309c6b0044d4facf2451d6c4a62078fe.ppt
- Количество слайдов: 46

ONCOLOGY Ramadhan T. Othman, MBCh. B, MSc, Ph. D Clinical Oncologist, Azadi Teaching Hospital Lecturer, University of Duhok 06/02/2017

• Lecture 1 Introduction, staging and investigations • Lecture 3 Cancer Treatment • Lecture 2 Oncologic emergency • Lecture 2 Palliative Treatment

Introduction • How cancer develop? چﻪﻭﺍ پﻪﻧﺠﻪﺷێﺮێ پﻪیﺪﺍ ﺩﺑیﺖ • What are the preventable and unpreventable causes of cancer? ﺋﻪگﻪﺭێﻦ پﻪﻧﺠﻪﺷێﺮێ • Can we reduce the risk of cancer? ﺧﻮ پﺎﺭﺍﺳﺘﻦ ژ پﻪﻧﺠﻪﺷێﺮێ
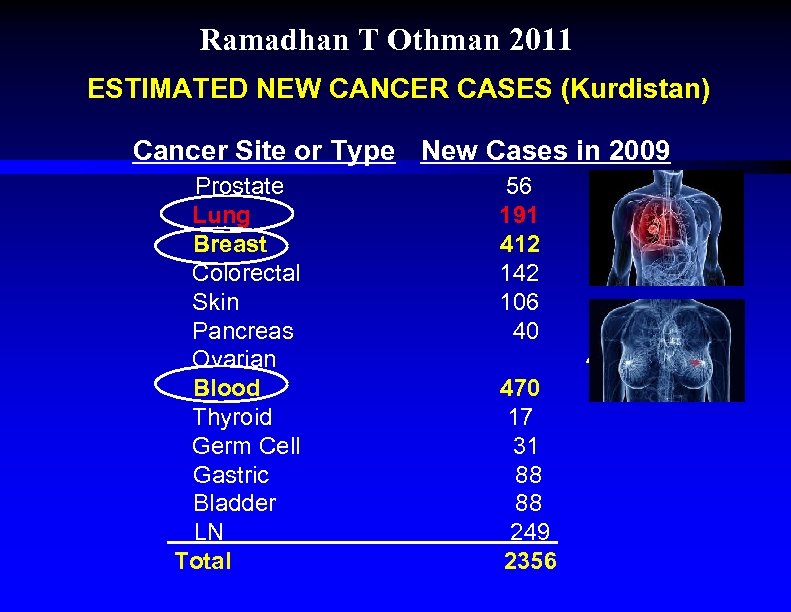
Ramadhan T Othman 2011 ESTIMATED NEW CANCER CASES (Kurdistan) Cancer Site or Type New Cases in 2009 Prostate Lung Breast Colorectal Skin Pancreas Ovarian Blood Thyroid Germ Cell Gastric Bladder LN Total 56 191 412 142 106 40 44 470 17 31 88 88 249 2356
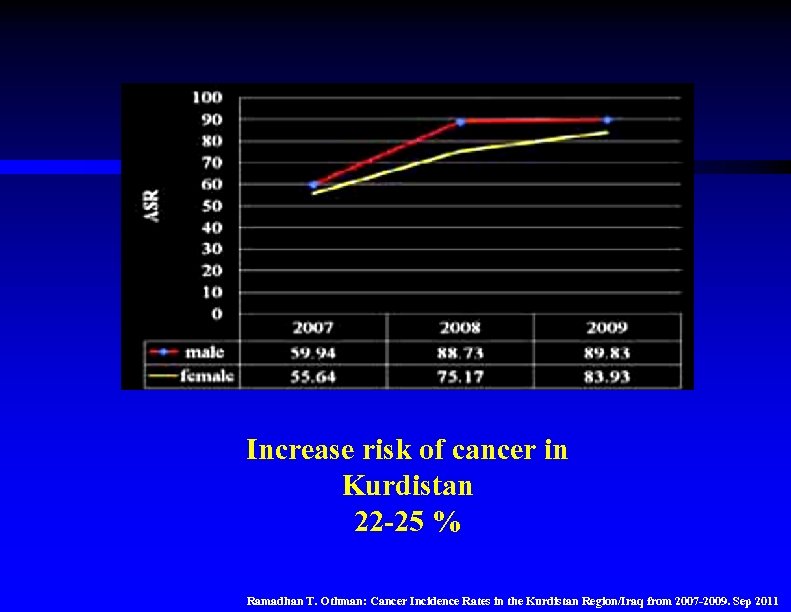
Increase risk of cancer in Kurdistan 22 -25 % Ramadhan T. Othman: Cancer Incidence Rates in the Kurdistan Region/Iraq from 2007 -2009. Sep 2011
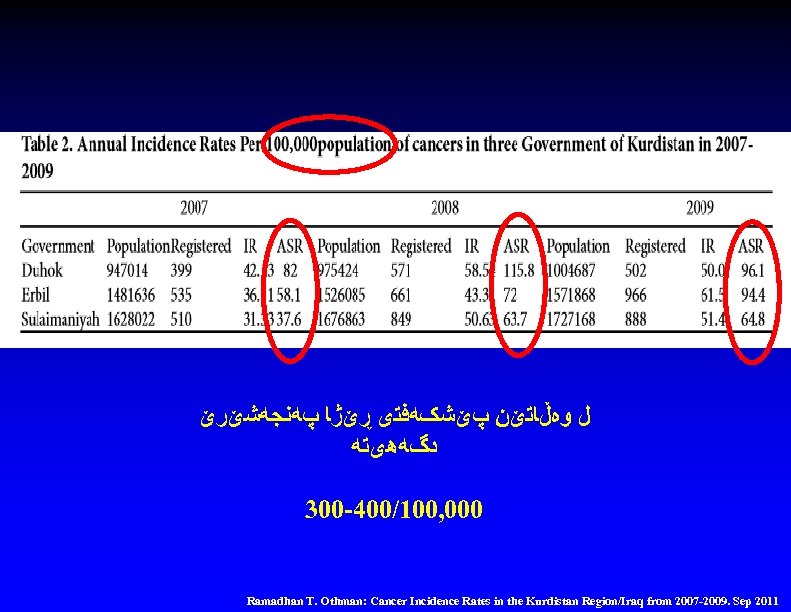
ﻝ ﻭﻩڵﺎﺗێﻦ پێﺸکﻪﻓﺘی ڕێژﺎ پﻪﻧﺠﻪﺷێﺮێ ﺩگﻪﻫیﺘﻪ 300 -400/100, 000 Ramadhan T. Othman: Cancer Incidence Rates in the Kurdistan Region/Iraq from 2007 -2009. Sep 2011
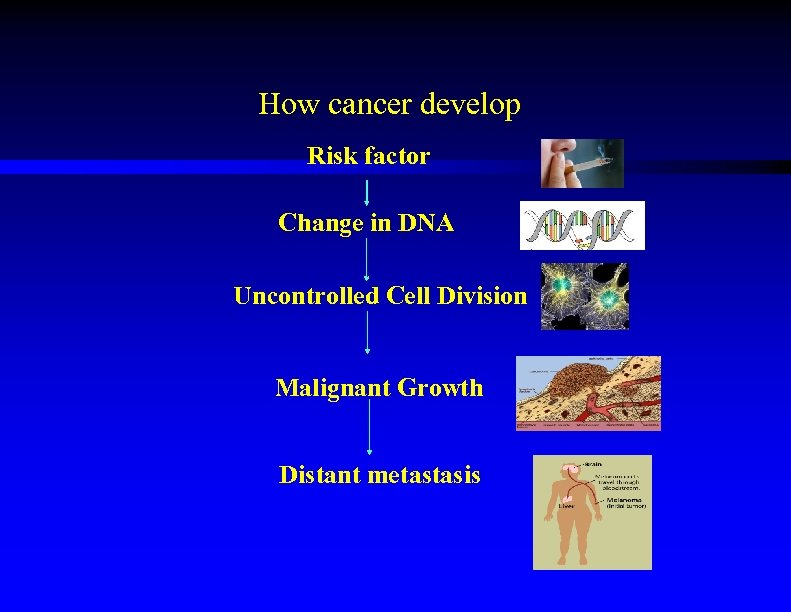
How cancer develop Risk factor Change in DNA Uncontrolled Cell Division Malignant Growth Distant metastasis

