e5670c441163d51bbc4242e37453c75f.ppt
- Количество слайдов: 40
 Opposites attract
Opposites attract
 attract
attract
 repel
repel
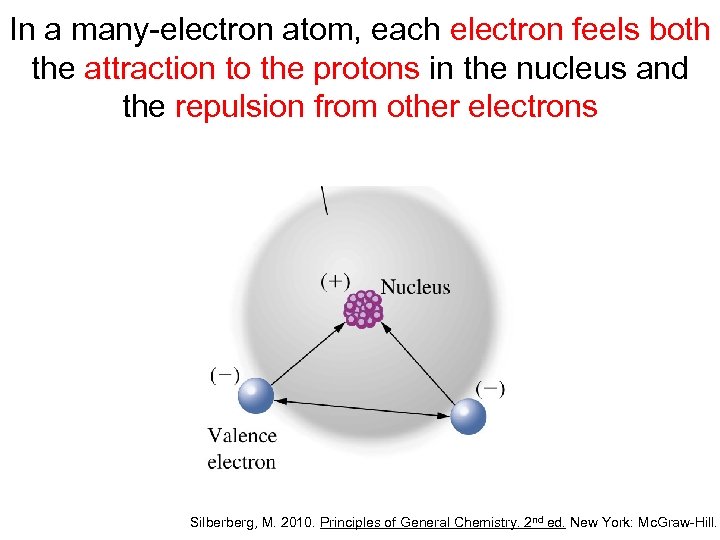 In a many-electron atom, each electron feels both the attraction to the protons in the nucleus and the repulsion from other electrons Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
In a many-electron atom, each electron feels both the attraction to the protons in the nucleus and the repulsion from other electrons Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
 Effective nuclear charge
Effective nuclear charge
 In a many-electron atom, each electron feels both the attraction to the protons in the nucleus and the repulsion from other electrons • Force of attraction increases as nuclear charge (# of protons) increases • Force of attraction decreases as the electron goes farther from the nucleus Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
In a many-electron atom, each electron feels both the attraction to the protons in the nucleus and the repulsion from other electrons • Force of attraction increases as nuclear charge (# of protons) increases • Force of attraction decreases as the electron goes farther from the nucleus Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
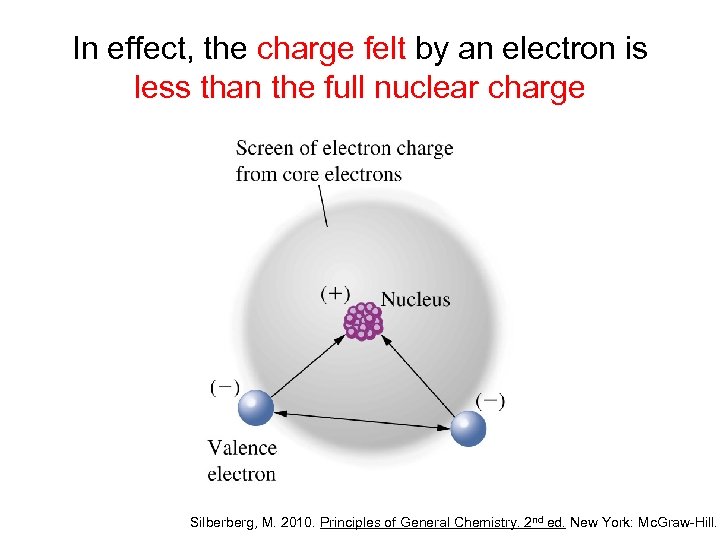 In effect, the charge felt by an electron is less than the full nuclear charge Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
