024449dbd6d5e214770d2a9a0d1f443e.ppt
- Количество слайдов: 40

OPPORTUNITIES IN HOMOGENEOUS AND SINGLE-SITE HETEROGENEOUS CATALYSIS Tobin Marks, DOE Catalysis Workshop May 2002 I. III. IV. V. VII. Current Drivers New Tools and Techniques Single-Site Polymerization. Catalysis Materials Multi-Site Catalysts and Cocatalysts Carbon-Heteroatom Bond Formation Homogeneous-Heterogeneous Interface Biomimetic/Supramolecular, Enantioselective Catalysis VIII. Opportunities and Needed Resources

CURRENT DRIVERS FOR RESEARCH IN HOMOGENEOUS (HETEROGENEOUS) CATALYSIS ENORMOUS ECONOMIC IMPORTANCE!! – Environmental (Green Chemistry, Atom Efficiency, Waste Remediation, Recycling) – Polymeric Materials (New Polymers and Polymer Architectures, New Monomers, New Processes) – Pharmaceuticals and Fine Chemicals (Demand for Greater Chemo-, Regio-, Stereo-, and Enantioselectivity) – Feedstocks (Practical Alternatives to Petroleum and Natural Gas) – Cost of Energy (More Efficient, Selective Processes) – Completely New Materials (e. g. , Carbon Nanotubes) – Cost Squeeze in Chemical Industry – Declining Corporate Investment in Basic Research

NEW TOOLS FOR HOMOGENEOUS (HETEROGENEOUS) CATALYSIS RESEARCH SIMPLE TO EXPENSIVE • New and In Situ Spectroscopies (NMR, EPR, IR/Raman, SPM, EM, X-Ray, EXAFS/XANES) • Synthetic Techniques (Exotic Ligands, New Elements, Solid State, Sol-Gel, Nanoscale) • Reaction Techniques (Combinatorial, High-Pressure, Polymerization) • Computational (DFT, ab initio, MD, combinations) • New Characterization Techniques (Calorimetry, Polymer, Isotopic, Stop-Flow, Chiral GC/HPLC)

A NEW GENERATION OF POLYOLEFINS
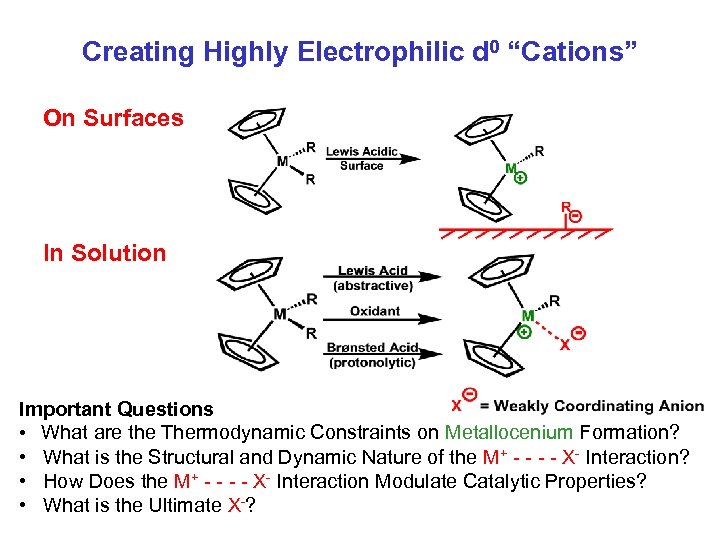
Creating Highly Electrophilic d 0 “Cations” On Surfaces In Solution Important Questions • What are the Thermodynamic Constraints on Metallocenium Formation? • What is the Structural and Dynamic Nature of the M+ - - X- Interaction? • How Does the M+ - - X- Interaction Modulate Catalytic Properties? • What is the Ultimate X-?

