124695e0c9aebc6ffa51372e97352fa7.ppt
- Количество слайдов: 82

Online Module: Traumatic Brain Injury and increased ICP Intracranial Hematomas Spontaneous Subarachnoid Hemorrhage Herniation Syndromes

Traumatic Brain Injury and Increased ICP

Practically speaking, the brain is protected by a rigid case, within which there is no “extra” space.

The Cranial Vault n Skull (holds three things) Brain n Blood n Cerebro-Spinal Fluid (CSF) n An increase in any one of these three “compartments” displaces the other two. n The principle buffer in the system is CSF. n Only small volume increases can be tolerated before intracranial pressure (ICP) begins to rise. n

The Monroe-Kellie Doctrine n Increase in one constituent necessitates a compensatory decrease in the volume of another constituent.

Compliance Because three constituents that take up residence within the cranial vault are housed within a relatively rigid container, small increases in volume can drastically increase the intracranial pressure n This is the concept of compliance. n n Compliance = change in volume/change in pressure

Normal Cerebral Metabolism Brain tissue relies on aerobic metabolism – gets starved for oxygen very quickly because it has no real backup energy reserve. n Normal cerebral metabolism requires a blood flow of approximately 50 m. L/100 g/min. n Serious neurological deficits begin to occur at 20 m. L/100 g/min. n Prolonged Cerebral Blood Flow > 12 m. L/100 g/min. results in cerebral infarction. n

MAP and ICP n MAP = “Mean Arterial Pressure” Defined as: [(2 x DBP) + (SBP)]/3 n This is literally the average arterial blood pressure over a single cardiac cycle. n n ICP = “Intra-Cranial Pressure” n n 15 mm. Hg is getting into the upper limits of normal in you and me. MAP – ICP = CPP

Cerebral Perfusion Pressure is key to brain tissue survival. n CPP = MAP – ICP n This takes measurable physiologic parameters into account to determine if the individual is able to adequately oxygenate brain tissue. It is the net pressure of blood delivery to the brain. n In a normal adult, CPP is generally regulated between 70 and 90 mm. Hg (can be more or less). n
Cerebral Metabolism n n In the uninjured brain, the arterioles regulate CBF to the brain over MAPs ranging from 50 -150 mm. Hg. Outside of this range, the arterioles lose the ability to autoregulate, and blood flow becomes dependent upon blood pressure (this is called pressure-passive flow). MAPs less than 50 run risk of ischemia due to decreased blood flow, while MAPs greater than 150 causes excess CBF that can contribute to increased intracranial pressure. Autoregulation is impaired in the injured brain.
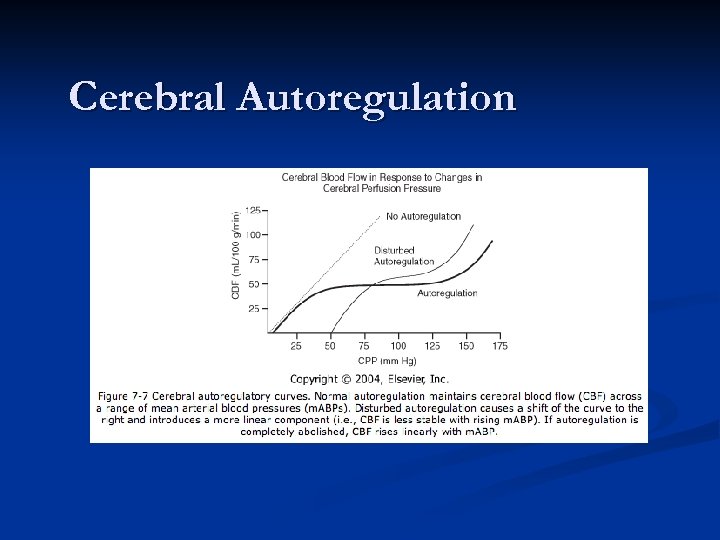
Cerebral Autoregulation

Summing that up Normal anatomy implies a fixed space in skull where small increase in volume causes large increase in pressure. n Perfusion of brain tissue is impaired by anything that’s taking up space or decreasing blood delivery to the tissue. n Normal autoregulation processes that help the brain maintain homeostasis are impaired after injury. n

The TRAUMATIC neuro exam n When dealing with “Emergency” neurological evaluation, you MUST MUST KNOW the concept of the Glascow Coma Scale!!!!!!!

Clinical Assessment: GCS

Diagnostic Imaging This is a great way to get past the handicap of having a relatively inflexible surface to evaluate. n Often provides you the information you need to ANTICIPATE a problem or PREVENT its occurrence before it becomes clinically apparent. n Newer diagnostic imaging modalities have REVOLUTIONIZED management of intracranial pathology (the benefit to traumatic brain injury is immeasurable)! n

“Reading head films 101” #1 – Identify the modality n #2 – Orient yourself n #3 – Describe what you see; don’t just try to find what’s abnormal n #4 – Pay attention to symmetry! n #5 – Pay attention to proportion n

The CT Scan n n The CT scan singlehandedly revolutionized the landscape for headtrauma. Allows quick evaluation of intracranial space (and skull). Good for acute. eval “Fancy X-ray”
Traumatic Brain Injury n Two general types of injury: n Primary – represents the direct result of the initial trauma. n n Fracture Cerebral contusion Vascular disruption Secondary – results from the evolution of the initial injury or complications. (This is what we aim to minimize) n n n Hypoxia and ischemia Cerebral edema Intracranial hypertension (ICH)

Cerebral Ischemia This is the mechanism that underlies “secondary” injury to the brain. n It all comes back around to Cerebral Perfusion Pressure. n Remember that you’ve got two opposing forces – the mean arterial pressure “pushing” oxygenated blood into brain tissue, and intracranial pressure pushing back. n CPP = MAP - ICP n

Bottom Line n n n You need adequate CPP to maintain viable brain tissue. Once past the primary injury, secondary injury sets in as the volume of any one (or more) of the three constituents starts to increase and cerebral ischemia occurs. ICP begins to rise relatively quickly once the extra volume increases (especially beyond ~100 cc’s. ) Generally, 60 – 70 mm. HG is the target CPP for patients with TBI. It is thought that ICP should be treated once it hits a threshhold of 20 mm. Hg.

