31e8937249cb8851a1df13f5729692d3.ppt
- Количество слайдов: 70
 Old groundwaters István Fórizs Ph. D. Institute for Geochemical Research, Hungarian Academy of Sciences Budapest
Old groundwaters István Fórizs Ph. D. Institute for Geochemical Research, Hungarian Academy of Sciences Budapest
 Why should we identify old groundwaters? • To determine the time and place of recharge (recharge may already be stopped) • Mean residence time • Exploitation induced recharge • To understand the geochemical and hydrological processes
Why should we identify old groundwaters? • To determine the time and place of recharge (recharge may already be stopped) • Mean residence time • Exploitation induced recharge • To understand the geochemical and hydrological processes
 Nomenclature • Old groundwaters are • Paleo-groundwaters (older than 10 000 a, infiltrated during the latest glaciation) • Sub-modern (older than 60 a)
Nomenclature • Old groundwaters are • Paleo-groundwaters (older than 10 000 a, infiltrated during the latest glaciation) • Sub-modern (older than 60 a)
 Stable isotopes and paleogroundwaters • These waters were infiltrated at cooler climatic conditions during the Ice Age. • Their d. D and d 18 O values are significantly more negative than those of Holocene infiltrated ones. Temperature effect!! • Shift in d-excess. The effect of relative humidity of (h) air on the primary evaporation. Characteristic for arid regions, Eastern Mediterranean and North Africa. • There are some areas where paleo-groundwaters postdate the glaciation, because during the Ice Age there was a permanent ice cover. The melted water infiltrated during the deglaciation (early Holocene), e. g. in Canada.
Stable isotopes and paleogroundwaters • These waters were infiltrated at cooler climatic conditions during the Ice Age. • Their d. D and d 18 O values are significantly more negative than those of Holocene infiltrated ones. Temperature effect!! • Shift in d-excess. The effect of relative humidity of (h) air on the primary evaporation. Characteristic for arid regions, Eastern Mediterranean and North Africa. • There are some areas where paleo-groundwaters postdate the glaciation, because during the Ice Age there was a permanent ice cover. The melted water infiltrated during the deglaciation (early Holocene), e. g. in Canada.
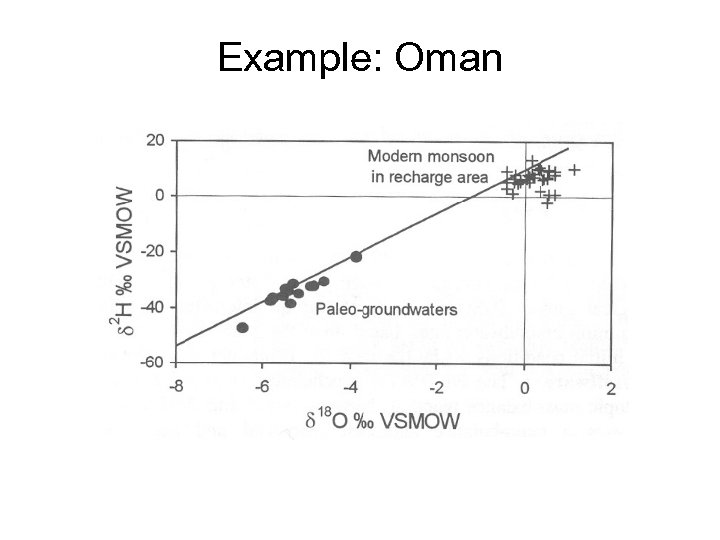 Example: Oman
Example: Oman

 Shift in deuterim-excess (d-excess) • Effect of primary evaporation • Effect of secondary evaporation • Definition: d = d. D – 8*d 18 O
Shift in deuterim-excess (d-excess) • Effect of primary evaporation • Effect of secondary evaporation • Definition: d = d. D – 8*d 18 O
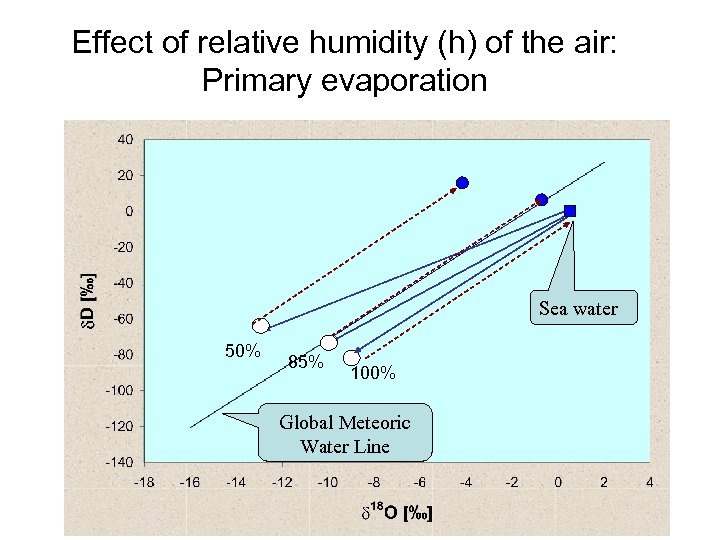 Effect of relative humidity (h) of the air: Primary evaporation Sea water 50% 85% 100% Global Meteoric Water Line
Effect of relative humidity (h) of the air: Primary evaporation Sea water 50% 85% 100% Global Meteoric Water Line