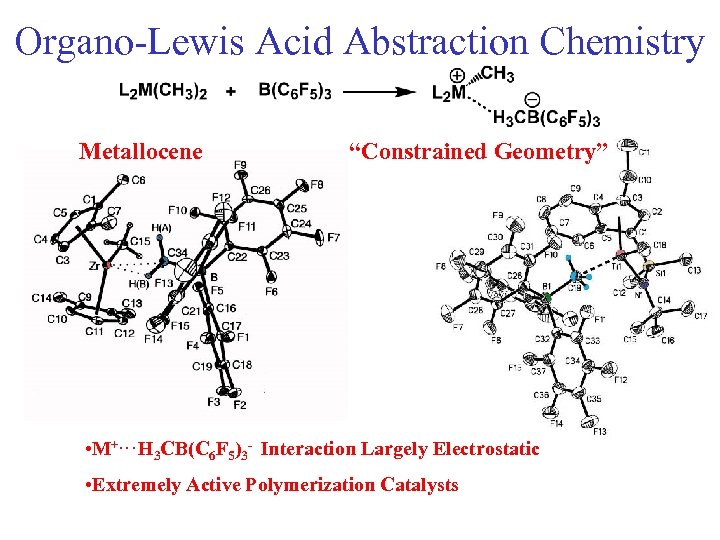
Organo-Lewis Acid Abstraction Chemistry Metallocene “Constrained Geometry” • M+. . . H 3 CB(C 6 F 5)3 - Interaction Largely Electrostatic • Extremely Active Polymerization Catalysts
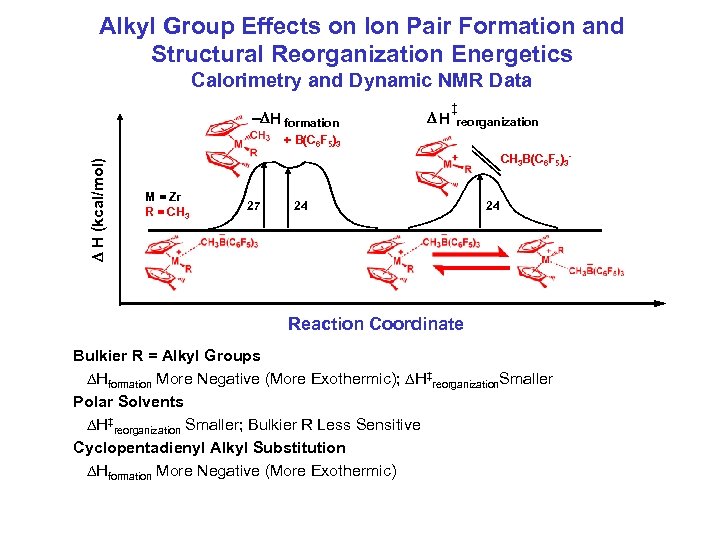
Alkyl Group Effects on Ion Pair Formation and Structural Reorganization Energetics Calorimetry and Dynamic NMR Data - H formation H ‡ reorganization H (kcal/mol) + B(C 6 F 5)3 CH 3 B(C 6 F 5)3 M = Zr R = CH 3 27 24 24 Reaction Coordinate Bulkier R = Alkyl Groups Hformation More Negative (More Exothermic); H‡reorganization. Smaller Polar Solvents H‡reorganization Smaller; Bulkier R Less Sensitive Cyclopentadienyl Alkyl Substitution Hformation More Negative (More Exothermic)

1. 32 1. 3 AB INITIO COMPUTED 2. 80 Å REACTION COORDINATE FOR OLEFIN INSERTION 2. 05 2. 0 2. 37 5 6. 49 1. 4 1 2. 1 4 2. 16 E (kcal/mol) 4. 72 Transition State 6 2. 37 Å Kinetic Product 1. 55 2. 09 1. 53 2. 93 6. 62 5. 48 8 3. 5 3. 0 2. 5 2. 0 1. 5 Reaction Coordinate in Benzene [Ethylene]—[CH 3 Ti] Distance (Å)