In effect, the charge felt by an electron is less than the full nuclear charge Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
 In effect, the charge felt by an electron is less than the full nuclear charge • The effective nuclear charge (Zeff) is the nuclear charge that is actually felt by an electron • An electron is shielded from the full charge of the proton – greatly by the inner electrons – only slightly by the other electrons in the same principal quantum number (n) – not at all by outer electrons Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
In effect, the charge felt by an electron is less than the full nuclear charge • The effective nuclear charge (Zeff) is the nuclear charge that is actually felt by an electron • An electron is shielded from the full charge of the proton – greatly by the inner electrons – only slightly by the other electrons in the same principal quantum number (n) – not at all by outer electrons Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
 The effective nuclear charge and electron configuration are key in understanding the periodic trends • Atomic radius Physical properties • Ionic radius – neutral vs. charged – isoelectronic series • Ionization energy Chemical properties – one atom vs. another – same atom • Electron affinity
The effective nuclear charge and electron configuration are key in understanding the periodic trends • Atomic radius Physical properties • Ionic radius – neutral vs. charged – isoelectronic series • Ionization energy Chemical properties – one atom vs. another – same atom • Electron affinity
 Atomic radius
Atomic radius
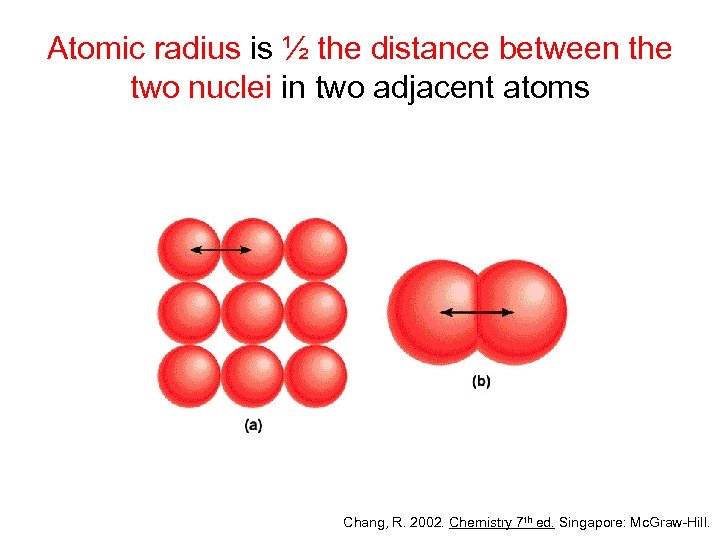 Atomic radius is ½ the distance between the two nuclei in two adjacent atoms Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Atomic radius is ½ the distance between the two nuclei in two adjacent atoms Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
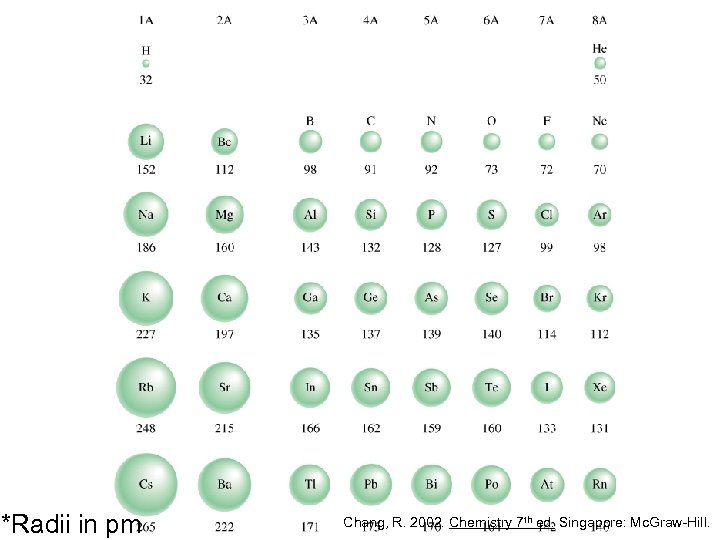 *Radii in pm Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
*Radii in pm Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
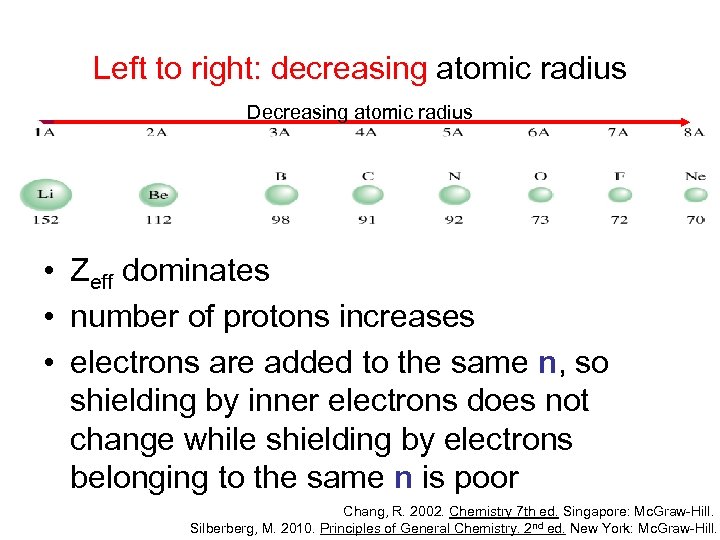 Left to right: decreasing atomic radius Decreasing atomic radius • Zeff dominates • number of protons increases • electrons are added to the same n, so shielding by inner electrons does not change while shielding by electrons belonging to the same n is poor Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Left to right: decreasing atomic radius Decreasing atomic radius • Zeff dominates • number of protons increases • electrons are added to the same n, so shielding by inner electrons does not change while shielding by electrons belonging to the same n is poor Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
 Top to bottom: increasing atomic radius • n dominates • going down the group, each member has one more level of inner electrons that shield the outer electrons very effectively Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Top to bottom: increasing atomic radius • n dominates • going down the group, each member has one more level of inner electrons that shield the outer electrons very effectively Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill. Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