Symptoms and Signs of Elevated ICP n Triad n Headache, nausea, vomiting n Cranial nerve palsies n Papilledema n Usually from a more chronic process n Vital sign changes n Cushing’s n Arterial hypertension and bradycardia n Respiratory changes
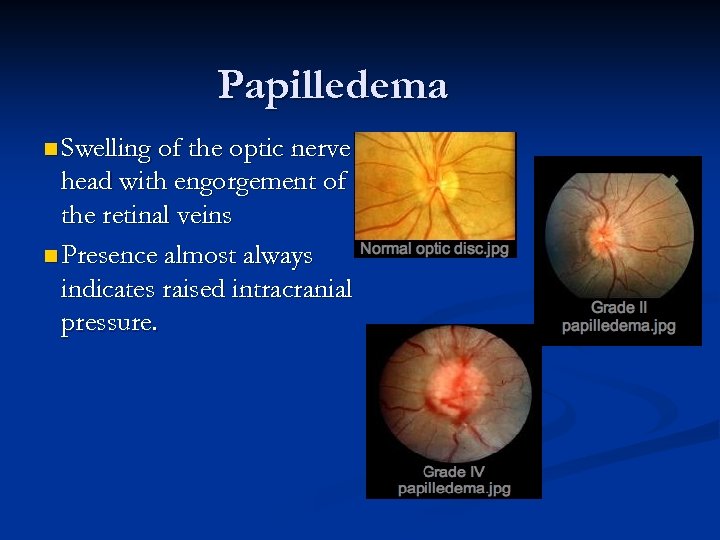
Papilledema n Swelling of the optic nerve head with engorgement of the retinal veins n Presence almost always indicates raised intracranial pressure.
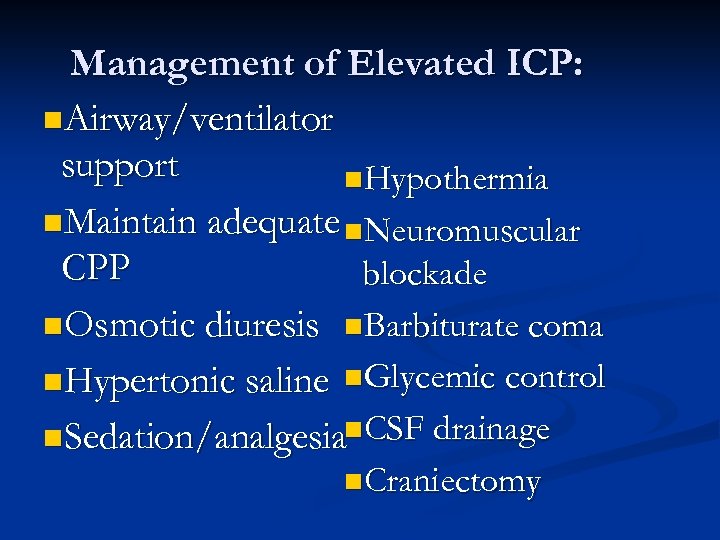
Management of Elevated ICP: n. Airway/ventilator support n. Hypothermia n. Maintain adequate n. Neuromuscular CPP blockade n. Osmotic diuresis n. Barbiturate coma n. Hypertonic saline n. Glycemic control n. Sedation/analgesian. CSF drainage n. Craniectomy
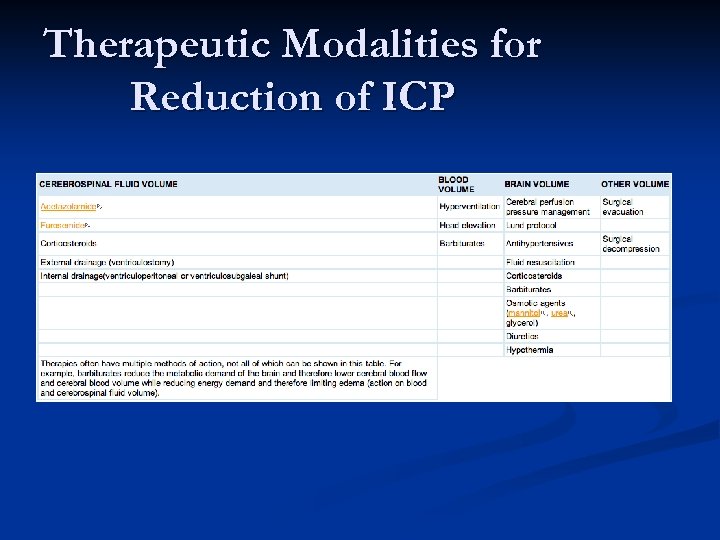
Therapeutic Modalities for Reduction of ICP

Intracranial Hematomas
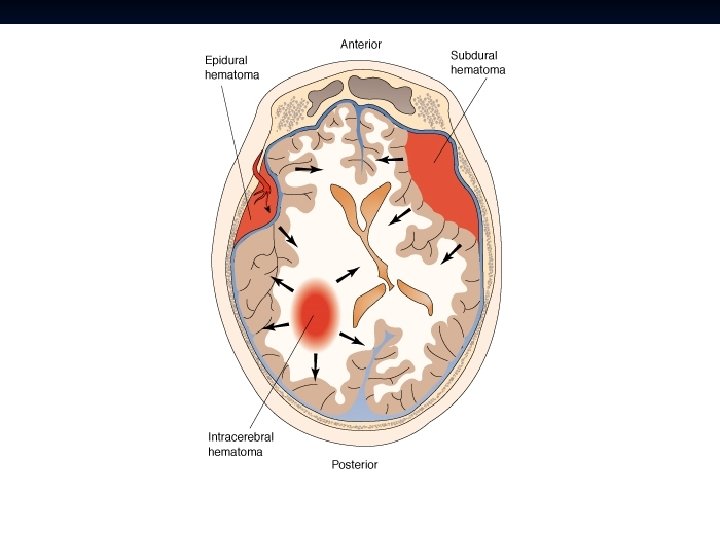
██████████████████

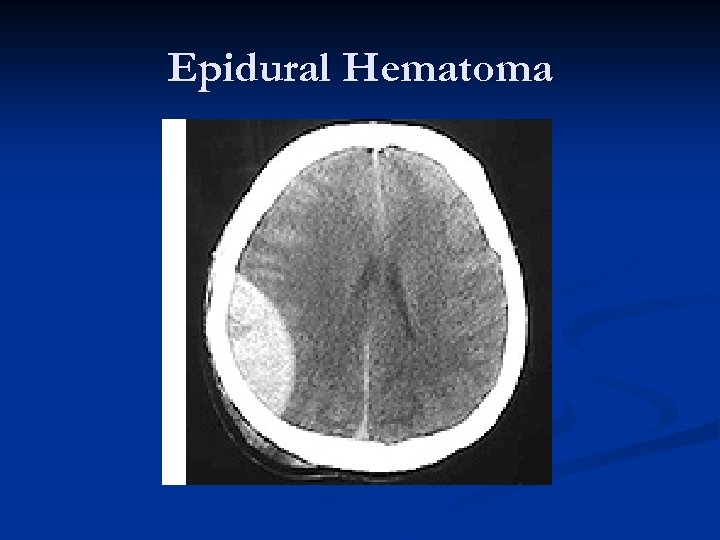
Epidural Hematoma Typically younger patients n Patients may present with alterations in consciousness, headache, nausea/vomiting, etc. n Evidence of trauma: Contusion, laceration, or bony step-off may be observed on the head (should have high clinical suspicion). n
Epidural Hematoma “Classic presentation”: Impact on side of head with “lucid interval, ” then CT scan demonstrating biconvex (lenticular – “lens shaped”) hematoma causing brain compression and midline shift. n Deterioration can be rapid. n Prognosis is generally excellent if managed aggressively!!! n Mortality estimated as high as 20%! n

