73e8b6ce7f570c7eb4e911265b2f390c.ppt
- Количество слайдов: 50
 Once upon a time excipients… Penny North-Lewis Paediatric Liver Pharmacist St James's University Hospital Leeds Teaching Hospitals Trust Dr Catherine Tuleu Senior Lecturer, Department of Pharmaceutics & Deputy Director, Centre for Paediatric Pharmacy Research
Once upon a time excipients… Penny North-Lewis Paediatric Liver Pharmacist St James's University Hospital Leeds Teaching Hospitals Trust Dr Catherine Tuleu Senior Lecturer, Department of Pharmaceutics & Deputy Director, Centre for Paediatric Pharmacy Research
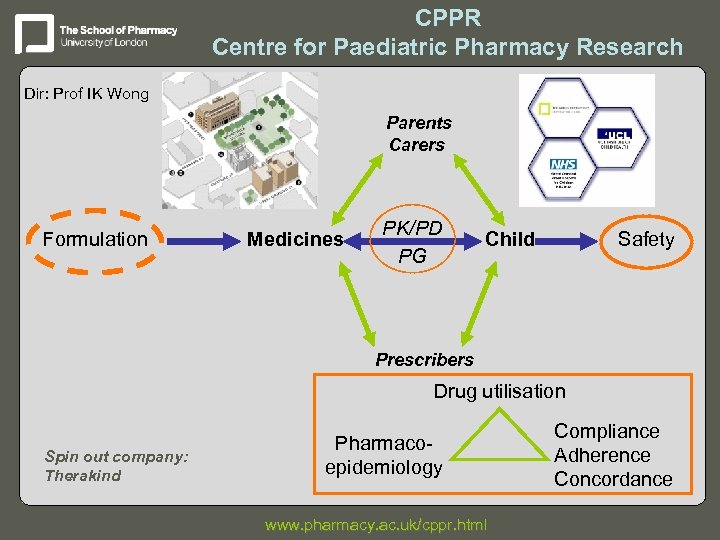 CPPR Centre for Paediatric Pharmacy Research Dir: Prof IK Wong Parents Carers Formulation Medicines PK/PD PG Child Safety Prescribers Drug utilisation Spin out company: Therakind Pharmacoepidemiology www. pharmacy. ac. uk/cppr. html Compliance Adherence Concordance
CPPR Centre for Paediatric Pharmacy Research Dir: Prof IK Wong Parents Carers Formulation Medicines PK/PD PG Child Safety Prescribers Drug utilisation Spin out company: Therakind Pharmacoepidemiology www. pharmacy. ac. uk/cppr. html Compliance Adherence Concordance
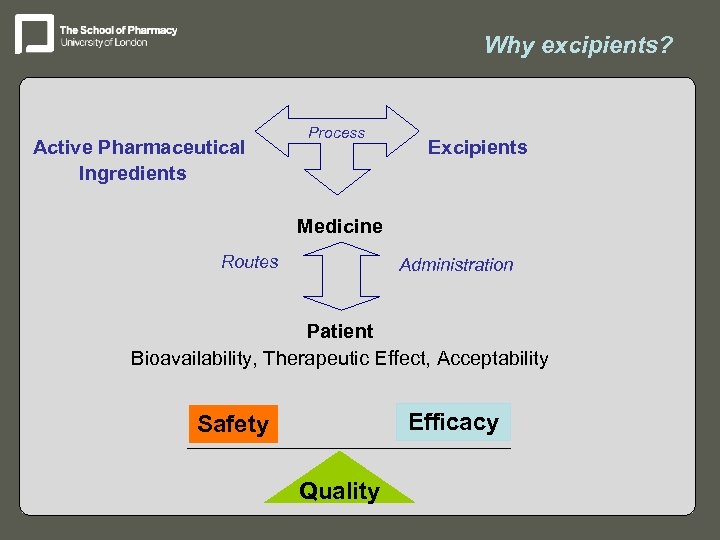 Why excipients? Active Pharmaceutical Ingredients Process Excipients Medicine Routes Administration Patient Bioavailability, Therapeutic Effect, Acceptability Efficacy Safety Quality
Why excipients? Active Pharmaceutical Ingredients Process Excipients Medicine Routes Administration Patient Bioavailability, Therapeutic Effect, Acceptability Efficacy Safety Quality
 The ideal paediatric formulation: holy grail? Paediatric single dose or safe and convenient withdrawal of paediatric single dose Age appropriate Minimal dose frequency ‘Non toxic’ excipients Good organoleptic characteristics Reliable safe and easy administration Good acceptability by children and parents/carers Optimised R&D (production vs cost) Developing countries
The ideal paediatric formulation: holy grail? Paediatric single dose or safe and convenient withdrawal of paediatric single dose Age appropriate Minimal dose frequency ‘Non toxic’ excipients Good organoleptic characteristics Reliable safe and easy administration Good acceptability by children and parents/carers Optimised R&D (production vs cost) Developing countries
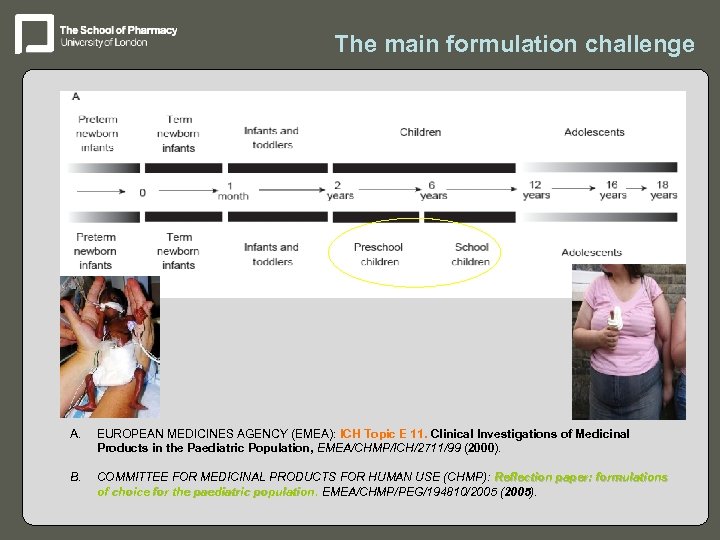 The main formulation challenge A. EUROPEAN MEDICINES AGENCY (EMEA): ICH Topic E 11. Clinical Investigations of Medicinal Products in the Paediatric Population, EMEA/CHMP/ICH/2711/99 (2000). B. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP): Reflection paper: formulations of choice for the paediatric population. EMEA/CHMP/PEG/194810/2005 (2005). population
