further to get better axial resolution if the signal strength is high enough. When the object is not a point, but has vertical extent, it is useful to be able to open up the detector aperture much more. • Variable apertures are important • Its better to set it too big than too small • Often, the fluorescence signal determines the ‘correct’ size
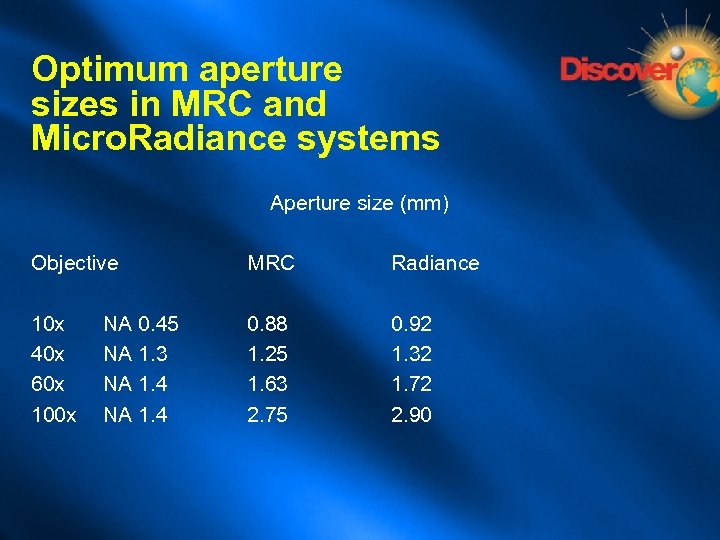 Optimum aperture sizes in MRC and Micro. Radiance systems Aperture size (mm) Objective MRC Radiance 10 x 40 x 60 x 100 x 0. 88 1. 25 1. 63 2. 75 0. 92 1. 32 1. 72 2. 90 NA 0. 45 NA 1. 3 NA 1. 4
Optimum aperture sizes in MRC and Micro. Radiance systems Aperture size (mm) Objective MRC Radiance 10 x 40 x 60 x 100 x 0. 88 1. 25 1. 63 2. 75 0. 92 1. 32 1. 72 2. 90 NA 0. 45 NA 1. 3 NA 1. 4
 Why does Bio-Rad use a variable iris as the detector aperture? Short answers: 1. It allows you to increase the signal strength and get an image, even when the specimen is too dim to provide a high enough signal-to-noise ratio for ‘true’ confocal imaging. 2. It’s a Bio-Rad patent (John White, 1985).
Why does Bio-Rad use a variable iris as the detector aperture? Short answers: 1. It allows you to increase the signal strength and get an image, even when the specimen is too dim to provide a high enough signal-to-noise ratio for ‘true’ confocal imaging. 2. It’s a Bio-Rad patent (John White, 1985).
 How does the optical design of the Micro. Radiance compare with the MRC series? The main difference is the way that the extra magnification at the aperture is obtained. In the MRC 500 -1024 series it was achieved by having a very long light path (1. 7 metres) from the eyepiece to the aperture, folded inside the casing of the instrument by means of mirrors. In the Micro. Radiance it is done by placing a telescope between the eyepiece and the aperture. This telescope, which contains highthroughput achromatic lenses, produces an enlarged image of the specimen at the level of the iris.
How does the optical design of the Micro. Radiance compare with the MRC series? The main difference is the way that the extra magnification at the aperture is obtained. In the MRC 500 -1024 series it was achieved by having a very long light path (1. 7 metres) from the eyepiece to the aperture, folded inside the casing of the instrument by means of mirrors. In the Micro. Radiance it is done by placing a telescope between the eyepiece and the aperture. This telescope, which contains highthroughput achromatic lenses, produces an enlarged image of the specimen at the level of the iris.
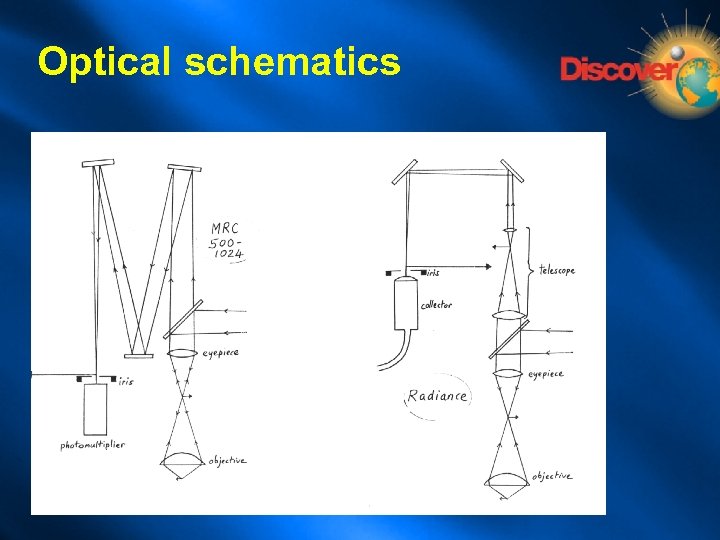 Optical schematics
Optical schematics
 Why were the MRC 500 -1024 series referred to as an ‘infinity optics’ design? Although often referred to as ‘infinity optics’ this design probably did not use infinite foci ( i. e. parallel beams). It probably owed its high performance to the fact that when the image was focussed on the iris (by a very small adjustment in the focus of the microscope), the iris was then at a conjugate focus with the beam waist in the laser, which was not situated at infinity. The micro. Radiance is an improved design, in the sense that the focal positions are under greater control: even the slight variations in beam waist positions between single-mode optical fibres are compensated for when the telescope is adjusted during manufacture.