Epidural Hematomas – 90% seen in head trauma with a skull fracture that crosses a portion of the middle meningeal artery/vein. n Middle meningeal artery is torn approximately 2/3 rds of the time. n Blood which collects in the epidural space is limited by the intracranial sutures, where the dural membrane is closely adherent to the inside of the calvarium. n
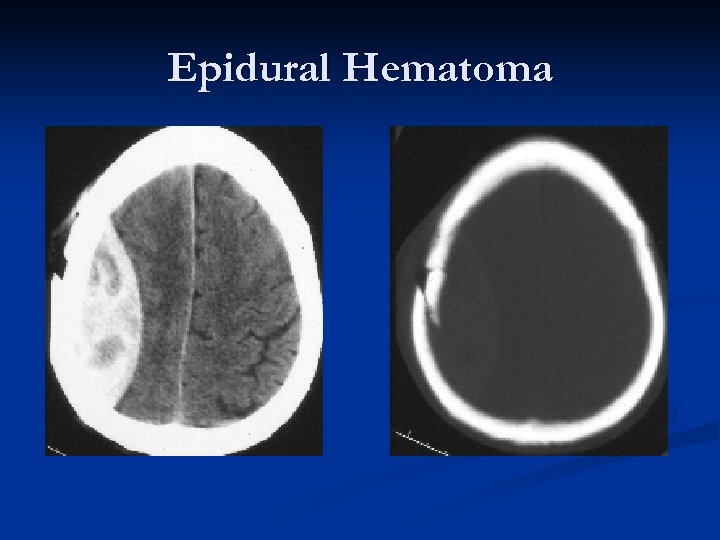
Epidural Hematoma
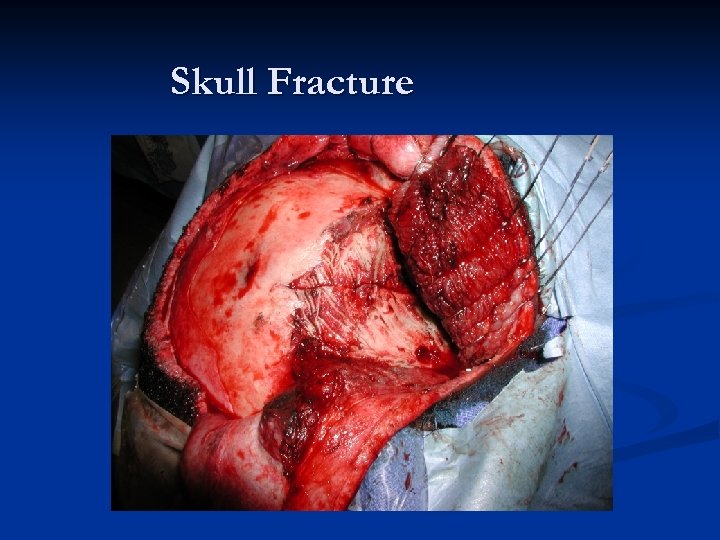
Skull Fracture
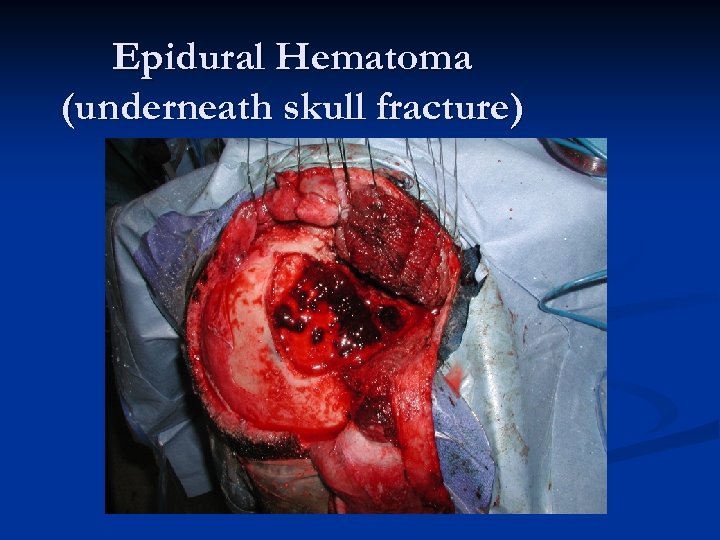
Epidural Hematoma (underneath skull fracture)

Epidural Hematoma Generally, treatment is surgical. n Can sometimes manage small EDHs conservatively if they are asymptomatic (but you still MUST get these patients to a Neurosurgeon so that if the EDH evolves or clinical picture worsens they can receive immediate treatment. n What really hurts these patients is delay in diagnosis, delay in transfer, i. e. anything that delays treatment!!! n
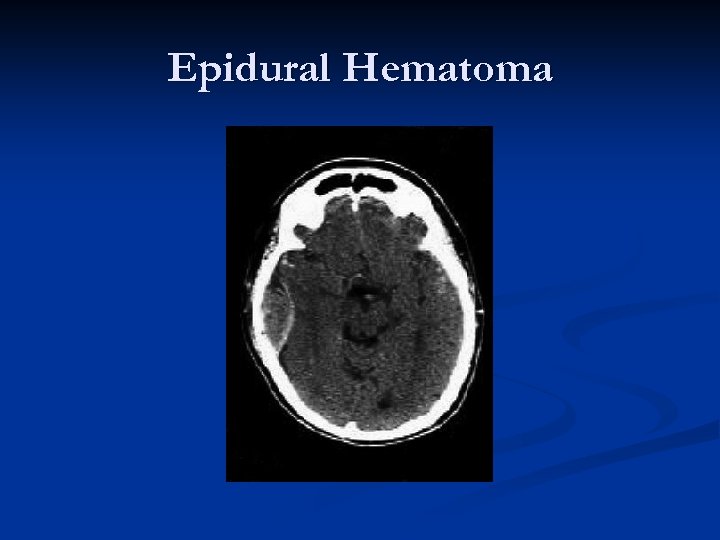
Epidural Hematoma
Epidural Hematoma

Subdural Hematoma n Come in three different “flavors”: Acute n Subacute n Chronic n n Don’t be fooled!!! Acute to Chronic SDHs are different clinical entities!
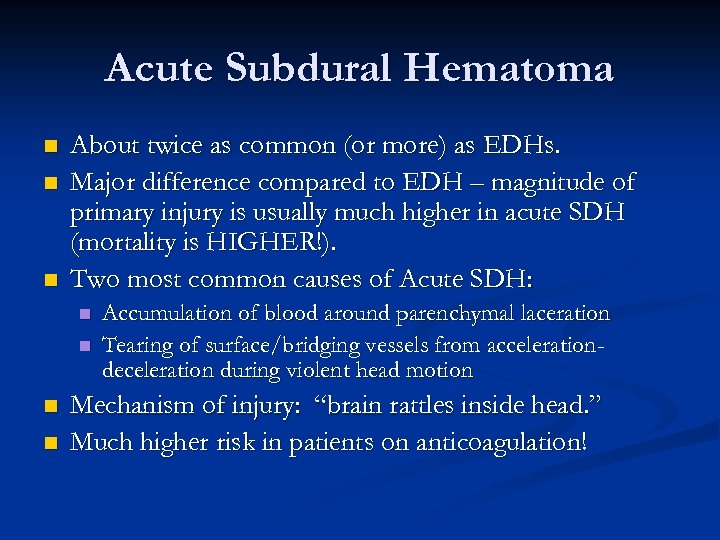
Acute Subdural Hematoma n n n About twice as common (or more) as EDHs. Major difference compared to EDH – magnitude of primary injury is usually much higher in acute SDH (mortality is HIGHER!). Two most common causes of Acute SDH: n n Accumulation of blood around parenchymal laceration Tearing of surface/bridging vessels from accelerationdeceleration during violent head motion Mechanism of injury: “brain rattles inside head. ” Much higher risk in patients on anticoagulation!

