 Old Registration System n The old system dealing with products under registration that acquired permission to proceed with product analysis at NODCAR & received NODCAR certificate
Old Registration System n The old system dealing with products under registration that acquired permission to proceed with product analysis at NODCAR & received NODCAR certificate
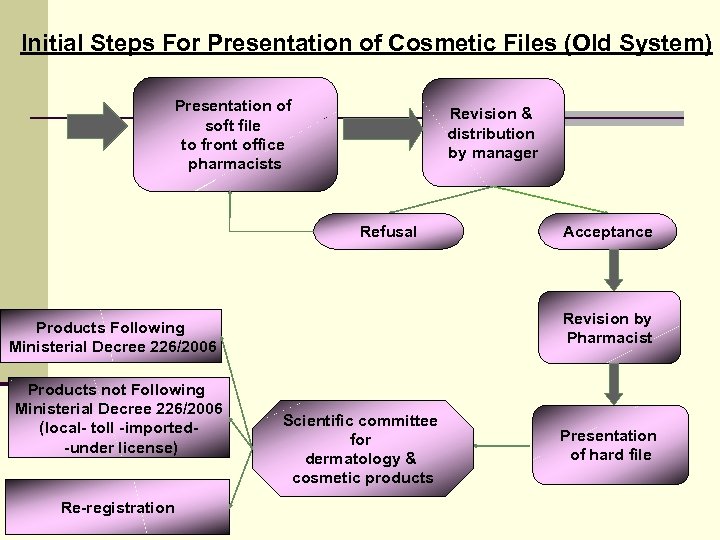 Initial Steps For Presentation of Cosmetic Files (Old System) Presentation of soft file to front office pharmacists Revision & distribution by manager Refusal Revision by Pharmacist Products Following Ministerial Decree 226/2006 Products not Following Ministerial Decree 226/2006 (local- toll -imported-under license) Re-registration Acceptance Scientific committee for dermatology & cosmetic products Presentation of hard file
Initial Steps For Presentation of Cosmetic Files (Old System) Presentation of soft file to front office pharmacists Revision & distribution by manager Refusal Revision by Pharmacist Products Following Ministerial Decree 226/2006 Products not Following Ministerial Decree 226/2006 (local- toll -imported-under license) Re-registration Acceptance Scientific committee for dermatology & cosmetic products Presentation of hard file
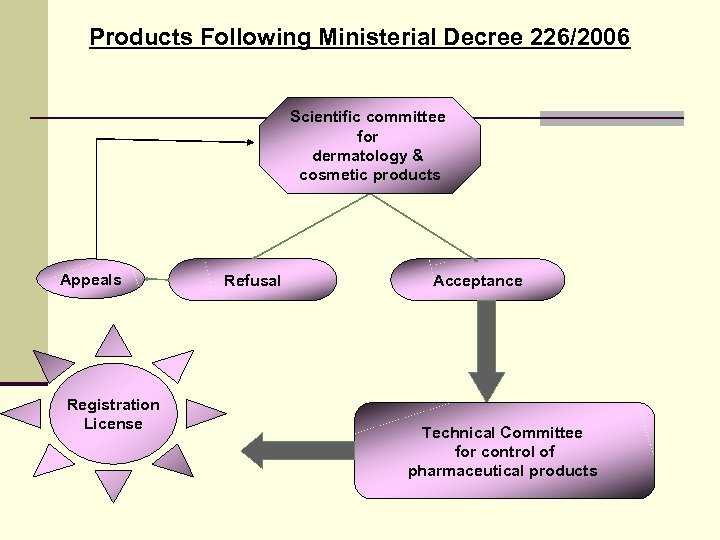 Products Following Ministerial Decree 226/2006 Scientific committee for dermatology & cosmetic products Appeals Registration License Refusal Acceptance Technical Committee for control of pharmaceutical products
Products Following Ministerial Decree 226/2006 Scientific committee for dermatology & cosmetic products Appeals Registration License Refusal Acceptance Technical Committee for control of pharmaceutical products