Why were the MRC 500 -1024 series referred to as an ‘infinity optics’ design? Although often referred to as ‘infinity optics’ this design probably did not use infinite foci ( i. e. parallel beams). It probably owed its high performance to the fact that when the image was focussed on the iris (by a very small adjustment in the focus of the microscope), the iris was then at a conjugate focus with the beam waist in the laser, which was not situated at infinity. The micro. Radiance is an improved design, in the sense that the focal positions are under greater control: even the slight variations in beam waist positions between single-mode optical fibres are compensated for when the telescope is adjusted during manufacture.
 Does a confocal microscope have better resolution than a conventional one? Short answer: Yes, it is improved by 40%. However, the big advantage of a confocal epifluorescence microscope is not improved resolution, but the improved contrast due to the elimination of out-of-focus glare.
Does a confocal microscope have better resolution than a conventional one? Short answer: Yes, it is improved by 40%. However, the big advantage of a confocal epifluorescence microscope is not improved resolution, but the improved contrast due to the elimination of out-of-focus glare.
 What do we mean by ‘resolution’ then? By far the best-known definition is the Rayleigh criterion. Two points are said to be resolved if their Airy disks interpenetrate in such a way that the first minimum of one overlaps the peak of the other (i. e. they are 3. 83 optical units apart). This is a rather pessimistic definition; in fact it is still possible to tell that a point is double by eye when closer than this. Another definition is the Sparrow criterion, which is the point at which the sum of the two patterns becomes flat between the two peaks. Yet another, commonly used, is the FWHM: the resolution is simply the width of the Airy disk at half its maximum height (this is coincidentally about the same as the radius of the first minimum). Which criterion you use makes a big difference to the assessment of optical systems. Confocal optics do not improve Rayleigh resolution at all, but do improve the Sparrow and FWHM resolution.
What do we mean by ‘resolution’ then? By far the best-known definition is the Rayleigh criterion. Two points are said to be resolved if their Airy disks interpenetrate in such a way that the first minimum of one overlaps the peak of the other (i. e. they are 3. 83 optical units apart). This is a rather pessimistic definition; in fact it is still possible to tell that a point is double by eye when closer than this. Another definition is the Sparrow criterion, which is the point at which the sum of the two patterns becomes flat between the two peaks. Yet another, commonly used, is the FWHM: the resolution is simply the width of the Airy disk at half its maximum height (this is coincidentally about the same as the radius of the first minimum). Which criterion you use makes a big difference to the assessment of optical systems. Confocal optics do not improve Rayleigh resolution at all, but do improve the Sparrow and FWHM resolution.
 What is a point spread function? If an infinitely small object, a geometrical point, is imaged by a microscope, the image consists of an Airy pattern. If the microscope is focussed through such an object, the image seems to exist in threedimensions around the original point. The apparent three-dimensional image of a point is called the ‘point spread function’. The horizontal section of the PSF for an ideal lens is the Airy pattern. The axial section is invariably stretched out more than the Airy disk radius, to an extent that depends on the numerical aperture of the objective lens. The ratio, always more than 1, is 3. 28 n / NA, where n is the refractive index of the medium around the object and NA = numerical aperture of the objective lens.
What is a point spread function? If an infinitely small object, a geometrical point, is imaged by a microscope, the image consists of an Airy pattern. If the microscope is focussed through such an object, the image seems to exist in threedimensions around the original point. The apparent three-dimensional image of a point is called the ‘point spread function’. The horizontal section of the PSF for an ideal lens is the Airy pattern. The axial section is invariably stretched out more than the Airy disk radius, to an extent that depends on the numerical aperture of the objective lens. The ratio, always more than 1, is 3. 28 n / NA, where n is the refractive index of the medium around the object and NA = numerical aperture of the objective lens.
 What is meant by numerical aperture? The numerical aperture of an objective lens is the refractive index of the medium times the sine of the semi-angle of the included cone. The latter means the angle to the optical axis of the extreme rays, those which only just get into the lens. The expression is normally written: NA = n sin The NA of a lens is a measure of both its light collecting and resolving capabilities.
What is meant by numerical aperture? The numerical aperture of an objective lens is the refractive index of the medium times the sine of the semi-angle of the included cone. The latter means the angle to the optical axis of the extreme rays, those which only just get into the lens. The expression is normally written: NA = n sin The NA of a lens is a measure of both its light collecting and resolving capabilities.
 The relationship between NA and resolution For lenses in air NA = 1 / 2 F, where F = the F number of the lens. (i. e. an objective of NA 0. 5 has an F number of one). Ernst Abbe introduced this term because he realised that it is proportional to the (lateral) resolving power of the lens, according to the famous equation r = 0. 61 / NA, where r = Rayleigh resolution and = vacuum wavelength of the light. Experts now dispute this, for very high numerical apertures, but still do not agree on the details.
The relationship between NA and resolution For lenses in air NA = 1 / 2 F, where F = the F number of the lens. (i. e. an objective of NA 0. 5 has an F number of one). Ernst Abbe introduced this term because he realised that it is proportional to the (lateral) resolving power of the lens, according to the famous equation r = 0. 61 / NA, where r = Rayleigh resolution and = vacuum wavelength of the light. Experts now dispute this, for very high numerical apertures, but still do not agree on the details.
 How are the lateral and axial resolutions changed by confocal operation? One way to think about this is to imagine the two stages of confocal operation as distinct events. The probability of illumination (i. e. of the arrival of a photon at a particular point in space) is proportional to the intensity of the point spread function at that point. The probability of detection (assuming ideal confocal performance) is also proportional to the point spread function intensity, since this corresponds to the PSF of the detector aperture projected back into object space. The resolution (neglecting any effects such a change in wavelength between illumination and emission) is determined by the product of the two probabilities, which is the square of the intensity of the PSF.
How are the lateral and axial resolutions changed by confocal operation? One way to think about this is to imagine the two stages of confocal operation as distinct events. The probability of illumination (i. e. of the arrival of a photon at a particular point in space) is proportional to the intensity of the point spread function at that point. The probability of detection (assuming ideal confocal performance) is also proportional to the point spread function intensity, since this corresponds to the PSF of the detector aperture projected back into object space. The resolution (neglecting any effects such a change in wavelength between illumination and emission) is determined by the product of the two probabilities, which is the square of the intensity of the PSF.
