3deb86f2508551c2156ed487aec068cb.ppt
- Количество слайдов: 78

OES Basics 1 Elemental

Informations OES n Basics of OES n Instrumentation n Calibration 2 Elemental
Basics of OES 3 Elemental

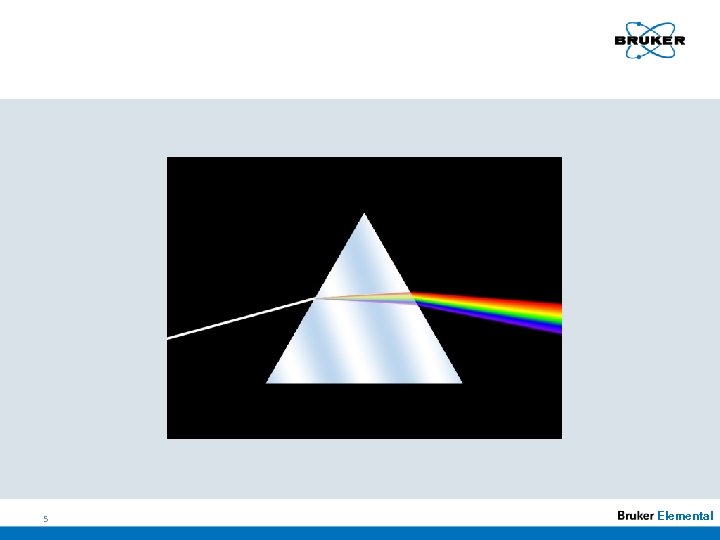
Historical Overview n 17 th century 1666– 1672): ( Isaak Newton Sunlight 1. Prism Spectral colors 2. Prism white light 4 Elemental
5 Elemental
Historical Overview n Isaak Newton: Light = Particle radiation 6 Christiaan Huygens: Light = Wave phenomenon (like sonic waves) Elemental
Historical Review n 1887: Heinrich Hertz Light = Small part of the electromagnetic spectrum n 1905: Albert Einstein Light = Particles (Photons) The 1921 physics Nobel prize was awarded to Einstein in most famous for his theory of relativity, but it is his discovery of photons that is mentioned by the Swedish Academy. 7 Elemental

Historical Review n Both is true: Light behaves somtimes like a Wave , and sometimes as a Particle ! 8 Elemental
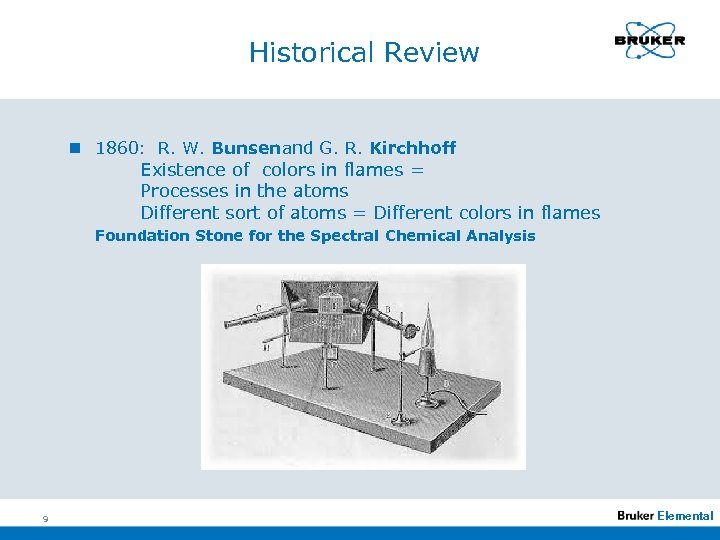
Historical Review n 1860: R. W. Bunsenand G. R. Kirchhoff Existence of colors in flames = Processes in the atoms Different sort of atoms = Different colors in flames Foundation Stone for the Spectral Chemical Analysis 9 Elemental
Basics OES n In the Optical Emission Spectroscopy, the atoms are exited by heat from an electrical discharge. The arising light is being dispersed into spectral wavelengths and the intensity of specific, atom related lines is measured. 10 Elemental
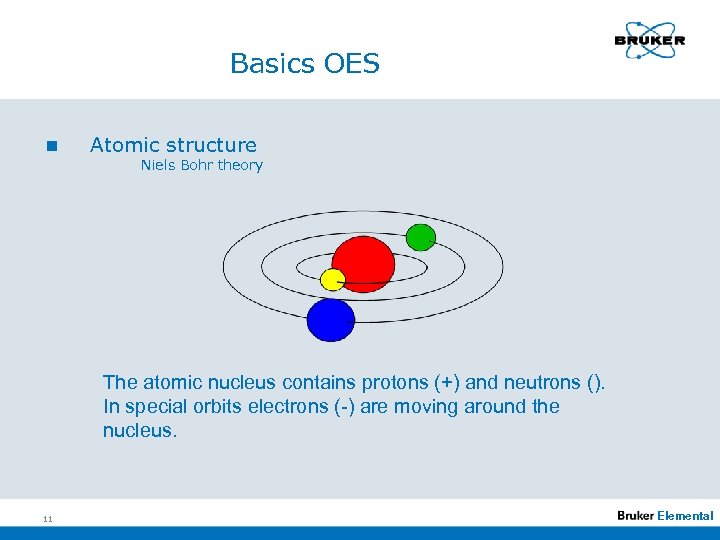
Basics OES n Atomic structure Niels Bohr theory The atomic nucleus contains protons (+) and neutrons (). In special orbits electrons (-) are moving around the nucleus. 11 Elemental
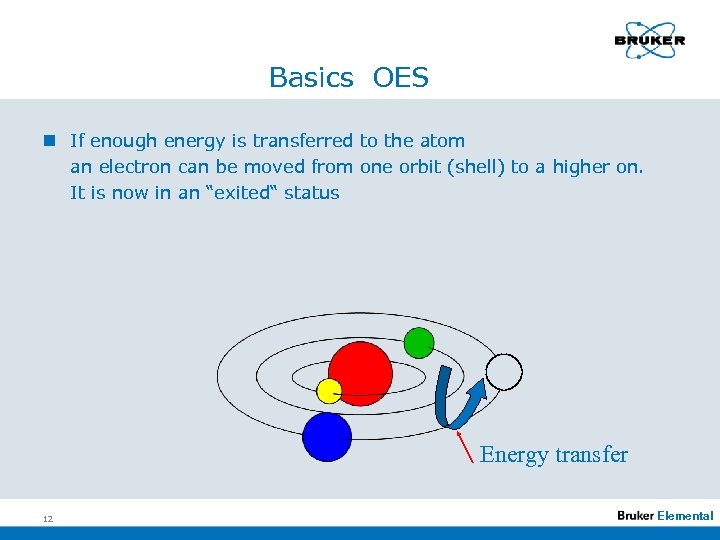
Basics OES n If enough energy is transferred to the atom an electron can be moved from one orbit (shell) to a higher on. It is now in an “exited“ status Energy transfer 12 Elemental
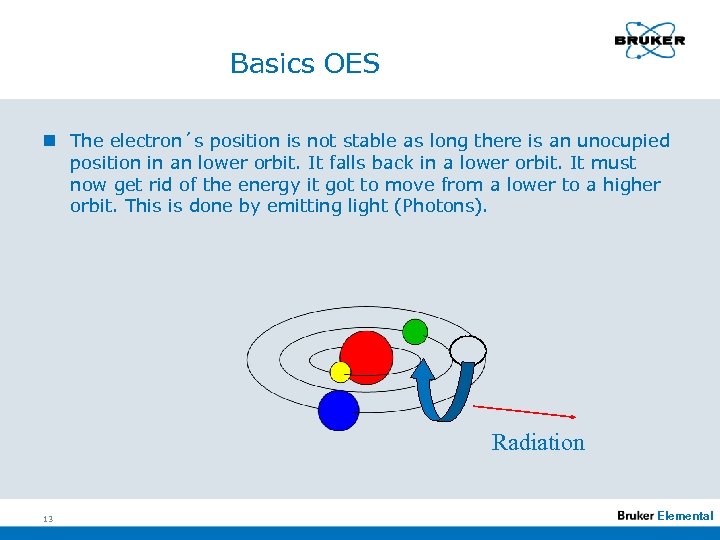
Basics OES n The electron´s position is not stable as long there is an unocupied position in an lower orbit. It falls back in a lower orbit. It must now get rid of the energy it got to move from a lower to a higher orbit. This is done by emitting light (Photons). Radiation 13 Elemental