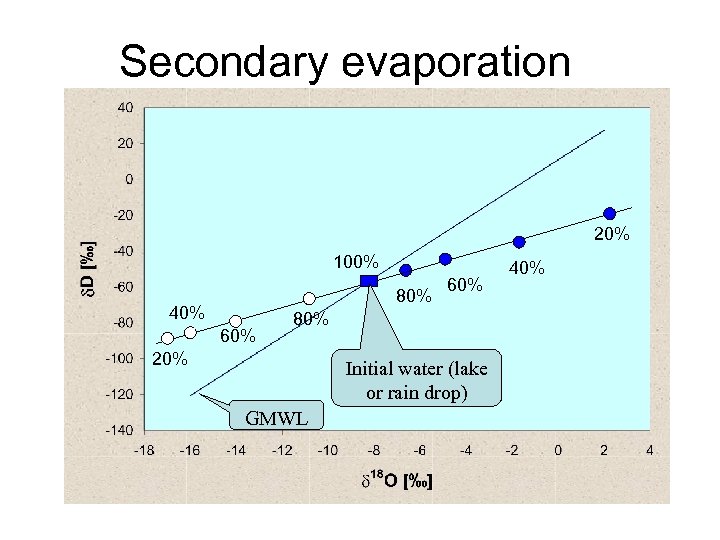 Secondary evaporation 20% 100% 80% 40% 60% 80% 20% Initial water (lake or rain drop) GMWL 40%
Secondary evaporation 20% 100% 80% 40% 60% 80% 20% Initial water (lake or rain drop) GMWL 40%
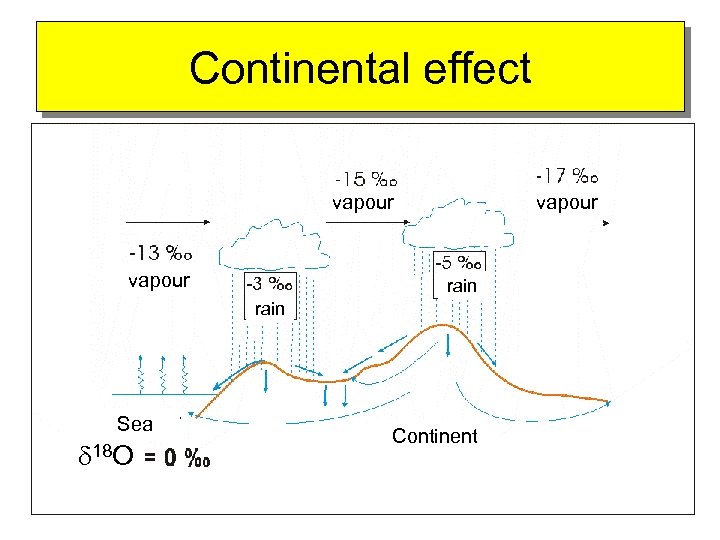 Continental effect vapour rain Sea d 18 O Continent
Continental effect vapour rain Sea d 18 O Continent
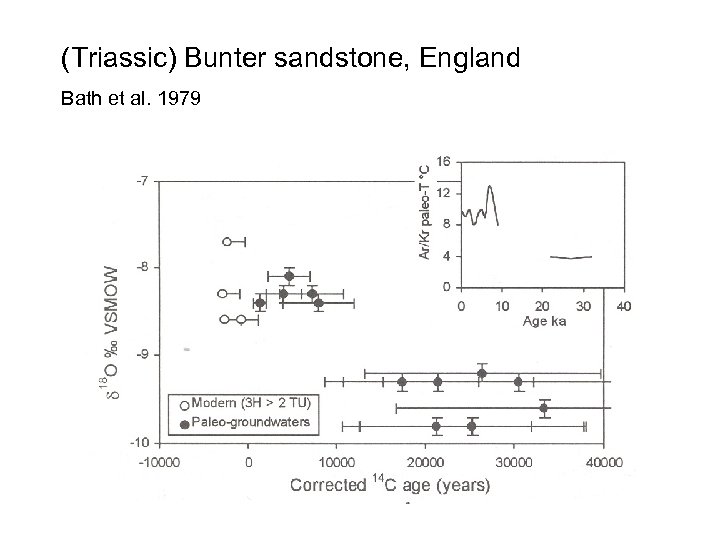 (Triassic) Bunter sandstone, England Bath et al. 1979
(Triassic) Bunter sandstone, England Bath et al. 1979

 Ice cores show well the climate change
Ice cores show well the climate change
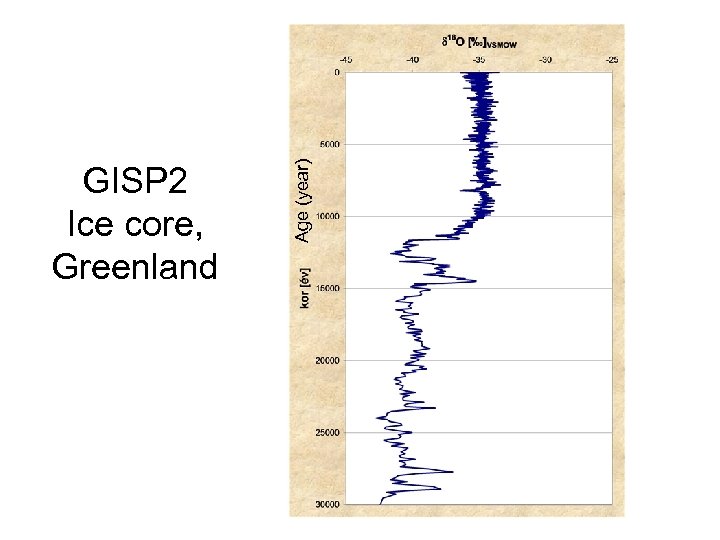 Age (year) GISP 2 Ice core, Greenland
Age (year) GISP 2 Ice core, Greenland
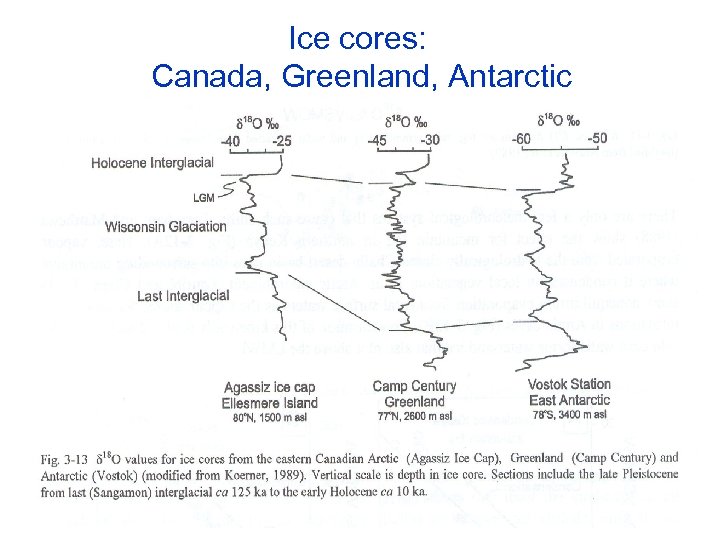 Ice cores: Canada, Greenland, Antarctic
Ice cores: Canada, Greenland, Antarctic
 Chemistry and paleogroundwaters
Chemistry and paleogroundwaters
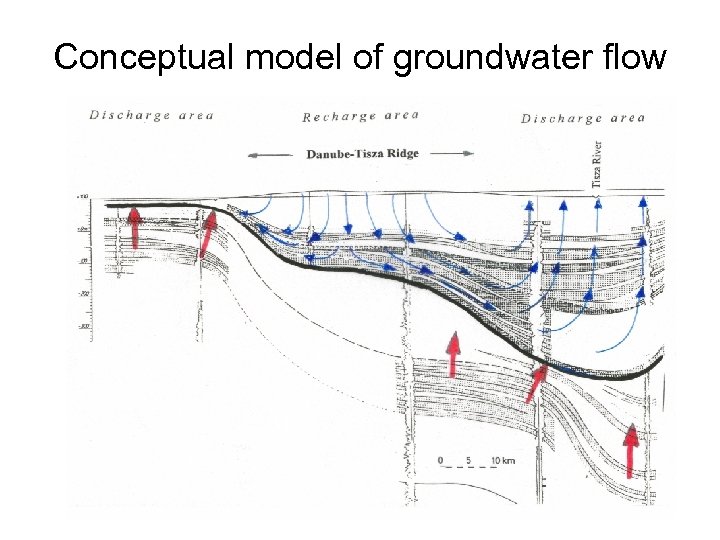 Conceptual model of groundwater flow
Conceptual model of groundwater flow
 Chemistry and paleo-groundwaters • Water-rock interaction may change the chemistry of water significatly • Recharge area: – low TDS – frequently Ca-HCO 3 type • Discharge area: – – high TDS frequently Na(-Ca)-HCO 3(-Cl-SO 4) type high p. H high trace element content
Chemistry and paleo-groundwaters • Water-rock interaction may change the chemistry of water significatly • Recharge area: – low TDS – frequently Ca-HCO 3 type • Discharge area: – – high TDS frequently Na(-Ca)-HCO 3(-Cl-SO 4) type high p. H high trace element content
 Groundwater dating methods
Groundwater dating methods
 Groundwater dating methods • • • Radiocarbon: 14 C Chlorine-36: 36 Cl The uranium decay series Helium ingrowth Krypton-81: 81 Kr
Groundwater dating methods • • • Radiocarbon: 14 C Chlorine-36: 36 Cl The uranium decay series Helium ingrowth Krypton-81: 81 Kr
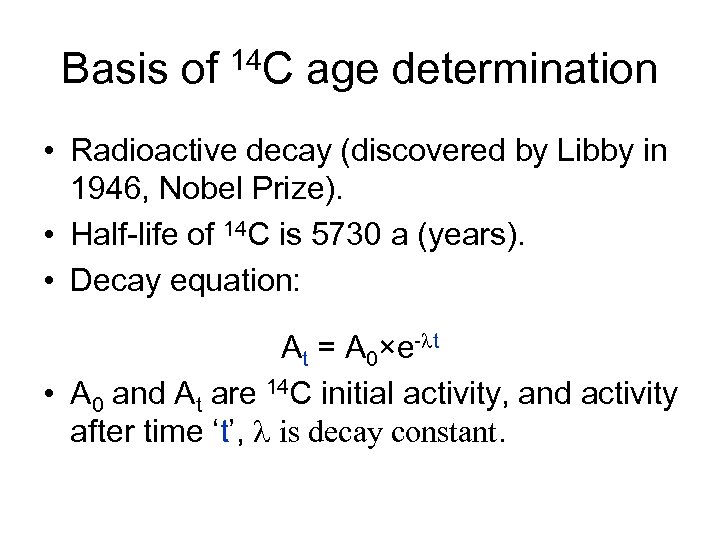 Basis of 14 C age determination • Radioactive decay (discovered by Libby in 1946, Nobel Prize). • Half-life of 14 C is 5730 a (years). • Decay equation: At = A 0×e-lt • A 0 and At are 14 C initial activity, and activity after time ‘t’, l is decay constant.
Basis of 14 C age determination • Radioactive decay (discovered by Libby in 1946, Nobel Prize). • Half-life of 14 C is 5730 a (years). • Decay equation: At = A 0×e-lt • A 0 and At are 14 C initial activity, and activity after time ‘t’, l is decay constant.
![Rearranged decay equation t = -8267×ln(At/A 0) [year] Rearranged decay equation t = -8267×ln(At/A 0) [year]](https://present5.com/presentation/31e8937249cb8851a1df13f5729692d3/image-22.jpg) Rearranged decay equation t = -8267×ln(At/A 0) [year]
Rearranged decay equation t = -8267×ln(At/A 0) [year]
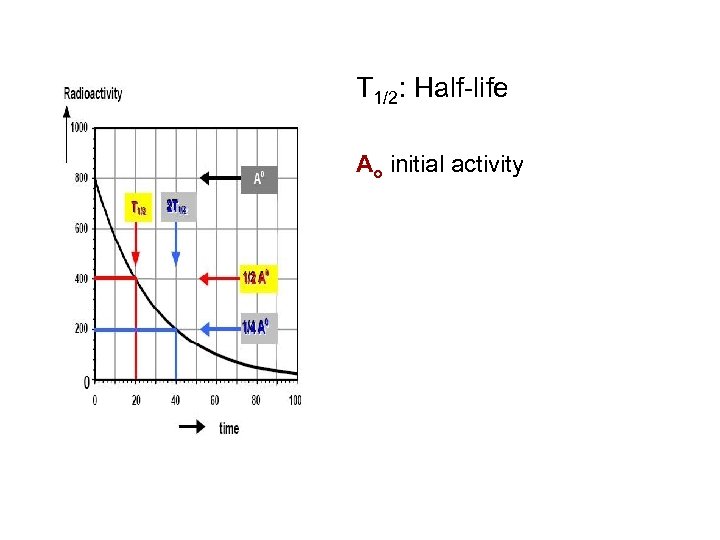 T 1/2: Half-life Ao initial activity
T 1/2: Half-life Ao initial activity
 Expression of 14 C activity • 14 C is expressed versus a reference, in percent modern carbon, pm. C. • Reference is the pre-industrial 14 C activity of atmospheric CO 2, that is regarded as 100%.