 Ionic radius: Neutral vs. charged
Ionic radius: Neutral vs. charged
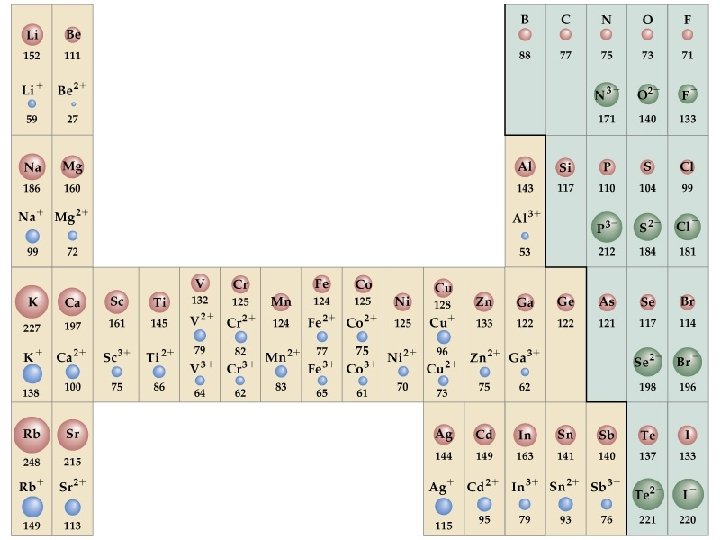
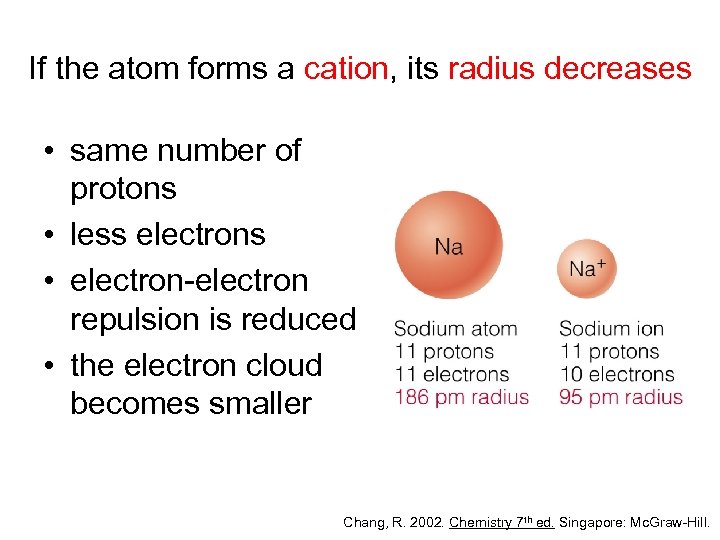 If the atom forms a cation, its radius decreases • same number of protons • less electrons • electron-electron repulsion is reduced • the electron cloud becomes smaller Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
If the atom forms a cation, its radius decreases • same number of protons • less electrons • electron-electron repulsion is reduced • the electron cloud becomes smaller Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
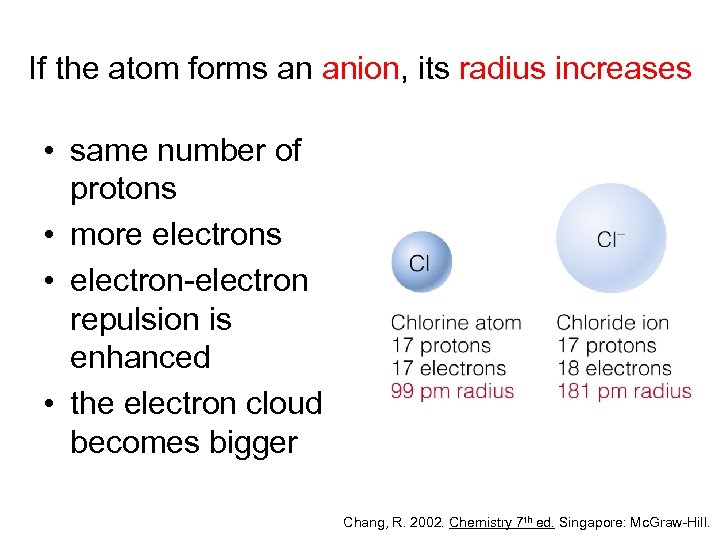 If the atom forms an anion, its radius increases • same number of protons • more electrons • electron-electron repulsion is enhanced • the electron cloud becomes bigger Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
If the atom forms an anion, its radius increases • same number of protons • more electrons • electron-electron repulsion is enhanced • the electron cloud becomes bigger Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Ionic radius: Isoelectronic series
Ionic radius: Isoelectronic series