Acute SDHs Typically an older patient who suffers some type of head injury (or suspected injury) and gets a CT scan in the ER. n Radiographically, what you typically see is a HYPER-dense CRESCENT-shaped fluid collection that CROSSES SUTURE LINES!!! n Associated parenchymal bleeding, even subarachnoid blood is not an uncommon finding. n
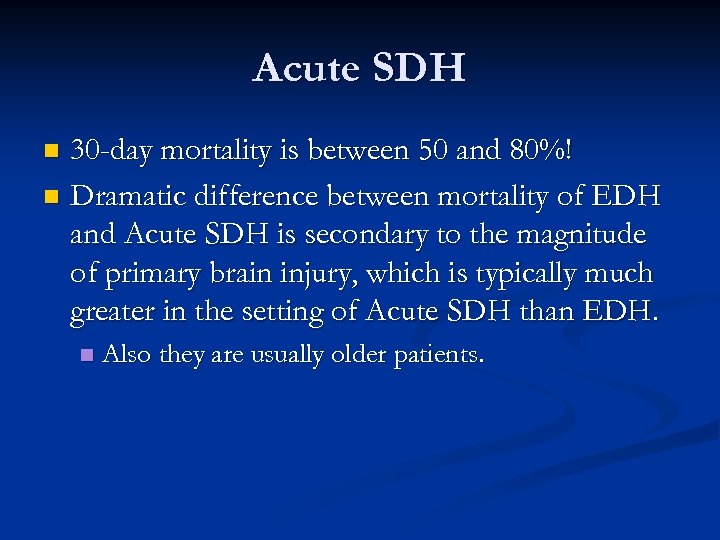
Acute SDH 30 -day mortality is between 50 and 80%! n Dramatic difference between mortality of EDH and Acute SDH is secondary to the magnitude of primary brain injury, which is typically much greater in the setting of Acute SDH than EDH. n n Also they are usually older patients.
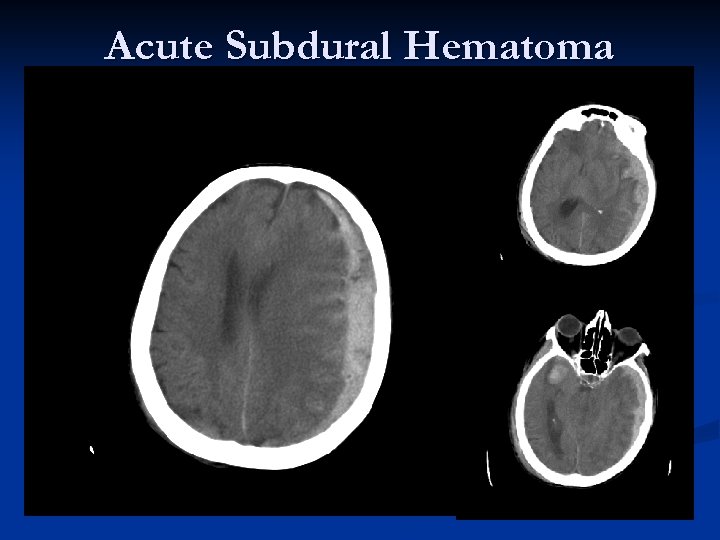
Acute Subdural Hematoma
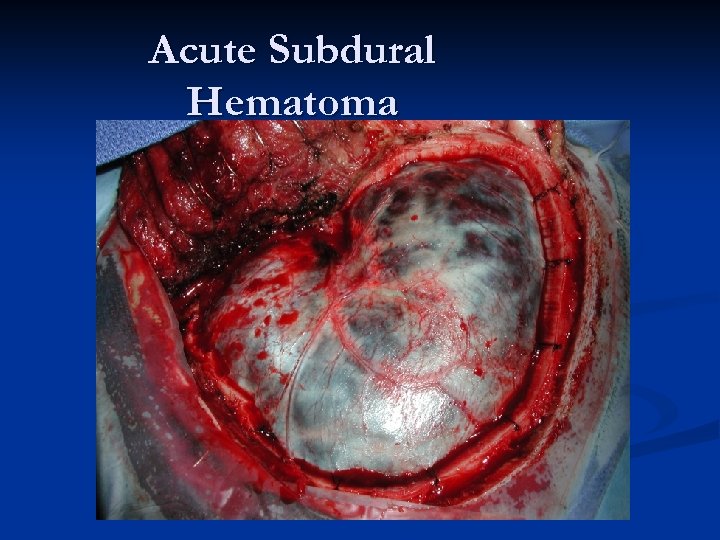
Acute Subdural Hematoma
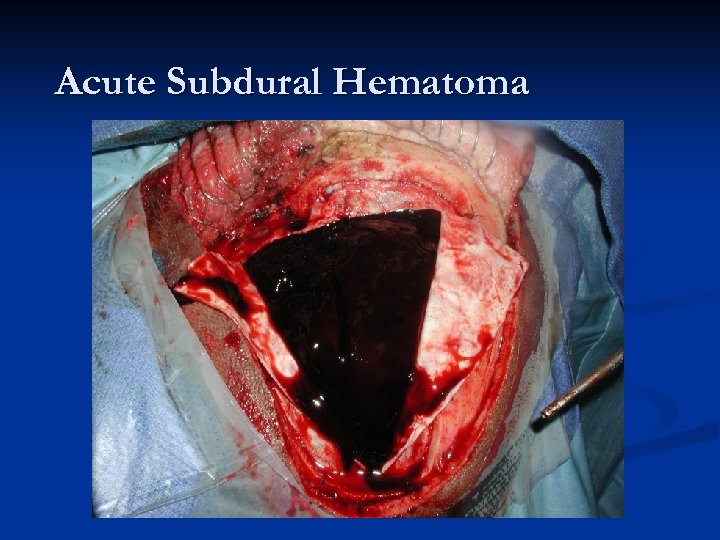
Acute Subdural Hematoma
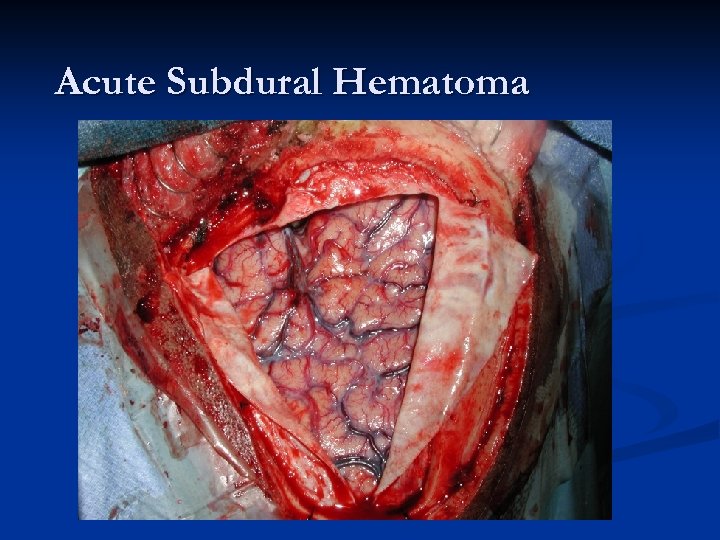
Acute Subdural Hematoma
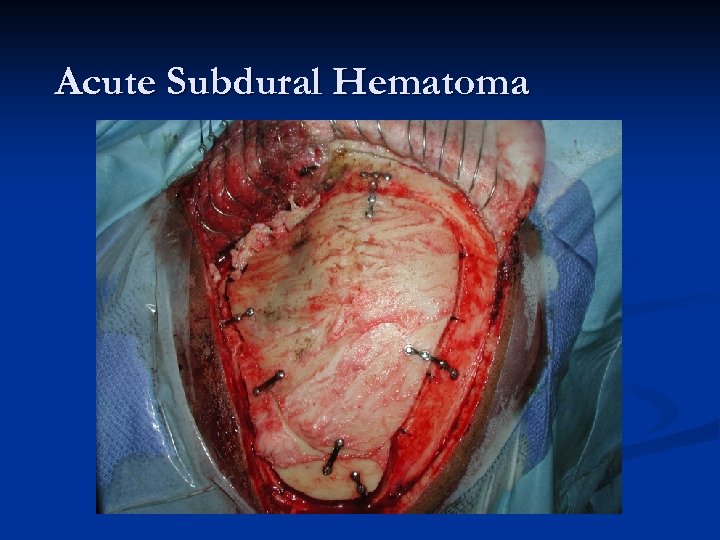
Acute Subdural Hematoma
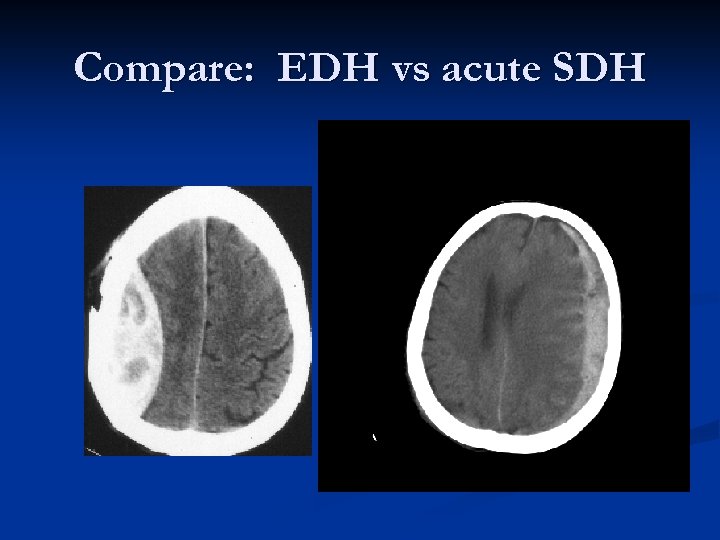
Compare: EDH vs acute SDH