Expression of 14 C activity • 14 C is expressed versus a reference, in percent modern carbon, pm. C. • Reference is the pre-industrial 14 C activity of atmospheric CO 2, that is regarded as 100%.
 Source of 14 C • Natural: 147 N + 10 n → 146 C + 11 p • Where n = neutron, p = proton • Anthropogenic: nuclear bomb tests starting in 1952.
Source of 14 C • Natural: 147 N + 10 n → 146 C + 11 p • Where n = neutron, p = proton • Anthropogenic: nuclear bomb tests starting in 1952.
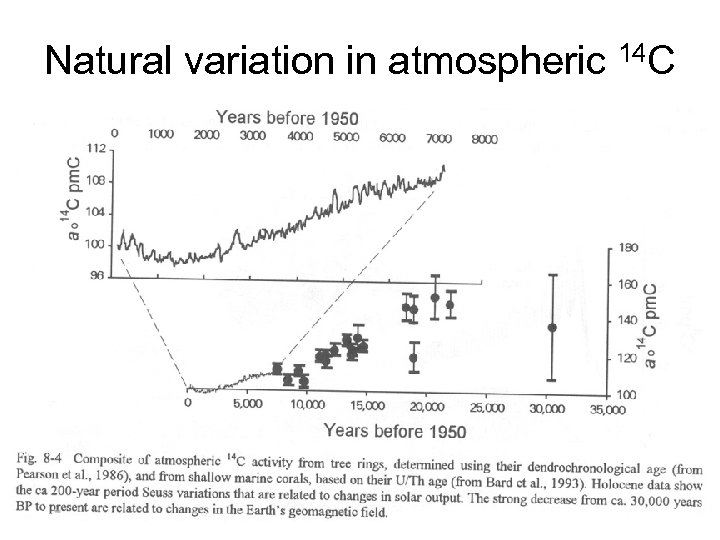 Natural variation in atmospheric 14 C
Natural variation in atmospheric 14 C
 The calculated age • If we disregard the natural variation in atmospheric 14 C (A 0 is regarded to have been constant, as 100%), then the calculated age is radiocarbon years and not in calendar years.
The calculated age • If we disregard the natural variation in atmospheric 14 C (A 0 is regarded to have been constant, as 100%), then the calculated age is radiocarbon years and not in calendar years.
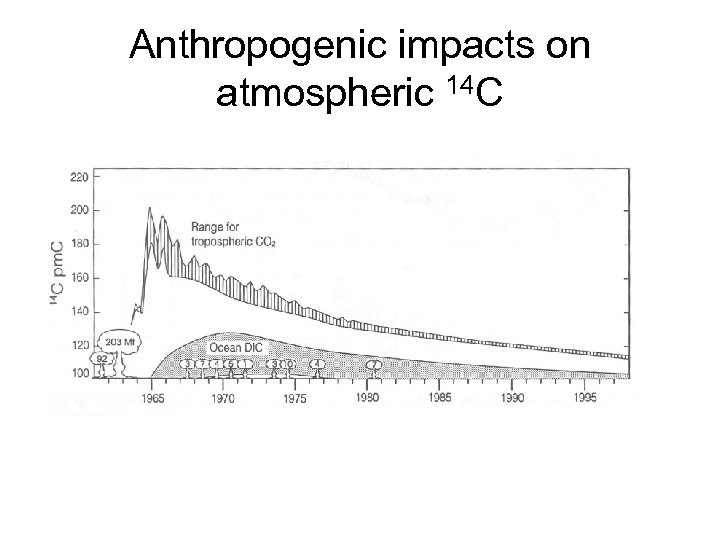 Anthropogenic impacts on atmospheric 14 C
Anthropogenic impacts on atmospheric 14 C
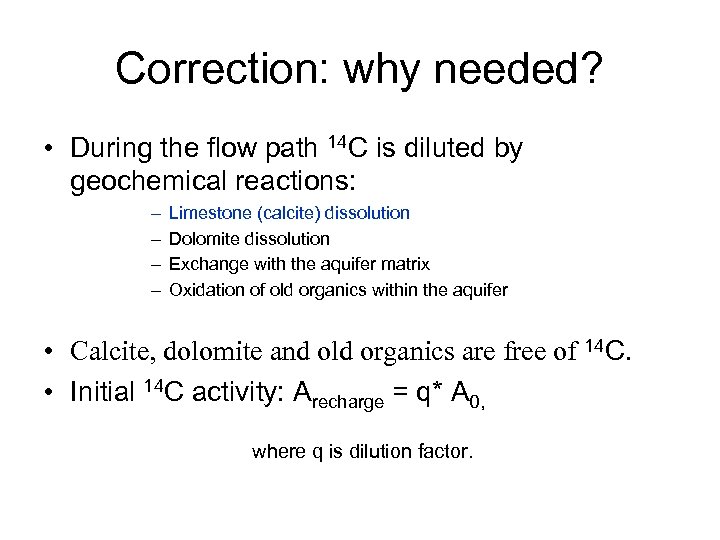 Correction: why needed? • During the flow path 14 C is diluted by geochemical reactions: – – Limestone (calcite) dissolution Dolomite dissolution Exchange with the aquifer matrix Oxidation of old organics within the aquifer • Calcite, dolomite and old organics are free of 14 C. • Initial 14 C activity: Arecharge = q* A 0, where q is dilution factor.
Correction: why needed? • During the flow path 14 C is diluted by geochemical reactions: – – Limestone (calcite) dissolution Dolomite dissolution Exchange with the aquifer matrix Oxidation of old organics within the aquifer • Calcite, dolomite and old organics are free of 14 C. • Initial 14 C activity: Arecharge = q* A 0, where q is dilution factor.