The main formulation challenge A. EUROPEAN MEDICINES AGENCY (EMEA): ICH Topic E 11. Clinical Investigations of Medicinal Products in the Paediatric Population, EMEA/CHMP/ICH/2711/99 (2000). B. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP): Reflection paper: formulations of choice for the paediatric population. EMEA/CHMP/PEG/194810/2005 (2005). population
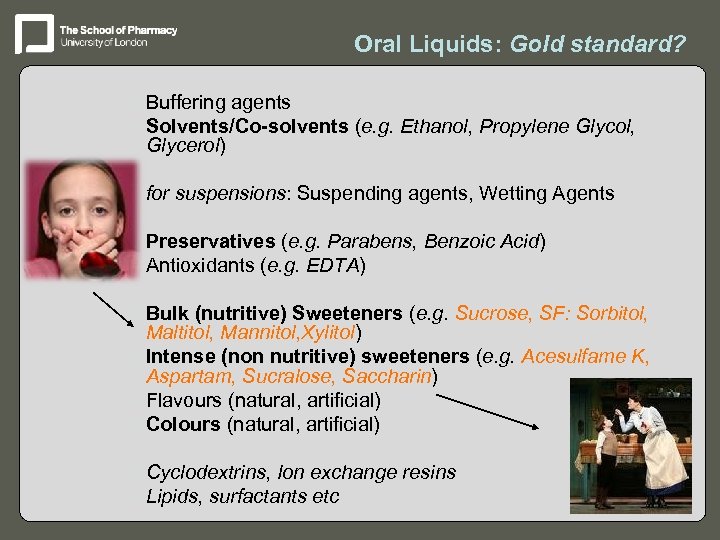 Oral Liquids: Gold standard? – Buffering agents – Solvents/Co-solvents (e. g. Ethanol, Propylene Glycol, Glycerol) – for suspensions: Suspending agents, Wetting Agents – Preservatives (e. g. Parabens, Benzoic Acid) – Antioxidants (e. g. EDTA) – Bulk (nutritive) Sweeteners (e. g. Sucrose, SF: Sorbitol, Maltitol, Mannitol, Xylitol) – Intense (non nutritive) sweeteners (e. g. Acesulfame K, Aspartam, Sucralose, Saccharin) – Flavours (natural, artificial) – Colours (natural, artificial) – Cyclodextrins, Ion exchange resins – Lipids, surfactants etc
Oral Liquids: Gold standard? – Buffering agents – Solvents/Co-solvents (e. g. Ethanol, Propylene Glycol, Glycerol) – for suspensions: Suspending agents, Wetting Agents – Preservatives (e. g. Parabens, Benzoic Acid) – Antioxidants (e. g. EDTA) – Bulk (nutritive) Sweeteners (e. g. Sucrose, SF: Sorbitol, Maltitol, Mannitol, Xylitol) – Intense (non nutritive) sweeteners (e. g. Acesulfame K, Aspartam, Sucralose, Saccharin) – Flavours (natural, artificial) – Colours (natural, artificial) – Cyclodextrins, Ion exchange resins – Lipids, surfactants etc
 Short case – 4 week old baby with prolonged jaundice is prescribed phenobarbital 5 mg/kg nocte for 5 days before a HIDA scan at a liver unit. This was dispensed by the DGH pharmacy. – Mum returns with the medicine the next day saying that she felt like her child was choking and stopped breathing when she gave the medicine and she is very worried about having to give another dose. – Why might this have happened? – What could you do instead?
Short case – 4 week old baby with prolonged jaundice is prescribed phenobarbital 5 mg/kg nocte for 5 days before a HIDA scan at a liver unit. This was dispensed by the DGH pharmacy. – Mum returns with the medicine the next day saying that she felt like her child was choking and stopped breathing when she gave the medicine and she is very worried about having to give another dose. – Why might this have happened? – What could you do instead?
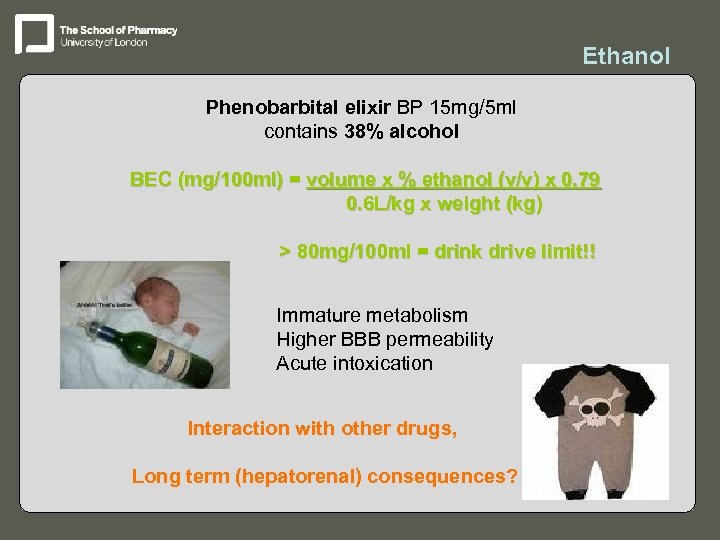 Ethanol Phenobarbital elixir BP 15 mg/5 ml contains 38% alcohol BEC (mg/100 ml) = volume x % ethanol (v/v) x 0. 79 0. 6 L/kg x weight (kg) > 80 mg/100 ml = drink drive limit!! Immature metabolism Higher BBB permeability Acute intoxication Interaction with other drugs, Long term (hepatorenal) consequences?
Ethanol Phenobarbital elixir BP 15 mg/5 ml contains 38% alcohol BEC (mg/100 ml) = volume x % ethanol (v/v) x 0. 79 0. 6 L/kg x weight (kg) > 80 mg/100 ml = drink drive limit!! Immature metabolism Higher BBB permeability Acute intoxication Interaction with other drugs, Long term (hepatorenal) consequences?