Basics OES n Wavelenghts and -ranges n Units 1 nm 1Å = = 10 -9 m 10 -10 m n Ranges Infrared range > 800 nm. . . Visible range: 400 -800 nm UV-range: 200 -400 nm VUV-range < 200 nm. . . 14 Elemental
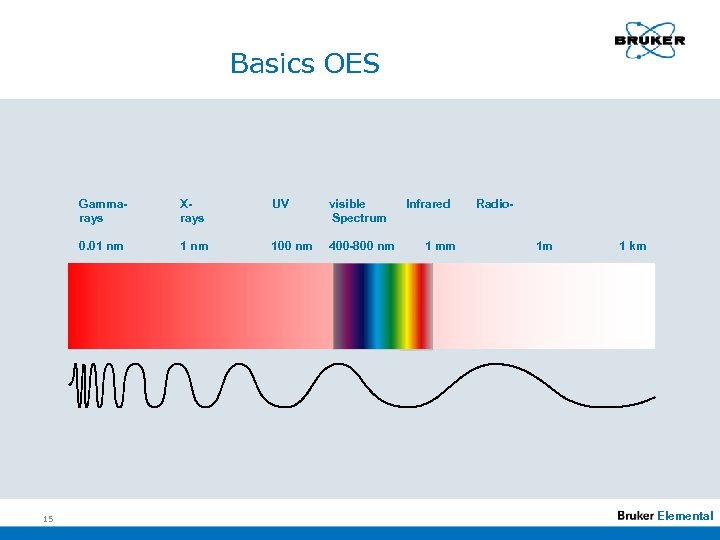
Basics OES Gammarays UV visible Spectrum 0. 01 nm 15 Xrays 1 nm 100 nm 400 -800 nm Infrared 1 mm Radio 1 m 1 km Elemental

Basics OES n Depending on the different possibilities of electron transfer between shells there are several specific wavelenght for an atom. n The OES uses the wavelength range 120 - 800 nm 16 Elemental

Basics OES Atomic lines and Ionic lines n Atomic lines • Exitation of electrons in neutral atoms n Ionic lines • Exitation of electrons in an ion (ionized Electron) (atom which lost one or more electrons) 17 Elemental