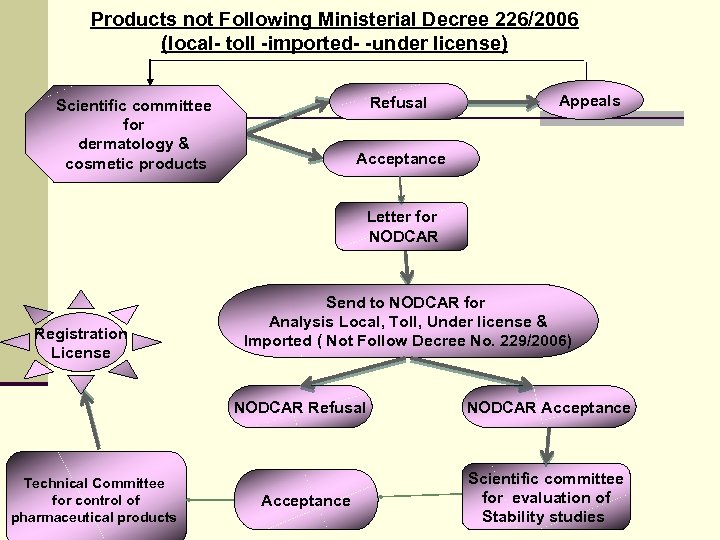 Products not Following Ministerial Decree 226/2006 (local- toll -imported- -under license) Refusal Scientific committee for dermatology & cosmetic products Appeals Acceptance Letter for NODCAR Registration License Send to NODCAR for Analysis Local, Toll, Under license & Imported ( Not Follow Decree No. 229/2006) NODCAR Refusal Technical Committee for control of pharmaceutical products 2 Acceptance NODCAR Acceptance Scientific committee for evaluation of Stability studies
Products not Following Ministerial Decree 226/2006 (local- toll -imported- -under license) Refusal Scientific committee for dermatology & cosmetic products Appeals Acceptance Letter for NODCAR Registration License Send to NODCAR for Analysis Local, Toll, Under license & Imported ( Not Follow Decree No. 229/2006) NODCAR Refusal Technical Committee for control of pharmaceutical products 2 Acceptance NODCAR Acceptance Scientific committee for evaluation of Stability studies
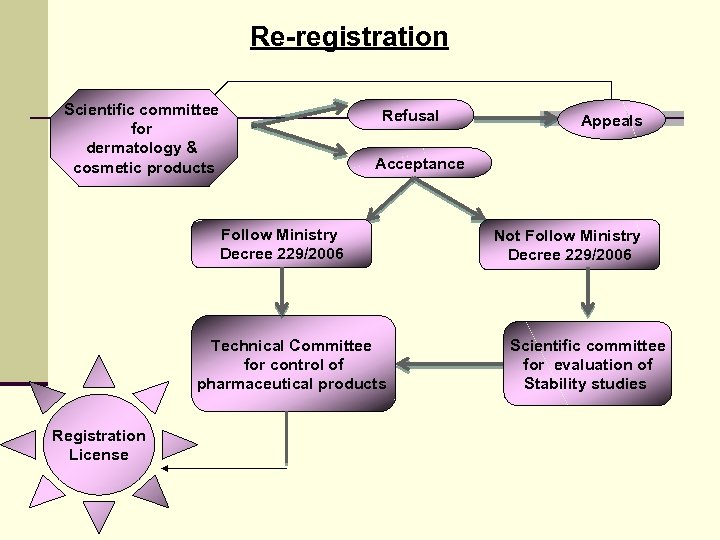 Re-registration Scientific committee for dermatology & cosmetic products Refusal Acceptance Follow Ministry Decree 229/2006 Technical Committee for control of pharmaceutical products Registration License Appeals Not Follow Ministry Decree 229/2006 Scientific committee for evaluation of Stability studies
Re-registration Scientific committee for dermatology & cosmetic products Refusal Acceptance Follow Ministry Decree 229/2006 Technical Committee for control of pharmaceutical products Registration License Appeals Not Follow Ministry Decree 229/2006 Scientific committee for evaluation of Stability studies