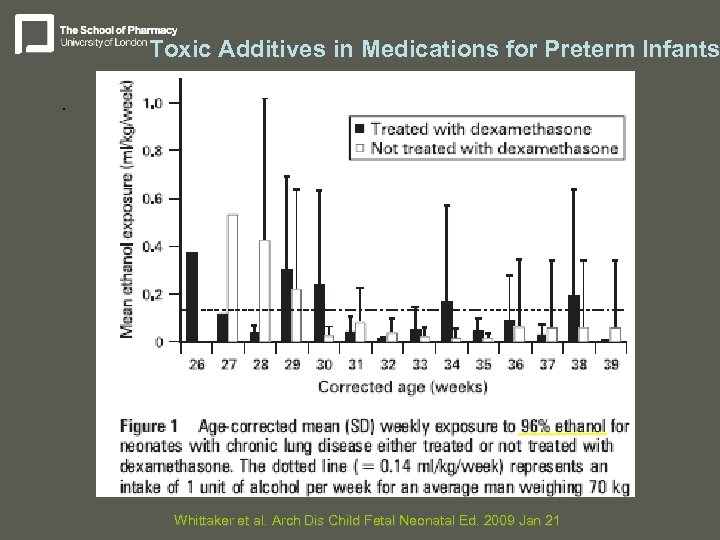 Toxic Additives in Medications for Preterm Infants. Whittaker et al. Arch Dis Child Fetal Neonatal Ed. 2009 Jan 21
Toxic Additives in Medications for Preterm Infants. Whittaker et al. Arch Dis Child Fetal Neonatal Ed. 2009 Jan 21
 Propylene Glycol
Propylene Glycol
 ADI <25 mg/Kg BW http: //jecfa. ilsi. org/search. cfm
ADI <25 mg/Kg BW http: //jecfa. ilsi. org/search. cfm
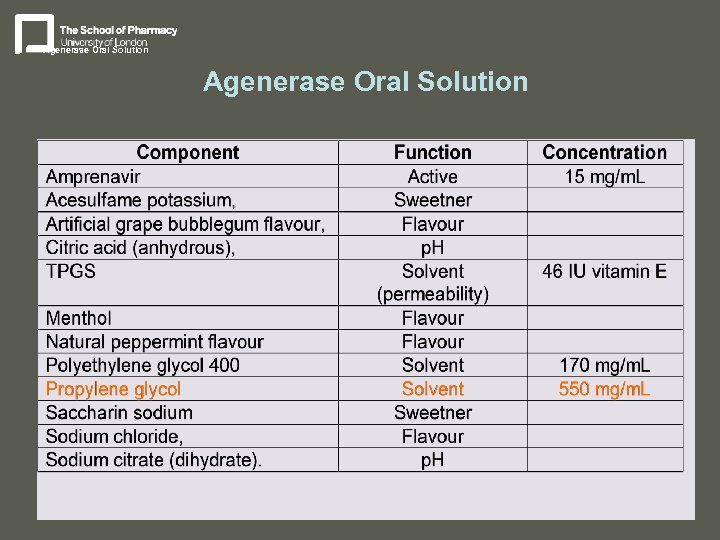 Agenerase Oral Solution
Agenerase Oral Solution
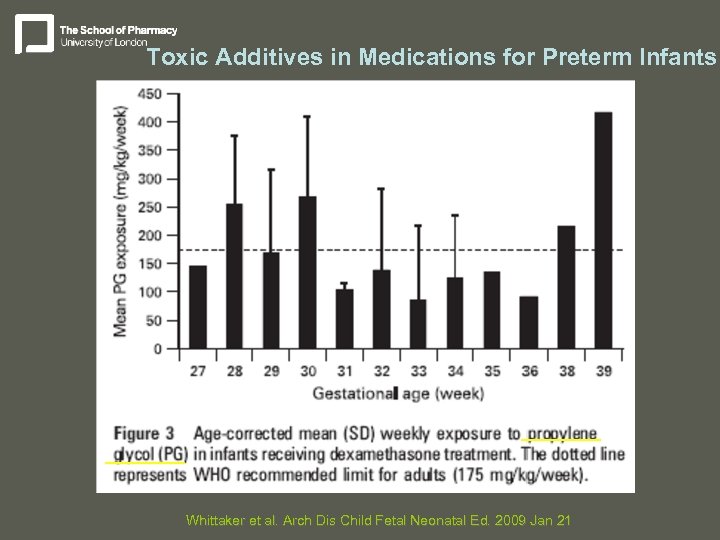 Toxic Additives in Medications for Preterm Infants Whittaker et al. Arch Dis Child Fetal Neonatal Ed. 2009 Jan 21
Toxic Additives in Medications for Preterm Infants Whittaker et al. Arch Dis Child Fetal Neonatal Ed. 2009 Jan 21
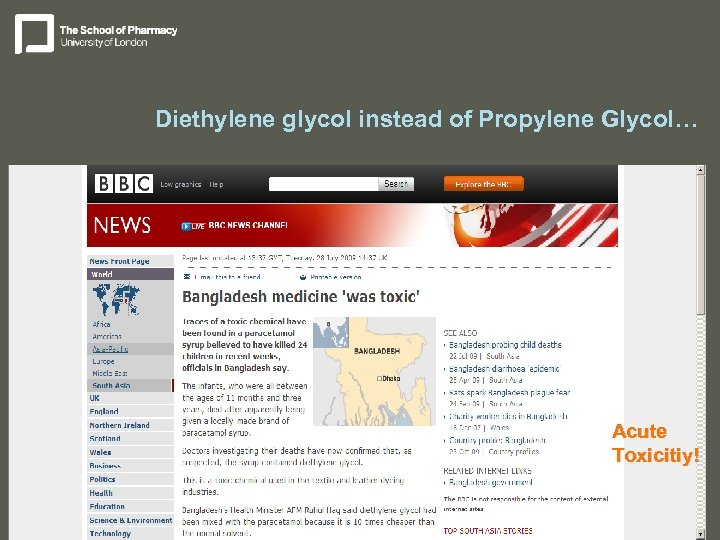 Diethylene glycol instead of Propylene Glycol… Acute Toxicitiy!
Diethylene glycol instead of Propylene Glycol… Acute Toxicitiy!