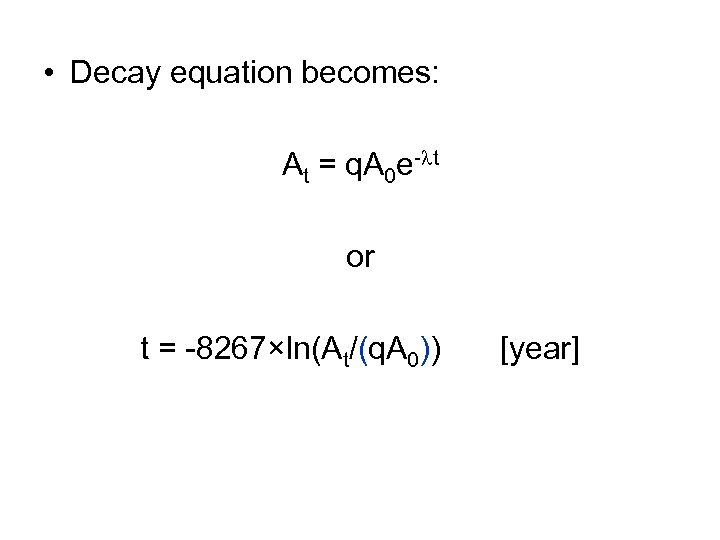 • Decay equation becomes: At = q. A 0 e-lt or t = -8267×ln(At/(q. A 0)) [year]
• Decay equation becomes: At = q. A 0 e-lt or t = -8267×ln(At/(q. A 0)) [year]
 Short introduction to carbon stable isotope geochemistry
Short introduction to carbon stable isotope geochemistry
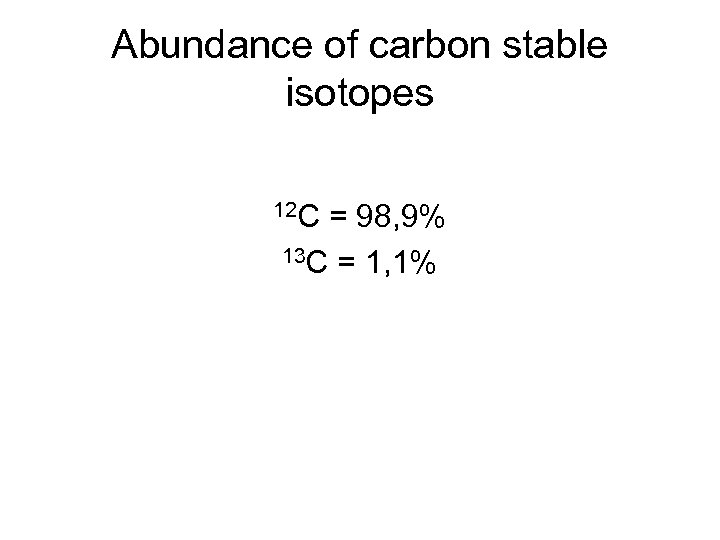 Abundance of carbon stable isotopes 12 C = 98, 9% 13 C = 1, 1%
Abundance of carbon stable isotopes 12 C = 98, 9% 13 C = 1, 1%
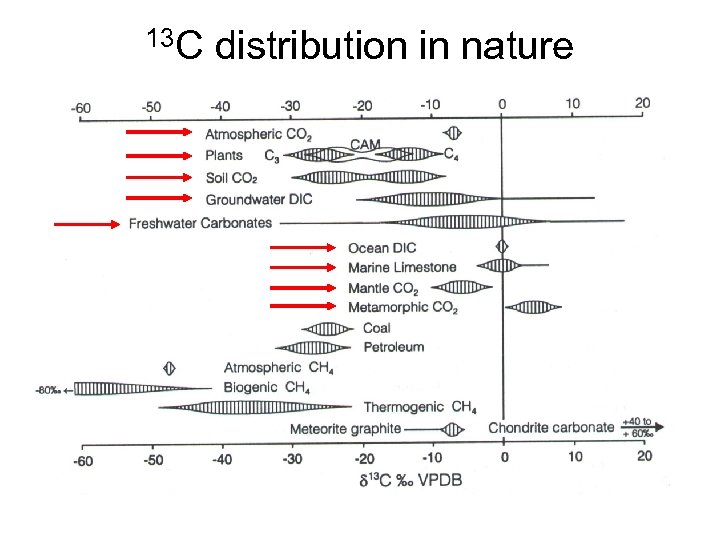 13 C distribution in nature
13 C distribution in nature
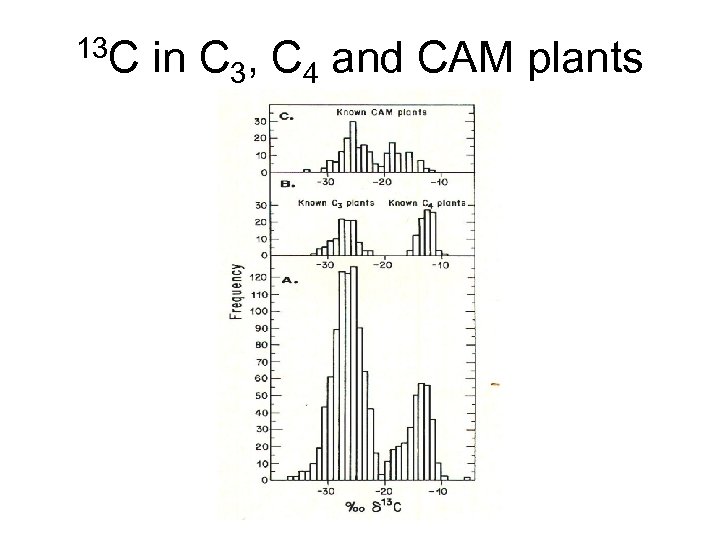 13 C in C 3, C 4 and CAM plants
13 C in C 3, C 4 and CAM plants
 Photosinthesis • C 3 plants (85%): Calvin cycle E. g. trees, cereals, legumes (bean), beet. • C 3 plants: d 13 C value is from -33 to -20 [‰]VPDB • Mean value= -27‰.
Photosinthesis • C 3 plants (85%): Calvin cycle E. g. trees, cereals, legumes (bean), beet. • C 3 plants: d 13 C value is from -33 to -20 [‰]VPDB • Mean value= -27‰.
 Photosinthesis • C 4 plants (5%): Hatch-Slack cycle E. g. cane, maize • d 13 C value is -16 to -9 [‰]VPDB • Mean value: -12, 5‰.
Photosinthesis • C 4 plants (5%): Hatch-Slack cycle E. g. cane, maize • d 13 C value is -16 to -9 [‰]VPDB • Mean value: -12, 5‰.
 13 C in soil CO 2 • Soil CO 2 originates from decomposition of organic material and root respiration. • The pressure of soil CO 2 gas is 10 -100 times higher than the atmospheric. • A part of soil CO 2 diffuses to the atmosphere causing isotopic fractionation: the remaining CO 2 is heavier by ca. 4‰. • The d 13 C value of soil CO 2: C 3 vegetation: ≈ -23 [‰]VPDB C 4 vegetation: ≈ -9 [‰]VPDB
13 C in soil CO 2 • Soil CO 2 originates from decomposition of organic material and root respiration. • The pressure of soil CO 2 gas is 10 -100 times higher than the atmospheric. • A part of soil CO 2 diffuses to the atmosphere causing isotopic fractionation: the remaining CO 2 is heavier by ca. 4‰. • The d 13 C value of soil CO 2: C 3 vegetation: ≈ -23 [‰]VPDB C 4 vegetation: ≈ -9 [‰]VPDB
![Carbon in water • Source: air CO 2 (d 13 C ≈ -7 [‰]VPDB), Carbon in water • Source: air CO 2 (d 13 C ≈ -7 [‰]VPDB),](https://present5.com/presentation/31e8937249cb8851a1df13f5729692d3/image-38.jpg) Carbon in water • Source: air CO 2 (d 13 C ≈ -7 [‰]VPDB), or soil CO 2 ( -9‰ — -23‰) or limestone (0± 2‰) • • Carbonate species in water CO 2(aq) (aquatic carbondioxide) H 2 CO 3 (carbonic acid) HCO 3 - (bicarbonate ion) CO 32 - (carbonate ion) } DIC