Instrumentation 18 Elemental

Video Automatic system with grinding 19 Elemental
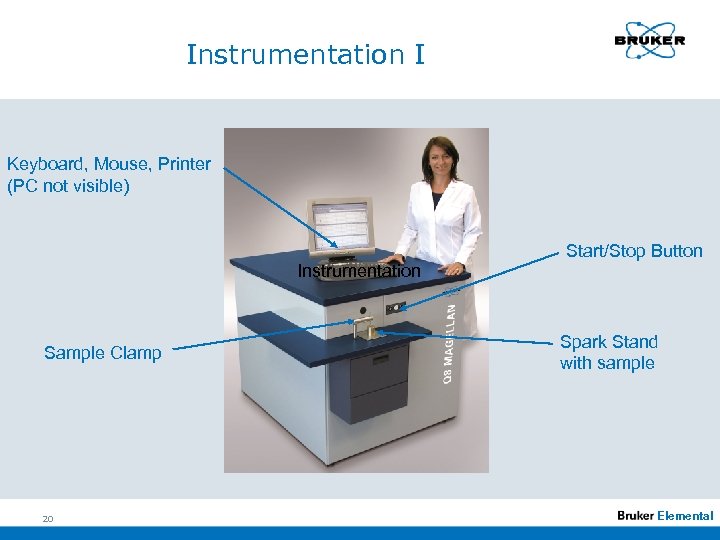
Instrumentation I Keyboard, Mouse, Printer (PC not visible) Instrumentation Sample Clamp 20 Start/Stop Button Spark Stand with sample Elemental
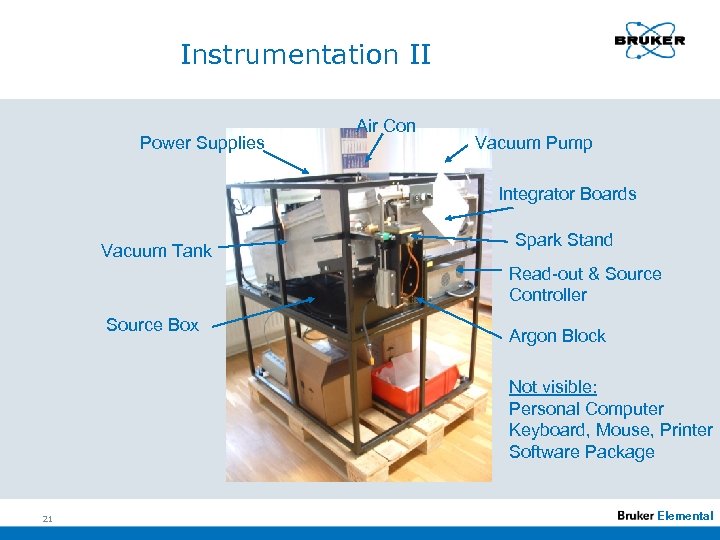
Instrumentation II Power Supplies Air Con Vacuum Pump Integrator Boards Vacuum Tank Spark Stand Read-out & Source Controller Source Box Argon Block Not visible: Personal Computer Keyboard, Mouse, Printer Software Package 21 Elemental
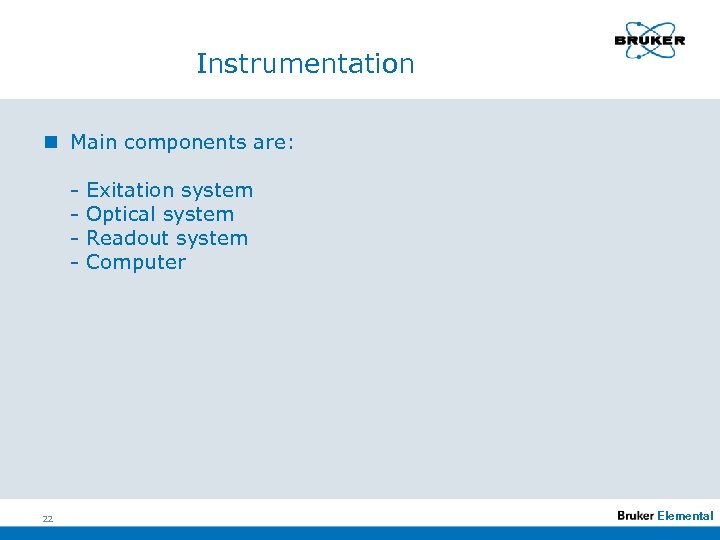
Instrumentation n Main components are: - 22 Exitation system Optical system Readout system Computer Elemental
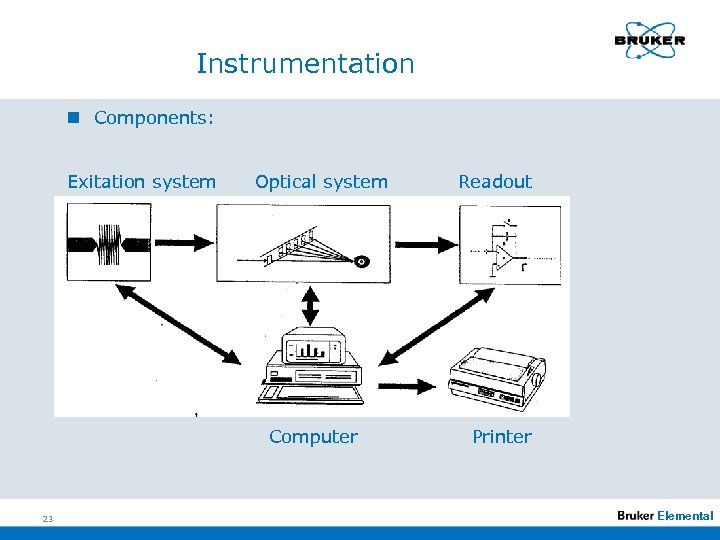
Instrumentation n Components: Exitation system Optical system Computer 23 Readout Printer Elemental
Instrumentation n Exitation system: - Between electrode and sample surface an electrical discharge is established - Material is being evapourated, partly atomized or ionized. - Atoms and ions are exited 24 Elemental
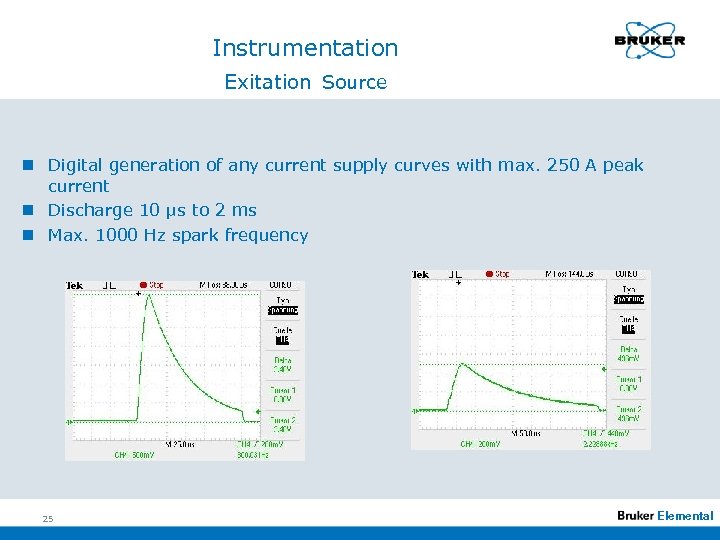
Instrumentation Exitation Source n Digital generation of any current supply curves with max. 250 A peak current n Discharge 10 µs to 2 ms n Max. 1000 Hz spark frequency 25 Elemental

Instrumentation n Optical System: - The exited light from the exitation source is transfered into the optical system - It is dispersed into the wavelengths contained in the exited light - The intensity of the atom dependend wavelenght is measured. 26 Elemental
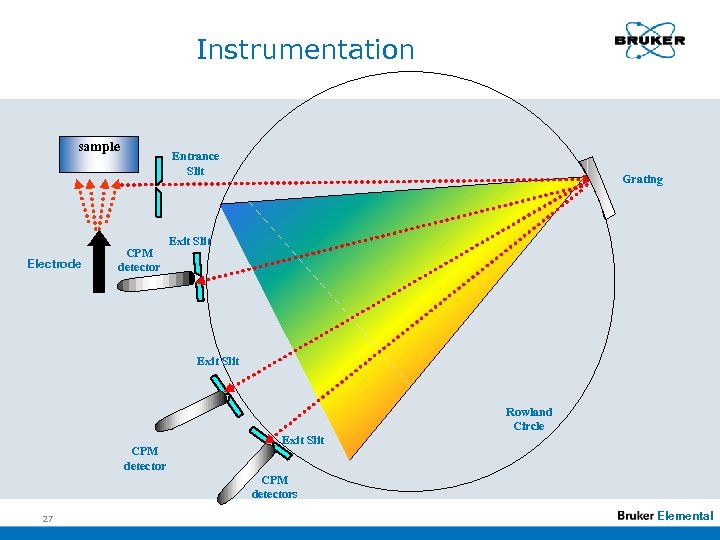
Instrumentation sample Electrode Entrance Slit CPM detector Grating Exit Slit Rowland Circle CPM detector Exit Slit CPM detectors 27 Elemental
Instrumentation OPTIC 28 Elemental