Causes of Cancer Unpreventable Causes ﺋﻪگﻪﺭێﻦ ﺗﻮ ﻧﻪﺷێی ﺧﻮ ژێ پﺎﺭێﺰی Age ژیێ ﻣﺮﻭڤ Sex ﺭﻩگﻪﺯ ﺑۆﻤﺎﻭﻩ )ﻭﺭﺍﺳی( Genetic Preventable Causes ﺋﻪگﻪﺭێﻦ ﺗﻮ ﺩﺷێی ﺧﻮ ژێ پﺎﺭێﺰی
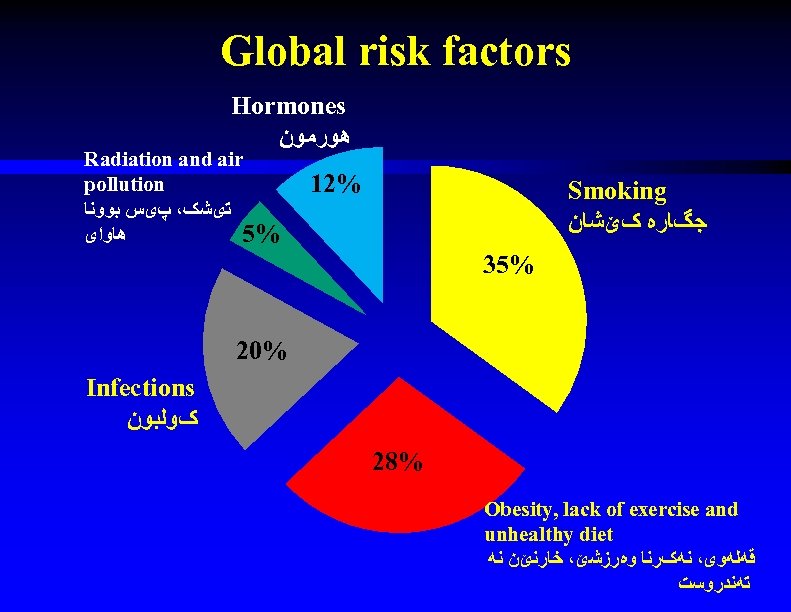
Global risk factors Hormones ﻫﻮﺭﻣﻮﻥ Radiation and air pollution ﺗیﺸک، پیﺲ ﺑﻮﻭﻧﺎ ﻫﺎﻭﺍی 5% 12% Smoking ﺟگﺎﺭﻩ کێﺸﺎﻥ 35% 20% Infections کﻮﻟﺒﻮﻥ 28% Obesity, lack of exercise and unhealthy diet ﻗﻪﻟﻪﻭی، ﻧﻪکﺮﻧﺎ ﻭﻩﺭﺯﺷێ، ﺧﺎﺭﻧێﻦ ﻧﻪ ﺗﻪﻧﺪﺭﻭﺳﺖ