Carbon in water • Source: air CO 2 (d 13 C ≈ -7 [‰]VPDB), or soil CO 2 ( -9‰ — -23‰) or limestone (0± 2‰) • • Carbonate species in water CO 2(aq) (aquatic carbondioxide) H 2 CO 3 (carbonic acid) HCO 3 - (bicarbonate ion) CO 32 - (carbonate ion) } DIC
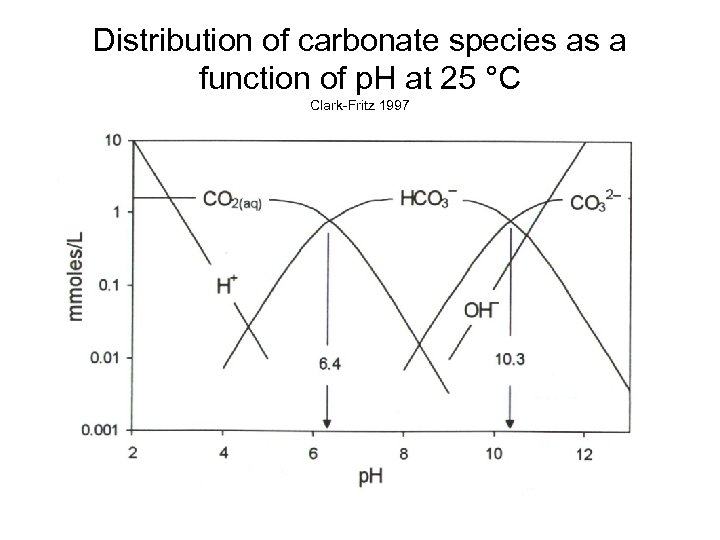 Distribution of carbonate species as a function of p. H at 25 °C Clark-Fritz 1997
Distribution of carbonate species as a function of p. H at 25 °C Clark-Fritz 1997
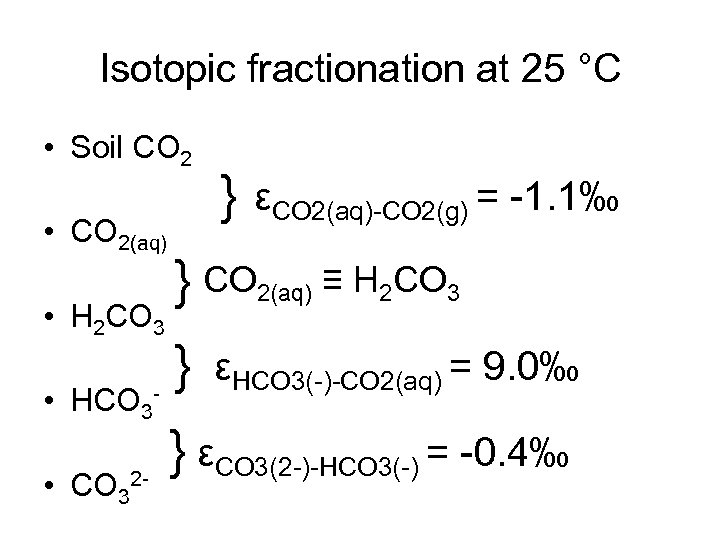 Isotopic fractionation at 25 °C • Soil CO 2 • CO 2(aq) • H 2 CO 3 • HCO 3 • CO 32 - } εCO 2(aq)-CO 2(g) = -1. 1‰ } CO 2(aq) ≡ H 2 CO 3 } εHCO 3(-)-CO 2(aq) = 9. 0‰ } εCO 3(2 -)-HCO 3(-) = -0. 4‰
Isotopic fractionation at 25 °C • Soil CO 2 • CO 2(aq) • H 2 CO 3 • HCO 3 • CO 32 - } εCO 2(aq)-CO 2(g) = -1. 1‰ } CO 2(aq) ≡ H 2 CO 3 } εHCO 3(-)-CO 2(aq) = 9. 0‰ } εCO 3(2 -)-HCO 3(-) = -0. 4‰
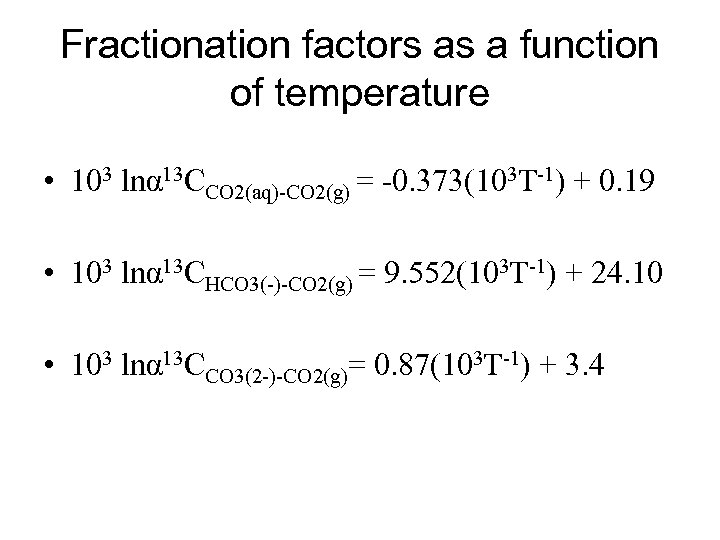 Fractionation factors as a function of temperature • 103 lnα 13 CCO 2(aq)-CO 2(g) = -0. 373(103 T-1) + 0. 19 • 103 lnα 13 CHCO 3(-)-CO 2(g) = 9. 552(103 T-1) + 24. 10 • 103 lnα 13 CCO 3(2 -)-CO 2(g)= 0. 87(103 T-1) + 3. 4
Fractionation factors as a function of temperature • 103 lnα 13 CCO 2(aq)-CO 2(g) = -0. 373(103 T-1) + 0. 19 • 103 lnα 13 CHCO 3(-)-CO 2(g) = 9. 552(103 T-1) + 24. 10 • 103 lnα 13 CCO 3(2 -)-CO 2(g)= 0. 87(103 T-1) + 3. 4
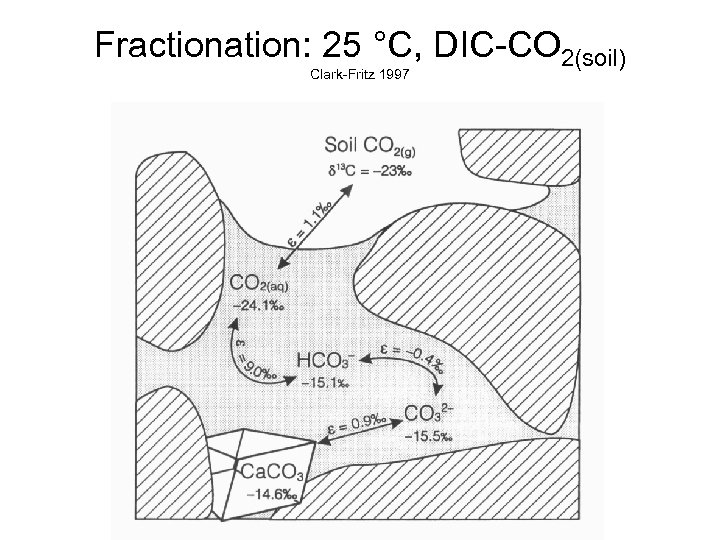 Fractionation: 25 °C, DIC-CO 2(soil) Clark-Fritz 1997
Fractionation: 25 °C, DIC-CO 2(soil) Clark-Fritz 1997
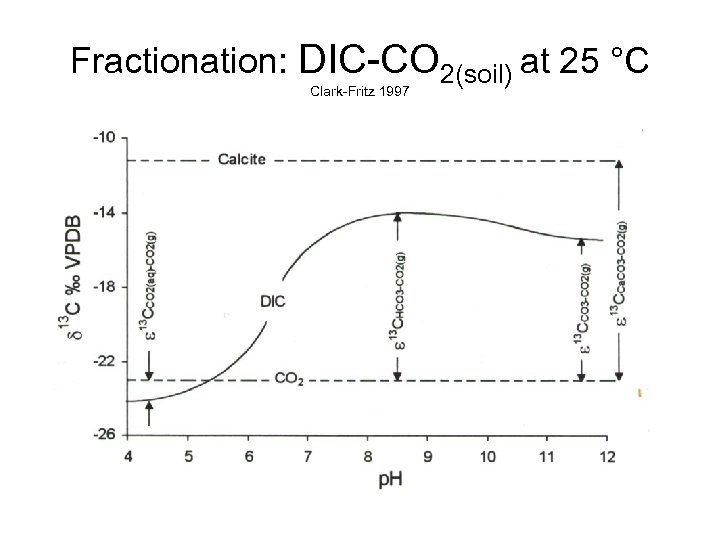 Fractionation: DIC-CO 2(soil) at 25 °C Clark-Fritz 1997
Fractionation: DIC-CO 2(soil) at 25 °C Clark-Fritz 1997
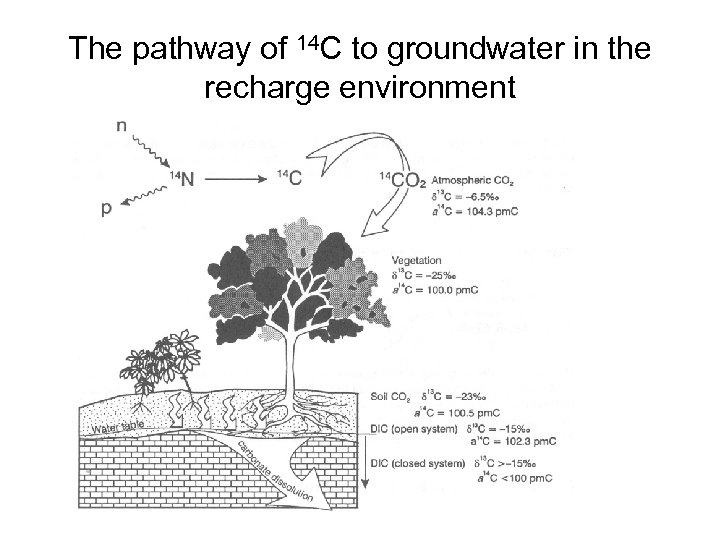 The pathway of 14 C to groundwater in the recharge environment
The pathway of 14 C to groundwater in the recharge environment
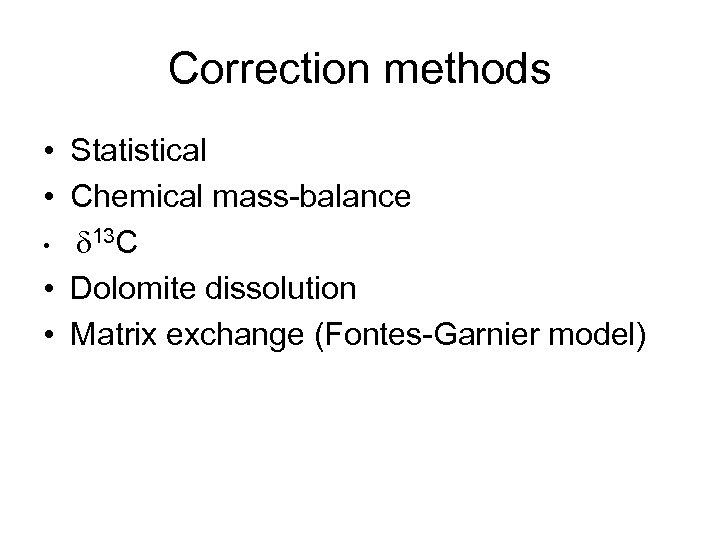 Correction methods • Statistical • Chemical mass-balance • d 13 C • Dolomite dissolution • Matrix exchange (Fontes-Garnier model)
Correction methods • Statistical • Chemical mass-balance • d 13 C • Dolomite dissolution • Matrix exchange (Fontes-Garnier model)