Instrumentation n Optical System: Grating and slits are mounted on a circle (Rowland circle), which diameter equals the concave radius of the grating. The spectral lines are images of the entrace slit on the position of a specific wavelength. They exist exactly on the Rowland circle. (Paschen Runge mounting) 29 Elemental
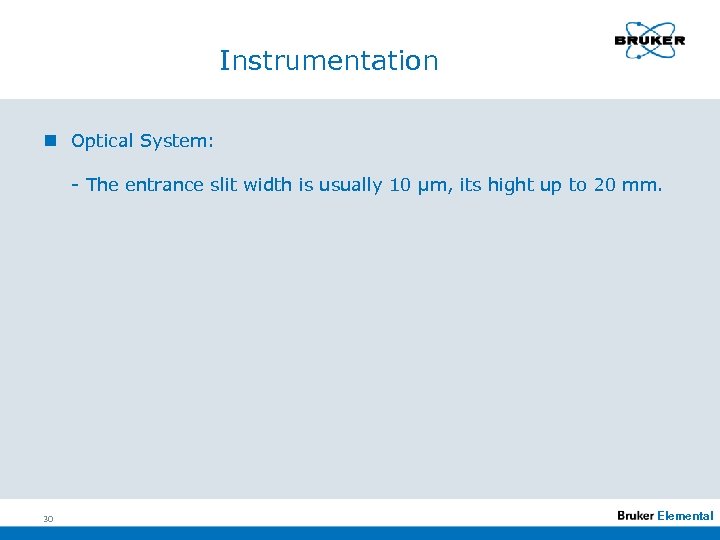
Instrumentation n Optical System: - The entrance slit width is usually 10 µm, its hight up to 20 mm. 30 Elemental
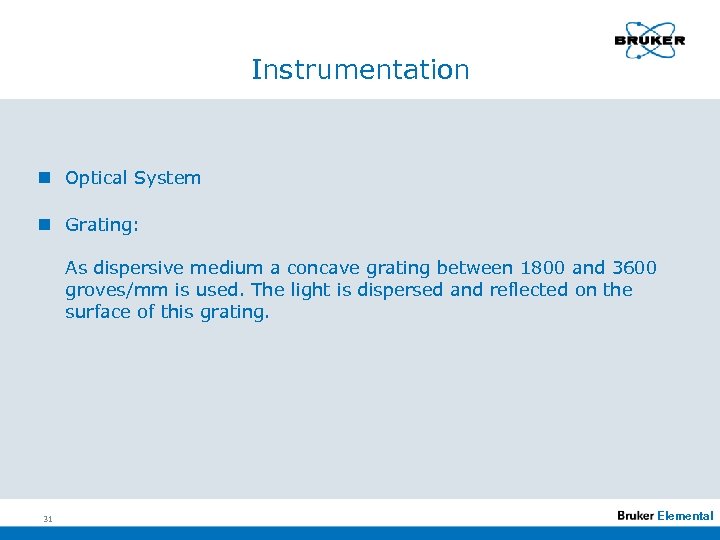
Instrumentation n Optical System n Grating: As dispersive medium a concave grating between 1800 and 3600 groves/mm is used. The light is dispersed and reflected on the surface of this grating. 31 Elemental
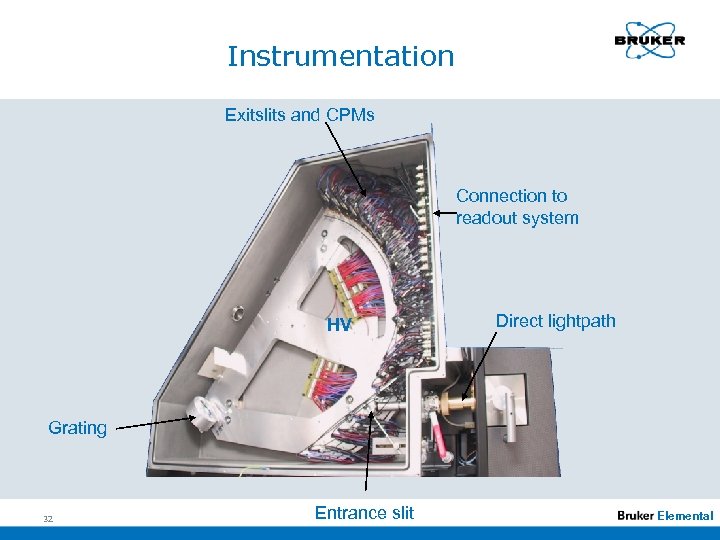
Instrumentation Exitslits and CPMs Connection to readout system HV Direct lightpath Grating 32 Entrance slit Elemental