Prevention ﺧﻮ پﺎﺭﺍﺳﺘﻦ ژ پﻪﻧﺠﻪﺷێﺮێ ﺯێﺪﻩکﺮﻧﺎ ﺑﻬﺎیێ ﺟگﺎﺭﺍ ﺯێﺪﻩکﺮﻧﺎ ﺯﻩﺭیﺒێ ﻝ ﺳﻪﺭکﻮﻣپﺎﻧیﺎ کﻮﻧﺘﺮﻭﻟکﺮﻧﺎ ﻓﺮﻭﺷﺘﻨﺎ ﺟگﺎﺭﺍ. ) ژیێ ۷۱ ﺳﺎﻟیێ( کﻮﻧﺘﺮﻭﻟکﺮﻧﺎ ﺟﻮﺭی

Prevention ﺧﻮ پﺎﺭﺍﺳﺘﻦ ژ پﻪﻧﺠﻪﺷێﺮێ Vaccination ڤﺎکﺴیﻨﺪﺍﻥ HBV HPV
Cancer Screening ﺯﻭی ﺩﻩﺳﺘﻨیﺸﺎکﺮﻥ • Breast cancer approved پﻪﻧﺠﻪﺷێﺮﺍ ﻣﻪﻣکی >40 YEARS • Colon cancer approved پﻪﻧﺠﻪﺷێﺮﺍ ﻗﻮﻟﻮﻧێ
• Cervical cancer approved پﻪﻧﺠﻪﺷێﺮﺍ ﻣﺎﻟﺒچیکی • Lung cancer approved for high risk group پﻪﻧﺠﻪﺷێﺮﺍ ﺳیﻬێ • Prostate cancer (You need to ask for it) پﻪﻧﺠﻪﺷێﺮﺍ پﺮﻭﺳﺘﺎﺗﺎ