 What is the effect of squaring the PSF? The position of the minima does not change, so the resolution, according to the strict Rayleigh definition, does not improve. However, the FWHM improves by a factor of 1. 4 (Brakenhoff, 1979). So, the lateral resolution becomes: r FWHM = 0. 44 / NA confocal (1) r FWHM = 0. 61 / NA conventional (2) compared with:
What is the effect of squaring the PSF? The position of the minima does not change, so the resolution, according to the strict Rayleigh definition, does not improve. However, the FWHM improves by a factor of 1. 4 (Brakenhoff, 1979). So, the lateral resolution becomes: r FWHM = 0. 44 / NA confocal (1) r FWHM = 0. 61 / NA conventional (2) compared with:
 For axial resolution the corresponding equations are: z FWHM = 2 / n (sin 2 ) = 2 / n (sin 2 sin-1(NA/n) ) (Brakenhoff et al. 1989) (3) At 488 nm, using an oil immersion objective (n=1. 515) of N. A. 1. 4, the values for equations (1) , (2) and (3) are 0. 15, 0. 21 and 0. 76 mm.
For axial resolution the corresponding equations are: z FWHM = 2 / n (sin 2 ) = 2 / n (sin 2 sin-1(NA/n) ) (Brakenhoff et al. 1989) (3) At 488 nm, using an oil immersion objective (n=1. 515) of N. A. 1. 4, the values for equations (1) , (2) and (3) are 0. 15, 0. 21 and 0. 76 mm.
 The plane spread function! If the microscope is imaging a plane surface (e. g. in reflection) the PSF may not be the most appropriate measure of the lens performance. A plane spread function, different from the point spread function, may be defined as the apparent structure of an ideal planar specimen. Xiao and Kino give an equation (without derivation) for the FWHM resolution in reflection imaging as follows: zref = 0. 45 / n (1 - cos ) = 0. 45 / n (1 - cos (sin-1(NA/n)) This gives a result of 0. 23 mm, best measured values being in the region of 0. 35 to 0. 4 mm.
The plane spread function! If the microscope is imaging a plane surface (e. g. in reflection) the PSF may not be the most appropriate measure of the lens performance. A plane spread function, different from the point spread function, may be defined as the apparent structure of an ideal planar specimen. Xiao and Kino give an equation (without derivation) for the FWHM resolution in reflection imaging as follows: zref = 0. 45 / n (1 - cos ) = 0. 45 / n (1 - cos (sin-1(NA/n)) This gives a result of 0. 23 mm, best measured values being in the region of 0. 35 to 0. 4 mm.
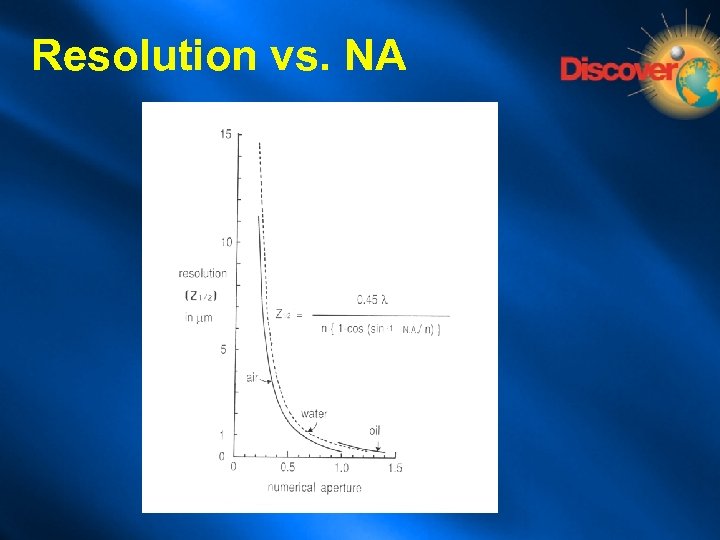 Resolution vs. NA
Resolution vs. NA
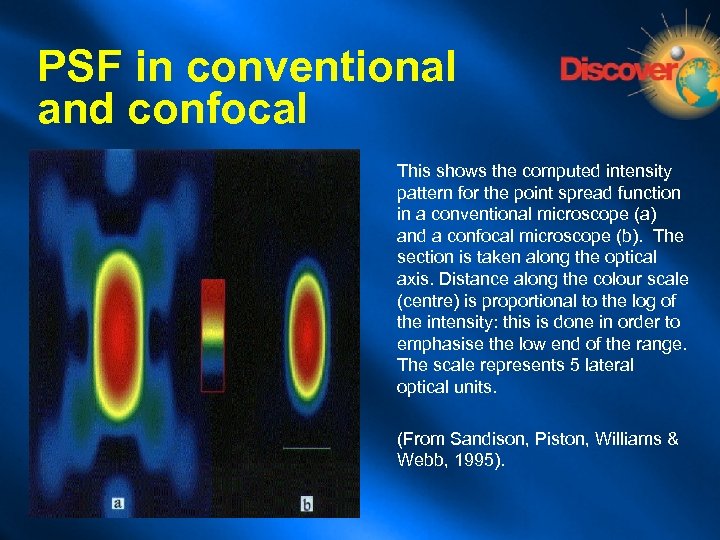 PSF in conventional and confocal This shows the computed intensity pattern for the point spread function in a conventional microscope (a) and a confocal microscope (b). The section is taken along the optical axis. Distance along the colour scale (centre) is proportional to the log of the intensity: this is done in order to emphasise the low end of the range. The scale represents 5 lateral optical units. (From Sandison, Piston, Williams & Webb, 1995).