Instrumentation n n n n 33 Channel - Photomultiplier (CPM) Since 1995 on the market Developed and produced in Germany Compact High sensitivity High dynamic range Extrem low dark current High amplification Wavelenght coverage: 110 -850 nm Elemental
Instrumentation n Photomultiplier: n CCD Detector (Charged Coupled Device) Both detectors convert light into an electrical signal (current). 34 Elemental
CCD (Charged-Couple-Device) n CCD detectors known from scanners and bar code readers or Cameras n Function based on semiconductor Technology n Cheap detector n Developed in early 1970‘s 35 Elemental
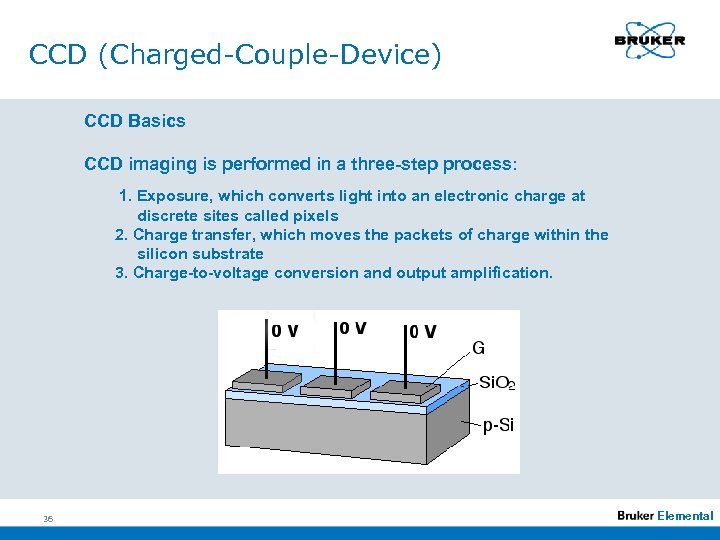
CCD (Charged-Couple-Device) CCD Basics CCD imaging is performed in a three-step process: 1. Exposure, which converts light into an electronic charge at discrete sites called pixels 2. Charge transfer, which moves the packets of charge within the silicon substrate 3. Charge-to-voltage conversion and output amplification. 36 Elemental
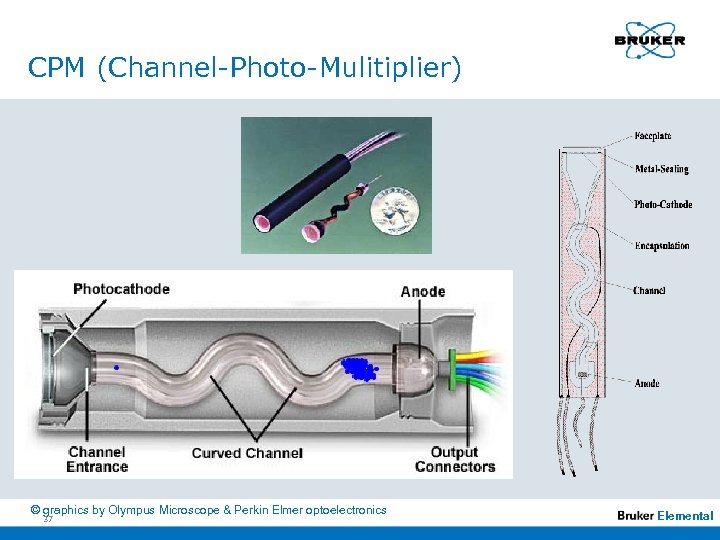
CPM (Channel-Photo-Mulitiplier) © graphics by Olympus Microscope & Perkin Elmer optoelectronics 37 Elemental
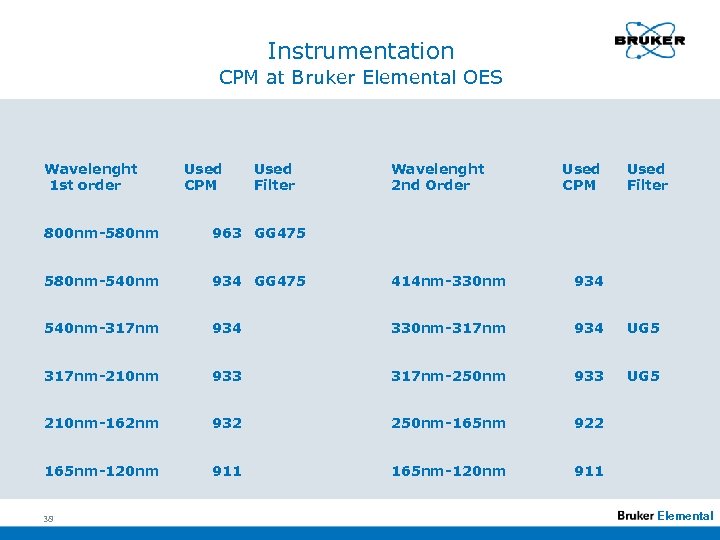
Instrumentation CPM at Bruker Elemental OES Wavelenght 1 st order Used CPM Used Filter Wavelenght 2 nd Order Used CPM Used Filter 800 nm-580 nm 963 GG 475 580 nm-540 nm 934 GG 475 414 nm-330 nm 934 540 nm-317 nm 934 330 nm-317 nm 934 UG 5 317 nm-210 nm 933 317 nm-250 nm 933 UG 5 210 nm-162 nm 932 250 nm-165 nm 922 165 nm-120 nm 911 38 Elemental
Instrumentation Readout n Developed by Bruker (Quantron) and Perkin Elmer n Optimized on CPM detectors n Frequency up to 250 k. Hz n Single Spark Evaluation (only with CPM) n Time Resolved Spectroscopy with up to 4 windows in any source parameter (only with CPM) 39 Elemental
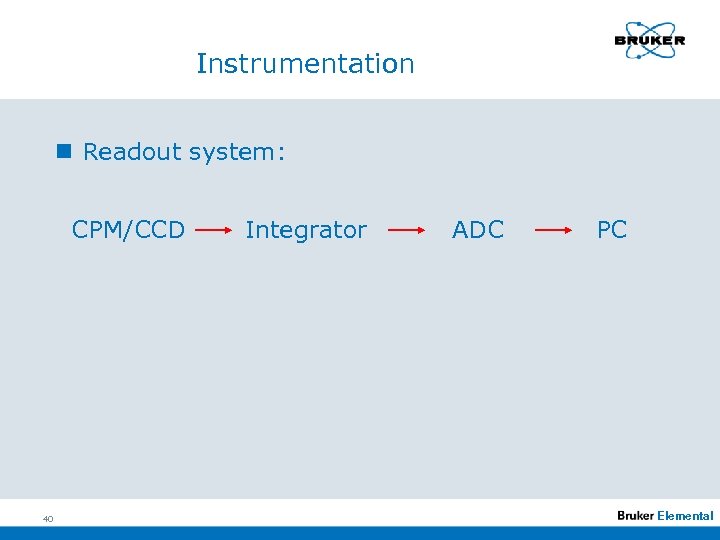
Instrumentation n Readout system: CPM/CCD 40 Integrator ADC PC Elemental
Instrumentation n Instrument to measure intensities of light - up to now the described instrument is able to measure intensities of light emitted by the source system, dispersed by the optical system and measured via the sensors by the readout system. - It is now an “Instrument to measure intensities of light“ 41 Elemental

Calibration 42 Elemental
Calibration An “Instrument to measure intensities of light“ only by calibration becomes an analytical instrument to analyze concentrations of Elements in an sample. 43 Elemental
Calibration n The intensity of light related to an element is proportional to the concentration of the element in the sample. n The calibration is established by using calibration samples with known concentration of elements inside. n The analysis of unknown samples is related to the calibration with the calibration samples. The method is a relative one. 44 Elemental
Calibration n Calibration samples should have the following properties: - The composition should be similar to the unknown sample(s) - They should be homogeneous - The concentration should be as “true“ as possible. This is the case when using CRMs (certified reference material) 45 Elemental

Calibration CRM: The composition is of such a sample is analyzed by 5 or more independent laboratories The manufacturer uses an international approved statistical procedure to calculate the best average and the deviation of this interlaboratory results. A certificate is part of the sample which describes all procedures used and the results. 46 Elemental
Calibration n With CRMs and possibly customer samples (secondary standards or RM) the instrument is calibrated. n For different elements different wavelenght are selected. n Rule: - for low concentrations a sensitive line is selected - for high concentrations a less sensitive line is used 47 Elemental
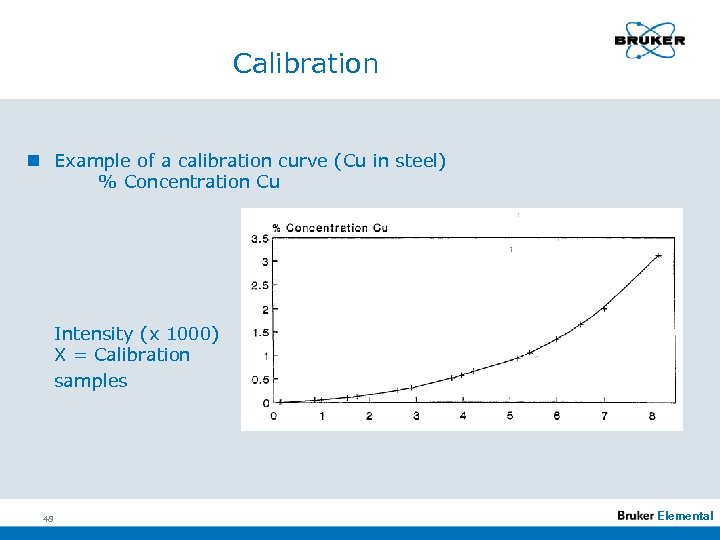
Calibration n Example of a calibration curve (Cu in steel) % Concentration Cu Intensity (x 1000) X = Calibration samples 48 Elemental