Chronic SDHs n n Generally occur in elderly (avg age of presentation is 63 yrs) Other risk factors: alcohol abuse, coagulopathies, fall risk, seizures, etc. n n n Anything that impairs blood clotting, coagulation, etc. Patients can present any of a multitude of different ways: TIA-type symptoms, headache, confusion, lethargy, speech trouble, weakness, etc. Often, no known history of trauma or event.

Chronic SDHs Prevailing thought is that Chronic SDHs evolve from small / asymptomatic acute SDHs. n Blood within the subdural space causes inflammatory response; fibroblasts invade, neomembranes form, neovascularization occurs. n Chronic SDHs can be very complex, multi-lobed structures. n Tend to gradually get larger over time. n
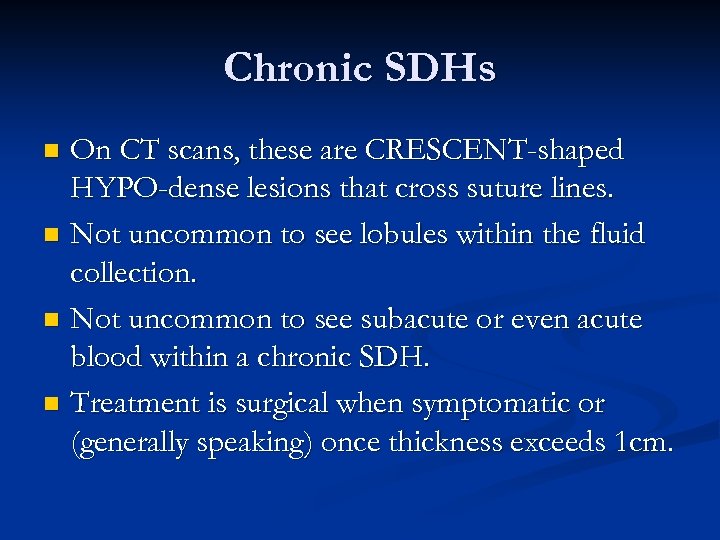
Chronic SDHs On CT scans, these are CRESCENT-shaped HYPO-dense lesions that cross suture lines. n Not uncommon to see lobules within the fluid collection. n Not uncommon to see subacute or even acute blood within a chronic SDH. n Treatment is surgical when symptomatic or (generally speaking) once thickness exceeds 1 cm. n
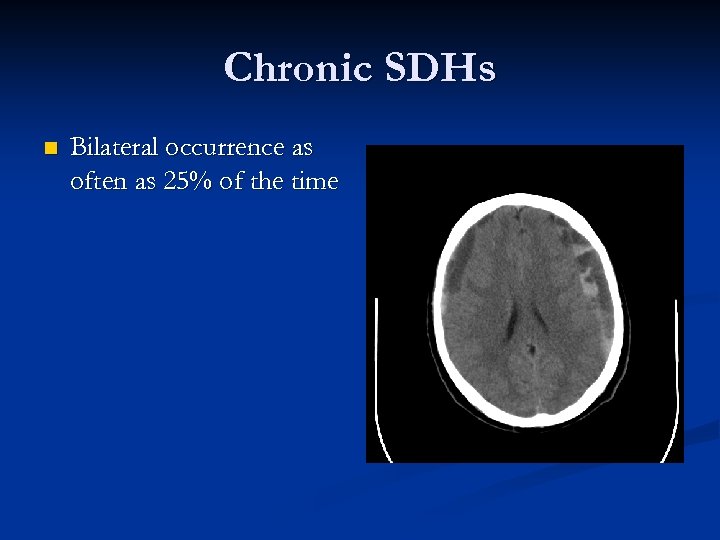
Chronic SDHs n Bilateral occurrence as often as 25% of the time
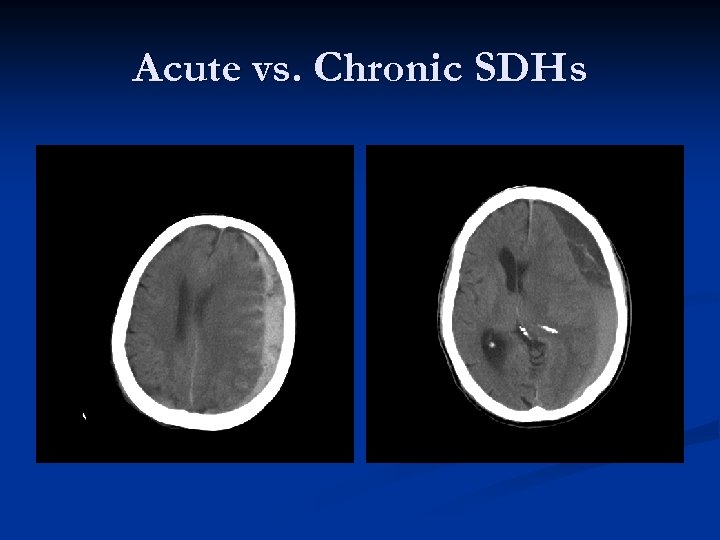
Acute vs. Chronic SDHs
Radiographic difference between SDH types: Timeframe Acute SDHs – hyperdense (less than one week old) n Subacute SDHs – isodense (between five days and three weeks old) n Chronic SDHs – hypodense (between three weeks and 3 -4 months old) n