Diagnosis 1. History 2. Clinical Examination 3. Laboratory investigation, blood test (Tumour marker) 4. Radiological diagnosis 5. Nuclear Medicine department (PET-CT scan 6. Biopsy and histopathology 8. Genetic study
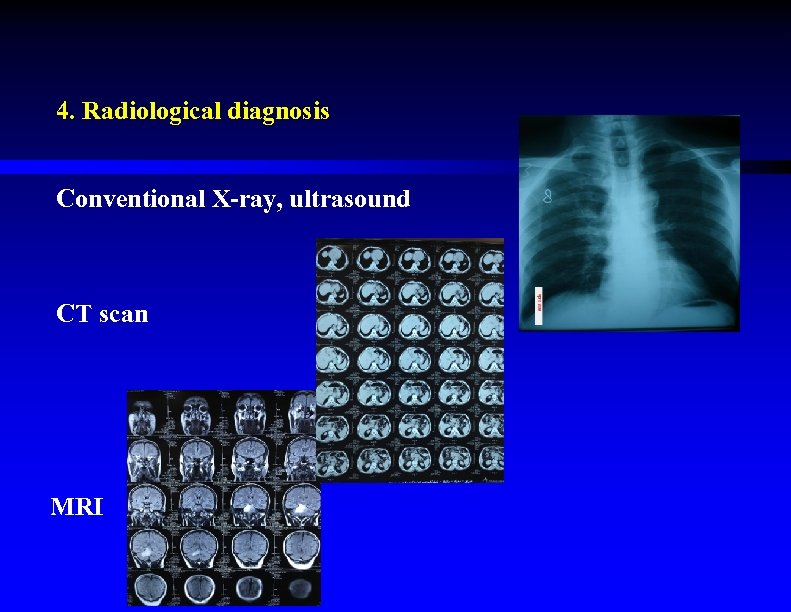
4. Radiological diagnosis Conventional X-ray, ultrasound CT scan MRI
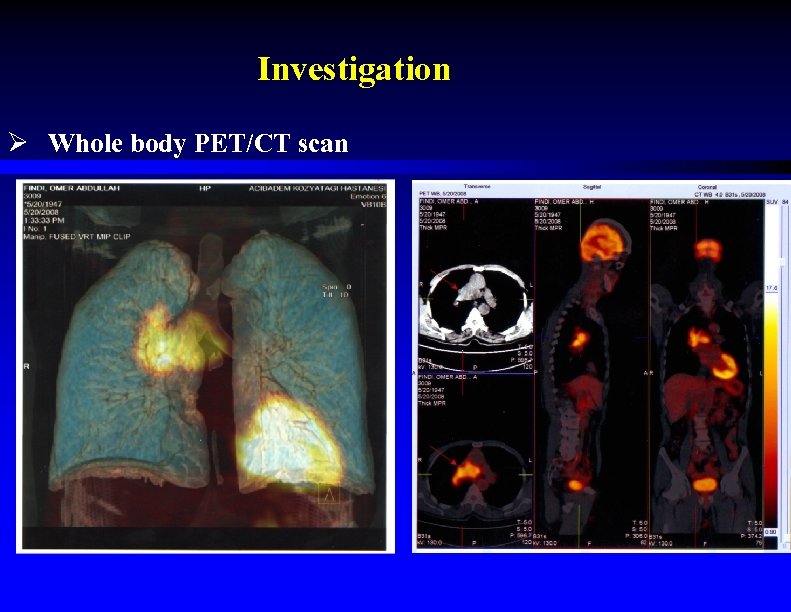
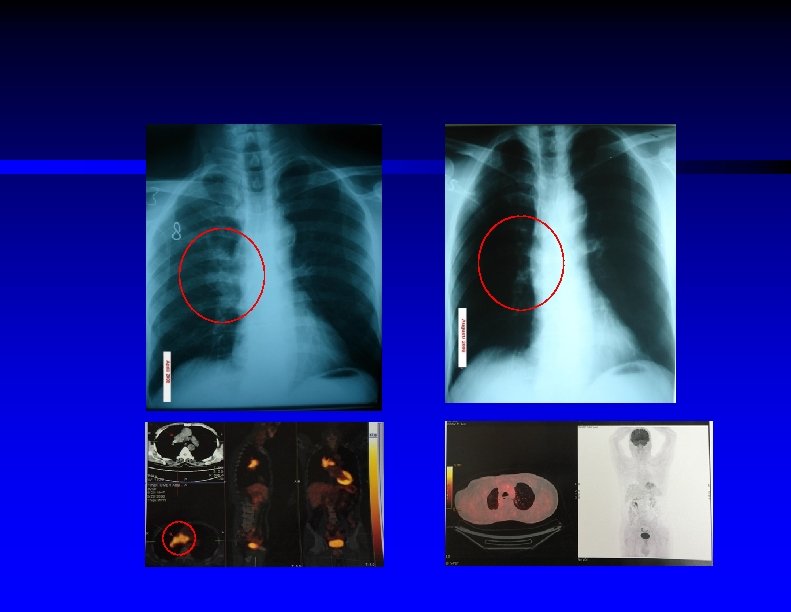
Investigation Ø Whole body PET/CT scan
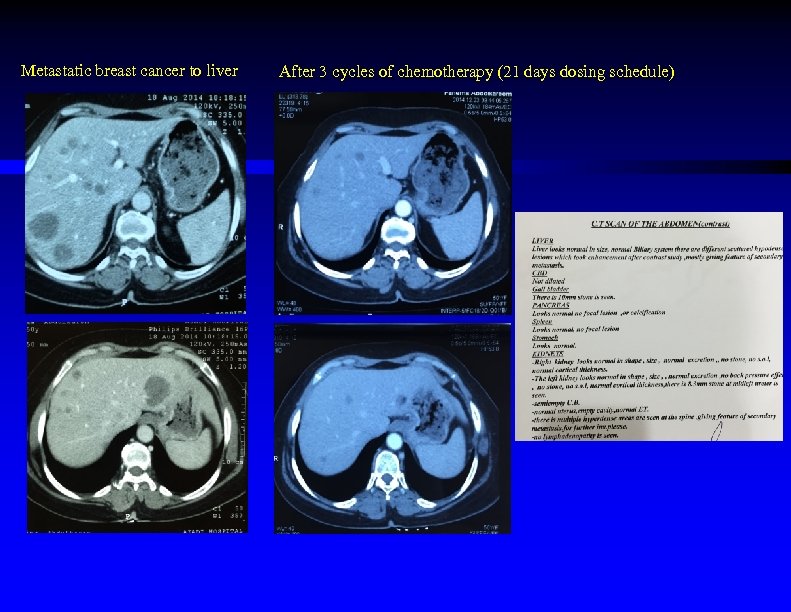
Metastatic breast cancer to liver After 3 cycles of chemotherapy (21 days dosing schedule)