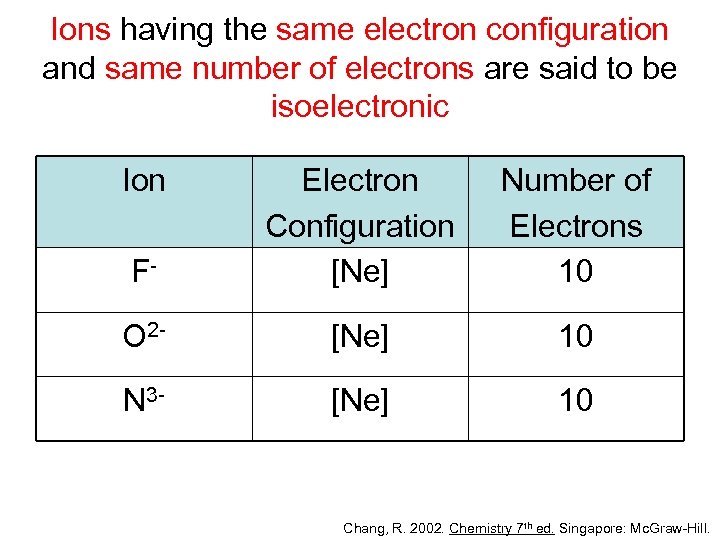 Ions having the same electron configuration and same number of electrons are said to be isoelectronic Ion F- Electron Configuration [Ne] Number of Electrons 10 O 2 - [Ne] 10 N 3 - [Ne] 10 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Ions having the same electron configuration and same number of electrons are said to be isoelectronic Ion F- Electron Configuration [Ne] Number of Electrons 10 O 2 - [Ne] 10 N 3 - [Ne] 10 Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
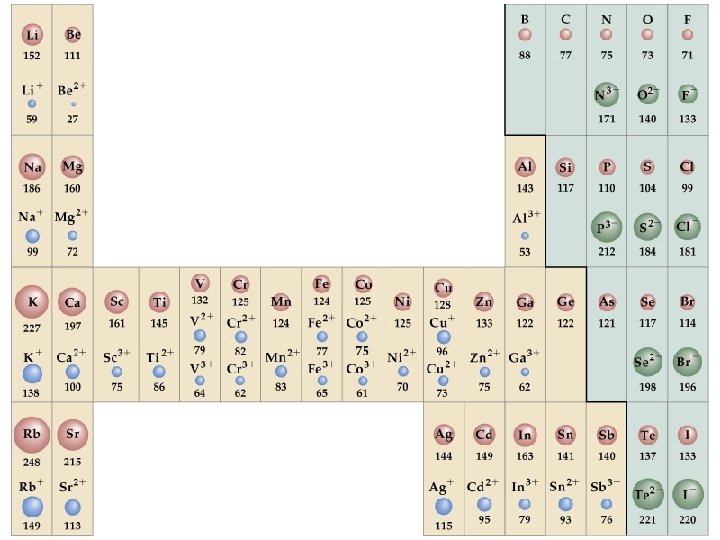
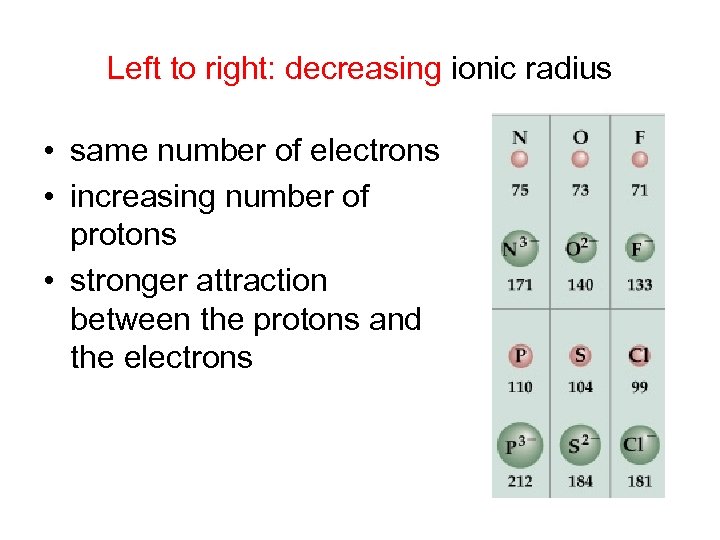 Left to right: decreasing ionic radius • same number of electrons • increasing number of protons • stronger attraction between the protons and the electrons
Left to right: decreasing ionic radius • same number of electrons • increasing number of protons • stronger attraction between the protons and the electrons
 Ionization energy: One atom vs. another
Ionization energy: One atom vs. another
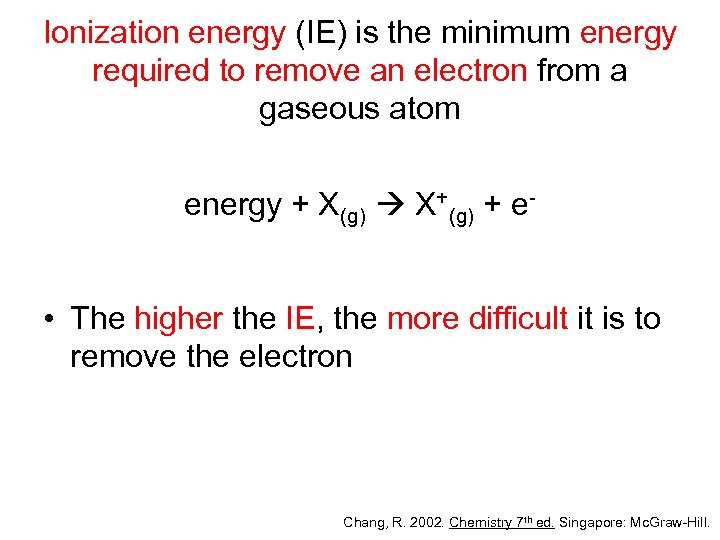 Ionization energy (IE) is the minimum energy required to remove an electron from a gaseous atom energy + X(g) X+(g) + e- • The higher the IE, the more difficult it is to remove the electron Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Ionization energy (IE) is the minimum energy required to remove an electron from a gaseous atom energy + X(g) X+(g) + e- • The higher the IE, the more difficult it is to remove the electron Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
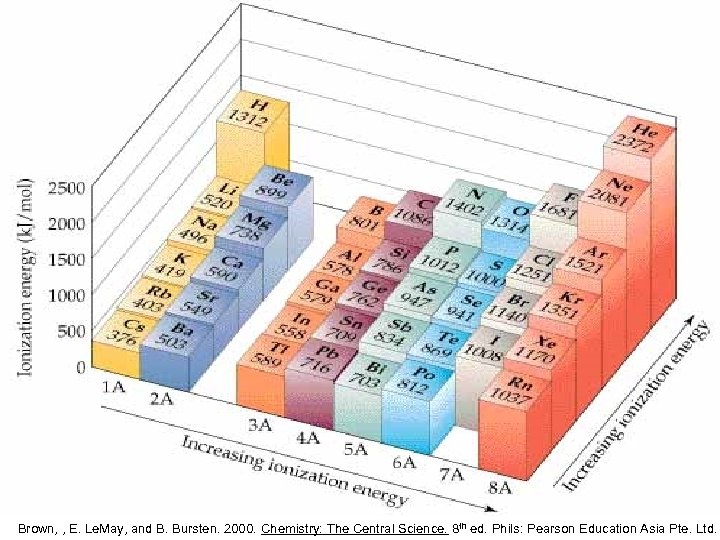 Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. 8 th ed. Phils: Pearson Education Asia Pte. Ltd.