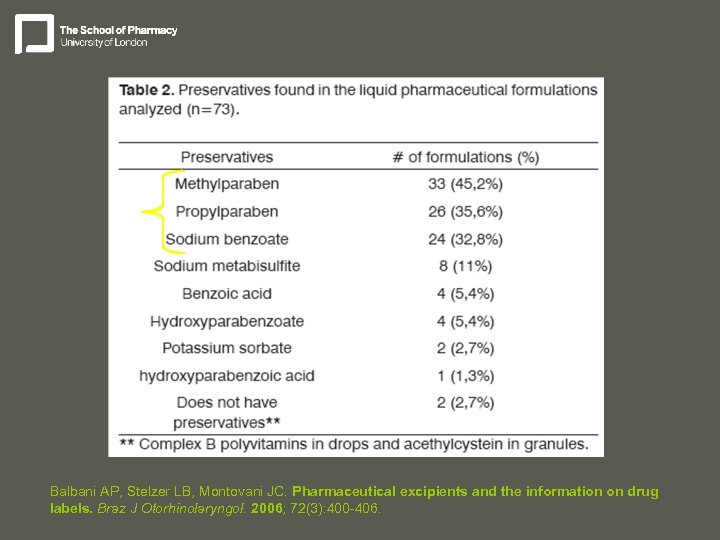 Balbani AP, Stelzer LB, Montovani JC. Pharmaceutical excipients and the information on drug labels. Braz J Otorhinolaryngol. 2006; 72(3): 400 -406.
Balbani AP, Stelzer LB, Montovani JC. Pharmaceutical excipients and the information on drug labels. Braz J Otorhinolaryngol. 2006; 72(3): 400 -406.
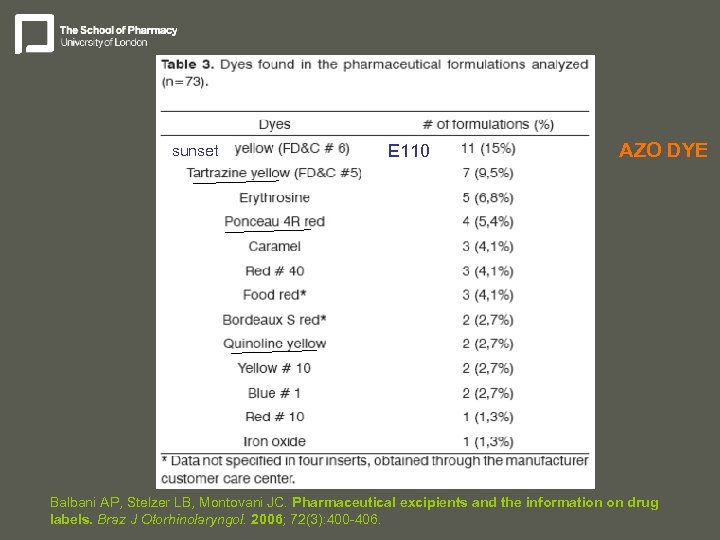 sunset E 110 AZO DYE Balbani AP, Stelzer LB, Montovani JC. Pharmaceutical excipients and the information on drug labels. Braz J Otorhinolaryngol. 2006; 72(3): 400 -406.
sunset E 110 AZO DYE Balbani AP, Stelzer LB, Montovani JC. Pharmaceutical excipients and the information on drug labels. Braz J Otorhinolaryngol. 2006; 72(3): 400 -406.
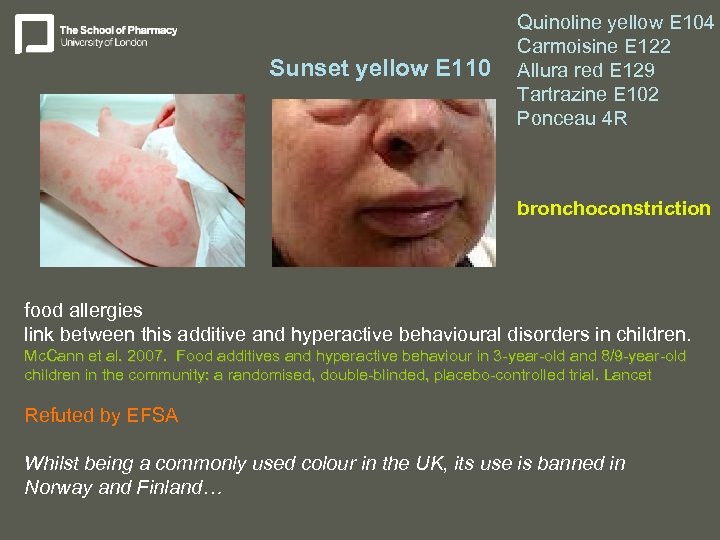 Sunset yellow E 110 Quinoline yellow E 104 Carmoisine E 122 Allura red E 129 Tartrazine E 102 Ponceau 4 R bronchoconstriction food allergies link between this additive and hyperactive behavioural disorders in children. Mc. Cann et al. 2007. Food additives and hyperactive behaviour in 3 -year-old and 8/9 -year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet Refuted by EFSA Whilst being a commonly used colour in the UK, its use is banned in Norway and Finland…
Sunset yellow E 110 Quinoline yellow E 104 Carmoisine E 122 Allura red E 129 Tartrazine E 102 Ponceau 4 R bronchoconstriction food allergies link between this additive and hyperactive behavioural disorders in children. Mc. Cann et al. 2007. Food additives and hyperactive behaviour in 3 -year-old and 8/9 -year-old children in the community: a randomised, double-blinded, placebo-controlled trial. Lancet Refuted by EFSA Whilst being a commonly used colour in the UK, its use is banned in Norway and Finland…
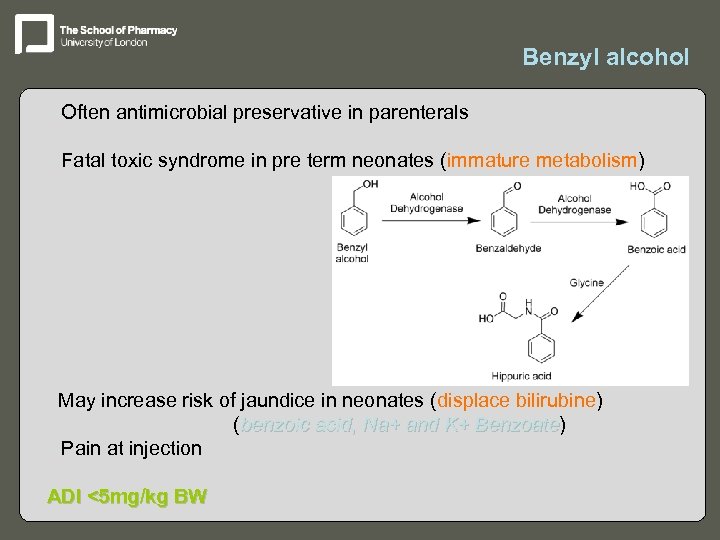 Benzyl alcohol – Often antimicrobial preservative in parenterals – Fatal toxic syndrome in pre term neonates (immature metabolism) May increase risk of jaundice in neonates (displace bilirubine) – (benzoic acid, Na+ and K+ Benzoate) Benzoate – Pain at injection ADI <5 mg/kg BW
Benzyl alcohol – Often antimicrobial preservative in parenterals – Fatal toxic syndrome in pre term neonates (immature metabolism) May increase risk of jaundice in neonates (displace bilirubine) – (benzoic acid, Na+ and K+ Benzoate) Benzoate – Pain at injection ADI <5 mg/kg BW
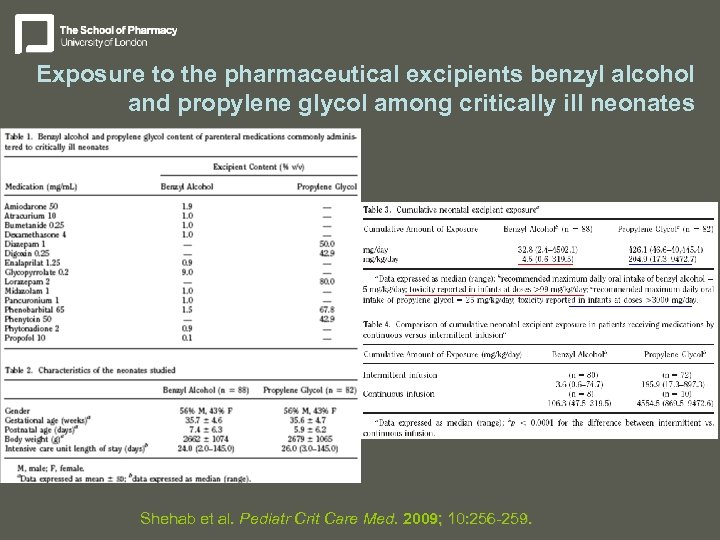 Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates Shehab et al. Pediatr Crit Care Med. 2009; 10: 256 -259.
Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates Shehab et al. Pediatr Crit Care Med. 2009; 10: 256 -259.
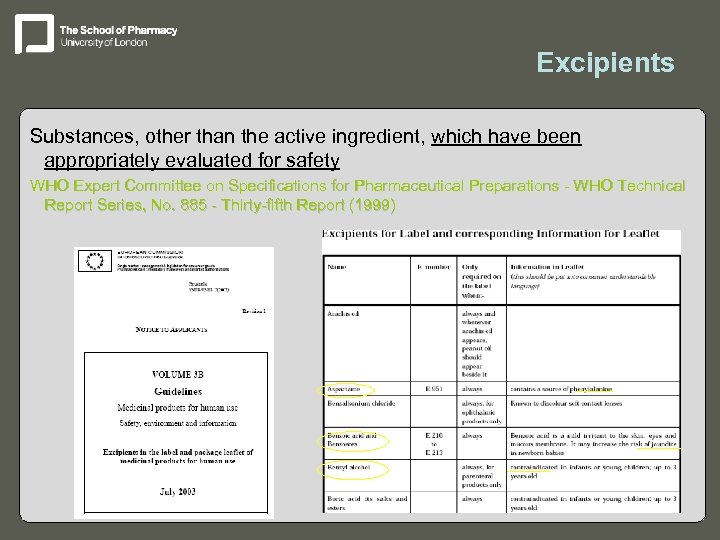 Excipients Substances, other than the active ingredient, which have been appropriately evaluated for safety WHO Expert Committee on Specifications for Pharmaceutical Preparations - WHO Technical Report Series, No. 885 - Thirty-fifth Report (1999)
Excipients Substances, other than the active ingredient, which have been appropriately evaluated for safety WHO Expert Committee on Specifications for Pharmaceutical Preparations - WHO Technical Report Series, No. 885 - Thirty-fifth Report (1999)
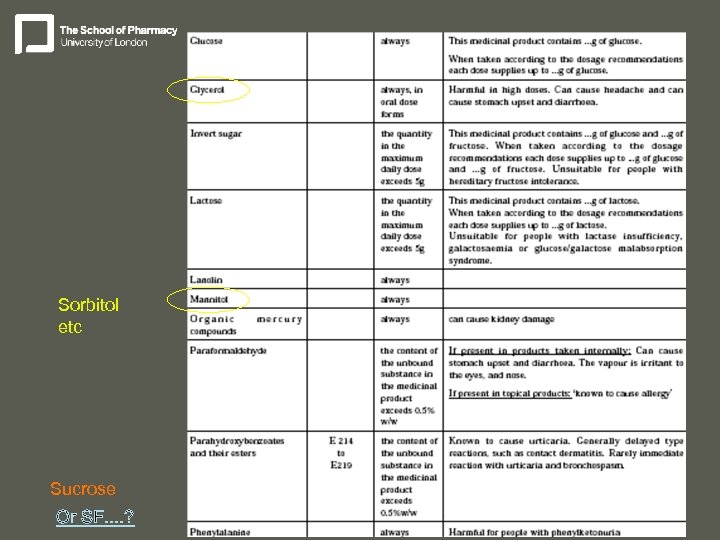 Sorbitol etc Sucrose Or SF. . ?
Sorbitol etc Sucrose Or SF. . ?