Cancer staging • • Stage and grade determine prognosis Staging reflects the clinical extent of the tumor Grading a tumor reflects its histologic subtype Of the two, staging is the primary indicator of prognosis
Grading • • • Degree of differentiation exhibited by cells How closely cells resemble normal tissue structure Grade I – low grade Grade II – moderately differentiated Grade III – poorly differentiated Neville, B. W. , Damm, D. D. , Allen, C. M. , & Bouquot, J. E. (2002). Oral and maxillofacial pathology (2 nd ed. ). Philadelphia: W. B. Saunders.
Staging • Based upon the size and extent of metastatic spread of the lesion • Tumor-node-metastasis (TNM) system used for most cancers
Staging – TNM system • Size, in cm, of the tumor (T) • Involvement of lymph nodes (N) • Presence or absence of distant metastasis (M)
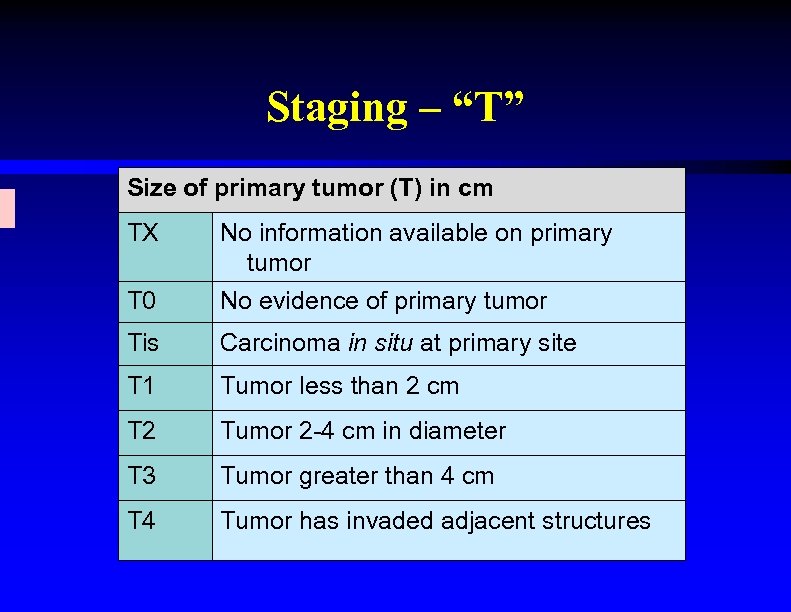
Staging – “T” Size of primary tumor (T) in cm TX No information available on primary tumor T 0 No evidence of primary tumor Tis Carcinoma in situ at primary site T 1 Tumor less than 2 cm T 2 Tumor 2 -4 cm in diameter T 3 Tumor greater than 4 cm T 4 Tumor has invaded adjacent structures
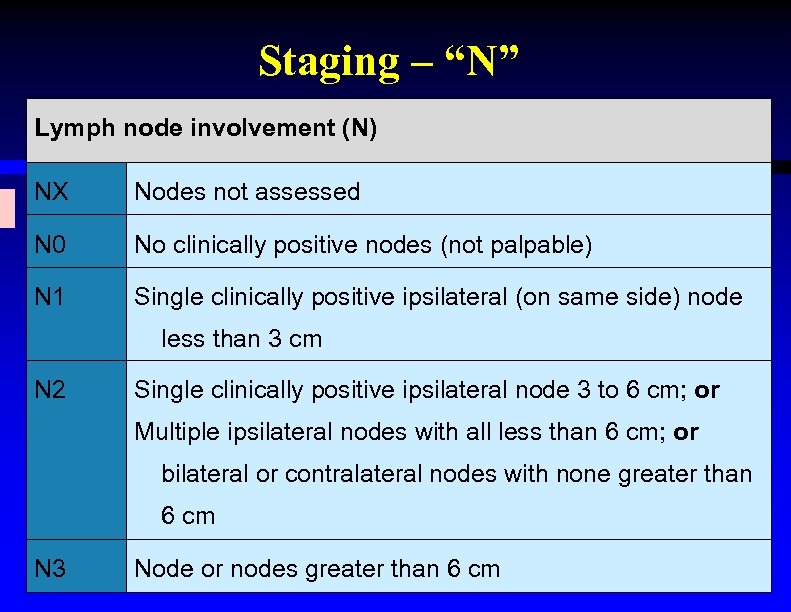
Staging – “N” Lymph node involvement (N) NX Nodes not assessed N 0 No clinically positive nodes (not palpable) N 1 Single clinically positive ipsilateral (on same side) node less than 3 cm N 2 Single clinically positive ipsilateral node 3 to 6 cm; or Multiple ipsilateral nodes with all less than 6 cm; or bilateral or contralateral nodes with none greater than 6 cm N 3 Node or nodes greater than 6 cm
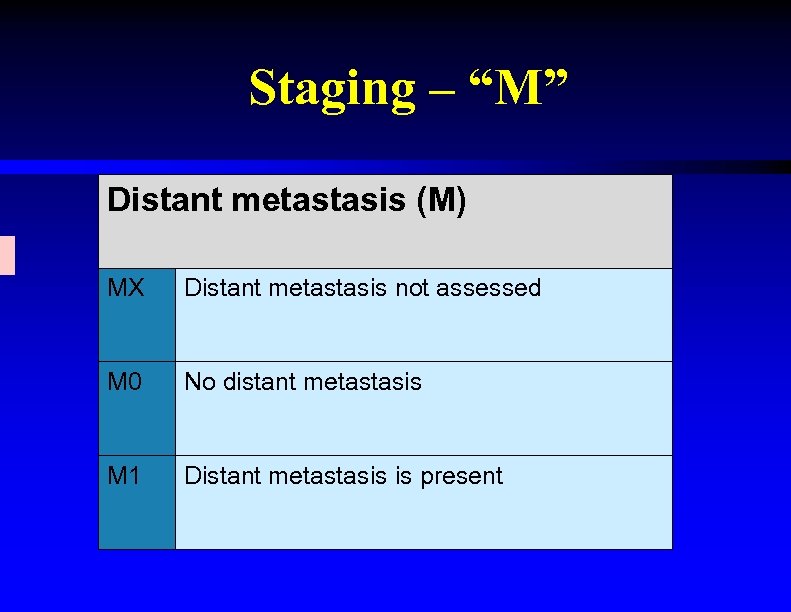
Staging – “M” Distant metastasis (M) MX Distant metastasis not assessed M 0 No distant metastasis M 1 Distant metastasis is present
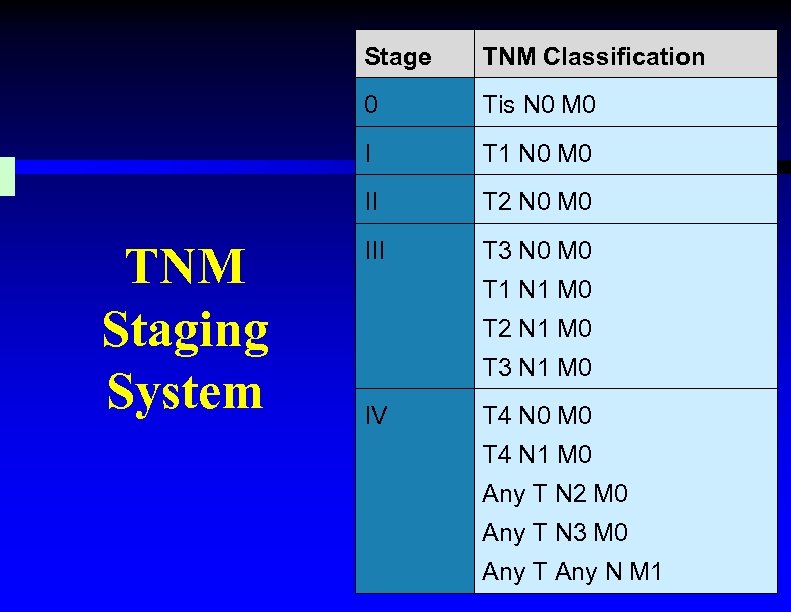
Stage 0 Tis N 0 M 0 I T 1 N 0 M 0 II TNM Staging System TNM Classification T 2 N 0 M 0 III T 3 N 0 M 0 T 1 N 1 M 0 T 2 N 1 M 0 T 3 N 1 M 0 IV T 4 N 0 M 0 T 4 N 1 M 0 Any T N 2 M 0 Any T N 3 M 0 Any T Any N M 1