Calibration n From measuring intensities to display the concentrations in %(weight) there are several steps of calculation. This steps are explained next: 49 Elemental
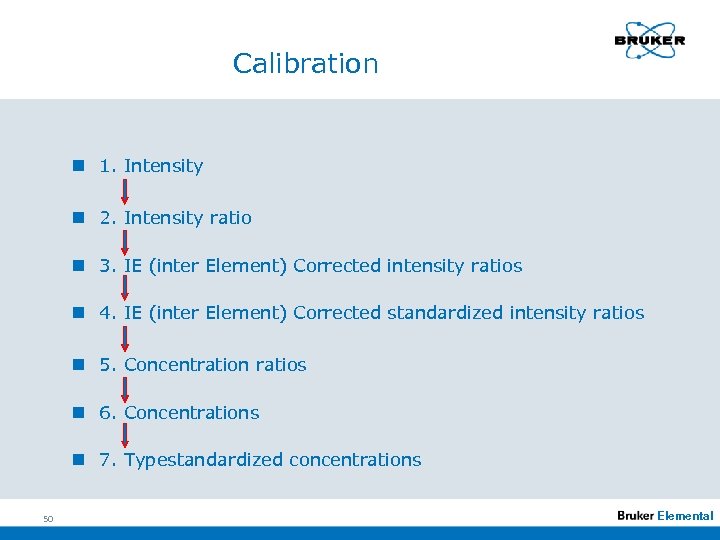
Calibration n 1. Intensity n 2. Intensity ratio n 3. IE (inter Element) Corrected intensity ratios n 4. IE (inter Element) Corrected standardized intensity ratios n 5. Concentration ratios n 6. Concentrations n 7. Typestandardized concentrations 50 Elemental
Calibration - Intensity ratio - The intensity of a spectral line is divided by the intensity of the „matrix element“. The matrix element is the element which is 50% or more in the sample. In steel its Fe. The intensity of the matrix element is called reference intensity. 51 Elemental
Calibration - Intensity ratio - n Why are ratios used? The rationing compensates changings of the status of the instrument during time. This changes are caused by: - Changes of the excitation system (i. e. change in the sample composition) - Pollution by condensate in spark stand - Pollution of optical components (windows, lenses etc. ) 52 Elemental
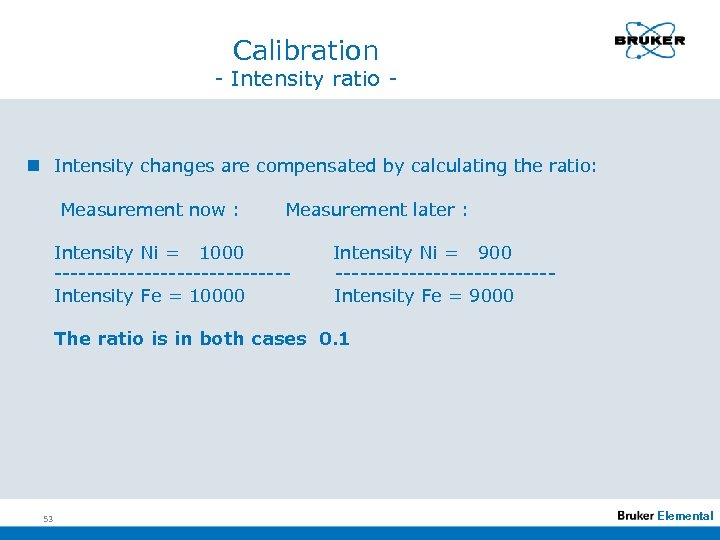
Calibration - Intensity ratio - n Intensity changes are compensated by calculating the ratio: Measurement now : Measurement later : Intensity Ni = 1000 --------------Intensity Fe = 10000 Intensity Ni = 900 -------------Intensity Fe = 9000 The ratio is in both cases 0. 1 53 Elemental
Calibration - Intensity ratios - n Intensity ratios : - The intensity ratio is multiplied by a so called “typical value“ to get numbers which are looking like intensities and not like concentrations. It is just a “cosmetic“ procedure. - The typical value is usually the intensity of the reference element line running the “pure sample“, that means pure Fe in steel matrix. 54 Elemental
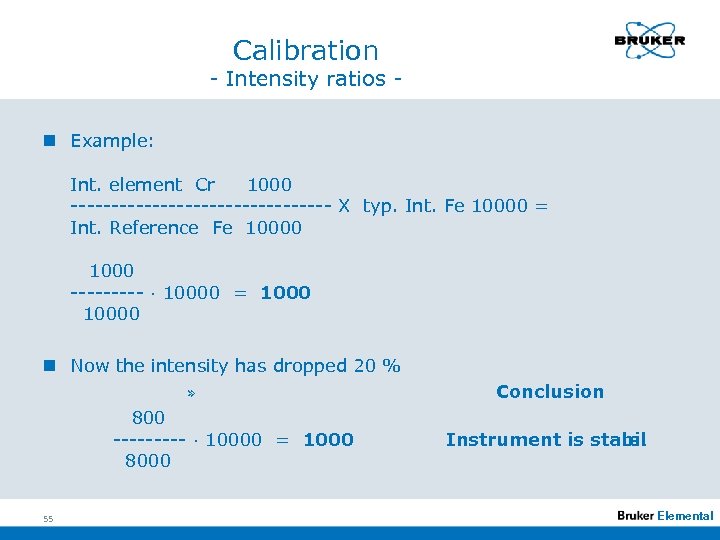
Calibration - Intensity ratios n Example: Int. element Cr 1000 ---------------- X typ. Int. Fe 10000 = Int. Reference Fe 10000 1000 ----- · 10000 = 10000 n Now the intensity has dropped 20 % » 800 ----- · 10000 = 1000 8000 55 Conclusion Instrument is stabil e! Elemental
Calibration n Corrected intensity ratio : So called additive and multiplicative corrections are done to the ratios: - Additive interferences caused by line interference - Multiplicative interferences caused by matrix effects n WHATS THAT? ? 56 Elemental