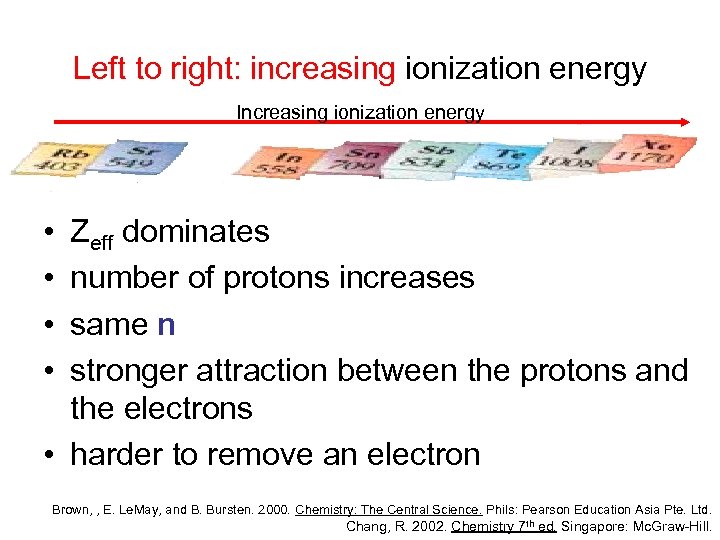 Left to right: increasing ionization energy Increasing ionization energy • • Zeff dominates number of protons increases same n stronger attraction between the protons and the electrons • harder to remove an electron Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Left to right: increasing ionization energy Increasing ionization energy • • Zeff dominates number of protons increases same n stronger attraction between the protons and the electrons • harder to remove an electron Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
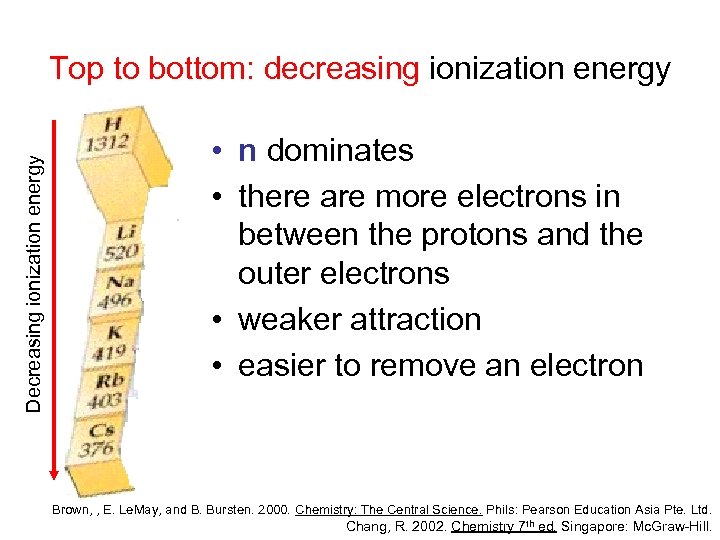 Decreasing ionization energy Top to bottom: decreasing ionization energy • n dominates • there are more electrons in between the protons and the outer electrons • weaker attraction • easier to remove an electron Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
Decreasing ionization energy Top to bottom: decreasing ionization energy • n dominates • there are more electrons in between the protons and the outer electrons • weaker attraction • easier to remove an electron Brown, , E. Le. May, and B. Bursten. 2000. Chemistry: The Central Science. Phils: Pearson Education Asia Pte. Ltd. Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Ionization energy: Same atom
Ionization energy: Same atom
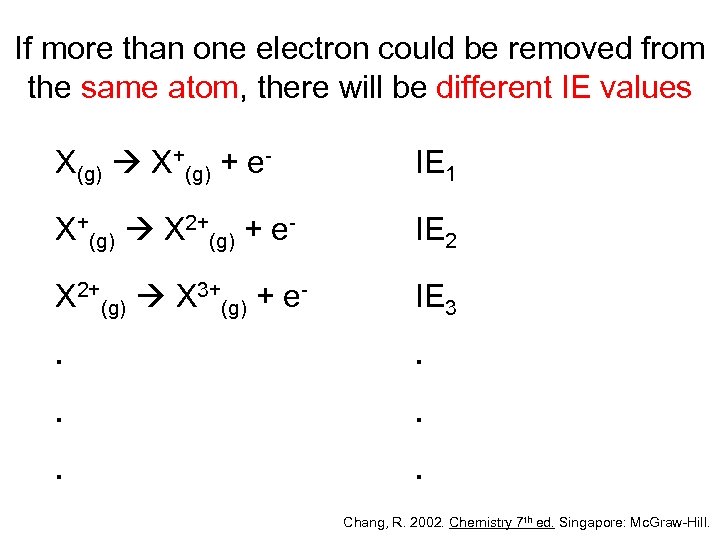 If more than one electron could be removed from the same atom, there will be different IE values X(g) X+(g) + e- IE 1 X+(g) X 2+(g) + e- IE 2 X 2+(g) X 3+(g) + e- IE 3 . . . Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