• Stage and grade of tumors indicates prognosis • Treatment plans based upon stage and grade, among other factors • TNM system used with most cancers

Tumour Markers: How We Can Use Them In The Clinical Practice

Objectives • To briefly introduce cancers incidence in Kurdistan • To define tumor markers (TMs) types, and characteristics of an ideal TM. • To know various applications of TMs detection Encouraging the wider dissemination and implementation of available guidelines is the primary goal

Summary • • European Society of Medical Oncology (ESMO) European Group on Tumour Markers (EGTM) American Society of Clinical Oncology (ASCO) National Academy of Clinical Biochemistry (NACB)
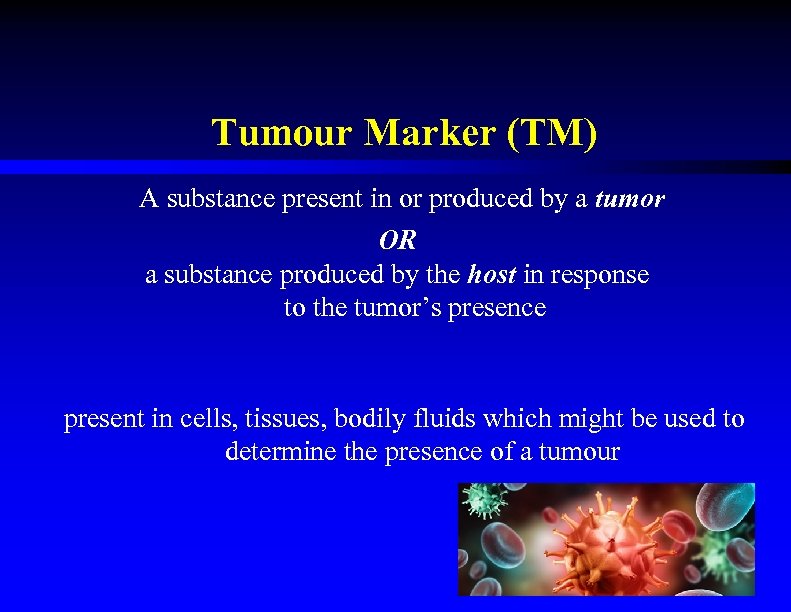
Tumour Marker (TM) A substance present in or produced by a tumor OR a substance produced by the host in response to the tumor’s presence present in cells, tissues, bodily fluids which might be used to determine the presence of a tumour
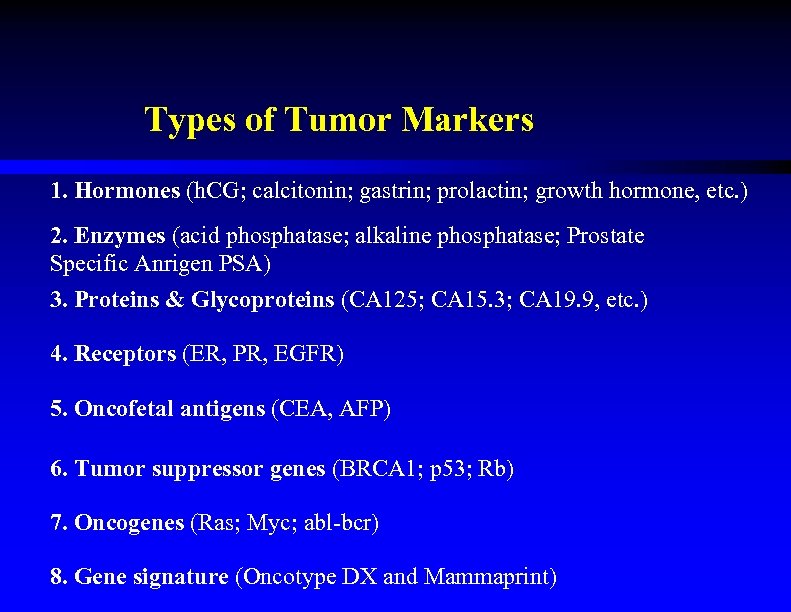
Types of Tumor Markers 1. Hormones (h. CG; calcitonin; gastrin; prolactin; growth hormone, etc. ) 2. Enzymes (acid phosphatase; alkaline phosphatase; Prostate Specific Anrigen PSA) 3. Proteins & Glycoproteins (CA 125; CA 15. 3; CA 19. 9, etc. ) 4. Receptors (ER, PR, EGFR) 5. Oncofetal antigens (CEA, AFP) 6. Tumor suppressor genes (BRCA 1; p 53; Rb) 7. Oncogenes (Ras; Myc; abl-bcr) 8. Gene signature (Oncotype DX and Mammaprint)
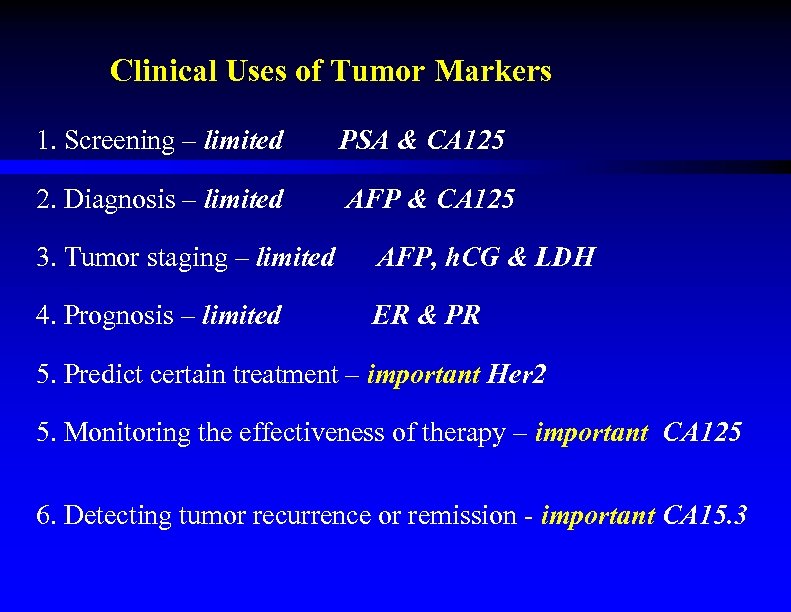
Clinical Uses of Tumor Markers 1. Screening – limited PSA & CA 125 2. Diagnosis – limited AFP & CA 125 3. Tumor staging – limited AFP, h. CG & LDH 4. Prognosis – limited ER & PR 5. Predict certain treatment – important Her 2 5. Monitoring the effectiveness of therapy – important CA 125 6. Detecting tumor recurrence or remission - important CA 15. 3