Calibration n Additive interference: The line of an other than the considert element is so close that it adds a part of ist intensity to the intensity considert. By carefull selection of the lines this can be reduced but never eliminated. n WHATS THAT? 57 Elemental
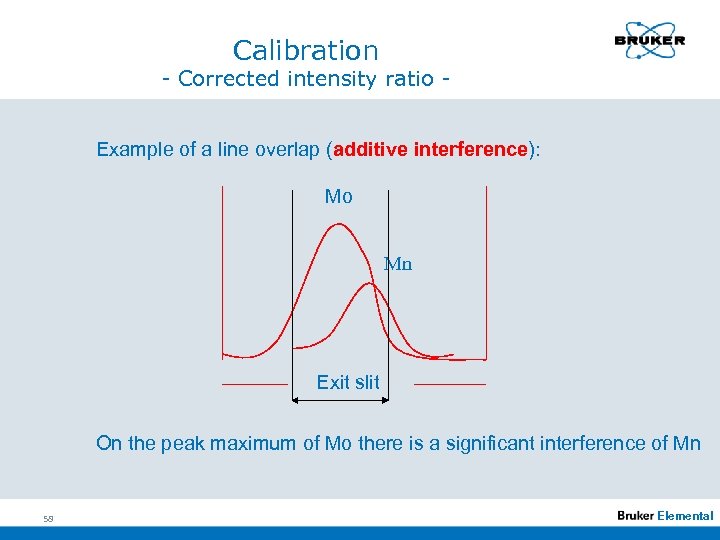
Calibration - Corrected intensity ratio Example of a line overlap (additive interference): Mo Mn Exit slit On the peak maximum of Mo there is a significant interference of Mn 58 Elemental
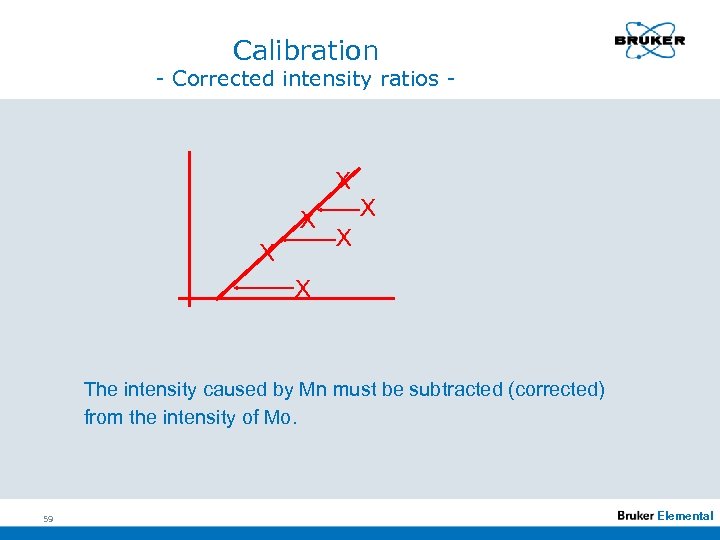
Calibration - Corrected intensity ratios - X X X The intensity caused by Mn must be subtracted (corrected) from the intensity of Mo. 59 Elemental
Calibration - Corrected intensity ratio n Multiplicative interference: Interference caused by physical and chemical properties of the sample which influences the discharged plasma. 60 Elemental
Calibration n Corrected standardized intensity ratio: During “standardisation“ the actual measured intensity ratios (actual values) are transformed by mathematical calculations into those measured during calibration (desired values). 61 Elemental
Calibration - Corrected standardized intensity ratios - n Why standardizing? Every spectrometer shows changing in the intensities with the time. This changes have the same reason why ratioing is neccessary: - Changes of the exitation system (i. e. change in the sample composition) - Pollution by condensate in spark stand - Pollution of optical components (windows, lenses etc. ) To be able to use the original calibration curves after those changes standardizing is neccessary. 62 Elemental
Calibration - Corrected intensity ratio - n For every calibration curve a sample with low concentration (low sample) and one with high element concentration (high sample) is selected. n This samples are measured during the calibration and the intensity ratios are stored as desired values. n Performing a standardisation later, the measured intensity ratios (actual values) are compared with the desired ones and a transformation equation is calculated. 63 Elemental
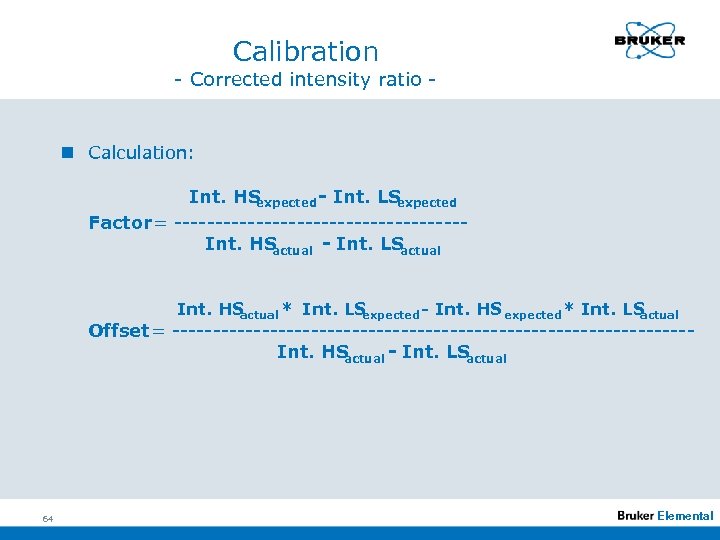
Calibration - Corrected intensity ratio - n Calculation: Int. HS expected - Int. LS expected Factor = ------------------Int. HS actual - Int. LS actual Int. HS actual * Int. LS expected - Int. HS expected * Int. LS actual Offset = --------------------------------Int. HS actual - Int. LS actual 64 Elemental
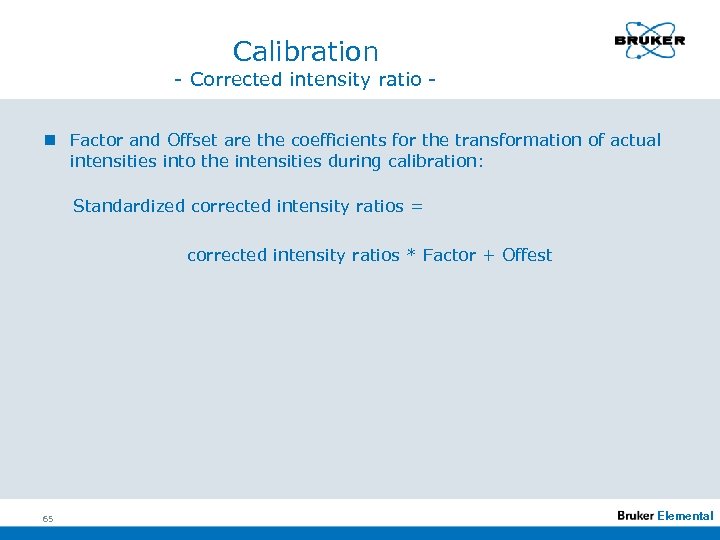
Calibration - Corrected intensity ratio n Factor and Offset are the coefficients for the transformation of actual intensities into the intensities during calibration: Standardized corrected intensity ratios = corrected intensity ratios * Factor + Offest 65 Elemental