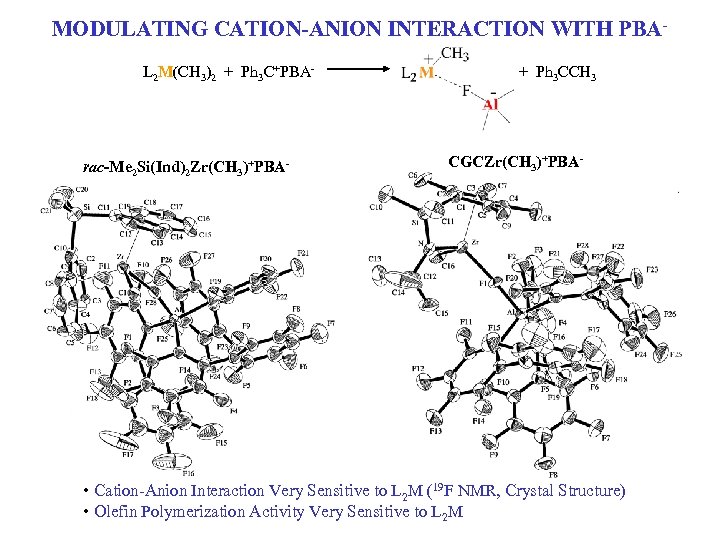
MODULATING CATION-ANION INTERACTION WITH PBAL 2 M(CH 3)2 + Ph 3 C+PBA- rac-Me 2 Si(Ind)2 Zr(CH 3)+PBA- + Ph 3 CCH 3 CGCZr(CH 3)+PBA- • Cation-Anion Interaction Very Sensitive to L 2 M (19 F NMR, Crystal Structure) • Olefin Polymerization Activity Very Sensitive to L 2 M

Are There Anion Effects on Me 2 C(Cp)(Flu)Zr. Me 2 -Mediated Propylene Polymerization ? Syndiospecific Enchainment Mechanism : Does chain swinging require ion pair reorganization ? An ideal system to evaluate ion pairing effects !
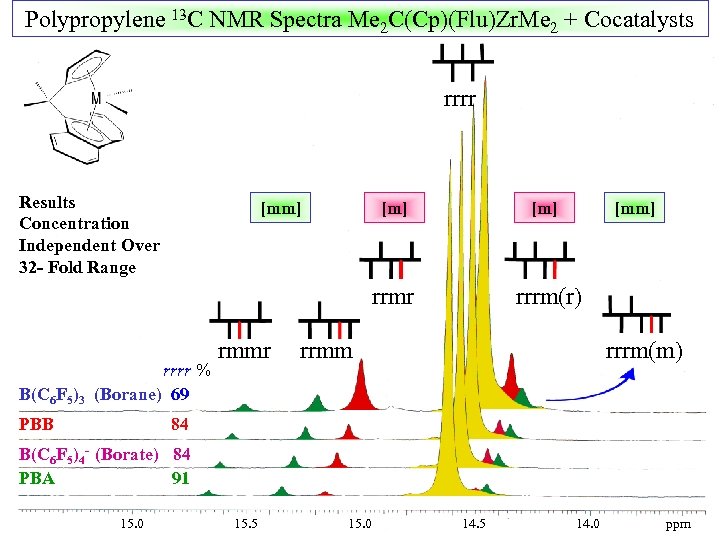
Polypropylene 13 C NMR Spectra Me 2 C(Cp)(Flu)Zr. Me 2 + Cocatalysts rrrr Results Concentration Independent Over 32 - Fold Range [mm] rrrr % B(C 6 F 5)3 (Borane) 69 PBB [m] rrmr rmmr [m] [mm] rrrm(r) rrmm rrrm(m) 84 B(C 6 F 5)4 - (Borate) 84 PBA 91 15. 0 15. 5 15. 0 14. 5 14. 0 ppm
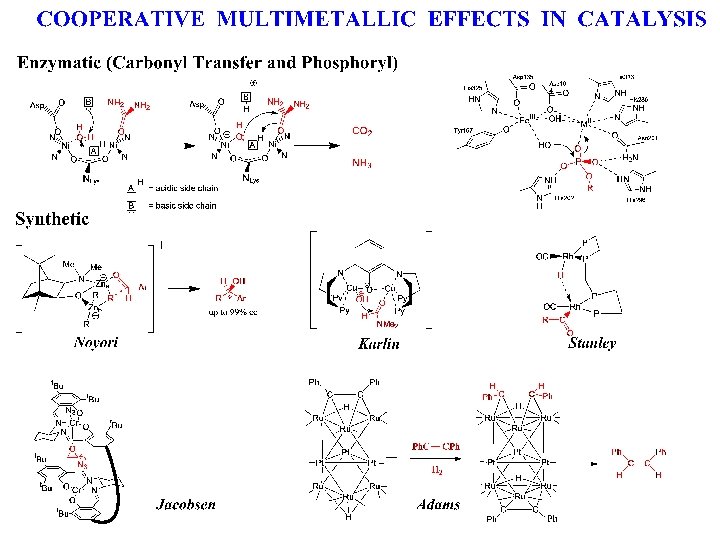
LONG CHAIN BRANCH FORMATION IN ETHYLENE POLYMERIZATION Macromonomer Branch Formation How to make reinsertion more probable ?
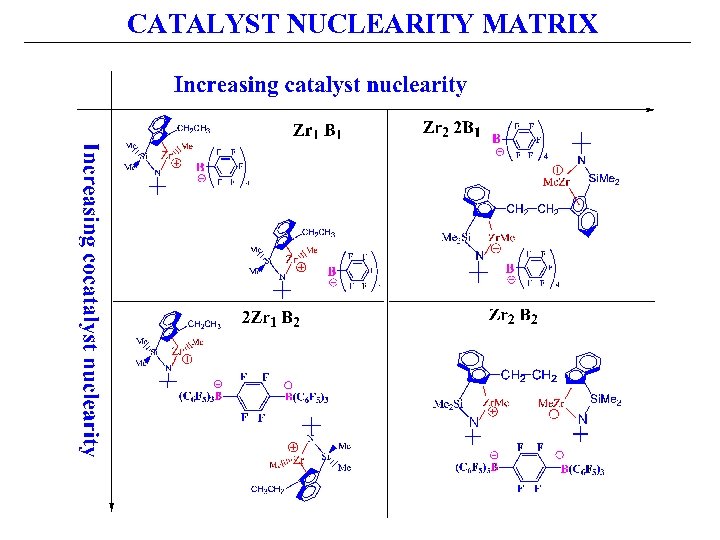
CATALYST NUCLEARITY MATRIX