PSF in conventional and confocal This shows the computed intensity pattern for the point spread function in a conventional microscope (a) and a confocal microscope (b). The section is taken along the optical axis. Distance along the colour scale (centre) is proportional to the log of the intensity: this is done in order to emphasise the low end of the range. The scale represents 5 lateral optical units. (From Sandison, Piston, Williams & Webb, 1995).
 How do the wrong mounting medium, wrong coverslip and focussing too deep ruin the image?
How do the wrong mounting medium, wrong coverslip and focussing too deep ruin the image?
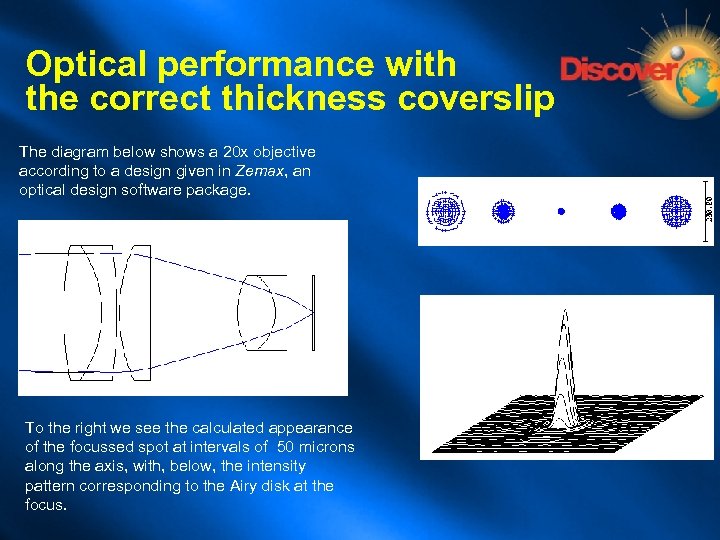 Optical performance with the correct thickness coverslip The diagram below shows a 20 x objective according to a design given in Zemax, an optical design software package. To the right we see the calculated appearance of the focussed spot at intervals of 50 microns along the axis, with, below, the intensity pattern corresponding to the Airy disk at the focus.
Optical performance with the correct thickness coverslip The diagram below shows a 20 x objective according to a design given in Zemax, an optical design software package. To the right we see the calculated appearance of the focussed spot at intervals of 50 microns along the axis, with, below, the intensity pattern corresponding to the Airy disk at the focus.
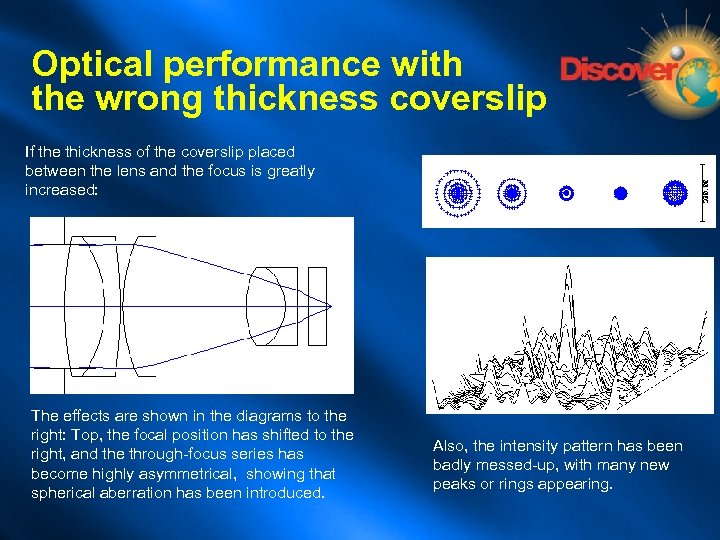 Optical performance with the wrong thickness coverslip If the thickness of the coverslip placed between the lens and the focus is greatly increased: The effects are shown in the diagrams to the right: Top, the focal position has shifted to the right, and the through-focus series has become highly asymmetrical, showing that spherical aberration has been introduced. Also, the intensity pattern has been badly messed-up, with many new peaks or rings appearing.
Optical performance with the wrong thickness coverslip If the thickness of the coverslip placed between the lens and the focus is greatly increased: The effects are shown in the diagrams to the right: Top, the focal position has shifted to the right, and the through-focus series has become highly asymmetrical, showing that spherical aberration has been introduced. Also, the intensity pattern has been badly messed-up, with many new peaks or rings appearing.
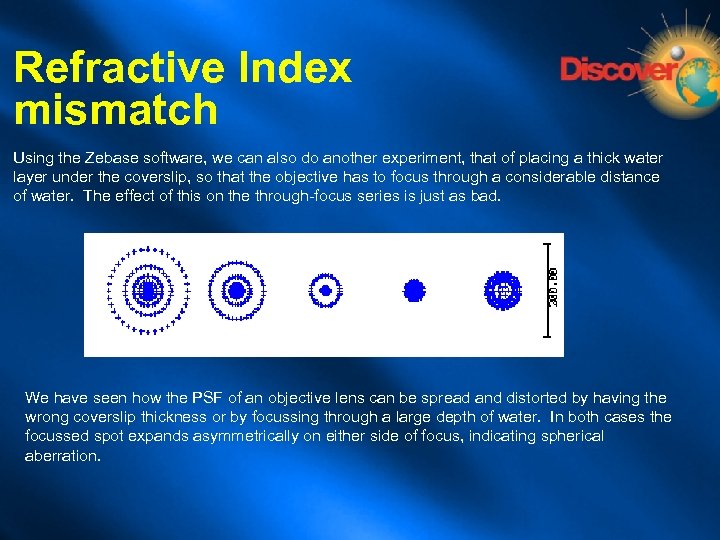 Refractive Index mismatch Using the Zebase software, we can also do another experiment, that of placing a thick water layer under the coverslip, so that the objective has to focus through a considerable distance of water. The effect of this on the through-focus series is just as bad. We have seen how the PSF of an objective lens can be spread and distorted by having the wrong coverslip thickness or by focussing through a large depth of water. In both cases the focussed spot expands asymmetrically on either side of focus, indicating spherical aberration.