Understand n Acute SDHs, like EDHs, need to be diagnosed immediately. If Neurosurgery service is not available, these patients must be transferred to hospital where it IS available. n Delays in diagnosis, transfer, treatment, etc. can (and do) kill patients! n
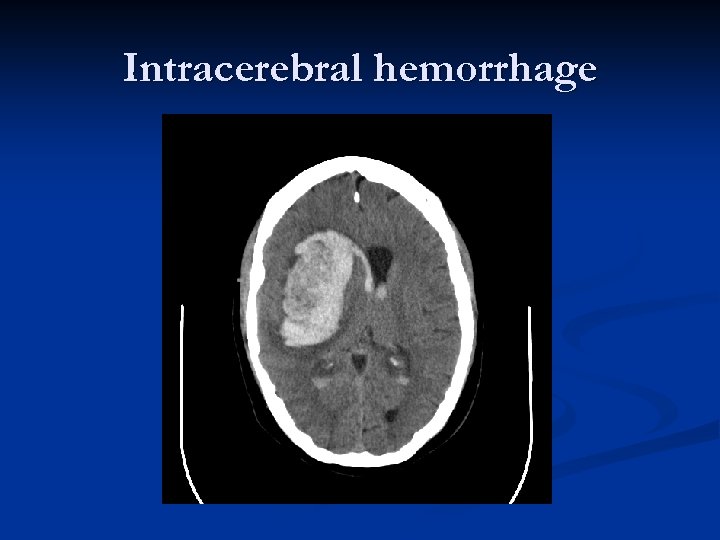
Intracerebral hemorrhage

ICH n n n n In adults, this is the second-most common form of stroke (~30%) and by far the most deadly. Typical onset during activity, with headache; also N/V Age > 55 Hypertension (greatest association) Drug use Coagulopathy, liver dysfunction, etc. , all associated with poorer outcomes. ANY prior CVA increases risk 23 times!

ICH n Four most common locations: n n n Basal Ganglia (Putamen, in particular) Thalamus Deep Cerebellar Nuclei Brainstem (Pons by far) Despite extensive study, the role of surgery in management of ICH is largely unclear. n Surgery never shown to improve long-term outcome over best medical management (Cerebellar hemorrhage is exception)
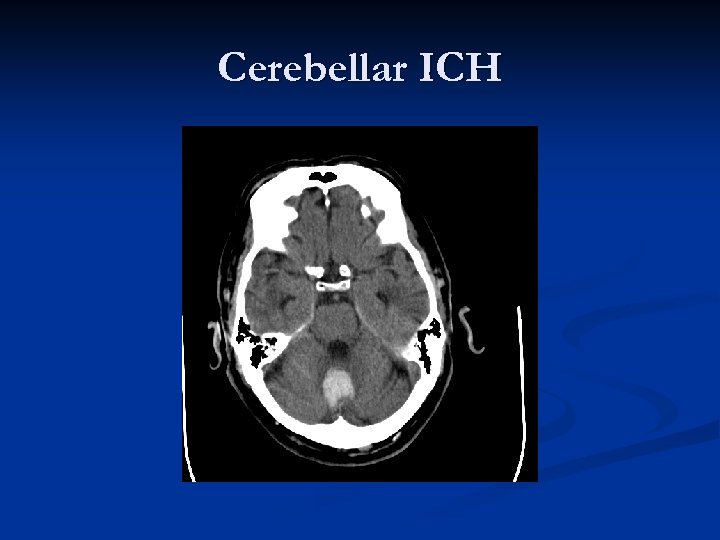
Cerebellar ICH
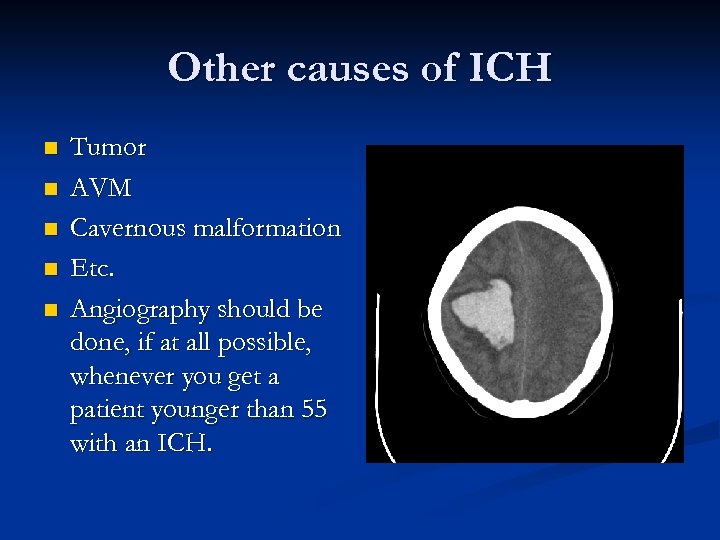
Other causes of ICH n n n Tumor AVM Cavernous malformation Etc. Angiography should be done, if at all possible, whenever you get a patient younger than 55 with an ICH.
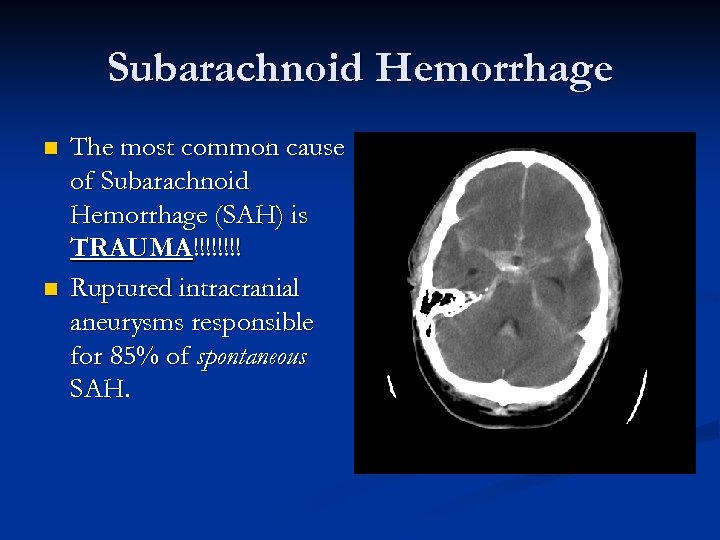
Subarachnoid Hemorrhage n n The most common cause of Subarachnoid Hemorrhage (SAH) is TRAUMA!!!! Ruptured intracranial aneurysms responsible for 85% of spontaneous SAH.
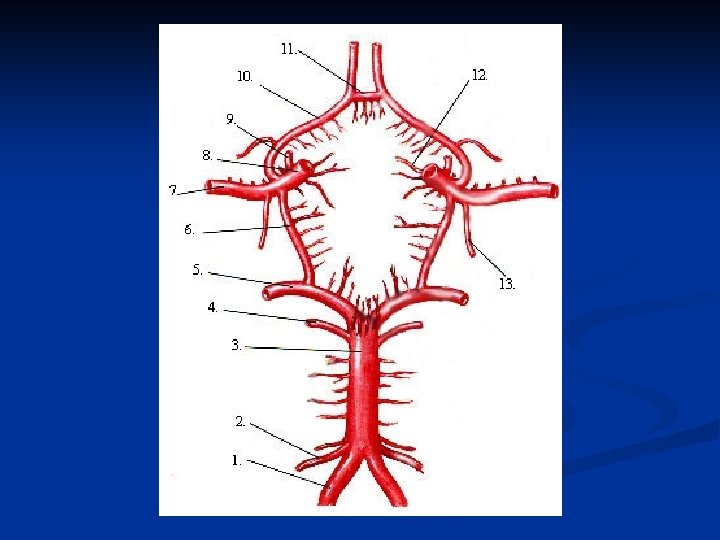
Aneurysmal SAH Aneurysms tend to occur at bifurcations or branching points of major cerebral vessels along the circle of Willis. n As a general rule, 30% in the anterior circulation (AComm), 30% in the middle circulation (ICA, MCA branching points), 30% in the posterior circulation (PComm), and 10% in the basilar circulation (Basilar bifurcation, PICA, etc). n