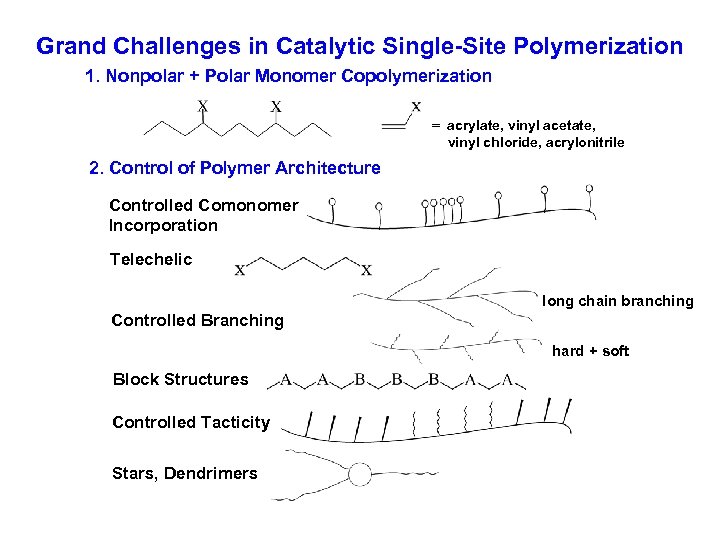
Grand Challenges in Catalytic Single-Site Polymerization 1. Nonpolar + Polar Monomer Copolymerization = acrylate, vinyl acetate, vinyl chloride, acrylonitrile 2. Control of Polymer Architecture Controlled Comonomer Incorporation Telechelic long chain branching Controlled Branching hard + soft Block Structures Controlled Tacticity Stars, Dendrimers