Refractive Index mismatch Using the Zebase software, we can also do another experiment, that of placing a thick water layer under the coverslip, so that the objective has to focus through a considerable distance of water. The effect of this on the through-focus series is just as bad. We have seen how the PSF of an objective lens can be spread and distorted by having the wrong coverslip thickness or by focussing through a large depth of water. In both cases the focussed spot expands asymmetrically on either side of focus, indicating spherical aberration.
 Why are they called ‘spherical’ aberrations? In lens optics, a plane is regarded as a special case of a spherical surface, with infinite radius. The rays passing from a single point through a planar boundary into a medium of different refractive index are bent at the interface in such a way that , when traced back, they no longer intersect in a single point. It is possible to correct for this by incorporating special spherical surfaces into a lens system, but this only works for one particular position of the object and the boundary and for one refractive index.
Why are they called ‘spherical’ aberrations? In lens optics, a plane is regarded as a special case of a spherical surface, with infinite radius. The rays passing from a single point through a planar boundary into a medium of different refractive index are bent at the interface in such a way that , when traced back, they no longer intersect in a single point. It is possible to correct for this by incorporating special spherical surfaces into a lens system, but this only works for one particular position of the object and the boundary and for one refractive index.
 How can you avoid spherical aberration problems? Start by considering the specimen. If the specimen can be examined dry, use an objective designed for use without a coverglass. These are often marked ‘NCG’ or ‘ 0’ in place of the usual ‘ 0. 17’, which is the coverslip thickness in millimetres. If the specimen must be examined under fluid, there are several possibilities:
How can you avoid spherical aberration problems? Start by considering the specimen. If the specimen can be examined dry, use an objective designed for use without a coverglass. These are often marked ‘NCG’ or ‘ 0’ in place of the usual ‘ 0. 17’, which is the coverslip thickness in millimetres. If the specimen must be examined under fluid, there are several possibilities:
 Homogeneous immersion with no coverglass So-called dipping objectives are available, designed for use in direct contact with physiological salines or even sea water. These are good for looking at whole animals under saline, but the N. A. is usually low, so they are usually not considered ‘good’ for confocal purposes.
Homogeneous immersion with no coverglass So-called dipping objectives are available, designed for use in direct contact with physiological salines or even sea water. These are good for looking at whole animals under saline, but the N. A. is usually low, so they are usually not considered ‘good’ for confocal purposes.
 Dry lenses with a coverslip Those with the highest available N. A. should be used (e. g. for a 10 x objective, an N. A. of 0. 45 is desirable, but expensive). Especially for the highest powers, the correct thickness of coverslip must be used. Coverslips of number 1 1/2 are often nearly correct, but should be checked with a micrometer. They are often thicker at one side than another. For 40 x dry lenses, an error of 10 m in coverslip thickness from the standard 0. 17 mm gives an appreciable deterioration in PSF. Some objectives have correction collars, sometimes for coverslips from zero to 2 mm. They are designed for looking into glass culture dishes through the base. Unfortunately the N. A. is usually too low for confocal use. Looking into a medium of higher index (n) than air introduces an axial distortion of approximately n-fold compression, which can be compensated for by entering the refractive index into the Bio-Rad software.
Dry lenses with a coverslip Those with the highest available N. A. should be used (e. g. for a 10 x objective, an N. A. of 0. 45 is desirable, but expensive). Especially for the highest powers, the correct thickness of coverslip must be used. Coverslips of number 1 1/2 are often nearly correct, but should be checked with a micrometer. They are often thicker at one side than another. For 40 x dry lenses, an error of 10 m in coverslip thickness from the standard 0. 17 mm gives an appreciable deterioration in PSF. Some objectives have correction collars, sometimes for coverslips from zero to 2 mm. They are designed for looking into glass culture dishes through the base. Unfortunately the N. A. is usually too low for confocal use. Looking into a medium of higher index (n) than air introduces an axial distortion of approximately n-fold compression, which can be compensated for by entering the refractive index into the Bio-Rad software.
 Oil immersion with a coverslip These objectives are the ones most often used for high-resolution confocal work. They have several problems, however. If used with live cells under the coverslip in an aqueous (i. e. low refractive index) medium such as a physiological saline, the PSF deteriorates markedly with distance through the aqueous layer. Beyond 50 mm, the image is poorly resolved and the brightness falls, making quantitation of fluorescence difficult. Also, the distance through which the focus has to be shifted is greater than the true height within the specimen, roughly in the ratio noil / nwater, so the zaxis of image datasets has to be compressed. Again, these corrections can be achieved by entering the medium refractive index and objective type into the Bio-Rad software. Another important point is that the working distance (i. e. the oil-filled space between the front of the objective and the coverslip) of immersion objectives varies greatly from one manufacturer to another. It is essential for researchers to consider this, especially if they are planning to do deep imaging in whole mounts of embryos, for example. Nikon have the longest available W. D. for a 60 x N. A. 1. 4 lens.
Oil immersion with a coverslip These objectives are the ones most often used for high-resolution confocal work. They have several problems, however. If used with live cells under the coverslip in an aqueous (i. e. low refractive index) medium such as a physiological saline, the PSF deteriorates markedly with distance through the aqueous layer. Beyond 50 mm, the image is poorly resolved and the brightness falls, making quantitation of fluorescence difficult. Also, the distance through which the focus has to be shifted is greater than the true height within the specimen, roughly in the ratio noil / nwater, so the zaxis of image datasets has to be compressed. Again, these corrections can be achieved by entering the medium refractive index and objective type into the Bio-Rad software. Another important point is that the working distance (i. e. the oil-filled space between the front of the objective and the coverslip) of immersion objectives varies greatly from one manufacturer to another. It is essential for researchers to consider this, especially if they are planning to do deep imaging in whole mounts of embryos, for example. Nikon have the longest available W. D. for a 60 x N. A. 1. 4 lens.