The Ideal Tumor Marker • Measured easily, reliably and cost-effectively • TM levels reflects tumor burden with high diagnostic sensitivity (few false negatives) with specificity (few false positives). • Test results influence especially outcome. • Predict recurrences before they are clinically detectable patient care and Unfortunately, all of the presently available TMs do not fit this ideal model
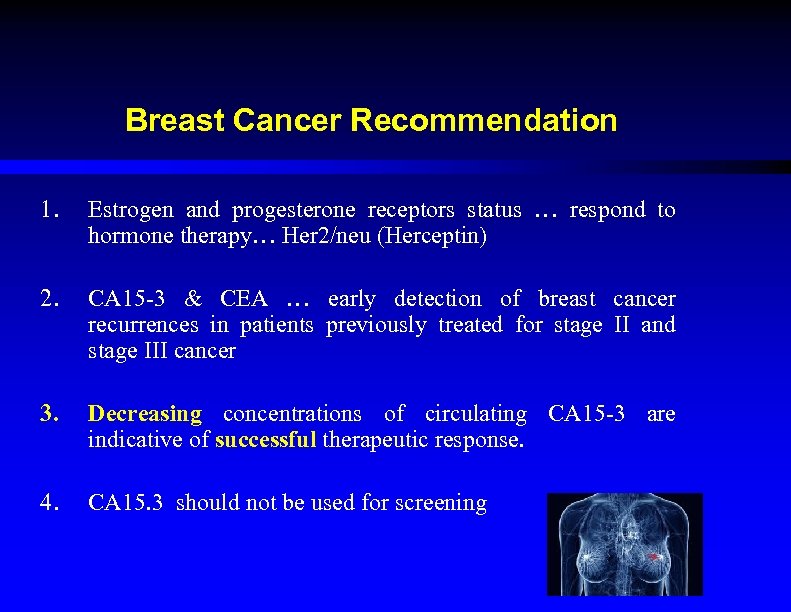
Breast Cancer Recommendation 1. Estrogen and progesterone receptors status … respond to hormone therapy… Her 2/neu (Herceptin) 2. CA 15 -3 & CEA … early detection of breast cancer recurrences in patients previously treated for stage II and stage III cancer 3. Decreasing concentrations of circulating CA 15 -3 are indicative of successful therapeutic response. 4. CA 15. 3 should not be used for screening
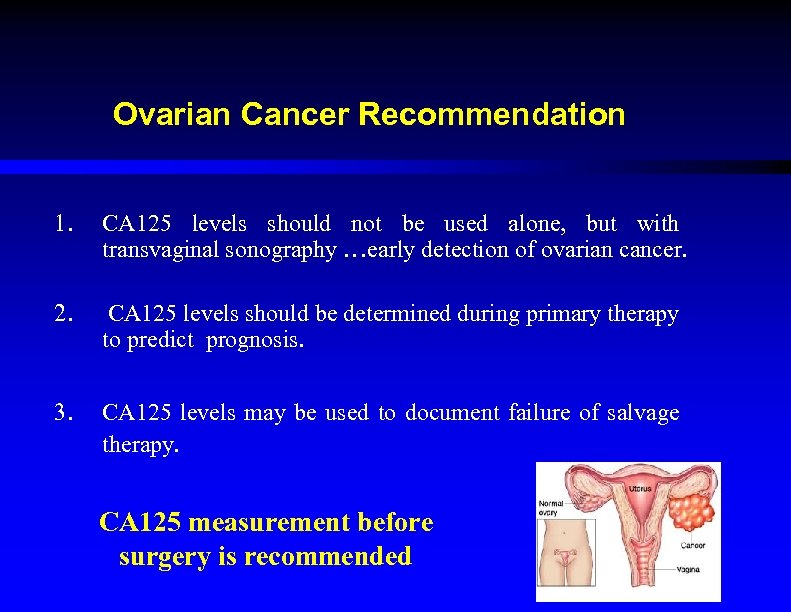
Ovarian Cancer Recommendation 1. CA 125 levels should not be used alone, but with transvaginal sonography …early detection of ovarian cancer. 2. CA 125 levels should be determined during primary therapy to predict prognosis. 3. CA 125 levels may be used to document failure of salvage therapy. CA 125 measurement before surgery is recommended
Prostate Cancer Recommendation 1. PSA must not be used alone, but should be evaluated in conjunction with DRE 2. PSA levels might be used to assess response to treatment and progression 3. It is recommended that blood be drawn before any manipulation of the prostate and several weeks after resolution of prostatitis PSA Analysis before surgery is recommended
Colorectal Cancer Recommendation 1. CEA has no role in either screening or early diagnosis 2. If an abnormal CEA is confirmed, additional evidence of metastatic disease should always be sought before initiating therapy 3. CEA monitoring in patients with advanced colorectal cancer …response to treatment CEA measurement before surgery is recommended
Germ Cell Cancer Recommendation 1. Determine AFP, h. CG and LDH as aids in the evaluation and staging of prior to orchiectomy 2. The level of TMs can change a patient’s clinical stage independent of the extent of metastases. 3. Normalization of TMs …. response to chemotherapy. Measurement before surgery is recommended