Palladium-Catalyzed Hydroamination of 1, 3 -Dienes Mechanism in the presence of acid Mechanism in the absence of acid Löber, O; Kawatsura, M. ; Hartwig, J. F. J. Am. Chem. Soc. 2001, 123, 4366 -4367
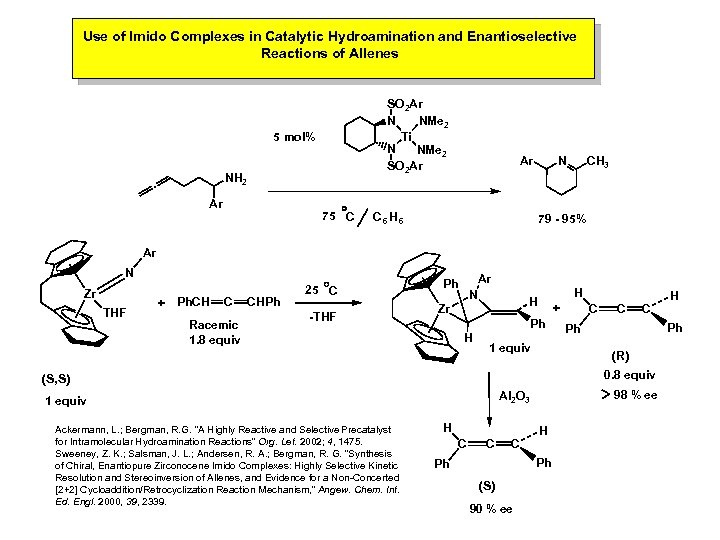
Use of Imido Complexes in Catalytic Hydroamination and Enantioselective Reactions of Allenes SO 2 Ar NMe 2 N Ti N NMe 2 SO 2 Ar 5 mol% NH 2 · Ar 75 C Ar N C 6 H 6 CH 3 79 - 95% Ar N Zr THF + Ph. CH C Racemic 1. 8 equiv CHPh 25 C -THF Ar N Ph H H Zr + Ph H 1 equiv C C 0. 8 equiv 98 % ee Al 2 O 3 1 equiv H C C C H Ph Ph (S) 90 % ee H Ph Ph (R) (S, S) Ackermann, L. ; Bergman, R. G. “A Highly Reactive and Selective Precatalyst for Intramolecular Hydroamination Reactions” Org. Let. 2002; 4, 1475. Sweeney, Z. K. ; Salsman, J. L. ; Andersen, R. A. ; Bergman, R. G. “Synthesis of Chiral, Enantiopure Zirconocene Imido Complexes: Highly Selective Kinetic Resolution and Stereoinversion of Allenes, and Evidence for a Non-Concerted [2+2] Cycloaddition/Retrocyclization Reaction Mechanism, ” Angew. Chem. Int. Ed. Engl. 2000, 39, 2339. C
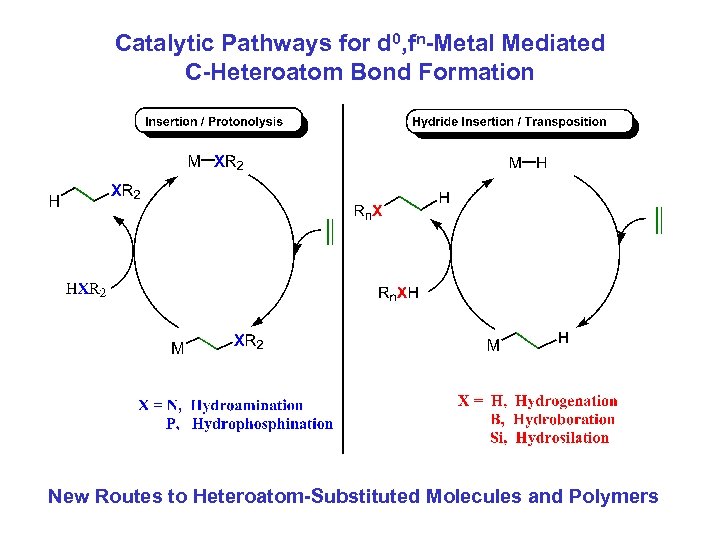
Catalytic Pathways for d 0, fn-Metal Mediated C-Heteroatom Bond Formation New Routes to Heteroatom-Substituted Molecules and Polymers