 Sample mounting considerations The problems of poor PSF, low brightness, distortion of z axis and limited working distance can all be alleviated if thin preparations can be placed in close contact with the coverslip: much better images can be obtained from cells grown on the coverslip than for the same cells grown in multiwell slides, covered with medium with a coverslip on top. If the cells can be fixed and permeated with a medium of high refractive index, the PSF remains good at considerable depth. Fluoromount (BDH/Gurr) is a suitable low-fluorescence resin mountant, but prone to large shrinkage as the solvent (xylene) evaporates. Methyl or Benzoyl salicylate or immersion oils are often used also. Surprisingly, the coverslip thickness still matters, possibly because neither the refractive index nor the dispersion of standard coverslip glass matches the corresponding value for standard immersion oil.
Sample mounting considerations The problems of poor PSF, low brightness, distortion of z axis and limited working distance can all be alleviated if thin preparations can be placed in close contact with the coverslip: much better images can be obtained from cells grown on the coverslip than for the same cells grown in multiwell slides, covered with medium with a coverslip on top. If the cells can be fixed and permeated with a medium of high refractive index, the PSF remains good at considerable depth. Fluoromount (BDH/Gurr) is a suitable low-fluorescence resin mountant, but prone to large shrinkage as the solvent (xylene) evaporates. Methyl or Benzoyl salicylate or immersion oils are often used also. Surprisingly, the coverslip thickness still matters, possibly because neither the refractive index nor the dispersion of standard coverslip glass matches the corresponding value for standard immersion oil.
 Multi-immersion objectives These are available from the main manufacturers in intermediate magnifications, e. g. 25 x, 40 x. Often the N. A. is rather low, but new designs are certainly worth trying for confocal purposes. Water, glycerol and oil may all be usable with the same objective. There is invariably a correction collar. If the focus is poor, the collar should be adjusted and the specimen refocussed, the process being repeated until an optimum is found. These objectives are invaluable for low power reflection imaging (reduced reflection off the coverglass).
Multi-immersion objectives These are available from the main manufacturers in intermediate magnifications, e. g. 25 x, 40 x. Often the N. A. is rather low, but new designs are certainly worth trying for confocal purposes. Water, glycerol and oil may all be usable with the same objective. There is invariably a correction collar. If the focus is poor, the collar should be adjusted and the specimen refocussed, the process being repeated until an optimum is found. These objectives are invaluable for low power reflection imaging (reduced reflection off the coverglass).
 Water/ coverglass/ water objectives of high NA These objectives are available with N. A. 1. 2 from the major manufacturers, at a cost of approximately 7 K sterling. They are truly remarkable in their ability to preserve the PSF even when focussing through as much as 200 mm of water. They have become established as the lenses of choice for use with whole mounts of living tissue, brain slices, embryos, plant roots etc. However, they should not be recommended to customers who study only thin monolayers of cells: there is no significant improvement over the 4 times-cheaper oil immersion objectives for specimens like this.
Water/ coverglass/ water objectives of high NA These objectives are available with N. A. 1. 2 from the major manufacturers, at a cost of approximately 7 K sterling. They are truly remarkable in their ability to preserve the PSF even when focussing through as much as 200 mm of water. They have become established as the lenses of choice for use with whole mounts of living tissue, brain slices, embryos, plant roots etc. However, they should not be recommended to customers who study only thin monolayers of cells: there is no significant improvement over the 4 times-cheaper oil immersion objectives for specimens like this.
 Chromatic aberration When white light is brought to a focus by a simple single-element lens, such as a magnifying glass, the blue component is refracted more by the glass and comes to a focus closer to the lens than the red. This separation of colours along the axis is called longitudinal chromatic aberration. An image of an object illuminated with white light may also show a chromatic difference in magnification or 'lateral colour'. This aberration gets worse further from the centre of the image. In a confocal microscope, chromatic aberration is a disaster. It has two obvious effects: Firstly, the laser light (e. g. blue) is focussed to a certain off-axis point, but the detector (sensitive to green or red) looks at a position shifted from the illumination by an amount that increases with distance from the centre of the image. So, lateral colour makes the signal strength fall with distance from the centre of the field. The way to test for this is to examine fluorescent beads distributed all over the field. Unlike the effect of curvature of focus, the lateral colour effect is much the same at all positions of focus. Lateral colour can also cause a radial mismatch between the scanned transmission image and the fluorescence image. Continued. . .
Chromatic aberration When white light is brought to a focus by a simple single-element lens, such as a magnifying glass, the blue component is refracted more by the glass and comes to a focus closer to the lens than the red. This separation of colours along the axis is called longitudinal chromatic aberration. An image of an object illuminated with white light may also show a chromatic difference in magnification or 'lateral colour'. This aberration gets worse further from the centre of the image. In a confocal microscope, chromatic aberration is a disaster. It has two obvious effects: Firstly, the laser light (e. g. blue) is focussed to a certain off-axis point, but the detector (sensitive to green or red) looks at a position shifted from the illumination by an amount that increases with distance from the centre of the image. So, lateral colour makes the signal strength fall with distance from the centre of the field. The way to test for this is to examine fluorescent beads distributed all over the field. Unlike the effect of curvature of focus, the lateral colour effect is much the same at all positions of focus. Lateral colour can also cause a radial mismatch between the scanned transmission image and the fluorescence image. Continued. . .