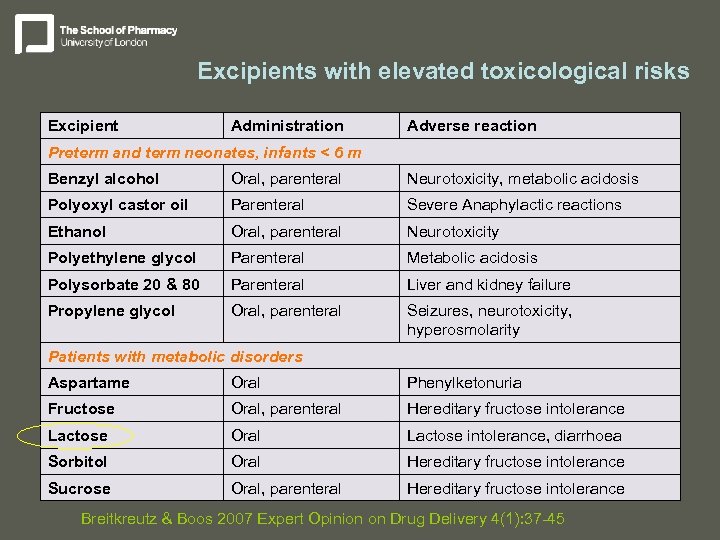 Excipients with elevated toxicological risks Excipient Administration Adverse reaction Preterm and term neonates, infants < 6 m Benzyl alcohol Oral, parenteral Neurotoxicity, metabolic acidosis Polyoxyl castor oil Parenteral Severe Anaphylactic reactions Ethanol Oral, parenteral Neurotoxicity Polyethylene glycol Parenteral Metabolic acidosis Polysorbate 20 & 80 Parenteral Liver and kidney failure Propylene glycol Oral, parenteral Seizures, neurotoxicity, hyperosmolarity Patients with metabolic disorders Aspartame Oral Phenylketonuria Fructose Oral, parenteral Hereditary fructose intolerance Lactose Oral Lactose intolerance, diarrhoea Sorbitol Oral Hereditary fructose intolerance Sucrose Oral, parenteral Hereditary fructose intolerance Breitkreutz & Boos 2007 Expert Opinion on Drug Delivery 4(1): 37 -45
Excipients with elevated toxicological risks Excipient Administration Adverse reaction Preterm and term neonates, infants < 6 m Benzyl alcohol Oral, parenteral Neurotoxicity, metabolic acidosis Polyoxyl castor oil Parenteral Severe Anaphylactic reactions Ethanol Oral, parenteral Neurotoxicity Polyethylene glycol Parenteral Metabolic acidosis Polysorbate 20 & 80 Parenteral Liver and kidney failure Propylene glycol Oral, parenteral Seizures, neurotoxicity, hyperosmolarity Patients with metabolic disorders Aspartame Oral Phenylketonuria Fructose Oral, parenteral Hereditary fructose intolerance Lactose Oral Lactose intolerance, diarrhoea Sorbitol Oral Hereditary fructose intolerance Sucrose Oral, parenteral Hereditary fructose intolerance Breitkreutz & Boos 2007 Expert Opinion on Drug Delivery 4(1): 37 -45
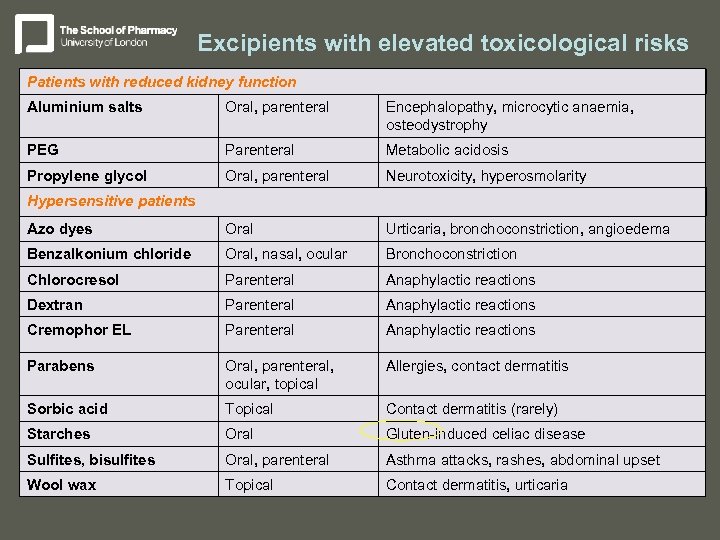 Excipients with elevated toxicological risks Patients with reduced kidney function Aluminium salts Oral, parenteral Encephalopathy, microcytic anaemia, osteodystrophy PEG Parenteral Metabolic acidosis Propylene glycol Oral, parenteral Neurotoxicity, hyperosmolarity Azo dyes Oral Urticaria, bronchoconstriction, angioedema Benzalkonium chloride Oral, nasal, ocular Bronchoconstriction Chlorocresol Parenteral Anaphylactic reactions Dextran Parenteral Anaphylactic reactions Cremophor EL Parenteral Anaphylactic reactions Parabens Oral, parenteral, ocular, topical Allergies, contact dermatitis Sorbic acid Topical Contact dermatitis (rarely) Starches Oral Gluten-induced celiac disease Sulfites, bisulfites Oral, parenteral Asthma attacks, rashes, abdominal upset Wool wax Topical Contact dermatitis, urticaria Hypersensitive patients
Excipients with elevated toxicological risks Patients with reduced kidney function Aluminium salts Oral, parenteral Encephalopathy, microcytic anaemia, osteodystrophy PEG Parenteral Metabolic acidosis Propylene glycol Oral, parenteral Neurotoxicity, hyperosmolarity Azo dyes Oral Urticaria, bronchoconstriction, angioedema Benzalkonium chloride Oral, nasal, ocular Bronchoconstriction Chlorocresol Parenteral Anaphylactic reactions Dextran Parenteral Anaphylactic reactions Cremophor EL Parenteral Anaphylactic reactions Parabens Oral, parenteral, ocular, topical Allergies, contact dermatitis Sorbic acid Topical Contact dermatitis (rarely) Starches Oral Gluten-induced celiac disease Sulfites, bisulfites Oral, parenteral Asthma attacks, rashes, abdominal upset Wool wax Topical Contact dermatitis, urticaria Hypersensitive patients
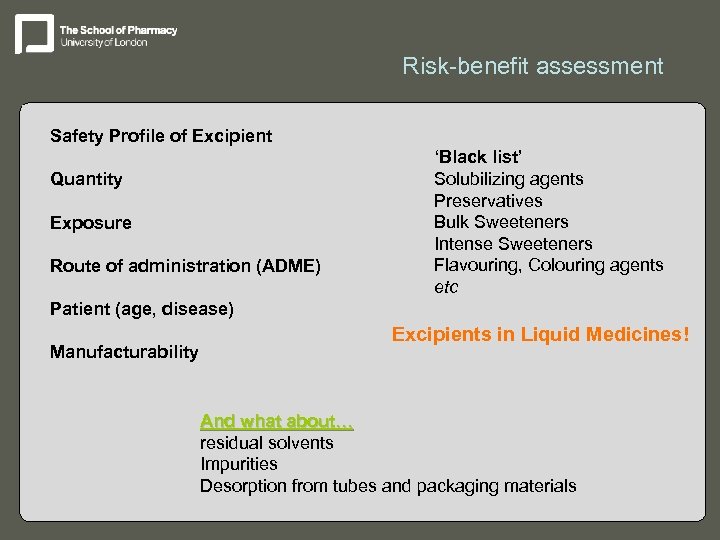 Risk-benefit assessment Safety Profile of Excipient Quantity Exposure Route of administration (ADME) ‘Black list’ Solubilizing agents Preservatives Bulk Sweeteners Intense Sweeteners Flavouring, Colouring agents etc Patient (age, disease) Manufacturability Excipients in Liquid Medicines! And what about… residual solvents Impurities Desorption from tubes and packaging materials
Risk-benefit assessment Safety Profile of Excipient Quantity Exposure Route of administration (ADME) ‘Black list’ Solubilizing agents Preservatives Bulk Sweeteners Intense Sweeteners Flavouring, Colouring agents etc Patient (age, disease) Manufacturability Excipients in Liquid Medicines! And what about… residual solvents Impurities Desorption from tubes and packaging materials
 And now, case scenarios
And now, case scenarios