THERMODYNAMICALLY BASED STRATEGIES FOR CATALYTIC HETEROATOM ADDITION EXAMPLE: Olefinic Substrates Intramolecular EXPECTATIONS • S, S‡ Favor Intramolecular Process • Hii < Hi • kii > ki • Hi (X): CH 3 H < Pr 2, NR 2 < SR, OR (X = Heteroatom Group) Intermolecular

Diastereoselectivity in Aminodiene Cyclization Good to excellent 2, 5 -trans (80% de), and 2, 6 -cis (99% de) diastereoselectivities Concise synthesis of (±)-pinidine with excellent stereocontrols (2, 6 cis and trans-alkene)

Is Hydrophosphination Analogous?

Metallocene – Metal Oxide Chemisorption 1. Lewis Acid Surfaces (Dehydroxylated Al 2 O 3, Mg. Cl 2) High Catalytic Activity Active Sites ~8% 2. Weak Brønsted Acid Surfaces (Si. O 2, Partially Dehydroxylated Al 2 O 3) Poorly Electrophilic Negligible Catalytic Activity

Catalysis with Organozirconium Hydrocarbyls Supported on Sulfated Zirconia Solid BrØnsted Super Acid Most active benzene hydrogenation catalyst known Polymerization activity varies with coordinative unsaturation: Zr. R 4 > Cp. Zr. R 3 > Cp 2 Zr. R 2
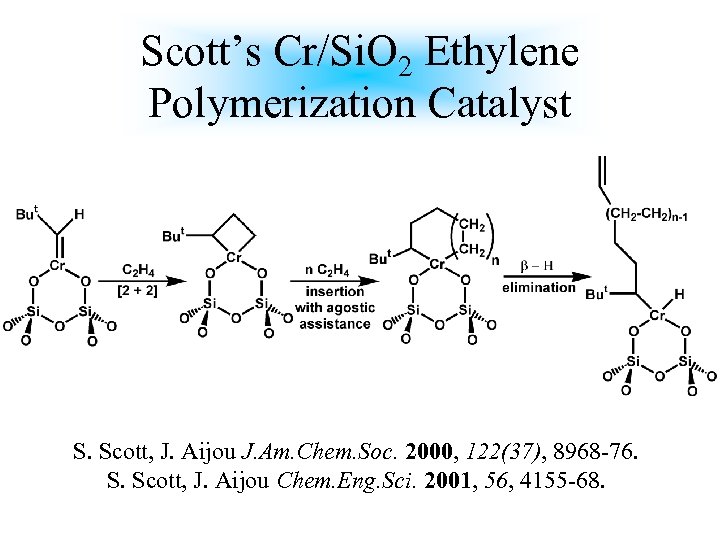
Scott’s Cr/Si. O 2 Ethylene Polymerization Catalyst S. Scott, J. Aijou J. Am. Chem. Soc. 2000, 122(37), 8968 -76. S. Scott, J. Aijou Chem. Eng. Sci. 2001, 56, 4155 -68.