If more than one electron could be removed from the same atom, there will be different IE values X(g) X+(g) + e- IE 1 X+(g) X 2+(g) + e- IE 2 X 2+(g) X 3+(g) + e- IE 3 . . . Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
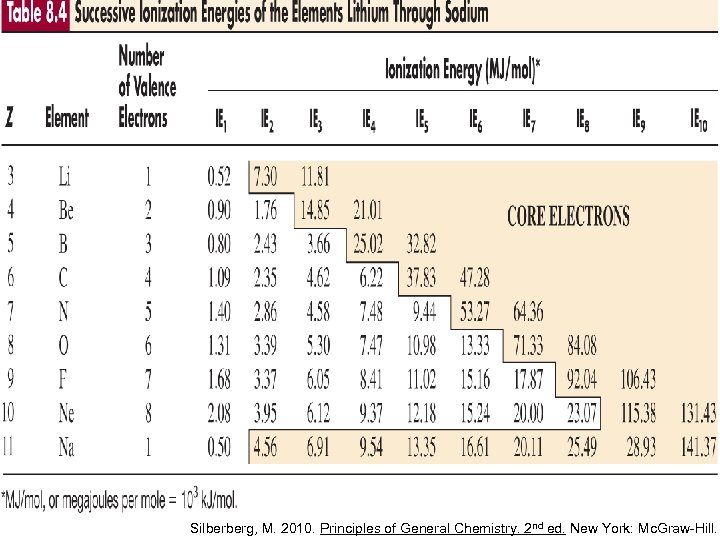
 For the same atom, IE 1 < IE 2
For the same atom, IE 1 < IE 2
 There is a dramatic increase in IE when an electron is removed from an atom/ion with a noble gas configuration • the noble gas configuration is stable • removing another electron from it will result in instability Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
There is a dramatic increase in IE when an electron is removed from an atom/ion with a noble gas configuration • the noble gas configuration is stable • removing another electron from it will result in instability Chang, R. 2002. Chemistry 7 th ed. Singapore: Mc. Graw-Hill.
 Electron affinity
Electron affinity
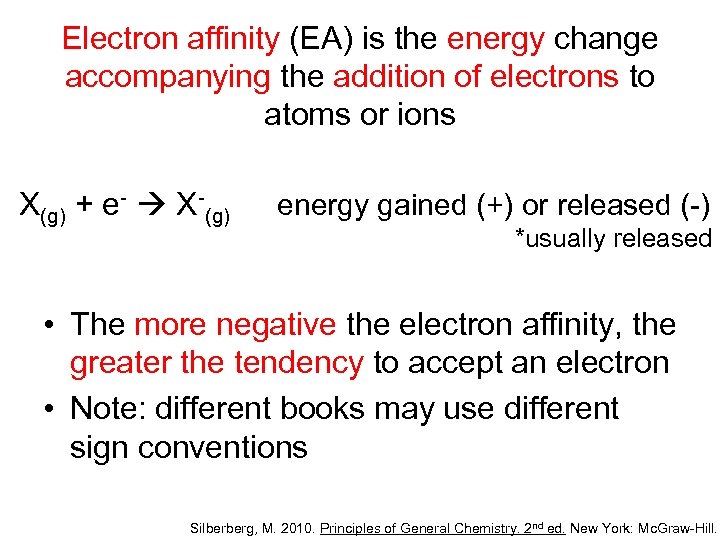 Electron affinity (EA) is the energy change accompanying the addition of electrons to atoms or ions X(g) + e- X-(g) energy gained (+) or released (-) *usually released • The more negative the electron affinity, the greater the tendency to accept an electron • Note: different books may use different sign conventions Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Electron affinity (EA) is the energy change accompanying the addition of electrons to atoms or ions X(g) + e- X-(g) energy gained (+) or released (-) *usually released • The more negative the electron affinity, the greater the tendency to accept an electron • Note: different books may use different sign conventions Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