Spontaneous aneurysmal SAH As a general rule, Neurosurgery patients don’t come any sicker than aneurysm ruptures. n 1/3 either don’t make it to the hospital or don’t survive the first week after rupture. n Of those who survive, half are left with serious neurological sequelae. Morbidity is high, and mortality in first six months approaches 50%. n

Aneurysmal SAH Classic presentation is the sudden onset of the “worst headache I’ve ever had in my life. ” n Nausea, Vomiting. n Decreased mental status n n Can be due to acute hydrocephalus, necessitating ventriculostomy/EVD placement

Three major complications from aneurysmal SAH An aneurysm can RE-bleed! Greatest risk is within first 24 hours, then drops exponentially n Vasospasm! This can lead to stroke. Greatest incidence of symptomatic spasm is between 4 and 10 days after rupture n Hydrocephalus, communicating type. Can present even months after a bleed. n n Acutely, you can have obstructive hydrocephalus.
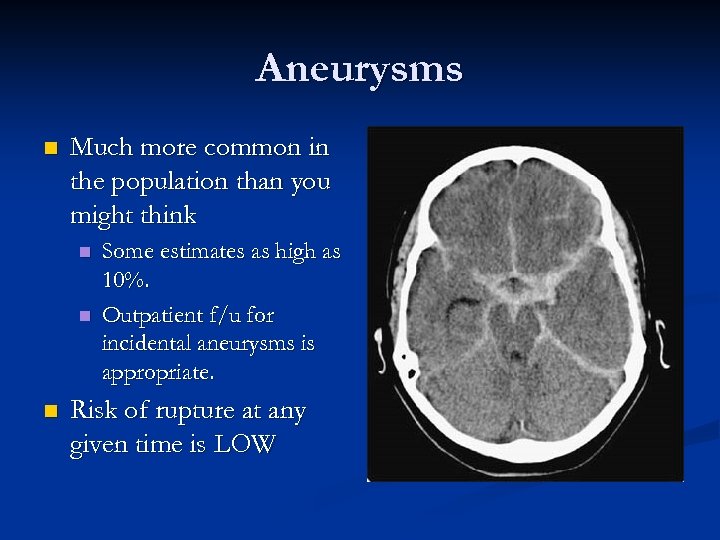
Aneurysms n Much more common in the population than you might think n n n Some estimates as high as 10%. Outpatient f/u for incidental aneurysms is appropriate. Risk of rupture at any given time is LOW

“Triple-H therapy” Hypertension n Hemodilution n Hypervolemia n Also, Nimotop 60 mg PO q 4 h is started immediately (for vasospasm prophylaxis) and continued through post-bleed day #21. n

Treatment of Aneurysms Incidentally found aneurysms do not necessitate treatment; patients may choose to seek treatment or not. n Ruptured aneurysms must be treated n Craniotomy and clipping n Endovascular treatment (newer treatment modality) n

What you can do n In any suspected intracranial hemorrhage, traumatic brain injury, etc. , there are some principles you can remember and things you can do…

Labs n In addition to all of the “normal” labs that are drawn in the ER setting, make sure you order coags (PT, INR, PTT) and start to correct any existing coagulopathy appropriately. FFP n Vitamin K n Platelets n

Blood Pressure n Take care not to “bottom out” blood pressure just because it’s high. Remember, the body is trying to maintain CPP. In the setting of an EDH or SDH, blindly reducing a patient’s BP can accelerate neural injury. n In the setting of intraparenchymal hemorrhage, shoot for a BP between 140 and 160 systolic; the idea is drawing a balance between decreasing BP to help stop the bleeding, and protecting the ischemic penumbra. n

Blood Pressure (cont’d) n In the setting of a ruptured aneurysm, allowing a patient to be extremely hypertensive can predispose to re-rupture of the aneurysm.

Herniation Syndromes

Remembering the “Box” n Increased ICP contributes to cerebral ischemia, but remember that an expanding mass lesion displaces its surrounding constituents. Since the intracranial volume is generally considered to be “fixed, ” past a certain point that extra volume has to go somewhere.

Pertinent Anatomy considering herniation syndromes n The intracranial compartment is divided into 3 compartments by 2 major dural structures, the falx cerebri and the tentorium cerebelli. The tentorium cerebelli divides the posterior fossa or infratentorial compartment (the cerebellum and the brainstem) from the supratentorial compartment (cerebral hemispheres).