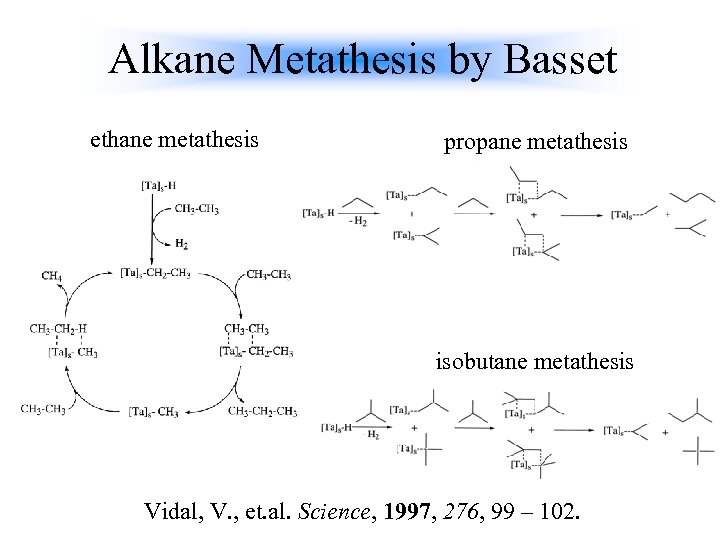
Alkane Metathesis by Basset ethane metathesis propane metathesis isobutane metathesis Vidal, V. , et. al. Science, 1997, 276, 99 – 102.
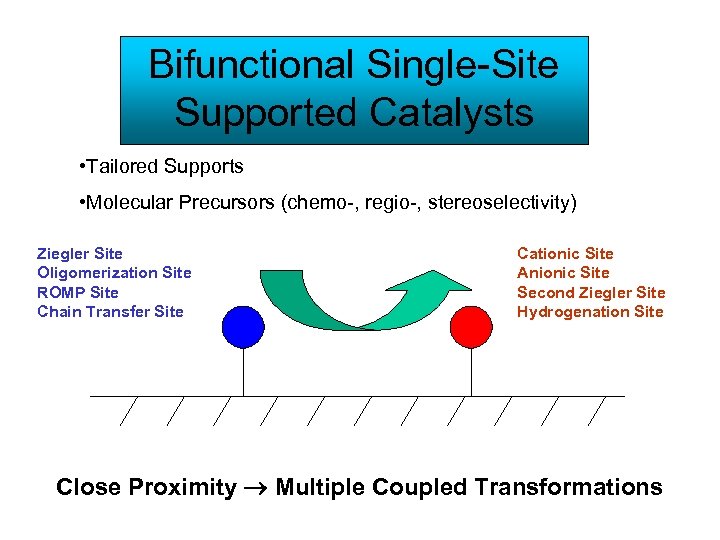
Bifunctional Single-Site Supported Catalysts • Tailored Supports • Molecular Precursors (chemo-, regio-, stereoselectivity) Ziegler Site Oligomerization Site ROMP Site Chain Transfer Site Cationic Site Anionic Site Second Ziegler Site Hydrogenation Site Close Proximity Multiple Coupled Transformations

Structure of Carbonic Anhydrase A Metalloenzyme CO 2 + H 2 O H 2 CO 3 Nt ~ 107 – 109 sec-1 Now with Cd: T. W. Lane and F. M. M Morel Proc. Nat. Acad. Sci. USA 2000, 97, 4627 -4631

Artificial Enzyme for Olefin Epoxidation º • Encapsulation of catalyst ==> 100 -fold increase in lifetime. • Incorporation of ligands predictably modifies the internal cavity size to induce substrate selectivity Nguyen, Hupp and coworkers
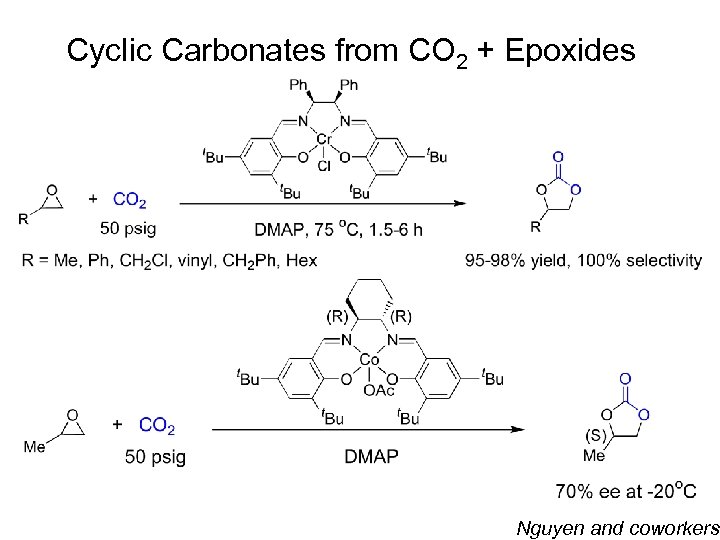
Cyclic Carbonates from CO 2 + Epoxides Nguyen and coworkers
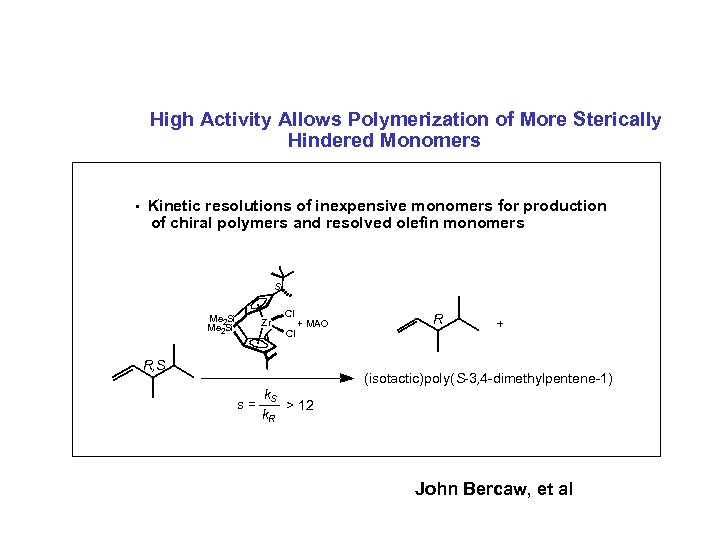
High Activity Allows Polymerization of More Sterically Hindered Monomers • Kinetic resolutions of inexpensive monomers for production of chiral polymers and resolved olefin monomers S Me 2 Si Zr Cl Cl + MAO R, S R + (isotactic)poly( S-3, 4 -dimethylpentene-1) s= k. S > 12 k. R John Bercaw, et al

