d471139ac36950cc18ec343eb3cdb19d.ppt
- Количество слайдов: 12

OCTOBER 27, 2015 • Objective: Demonstrate how the conservation of atoms in chemical reactions leads to the ability to calculate the mass of products and reactants using the mole concept. • Do Now: Brainstorm various counting units that can be used to group different items, or different groups of people. Record your ideas on the following table in your notebook: Counting unit Number of units Ex: Dozen 12

MOLES 3. 4

COUNTING UNITS • We buy things by the dozen • One dozen = 12 items • We use the unit mole to measure things in chemistry.

MOLES • The SI base unit that describes the amount of a substance.
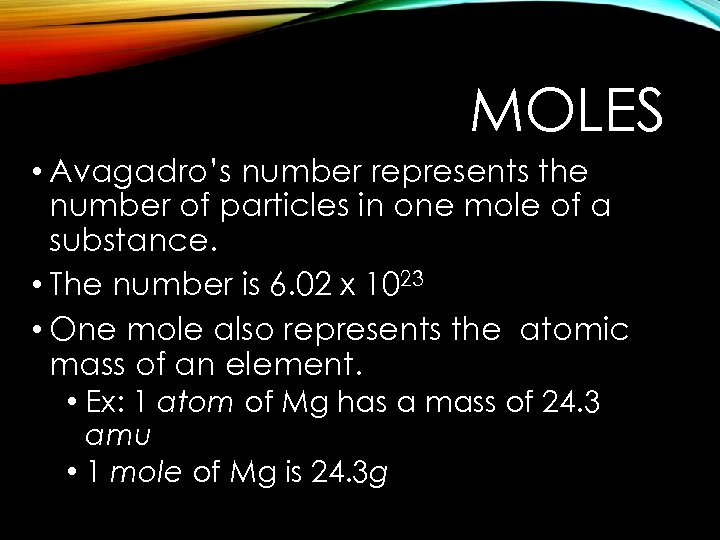
MOLES • Avagadro’s number represents the number of particles in one mole of a substance. • The number is 6. 02 x 1023 • One mole also represents the atomic mass of an element. • Ex: 1 atom of Mg has a mass of 24. 3 amu • 1 mole of Mg is 24. 3 g

CONVERSION FACTORS • Get into groups of 4. • Brainstorm units of measurement for your area of measurements. • Write each unit on a card. • Then, shuffle the cards and pick 2. Come up with a way to get from the Left unit to the Right unit. • 10 minutes. Lets see A)How many units, and B) how many conversion factors each group can get!

MOLAR MASS • Using the periodic chart you can determine the molar mass of any element. • It will equal the atomic mass of the element. • Example • 40 Ca has an atomic mass of 40 as well as a molar mass of 40. Therefore one mole of Ca equals 40 g. • Two moles would equal 80 g. 20

MOLES • You can determine how many moles of a substance you have if you know the mass of the substance • Example • If you have 92 grams of Na how many moles would you have.

SOLUTION • You divide the mass by the molar mass of the element. • 92 g/23 = 4 moles
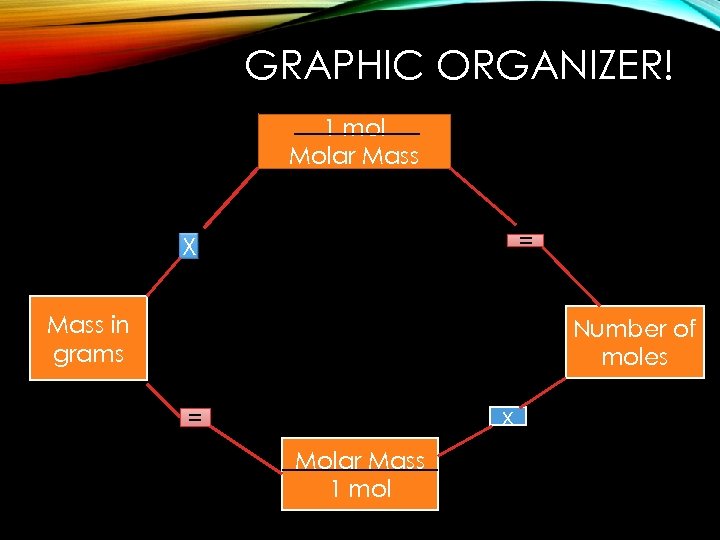
GRAPHIC ORGANIZER! 1 mol Molar Mass = X Mass in grams Number of moles x = Molar Mass 1 mol

PRACTICE! • What is the mass, in grams, of each of the following? • 2. 50 mol of Sulfur • 1. 80 mol of calcium • 0. 50 mol of carbon

SIGNIFICANT FIGURES (SIG FIGS) • Do Now: Determine how many Sig Figs are in the following: a) 1. 638 b) 900. 06 c) 0. 0004 d) 8. 1000 e) 0. 0230
d471139ac36950cc18ec343eb3cdb19d.ppt