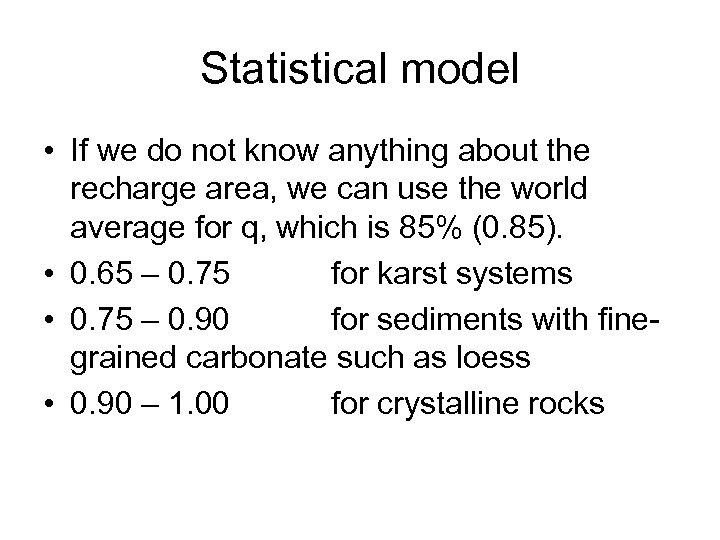 Statistical model • If we do not know anything about the recharge area, we can use the world average for q, which is 85% (0. 85). • 0. 65 – 0. 75 for karst systems • 0. 75 – 0. 90 for sediments with finegrained carbonate such as loess • 0. 90 – 1. 00 for crystalline rocks
Statistical model • If we do not know anything about the recharge area, we can use the world average for q, which is 85% (0. 85). • 0. 65 – 0. 75 for karst systems • 0. 75 – 0. 90 for sediments with finegrained carbonate such as loess • 0. 90 – 1. 00 for crystalline rocks
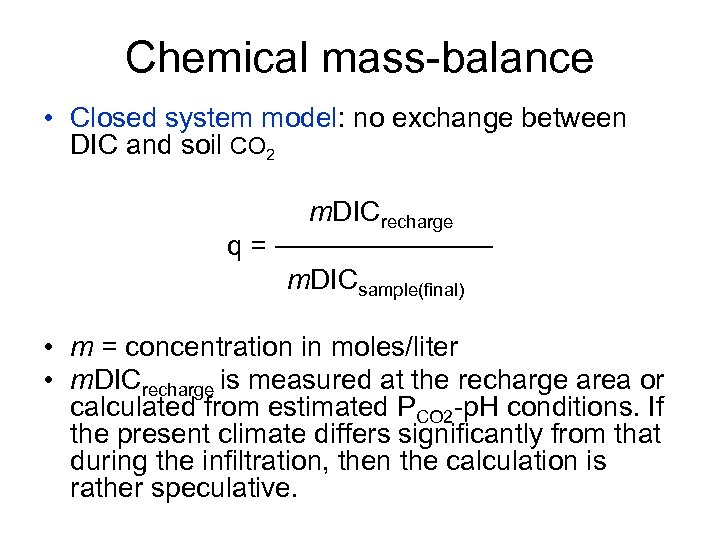 Chemical mass-balance • Closed system model: no exchange between DIC and soil CO 2 m. DICrecharge q = ────── m. DICsample(final) • m = concentration in moles/liter • m. DICrecharge is measured at the recharge area or calculated from estimated PCO 2 -p. H conditions. If the present climate differs significantly from that during the infiltration, then the calculation is rather speculative.
Chemical mass-balance • Closed system model: no exchange between DIC and soil CO 2 m. DICrecharge q = ────── m. DICsample(final) • m = concentration in moles/liter • m. DICrecharge is measured at the recharge area or calculated from estimated PCO 2 -p. H conditions. If the present climate differs significantly from that during the infiltration, then the calculation is rather speculative.
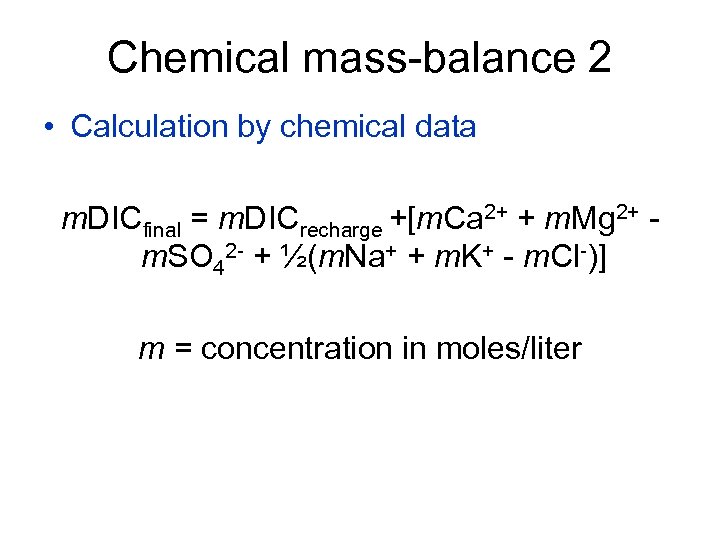 Chemical mass-balance 2 • Calculation by chemical data m. DICfinal = m. DICrecharge +[m. Ca 2+ + m. Mg 2+ m. SO 42 - + ½(m. Na+ + m. K+ - m. Cl-)] m = concentration in moles/liter
Chemical mass-balance 2 • Calculation by chemical data m. DICfinal = m. DICrecharge +[m. Ca 2+ + m. Mg 2+ m. SO 42 - + ½(m. Na+ + m. K+ - m. Cl-)] m = concentration in moles/liter
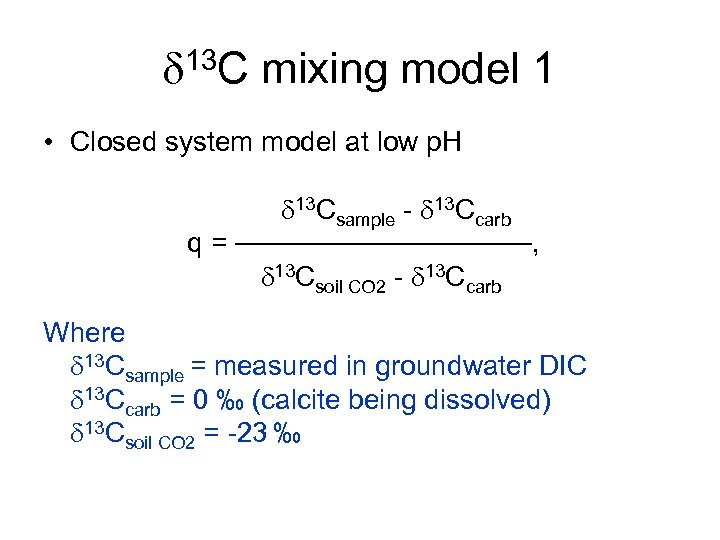 d 13 C mixing model 1 • Closed system model at low p. H d 13 Csample - d 13 Ccarb q = ────────, d 13 Csoil CO 2 - d 13 Ccarb Where d 13 Csample = measured in groundwater DIC d 13 Ccarb = 0 ‰ (calcite being dissolved) d 13 Csoil CO 2 = -23 ‰
d 13 C mixing model 1 • Closed system model at low p. H d 13 Csample - d 13 Ccarb q = ────────, d 13 Csoil CO 2 - d 13 Ccarb Where d 13 Csample = measured in groundwater DIC d 13 Ccarb = 0 ‰ (calcite being dissolved) d 13 Csoil CO 2 = -23 ‰