Thermal, Catalytic, Regiospecific Functionalization of Alkanes (RBpin) • terminal product only steric preference for a linear metal-alkyl complex Chen, H. ; Schlecht, S. ; Semple, T. C. ; Hartwig, J. F. Science 2000, 287(5460), 1995 -1997
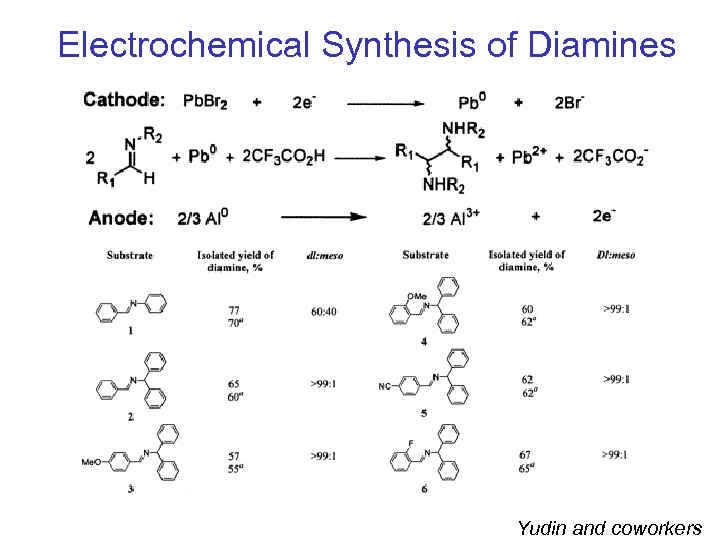
Electrochemical Synthesis of Diamines Yudin and coworkers

SUMMARY. FUTURE OPPORTUNITIES • MULTINUCLEAR / MULTIFUNCTIONAL CATALYSTS – Multisite Substrate Activation, Conversion – New Polymer Architectures, Modifications • NEW SURFACES – New Molecular Catalyst Activation Routes – Single-Site Ensembles • NEW OR IMPROVED TRANSFORMATIONS – Improved Selectivity (Chemo-, Regio-, Enantio-) – C-Heteroatom Formation (C-O, C-N, C-P, C-S, etc. ) – Abundant Feedstocks (CO 2, Si. O 2, Saturated Hydrocarbons, Biomass, Bioproducts, Waste) – Atom-Efficient, Heat-Efficient Transformations • NEW ELEMENTS, LIGANDS, COCATALYSTS – Early Transition Metals, Lanthanides, Actinides – Ligand Engineering – Cocatalyst Engineering

Catalytic Cycle for Aryl Ether Synthesis

024449dbd6d5e214770d2a9a0d1f443e.ppt