Thyroid Cancer Recommendation • Calcitonin - Medullary Ca • Thyroglobulin - Follicular Ca • TSH Analysis before surgery is recommended
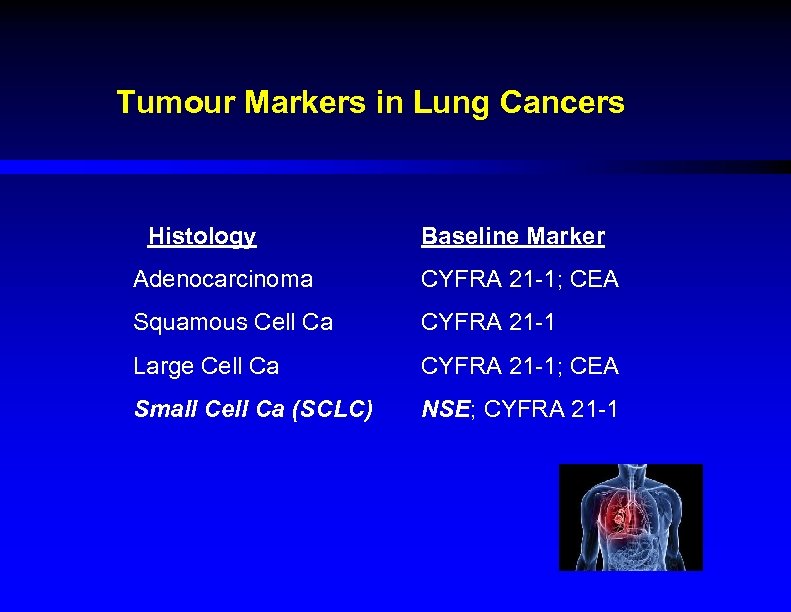
Tumour Markers in Lung Cancers Histology Baseline Marker Adenocarcinoma CYFRA 21 -1; CEA Squamous Cell Ca CYFRA 21 -1 Large Cell Ca CYFRA 21 -1; CEA Small Cell Ca (SCLC) NSE; CYFRA 21 -1

Recommendation for Lung Cancer 1. Where inoperable lung cancer is suspected but no histology is available, raised serum NSE is suggestive of SCLC. 2. NSE can be measured during systemic treatment of SCLC to reflect response to therapy and to document progressive disease.
Lymphoma HL & NHL • LDH Malignant Bone Tumour • LDH • ALP

Summary 1. TMs are produced either by the tumor or as an effect of the tumor on the host (serum proteins, oncofetal antigens, hormones, metabolites, receptors, and enzymes) 2. Ideally, a TM would be: tumor specific, absent in healthy individuals, & readily detectable in body fluids. 3. No TM alone can be used for diagnosis of tumors 4. TMs with high specificity & sensitivity might be used in: screening, prognosis, detection of recurrence & monitoring response to treatment. 5. Measurement of TMs levels is recommended before surgery especially for germ cell tumours, colorectal, ovarian, prostate cancer & Thyroıd cancer
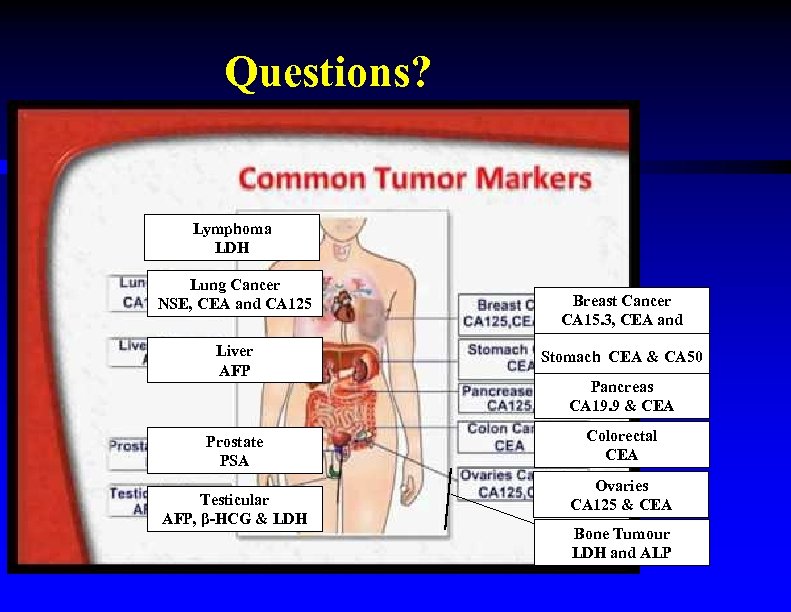
Questions? Lymphoma LDH Lung Cancer NSE, CEA and CA 125 Liver AFP Breast Cancer CA 15. 3, CEA and CA 125 Stomach CEA & CA 50 Pancreas CA 19. 9 & CEA Prostate PSA Colorectal CEA Testicular LDH AFP, β-HCG & LDH Ovaries CA 125 & CEA Bone Tumour LDH and ALP

Therapeutics in oncology Cancer Treatment
309c6b0044d4facf2451d6c4a62078fe.ppt