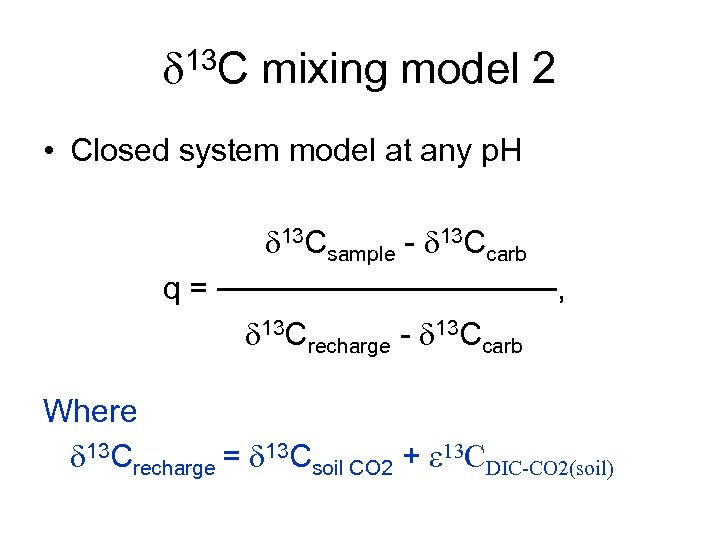 d 13 C mixing model 2 • Closed system model at any p. H d 13 Csample - d 13 Ccarb q = ────────, d 13 Crecharge - d 13 Ccarb Where d 13 Crecharge = d 13 Csoil CO 2 + e 13 CDIC-CO 2(soil)
d 13 C mixing model 2 • Closed system model at any p. H d 13 Csample - d 13 Ccarb q = ────────, d 13 Crecharge - d 13 Ccarb Where d 13 Crecharge = d 13 Csoil CO 2 + e 13 CDIC-CO 2(soil)
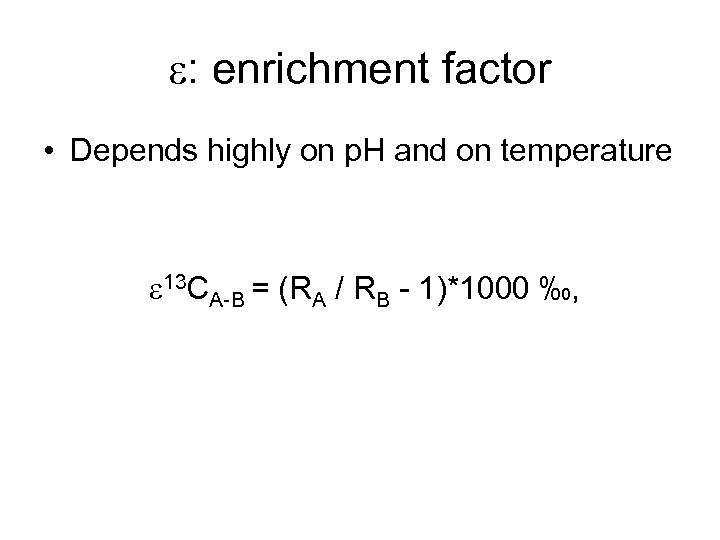 e: enrichment factor • Depends highly on p. H and on temperature e 13 CA-B = (RA / RB - 1)*1000 ‰,
e: enrichment factor • Depends highly on p. H and on temperature e 13 CA-B = (RA / RB - 1)*1000 ‰,
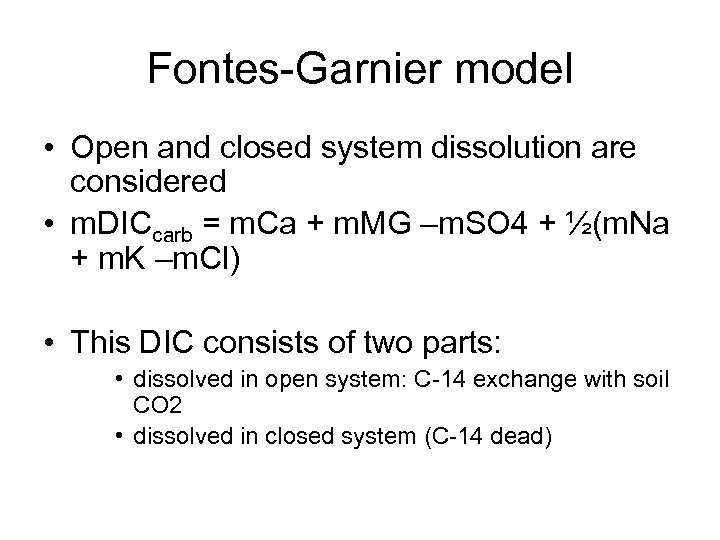 Fontes-Garnier model • Open and closed system dissolution are considered • m. DICcarb = m. Ca + m. MG –m. SO 4 + ½(m. Na + m. K –m. Cl) • This DIC consists of two parts: • dissolved in open system: C-14 exchange with soil CO 2 • dissolved in closed system (C-14 dead)
Fontes-Garnier model • Open and closed system dissolution are considered • m. DICcarb = m. Ca + m. MG –m. SO 4 + ½(m. Na + m. K –m. Cl) • This DIC consists of two parts: • dissolved in open system: C-14 exchange with soil CO 2 • dissolved in closed system (C-14 dead)
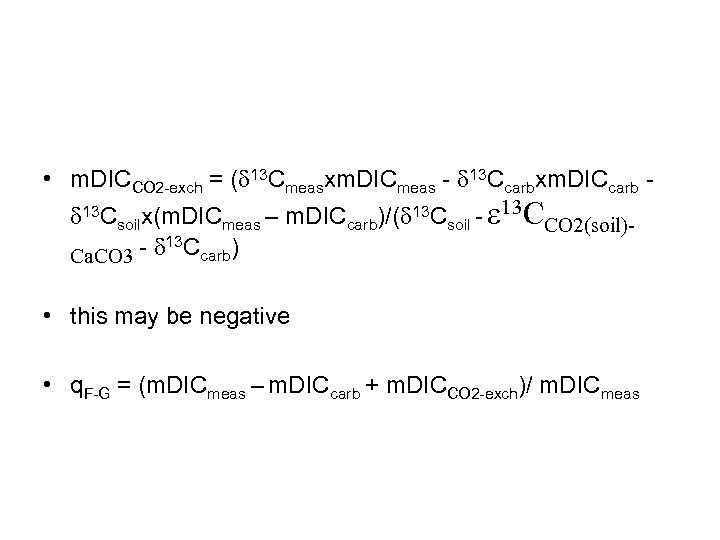 • m. DICCO 2 -exch = (d 13 Cmeasxm. DICmeas - d 13 Ccarbxm. DICcarb d 13 Csoilx(m. DICmeas – m. DICcarb)/(d 13 Csoil - e 13 CCO 2(soil)- d 13 Ccarb) Ca. CO 3 • this may be negative • q. F-G = (m. DICmeas – m. DICcarb + m. DICCO 2 -exch)/ m. DICmeas
• m. DICCO 2 -exch = (d 13 Cmeasxm. DICmeas - d 13 Ccarbxm. DICcarb d 13 Csoilx(m. DICmeas – m. DICcarb)/(d 13 Csoil - e 13 CCO 2(soil)- d 13 Ccarb) Ca. CO 3 • this may be negative • q. F-G = (m. DICmeas – m. DICcarb + m. DICCO 2 -exch)/ m. DICmeas
 Uncertainity
Uncertainity
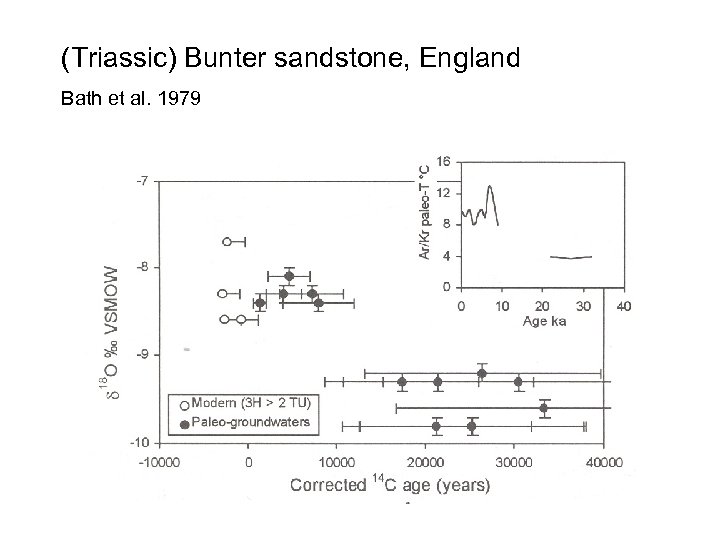 (Triassic) Bunter sandstone, England Bath et al. 1979
(Triassic) Bunter sandstone, England Bath et al. 1979
 Problem Data got on well water in Hungary • Tritium: 3 TU • d 18 O = -10, 7 [‰]VSMOW • 14 C-content: 30 pm. C • What is your opinion about this water?
Problem Data got on well water in Hungary • Tritium: 3 TU • d 18 O = -10, 7 [‰]VSMOW • 14 C-content: 30 pm. C • What is your opinion about this water?