Calibration n Concentration ratio: - Since the calibration is done using concentration ratios instead of concentrations the first result using the calibration curve is concentration ratio. - It is calculated: % Element -------- · 100 % Matrix 66 Elemental
Calibration n Concentration ratio: - The concentration of the matrix element is calculates as 100% - Sum(% elements) - To calculate the matrix concentration it is neccessary that almost all elements are analyzed by the instrument 67 Elemental
Calibration - Concentration n Concentration: After calculating the matrix concentration the software calculates each element concentration interactively for its concentration ratio. n Now the final CONCENTRATION is displayed 68 Elemental

Example on instruments Q 2 ION Q 4 TASMAN Q 8 MAGELLAN Q 4 MOBILE 69 Elemental

Automation, possible configurations. 70 Elemental

Future? Inclusion Analysis / Steel Cleanliness Determination by Spark OES Characterization of inclusions in steel by OES Pulse Discrimination Analysis (OES-PDA) 71 Elemental

Reference Method for Inclusion Analysis: SEM/EDS with Bruker Quantax 400 EDS Scanning electron microscope with energy dispersive x-ray spectroscopy Universal method: differentiation of carbides, oxides, nitrides, sulfides Large observation area Imaging method Highest accuracy Surface method, low penetration depth (~1µm) Costly, long measurement time (~310 h) Highly educated operating staff 72 Elemental
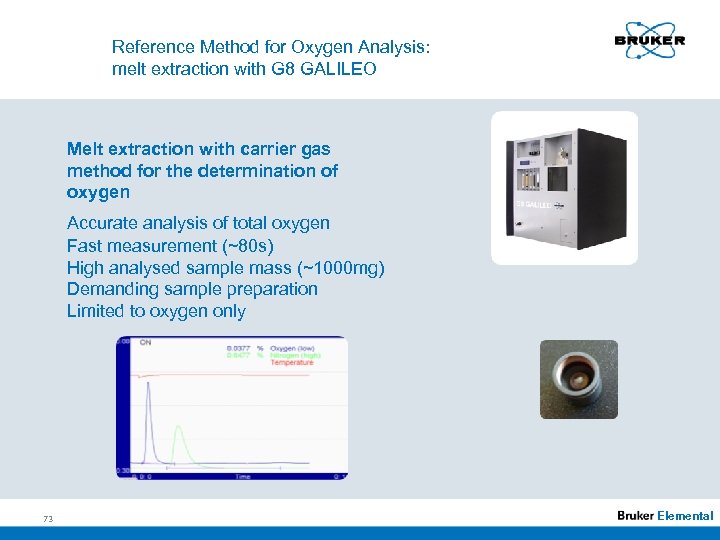
Reference Method for Oxygen Analysis: melt extraction with G 8 GALILEO Melt extraction with carrier gas method for the determination of oxygen Accurate analysis of total oxygen Fast measurement (~80 s) High analysed sample mass (~1000 mg) Demanding sample preparation Limited to oxygen only 73 Elemental
Rapid Method for Inclusions & Oxygen: OES-PDA = MCI = Metal Cleanliness Inspection Inclusion characterization & oxygen determination by Optical Emission Spectrometry with Pulse Discrimination Analysis Complete elemental analysis Determination of various oxide and sulfide inclusions Calculation of total oxygen Simple sample preparation (grinding w/ Si. C paper or milling) Fast measurement (~5 s/burn, multiple burns recommended, e. g. 5 x) User-friendly software for „normal“ OES operator Feasibility study advisable 74 Elemental
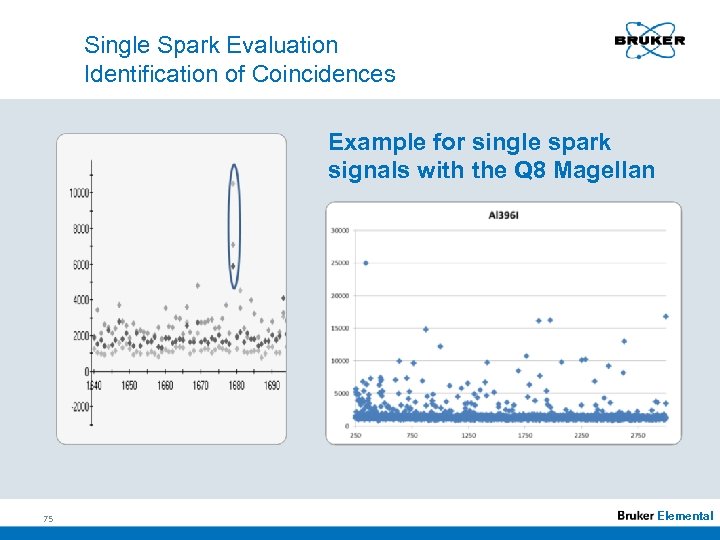
Single Spark Evaluation Identification of Coincidences Example for single spark signals with the Q 8 Magellan 75 Elemental
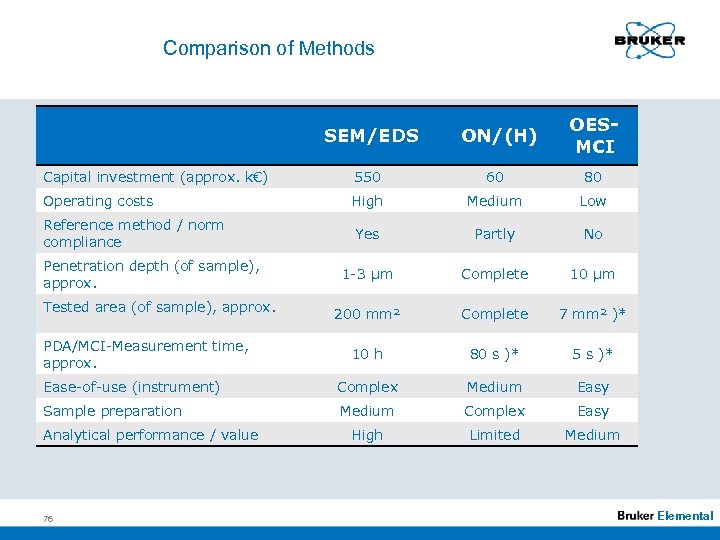
Comparison of Methods SEM/EDS ON/(H) OESMCI Capital investment (approx. k€) 550 60 80 Operating costs High Medium Low Reference method / norm compliance Yes Partly No 1 -3 µm Complete 10 µm 200 mm² Complete 7 mm² )* 10 h 80 s )* 5 s )* Ease-of-use (instrument) Complex Medium Easy Sample preparation Medium Complex Easy High Limited Medium Penetration depth (of sample), approx. Tested area (of sample), approx. PDA/MCI-Measurement time, approx. Analytical performance / value 76 Elemental

Thank you very much for your attention! 77 Elemental

www. bruker-elemental. com 78 Elemental
3deb86f2508551c2156ed487aec068cb.ppt