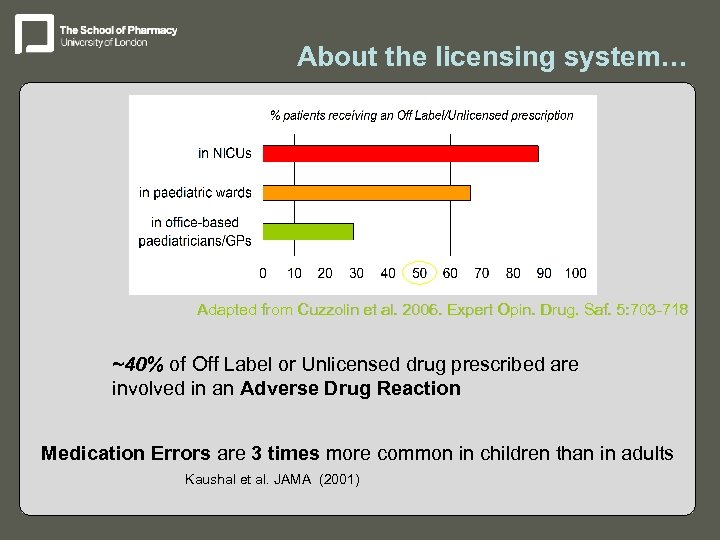 About the licensing system… Adapted from Cuzzolin et al. 2006. Expert Opin. Drug. Saf. 5: 703 -718 ~40% of Off Label or Unlicensed drug prescribed are involved in an Adverse Drug Reaction Medication Errors are 3 times more common in children than in adults Kaushal et al. JAMA (2001)
About the licensing system… Adapted from Cuzzolin et al. 2006. Expert Opin. Drug. Saf. 5: 703 -718 ~40% of Off Label or Unlicensed drug prescribed are involved in an Adverse Drug Reaction Medication Errors are 3 times more common in children than in adults Kaushal et al. JAMA (2001)
 The EU paediatric regulation Since January 2007 3 pilars: Obligation : Paediatric Investigation Plan (PIP) Paediatric Committee (PDCO) at the EMEA Reward (incentives) for studies conducted results are included in the product information (SPC) = medicine eligible for six months’ patent extension even if clinical trial do not show efficacy
The EU paediatric regulation Since January 2007 3 pilars: Obligation : Paediatric Investigation Plan (PIP) Paediatric Committee (PDCO) at the EMEA Reward (incentives) for studies conducted results are included in the product information (SPC) = medicine eligible for six months’ patent extension even if clinical trial do not show efficacy
 The future is bright(er) PIP Overall strategy Review any information on the product such as: Suitability of the existing pharmaceutical form (tablets vs liquid, new strength…) Off label or unlicensed use Strategy in relation to quality aspect Address Critical issue such as: The need for a specific formulation or dosage form in relation to age group – discussion of the benefit Availability/timeframe for the development Potential issues (e. g. excipients) Administration (use of specific device, ability to mix with food, socio-cultural issues, packaging)
The future is bright(er) PIP Overall strategy Review any information on the product such as: Suitability of the existing pharmaceutical form (tablets vs liquid, new strength…) Off label or unlicensed use Strategy in relation to quality aspect Address Critical issue such as: The need for a specific formulation or dosage form in relation to age group – discussion of the benefit Availability/timeframe for the development Potential issues (e. g. excipients) Administration (use of specific device, ability to mix with food, socio-cultural issues, packaging)
 www. emea. europa. eu/pdfs/hu man/qwp/13893108 en. pdf Guideline being prepared by Quality Working Party drafting group – consultation expected soon
www. emea. europa. eu/pdfs/hu man/qwp/13893108 en. pdf Guideline being prepared by Quality Working Party drafting group – consultation expected soon
 ERANET PRIOMEDCHILD To support researchers working together internationally allowing them to address important research questions that they could not address otherwise - The development or use of innovative methodology in medicines for children research - Innovation of paediatric formulations and drug delivery systems The studies on novel modes of drug delivery (buccal, nasal, transdermal), age specific formulations, Innovation on taste masking and improved dosing devices Risk assessment and data collection on excipients. 8. 5 -10 million Euros www. priomedchild. eu/index. php? id=6574
ERANET PRIOMEDCHILD To support researchers working together internationally allowing them to address important research questions that they could not address otherwise - The development or use of innovative methodology in medicines for children research - Innovation of paediatric formulations and drug delivery systems The studies on novel modes of drug delivery (buccal, nasal, transdermal), age specific formulations, Innovation on taste masking and improved dosing devices Risk assessment and data collection on excipients. 8. 5 -10 million Euros www. priomedchild. eu/index. php? id=6574
 Data base on Excipients for paediatric medicines Eu. PFi and PFI (US) are building a database which will be available freely on a website Thanks for your attention And now, the Sunday morning QUIZ!!!
Data base on Excipients for paediatric medicines Eu. PFi and PFI (US) are building a database which will be available freely on a website Thanks for your attention And now, the Sunday morning QUIZ!!!