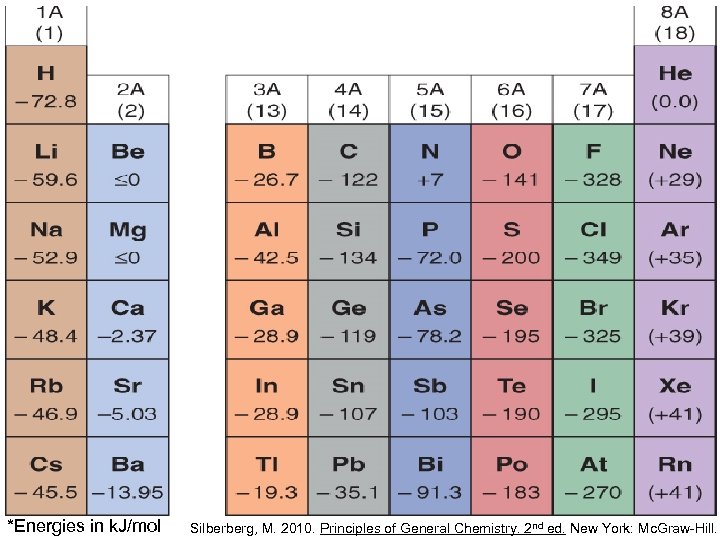 *Energies in k. J/mol Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
*Energies in k. J/mol Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
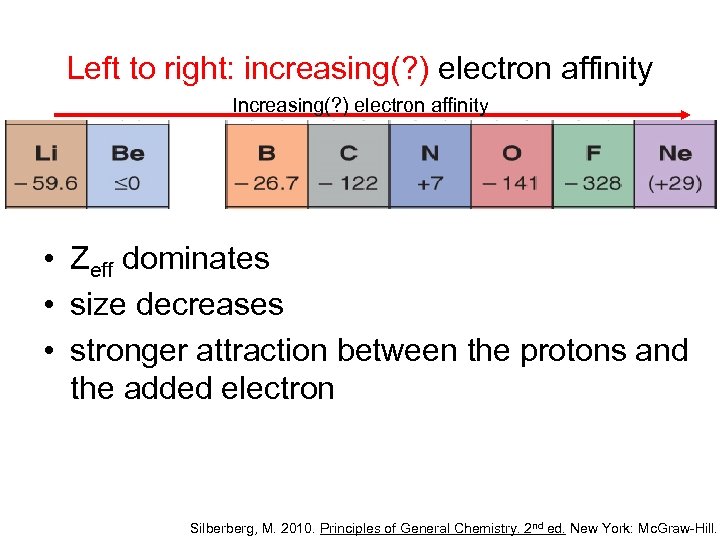 Left to right: increasing(? ) electron affinity Increasing(? ) electron affinity • Zeff dominates • size decreases • stronger attraction between the protons and the added electron Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Left to right: increasing(? ) electron affinity Increasing(? ) electron affinity • Zeff dominates • size decreases • stronger attraction between the protons and the added electron Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
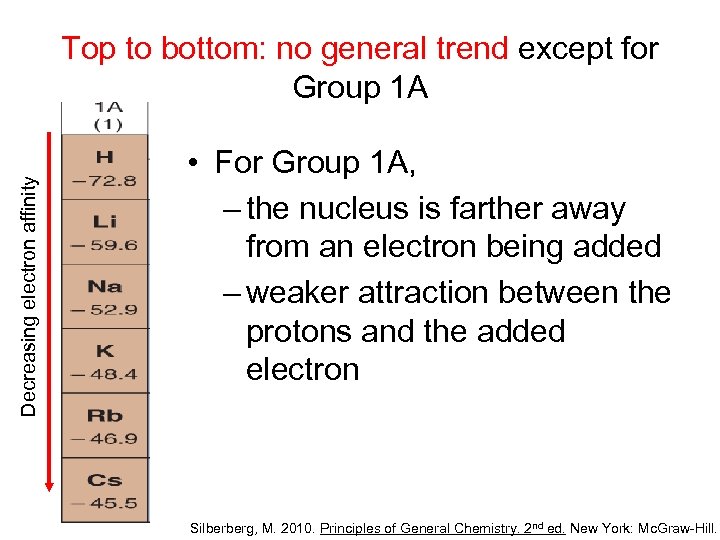 Decreasing electron affinity Top to bottom: no general trend except for Group 1 A • For Group 1 A, – the nucleus is farther away from an electron being added – weaker attraction between the protons and the added electron Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
Decreasing electron affinity Top to bottom: no general trend except for Group 1 A • For Group 1 A, – the nucleus is farther away from an electron being added – weaker attraction between the protons and the added electron Silberberg, M. 2010. Principles of General Chemistry. 2 nd ed. New York: Mc. Graw-Hill.
 Factors other than Zeff and atomic size affect electron affinities, so trends are not as regular as those for the other properties
Factors other than Zeff and atomic size affect electron affinities, so trends are not as regular as those for the other properties