 Chromatic aberration cont’d. Secondly, an object which emits over a wide range of fluorescence colours will, in the presence of longitudinal chromatic aberration, appear as a smeared or multiple image at a range of depths. The test for this is to image beads which fluoresce both red and green (available from Polysciences at 1 mm and 2 mm diameter. If you make a z-section through these beads, even the best objective lenses are likely to show image duplication. In the merged image of red and green emission channels, the upper part of each bead is likely to be coloured differently from the lower. This is quite a problem for researchers who wish to test the colocalisation of, say, a red and a green-emitting fluorochrome. The aberration may, at a particular focal level, give a false single positive result for a small object which is, in reality, doubly positive (i. e. stained with both stains).
Chromatic aberration cont’d. Secondly, an object which emits over a wide range of fluorescence colours will, in the presence of longitudinal chromatic aberration, appear as a smeared or multiple image at a range of depths. The test for this is to image beads which fluoresce both red and green (available from Polysciences at 1 mm and 2 mm diameter. If you make a z-section through these beads, even the best objective lenses are likely to show image duplication. In the merged image of red and green emission channels, the upper part of each bead is likely to be coloured differently from the lower. This is quite a problem for researchers who wish to test the colocalisation of, say, a red and a green-emitting fluorochrome. The aberration may, at a particular focal level, give a false single positive result for a small object which is, in reality, doubly positive (i. e. stained with both stains).
 What can be done about chromatic aberration in confocal imaging? Use the right type of lens: Severe chromatic effects may be due to the use of the wrong type of objective. Do not use old-style non-compensated objectives such as the Olympus S-Plan series: the Bio-Rad scan head is designed for modern objectives which give an achromatic intermediate image. The S-Plans were designed to be used with an equal and opposite chromatic aberration in the eyepiece to cancel their own.
What can be done about chromatic aberration in confocal imaging? Use the right type of lens: Severe chromatic effects may be due to the use of the wrong type of objective. Do not use old-style non-compensated objectives such as the Olympus S-Plan series: the Bio-Rad scan head is designed for modern objectives which give an achromatic intermediate image. The S-Plans were designed to be used with an equal and opposite chromatic aberration in the eyepiece to cancel their own.
 Collect a 3 D data set: Researchers who are doing colocalisation experiments involving small intracellular particles, bacteria etc. should test for aberration by collecting complete 3 D datasets. It should then be easy to establish whether apparent single positives are real or simply a focus artefact. Set UV correction lens: Strong chromatic aberration is invariably present in objectives over the range from ultra-violet to visible. The Bio-Rad UV confocal apparatus contains lenses designed to counteract this effect. The range of objectives that can be used is strictly limited.
Collect a 3 D data set: Researchers who are doing colocalisation experiments involving small intracellular particles, bacteria etc. should test for aberration by collecting complete 3 D datasets. It should then be easy to establish whether apparent single positives are real or simply a focus artefact. Set UV correction lens: Strong chromatic aberration is invariably present in objectives over the range from ultra-violet to visible. The Bio-Rad UV confocal apparatus contains lenses designed to counteract this effect. The range of objectives that can be used is strictly limited.
 Use a reflecting objective lens? Reflecting objectives of the Schwartzchild type (a combination of a convex mirror and a catadioptric concave mirror (i. e. one with a hole in it) have been suggested as a cure for chromatic problems. They have the additional appeal that spherical aberration can be compensated by adjustment of the mirror separation. However, the PSF is badly disturbed by the central occlusion and they are useless for all high-resolution work. Buy a 2 -photon system! 2 -photon imaging with non-confocal detection provides a cure. Here, it does not matter if the lens is strongly chromatically aberrant, because the resolution is determined entirely by the (monochromatic) illumination and the emission is collected but not focussed into an aperture. This will probably become the method of choice for colocalisation studies in the future. However, this will demand the use of fluorochromes that can be separated completely by emission windowing alone: FITC and TRITC will not do!
Use a reflecting objective lens? Reflecting objectives of the Schwartzchild type (a combination of a convex mirror and a catadioptric concave mirror (i. e. one with a hole in it) have been suggested as a cure for chromatic problems. They have the additional appeal that spherical aberration can be compensated by adjustment of the mirror separation. However, the PSF is badly disturbed by the central occlusion and they are useless for all high-resolution work. Buy a 2 -photon system! 2 -photon imaging with non-confocal detection provides a cure. Here, it does not matter if the lens is strongly chromatically aberrant, because the resolution is determined entirely by the (monochromatic) illumination and the emission is collected but not focussed into an aperture. This will probably become the method of choice for colocalisation studies in the future. However, this will demand the use of fluorochromes that can be separated completely by emission windowing alone: FITC and TRITC will not do!
 How do multiphoton optics differ from confocal? In any laser scanning microscope the beam is focused into a cone where the intensity of illumination above or below the focal plane is proportional to the square of the distance from focus and absorption is directly proportional to intensity. However, in the 2 -photon case, because of the need for two photons to arrive in a short time interval, absorption is proportional to the square of the intensity and falls off very rapidly away from the focus (according to an inverse fourth-power rule). The fall-off is even more rapid with 3 -photon and higher order processes. Analysis (Williams et al. ) yields the perhaps unexpected result that, provided the illuminated volume is uniformly filled with fluorophores, the total fluorescent emission signal strength is independent of the numerical aperture of the objective lens. Unlike confocal microscopy, multiphoton imaging is quite effective with low-magnification lenses of moderate numerical aperture, provided wide-angle detection is used.
How do multiphoton optics differ from confocal? In any laser scanning microscope the beam is focused into a cone where the intensity of illumination above or below the focal plane is proportional to the square of the distance from focus and absorption is directly proportional to intensity. However, in the 2 -photon case, because of the need for two photons to arrive in a short time interval, absorption is proportional to the square of the intensity and falls off very rapidly away from the focus (according to an inverse fourth-power rule). The fall-off is even more rapid with 3 -photon and higher order processes. Analysis (Williams et al. ) yields the perhaps unexpected result that, provided the illuminated volume is uniformly filled with fluorophores, the total fluorescent emission signal strength is independent of the numerical aperture of the objective lens. Unlike confocal microscopy, multiphoton imaging is quite effective with low-magnification lenses of moderate numerical aperture, provided wide-angle detection is used.