Pertinent Anatomy considering herniation syndromes n Both the falx and the tentorium have central openings and prominent edges at the borders of each of these openings. When a significant increase in ICP occurs, caused by either a large mass lesion or significant cerebral edema, the brain can slide through these openings, a phenomenon known as herniation. As the brain slides over the free dural edges of the tentorium or the falx, it can sustain injuies caused by the dural edge.
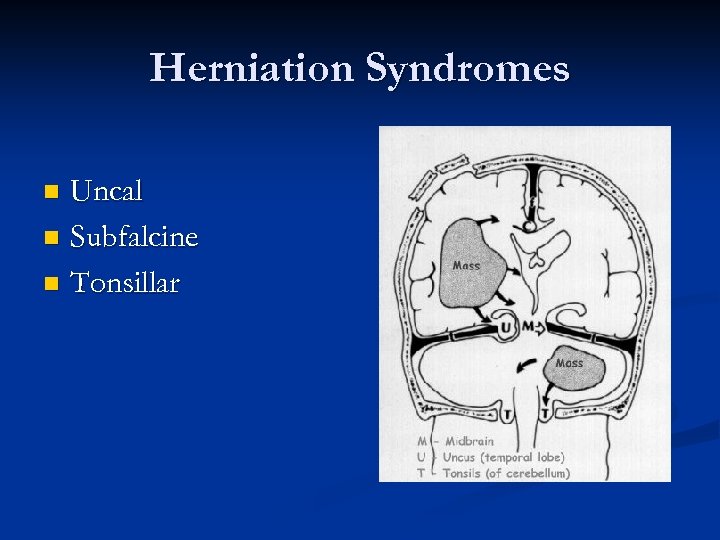
Herniation Syndromes Uncal n Subfalcine n Tonsillar n

Uncal Herniation When people talk “herniation”, this is it. n The uncus of the hippocampus is pushed across the tentorial notch into the posterior fossa. n Clinical Presentation n Ipsilateral “blown pupil. ” n Compression of ipsilateral cerebral peduncle (Contralateral hemiparesis). n Compression of ipsilateral PCA (Occipital infarction). n
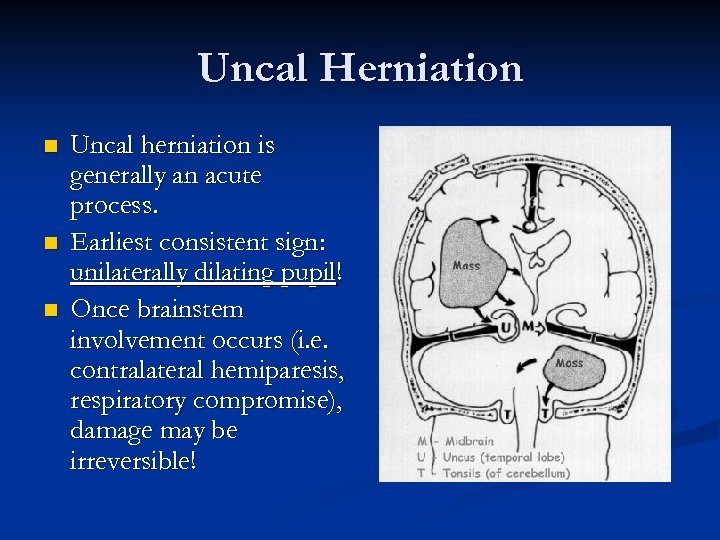
Uncal Herniation n Uncal herniation is generally an acute process. Earliest consistent sign: unilaterally dilating pupil! Once brainstem involvement occurs (i. e. contralateral hemiparesis, respiratory compromise), damage may be irreversible!

Uncal herniation n Preventing uncal herniation is the general idea behind rapid diagnosis of EDHs and aggressive management.
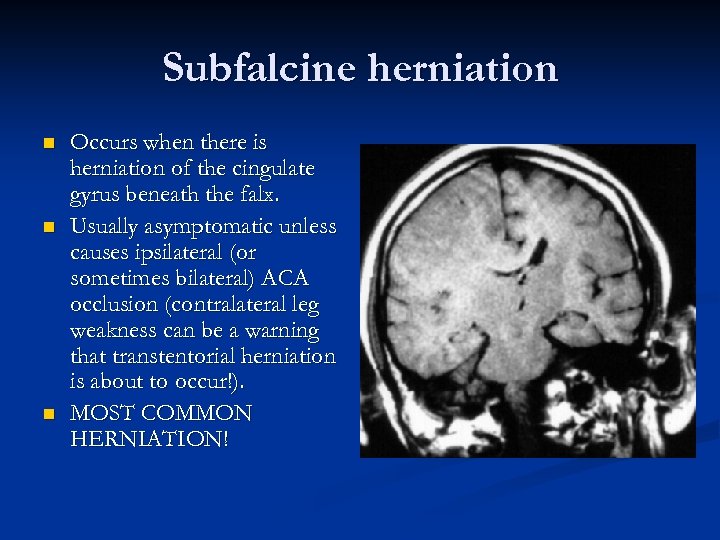
Subfalcine herniation n Occurs when there is herniation of the cingulate gyrus beneath the falx. Usually asymptomatic unless causes ipsilateral (or sometimes bilateral) ACA occlusion (contralateral leg weakness can be a warning that transtentorial herniation is about to occur!). MOST COMMON HERNIATION!
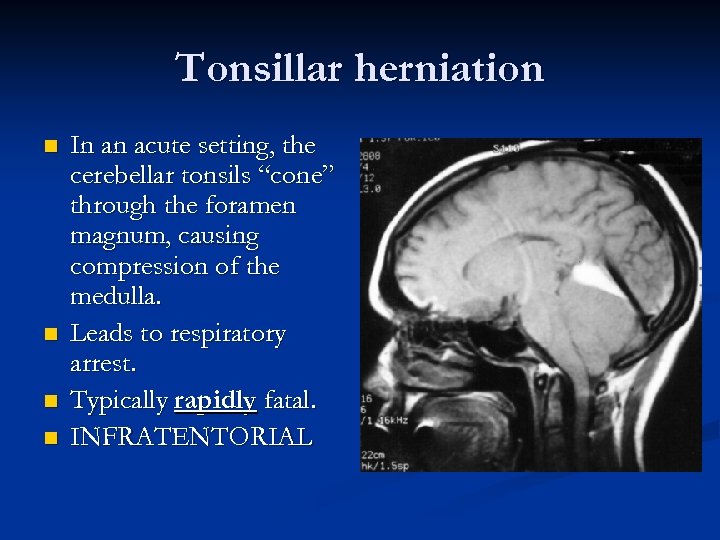
Tonsillar herniation n n In an acute setting, the cerebellar tonsils “cone” through the foramen magnum, causing compression of the medulla. Leads to respiratory arrest. Typically rapidly fatal. INFRATENTORIAL

Tonsillar herniation Can occur with either supra or infra-tentorial lesions, or with elevated ICP. n Can be precipitated by Lumbar Puncture! n Same type of anatomical finding demonstrated in patients with Chiari malformation (but chronic rather than acute). n

Summary n This online module contained information from four of your “topics. ” Traumatic Brain Injury and increased ICP n Intracranial hematomas n Spontaneous Subarachnoid Hemorrhage n Herniation Syndromes n
124695e0c9aebc6ffa51372e97352fa7.ppt