 Question 1 – The ideal paediatric formulation should have all of the following characteristics EXCEPT: –A –B –C –D A full range of dosage formulations Non-toxic excipients A minimal dosage frequency Not easily reproduced Paediatric Formulations – Pharmaceutical Excipients and Adverse Reactions This MCQs have been prepared from the PEP (Pharmacist Education programme) developed by IDIS
Question 1 – The ideal paediatric formulation should have all of the following characteristics EXCEPT: –A –B –C –D A full range of dosage formulations Non-toxic excipients A minimal dosage frequency Not easily reproduced Paediatric Formulations – Pharmaceutical Excipients and Adverse Reactions This MCQs have been prepared from the PEP (Pharmacist Education programme) developed by IDIS
 Question 2 – What is the recommended target dose volume for children under the age of 5 years? –A –B –C –D 2. 5 ml or less 7. 5 ml or less 10 ml or less
Question 2 – What is the recommended target dose volume for children under the age of 5 years? –A –B –C –D 2. 5 ml or less 7. 5 ml or less 10 ml or less
 Question 3 – Effervescent tablets are unsuitable for which of the following patient groups? –A –B –C –D Children with renal impairment Epileptic patients/children calcium restricted Infants Adolescents
Question 3 – Effervescent tablets are unsuitable for which of the following patient groups? –A –B –C –D Children with renal impairment Epileptic patients/children calcium restricted Infants Adolescents
 Question 4 – Which of the following excipients can cause jaundice in neonates? –A –B –C –D Benzoic acid Aspartame Lactose Propylene glycol
Question 4 – Which of the following excipients can cause jaundice in neonates? –A –B –C –D Benzoic acid Aspartame Lactose Propylene glycol
 Question 5 – Which of the following excipients should be avoided in children under three years old due to the risk of Central Nervous System (CNS) depression? –A –B –C –D Benzyl alcohol Aspartame Lactose Propylene glycol
Question 5 – Which of the following excipients should be avoided in children under three years old due to the risk of Central Nervous System (CNS) depression? –A –B –C –D Benzyl alcohol Aspartame Lactose Propylene glycol
 Question 6 – Which of the following excipients is associated with hyperactive behaviour? –A –B –C –D Sucrose Sorbitol Azodyes Fructose
Question 6 – Which of the following excipients is associated with hyperactive behaviour? –A –B –C –D Sucrose Sorbitol Azodyes Fructose
 Question 7 – The use of which of the following orally toxic excipients was linked to death when used as a solvent? –A –B –C –D Lactose Aspartame Propylene glycol Diethylene glycol
Question 7 – The use of which of the following orally toxic excipients was linked to death when used as a solvent? –A –B –C –D Lactose Aspartame Propylene glycol Diethylene glycol
 Question 78 – Which of the following is used as a solvent and preservative? –A –B –C –D Benzyl alcohol Arachis oil Propylene glycol Diethylene glycol
Question 78 – Which of the following is used as a solvent and preservative? –A –B –C –D Benzyl alcohol Arachis oil Propylene glycol Diethylene glycol
 Question 9 – Which of the following excipients is NOT used as a solvent? –A –B –C –D Propylene glycol Benzyl alcohol Ethanol Arachis oil
Question 9 – Which of the following excipients is NOT used as a solvent? –A –B –C –D Propylene glycol Benzyl alcohol Ethanol Arachis oil
 Question 10 – Which of the following is a source of phenylalanine? –A –B –C –D Saccharin Aspartame Sorbitol Fructose
Question 10 – Which of the following is a source of phenylalanine? –A –B –C –D Saccharin Aspartame Sorbitol Fructose
 Question 11 – Which of the following sweeteners should be avoided in patients with a sulphonamide allergy? –A –B –C –D Saccharin Aspartame Sorbitol Fructose
Question 11 – Which of the following sweeteners should be avoided in patients with a sulphonamide allergy? –A –B –C –D Saccharin Aspartame Sorbitol Fructose
 Question 12 – Which of the following preservatives is associated with a potentially fatal toxic syndrome in neonates? –A –B –C –D Benzyl alcohol Sodium benzoate Benzoic acid Propylene glycol
Question 12 – Which of the following preservatives is associated with a potentially fatal toxic syndrome in neonates? –A –B –C –D Benzyl alcohol Sodium benzoate Benzoic acid Propylene glycol
 Question 13 – Which of the following preservatives is NOT associated with jaundice in neonates? –A –B –C –D Thiomersal Sodium benzoate Benzoic acid Potassium benzoate
Question 13 – Which of the following preservatives is NOT associated with jaundice in neonates? –A –B –C –D Thiomersal Sodium benzoate Benzoic acid Potassium benzoate
 Question 14 – In neonates the daily benzyl alcohol dose should be limited to: –A –B –C –D 5 mg/kg or less 10 mg/kg or less 15 mg/kg or less 20 mg/kg or less
Question 14 – In neonates the daily benzyl alcohol dose should be limited to: –A –B –C –D 5 mg/kg or less 10 mg/kg or less 15 mg/kg or less 20 mg/kg or less
 Question 15 – In the event that a repeat prescription for a paediatric medicine is presented to the pharmacy for a child with severe allergy to a pharmaceutical excipient, the most appropriate course of action would be: –A Confirm with the carer which product was used previously –B Check the Summary of Product Characteristics (SPC) for details on excipients –C Check the BNFc for details on excipients –D Contact the manufacturer for details on excipients
Question 15 – In the event that a repeat prescription for a paediatric medicine is presented to the pharmacy for a child with severe allergy to a pharmaceutical excipient, the most appropriate course of action would be: –A Confirm with the carer which product was used previously –B Check the Summary of Product Characteristics (SPC) for details on excipients –C Check the BNFc for details on excipients –D Contact the manufacturer for details on excipients
 Question 16 – What average percentage of medicines used by children in Europe are untested or unauthorised for paediatric use, according to the European Medicines Agency (EMEA)? –A –B –C –D At least 5% At least 10% At least 25% At least 50%
Question 16 – What average percentage of medicines used by children in Europe are untested or unauthorised for paediatric use, according to the European Medicines Agency (EMEA)? –A –B –C –D At least 5% At least 10% At least 25% At least 50%
 Question 17 – The European regulations on medicinal products for children came into force in which year? –A –B –C –D 2005 2006 2007 2008
Question 17 – The European regulations on medicinal products for children came into force in which year? –A –B –C –D 2005 2006 2007 2008
 Question 18 – According to the International Conference on Harmonisation, the term ‘child’ applies to which age range? –A –B –C –D Birth to one month One month to two years Two to 12 years 12 to 18 years
Question 18 – According to the International Conference on Harmonisation, the term ‘child’ applies to which age range? –A –B –C –D Birth to one month One month to two years Two to 12 years 12 to 18 years
 Question 19 - Under the new European Regulation, studying medicines for use in children will be rewarded by –A –B –C –D Funding incentives Patent extension of 6 months Patent extension for 12 months Patent extension for 18 months
Question 19 - Under the new European Regulation, studying medicines for use in children will be rewarded by –A –B –C –D Funding incentives Patent extension of 6 months Patent extension for 12 months Patent extension for 18 months