 Resolution in multi-photon - theory The resolution in a 2 -photon microscope is determined entirely by the restriction of excitation mentioned above. Williams et al (1994) show that the lateral resolution is given by: rlat = 0. 37 / n sin and the axial by: rax = 0. 32 / n sin 2 /2 where is the exciting wavelength and n sin is the numerical aperture of the objective lens. These expressions suggest approximately twice better resolution than a conventional microscope, but this is counterbalanced by the need to use a twice-longer wavelength.
Resolution in multi-photon - theory The resolution in a 2 -photon microscope is determined entirely by the restriction of excitation mentioned above. Williams et al (1994) show that the lateral resolution is given by: rlat = 0. 37 / n sin and the axial by: rax = 0. 32 / n sin 2 /2 where is the exciting wavelength and n sin is the numerical aperture of the objective lens. These expressions suggest approximately twice better resolution than a conventional microscope, but this is counterbalanced by the need to use a twice-longer wavelength.
 Resolution in multi-photon - practice In practice, the resolution in 2 -photon micrographs looks similar to that of conventional epifluorescence, or very slightly inferior if a 1047 nm wavelength is used. A combination of multiphoton excitation and confocal detection is often suggested as a means of improving resolution, but this is seldom used, because of the loss of signal observed when the confocal aperture is inserted. The failure of this combination is interesting; it is probably due to scattering of the emitted light in the specimen or chromatic aberration. In the Bio-Rad multiphoton system conventional objectives are used; their transmission in the infra-red is quite good. The reflectors in the scan head are designed to reflect both IR and visible light (and, sometimes, near UV as well). Special apparatus is used to pick off the light coming from the specimen before it enters the scan head, and so achieve nondescanned detection. This increases detection efficiency by at least a factor of three and usually much more (greater for scattering specimens).
Resolution in multi-photon - practice In practice, the resolution in 2 -photon micrographs looks similar to that of conventional epifluorescence, or very slightly inferior if a 1047 nm wavelength is used. A combination of multiphoton excitation and confocal detection is often suggested as a means of improving resolution, but this is seldom used, because of the loss of signal observed when the confocal aperture is inserted. The failure of this combination is interesting; it is probably due to scattering of the emitted light in the specimen or chromatic aberration. In the Bio-Rad multiphoton system conventional objectives are used; their transmission in the infra-red is quite good. The reflectors in the scan head are designed to reflect both IR and visible light (and, sometimes, near UV as well). Special apparatus is used to pick off the light coming from the specimen before it enters the scan head, and so achieve nondescanned detection. This increases detection efficiency by at least a factor of three and usually much more (greater for scattering specimens).
 References Amos, W. B. (1995) A note on optical units. Appendix I, pp 579 -580. in Handbook of Biological Confocal Microscopy. J. B. Pawley, ed. IInd Edn. Plenum Press. New York. Denk, W. , Strickler, J. H. & Webb, W. W. (1990) Two-photon laser scanning fluorescence microscopy. Science 248, 73 -76. Kapitza, H. G. (1986) Oral presentation at Seventh Congress of the International Society for Analytical Cytology, Cambridge UK. van der Voort, H. T. M. & Brakenhoff, G. J. (1990) Three dimensional image formation in high-aperture fluorescence confocal microscopy: a numerical analysis. J. Microscopy Minsky, M. (1957) U. S. Patent 3013467. Microscopy Apparatus. Sandison, D. R. Piston, D. W. , Williams, R. M. & Webb, W. W. (1995) Quantitative comparison of background rejection, signal-to-noise ratio and resolution in confocal and full-field laser scanning microscopes. Applied Optics 34, 3576 -3588. Visser, T. D. , Oud, J. L. & Brakenhoff, G. J. (1992) Refractive index and axial distance measurements in 3 D microscopy. Optik. 90, 17 -19. White, J. G. (1985) UK Patent Application 2 184 321 A Confocal Scanning Microscope. Xiao, G. Q. and Kino, G. S. (1987) A real-time confocal scanning optical microscope. Proc. SPIE 809 , Scanning Image Technology. T. Wilson and L. Balk. eds. pp 107 -113.
References Amos, W. B. (1995) A note on optical units. Appendix I, pp 579 -580. in Handbook of Biological Confocal Microscopy. J. B. Pawley, ed. IInd Edn. Plenum Press. New York. Denk, W. , Strickler, J. H. & Webb, W. W. (1990) Two-photon laser scanning fluorescence microscopy. Science 248, 73 -76. Kapitza, H. G. (1986) Oral presentation at Seventh Congress of the International Society for Analytical Cytology, Cambridge UK. van der Voort, H. T. M. & Brakenhoff, G. J. (1990) Three dimensional image formation in high-aperture fluorescence confocal microscopy: a numerical analysis. J. Microscopy Minsky, M. (1957) U. S. Patent 3013467. Microscopy Apparatus. Sandison, D. R. Piston, D. W. , Williams, R. M. & Webb, W. W. (1995) Quantitative comparison of background rejection, signal-to-noise ratio and resolution in confocal and full-field laser scanning microscopes. Applied Optics 34, 3576 -3588. Visser, T. D. , Oud, J. L. & Brakenhoff, G. J. (1992) Refractive index and axial distance measurements in 3 D microscopy. Optik. 90, 17 -19. White, J. G. (1985) UK Patent Application 2 184 321 A Confocal Scanning Microscope. Xiao, G. Q. and Kino, G. S. (1987) A real-time confocal scanning optical microscope. Proc. SPIE 809 , Scanning Image Technology. T. Wilson and L. Balk. eds. pp 107 -113.
 THE END
THE END