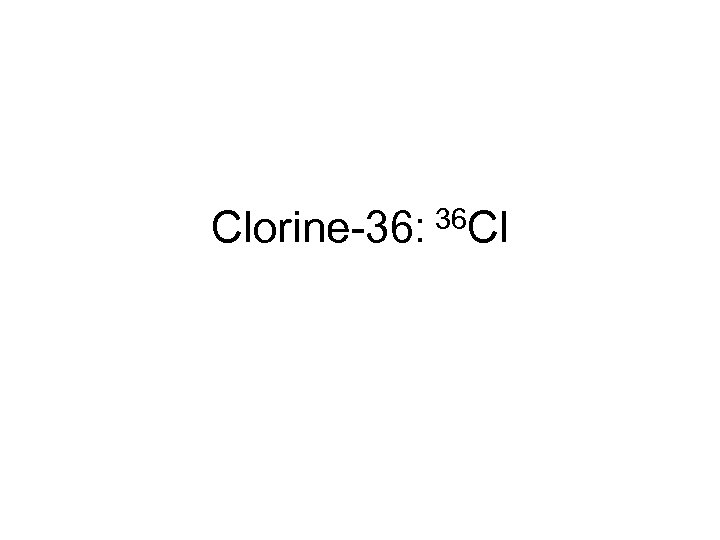 Clorine-36: 36 Cl
Clorine-36: 36 Cl
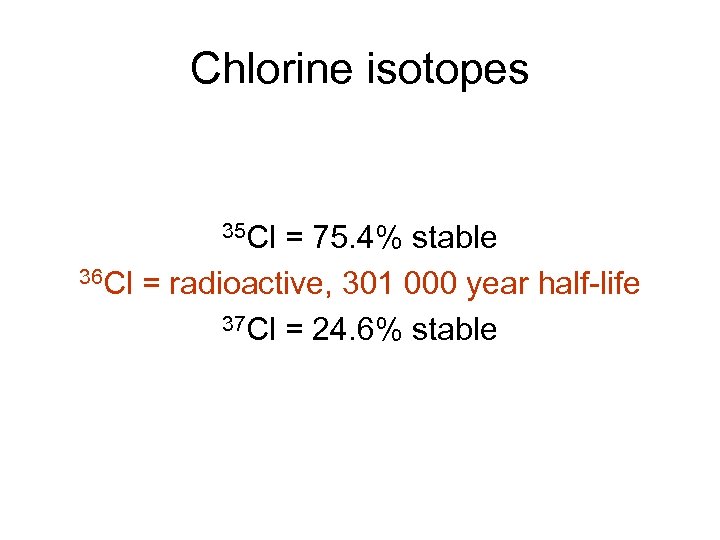 Chlorine isotopes 35 Cl = 75. 4% stable 36 Cl = radioactive, 301 000 year half-life 37 Cl = 24. 6% stable
Chlorine isotopes 35 Cl = 75. 4% stable 36 Cl = radioactive, 301 000 year half-life 37 Cl = 24. 6% stable
 Sources of 36 Cl • Natural: collision of cosmic neutron and 35 Cl atom. • Subsurface or epigenic production? • Anthropogene: mostly nuclear bomb tests in sea water.
Sources of 36 Cl • Natural: collision of cosmic neutron and 35 Cl atom. • Subsurface or epigenic production? • Anthropogene: mostly nuclear bomb tests in sea water.
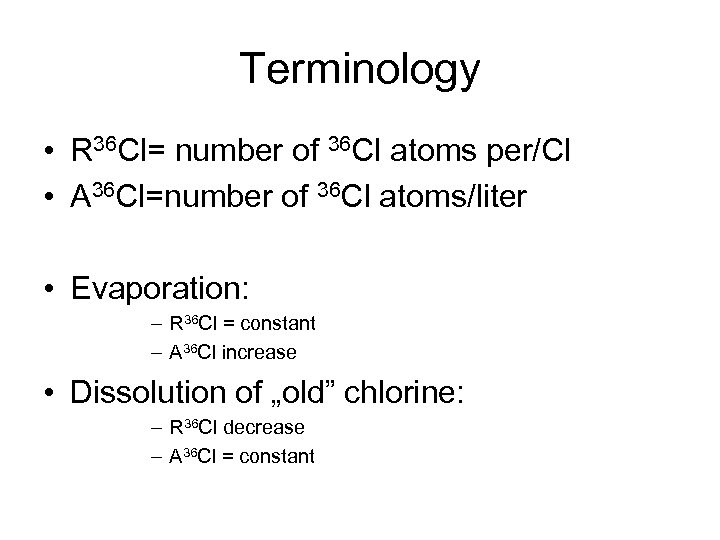 Terminology • R 36 Cl= number of 36 Cl atoms per/Cl • A 36 Cl=number of 36 Cl atoms/liter • Evaporation: – R 36 Cl = constant – A 36 Cl increase • Dissolution of „old” chlorine: – R 36 Cl decrease – A 36 Cl = constant
Terminology • R 36 Cl= number of 36 Cl atoms per/Cl • A 36 Cl=number of 36 Cl atoms/liter • Evaporation: – R 36 Cl = constant – A 36 Cl increase • Dissolution of „old” chlorine: – R 36 Cl decrease – A 36 Cl = constant
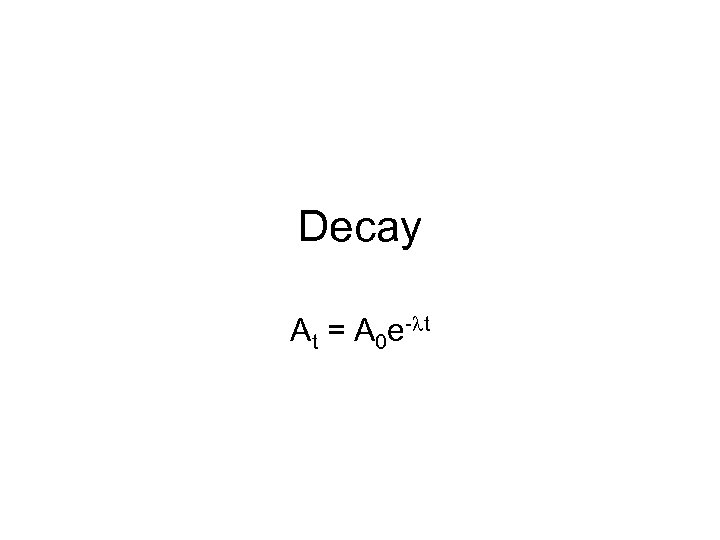 Decay At = A 0 e-lt
Decay At = A 0 e-lt
 Initial activity of 36 Cl • A 0 is determined by the geomagnetic latitude • Minimum at 0 and 90 degrees • Maximum at 40 degrees • You must take into account the distance from the sea • You have to create 36 Cl/Cl in precipitation map
Initial activity of 36 Cl • A 0 is determined by the geomagnetic latitude • Minimum at 0 and 90 degrees • Maximum at 40 degrees • You must take into account the distance from the sea • You have to create 36 Cl/Cl in precipitation map
 • AMS is used for the measurement • Sampling is very simple • Geochemical modelling is necessary: dissolution of 36 Cl-free chlorine (this is a most problematic part) • Age range up to 1. 5 million years
• AMS is used for the measurement • Sampling is very simple • Geochemical modelling is necessary: dissolution of 36 Cl-free chlorine (this is a most problematic part) • Age range up to 1. 5 million years
 Krypton-81: 81 Kr
Krypton-81: 81 Kr
 Krypton-81: 81 Kr • 81 Kr • • is produced in the upper atmosphere by cosmic-ray-induced spallation of five heavier Kr isotopes, i. e. from 82 Kr to 86 Kr. Or by neutron capture: 80 Kr + n → 81 Kr + g 36 36 No significant subsurface production. No appreciable anthropogenic source. Half-life is 229 000 years. Age range: from 35 000 to 670 000 years.
Krypton-81: 81 Kr • 81 Kr • • is produced in the upper atmosphere by cosmic-ray-induced spallation of five heavier Kr isotopes, i. e. from 82 Kr to 86 Kr. Or by neutron capture: 80 Kr + n → 81 Kr + g 36 36 No significant subsurface production. No appreciable anthropogenic source. Half-life is 229 000 years. Age range: from 35 000 to 670 000 years.
 Krypton-81: 81 Kr (cont. ) • The decay equation is: 81 Kr = 81 Kr ×e-lt t 0 • The 81 Kr concentration is expressed as number of atoms/liter • 81 Kr = 1100 atoms/L: initial value in 0 modern groundwater • E. g. 81 Kr = 900 atoms/L • t = -(ln(900/1100)/l = 66 297 a
Krypton-81: 81 Kr (cont. ) • The decay equation is: 81 Kr = 81 Kr ×e-lt t 0 • The 81 Kr concentration is expressed as number of atoms/liter • 81 Kr = 1100 atoms/L: initial value in 0 modern groundwater • E. g. 81 Kr = 900 atoms/L • t = -(ln(900/1100)/l = 66 297 a
 Krypton-81: 81 Kr (cont. ) • The 81 Kr concentration can be expressed as percent of modern atmosphere (similar to 14 C) • R/Rair = (81 Kr/Kr)sample/(81 Kr/Kr)air in percent • E. g. 81 Kr = 40% • t = -(ln(40%/100%)/l = (ln(0. 4)/(3. 03*10 -6) = 302 722 a
Krypton-81: 81 Kr (cont. ) • The 81 Kr concentration can be expressed as percent of modern atmosphere (similar to 14 C) • R/Rair = (81 Kr/Kr)sample/(81 Kr/Kr)air in percent • E. g. 81 Kr = 40% • t = -(ln(40%/100%)/l = (ln(0. 4)/(3. 03*10 -6) = 302 722 a
 Krypton-81: 81 Kr (cont. ) • Advantages: – Anthropogenic sources are minimal. – 81 Kr is inert (no chemical reactions envolved) • Disadvantages: – Technical difficulties, 1 or 2 labs in the world. – Limited experience (only 3 case studies worldwide)
Krypton-81: 81 Kr (cont. ) • Advantages: – Anthropogenic sources are minimal. – 81 Kr is inert (no chemical reactions envolved) • Disadvantages: – Technical difficulties, 1 or 2 labs in the world. – Limited experience (only 3 case studies worldwide)
 Brines
Brines



