cb7a0394d82b6eb294564d78c53b7719.ppt
- Количество слайдов: 11

ÖBIG Pharmaceutical Pricing & Reimbursement Information List of reimbursable pharmaceuticals PPRI Project Christine Leopold Gesundheit Österreich Gmb. H PPRI Conference Warsaw, 29 October 2007 Warsaw 29 October 2007 1
Pharmaceutical Pricing & Reimbursement Information ÖBIG Type of reimbursement - eligibility criteria Ø Product-specific Ø most common (e. g. AT, EL, IT, SK, SI) Ø Example Austria Ø main criterion is the product Ø assessment of the product by pharmacological evaluation, medical-therapeutic evaluation, health-economic evaluation Ø Disease-specific Ø Baltic states, sub-scheme in some countries (BG, CZ, FR, IE) Ø Example Latvia Ø Main criterion is the underlying disease which shall be treated Ø „List of reimbursable diseases“ Ø Same pharmaceuticals may be reimbursed at different reimbursement rates for the treatment of different diseases Warsaw 29 October 2007 2

Pharmaceutical Pricing & Reimbursement Information ÖBIG Type of reimbursement - eligibility criteria Ø Patient-group-specific Ø CY, IE, MT; TR Ø Example Malta and Cyprus Ø Specific population groups are eligible for reimbursement e. g. children, elderly person, handicapped persons or low income population. Ø Non-eligible persons have to buy health services and pharmaceuticals out-of-pocket Ø Consumption-based Ø DK and SE Ø Example DK Ø Level of reimbursement depends on the expenses for pharmaceuticals of a patient within a certain period of time (e. g. a year) Ø Patient pays the full cost of his/her reimbursable medication up to a threshold of about € 64. per a 12 month period. After passing this threshold, the reimbursement rate rises gradually Warsaw 29 October 2007 3
Pharmaceutical Pricing & Reimbursement Information ÖBIG Pricing of reimbursable pharmaceuticals – pricing procedures Pricing procedures Ø External price referencing Ø Internal price referencing Ø Cost-plus Ø Others (e. g. indirect price control) See PPRI Glossary, http: //ppri. oebig. at Warsaw 29 October 2007 4
Pharmaceutical Pricing & Reimbursement Information ÖBIG Pricing procedures Methodology to determine a price Ø External price referencing (international price comparisons) Ø Used in nearly all Member States Ø For at least a range of pharmaceuticals Focus on a few countries (exceptions: AT, BE), mostly MS/neighbouring countries Ø Framework Ø Relevance Ø Methodological issues Warsaw 29 October 2007 5
Pharmaceutical Pricing & Reimbursement Information ÖBIG Pricing procedures Methodology to determine a price Ø Internal price referencing Ø often in connection with reimbursement Ø Cost-plus Ø For locally-produced pharmaceuticals (CZ, CY, EL, SK, exceptionally - UK) Ø Others Ø Indirect price control - PPRS (UK) Ø Agreed prices - Price competition (SK) Warsaw 29 October 2007 6

ÖBIG Pharmaceutical Pricing & Reimbursement Information Reimbursement lists Ø Define which pharmaceuticals are considered as reimbursable Ø Positive lists Ø in all EU Member States (exemption EL) Ø Negative lists Ø seldom used (FI - not implemented; ES and UK) Ø Updating of reimbursement lists Ø Annually Ø Quarterly Ø Every three months Ø As soon as a new pharmaceutical enters the market Warsaw 29 October 2007 7
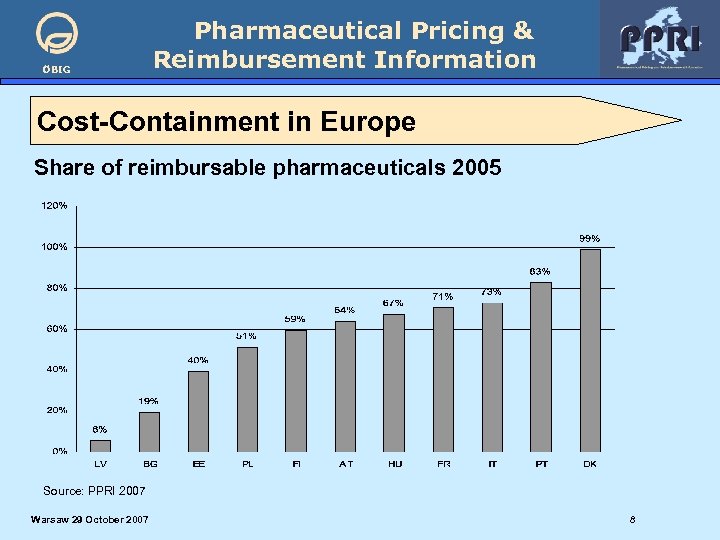
ÖBIG Pharmaceutical Pricing & Reimbursement Information Cost-Containment in Europe Share of reimbursable pharmaceuticals 2005 Source: PPRI 2007 Warsaw 29 October 2007 8
Pharmaceutical Pricing & Reimbursement Information ÖBIG What does reimbursable mean? Ø All pharmaceuticals on the positive list(s) are 100% reimbursed Ø only in AT, IE, MT, NL, UK Ø Percentage reimbursement rates Ø according to severity of disease for which the pharmaceutical is used Ø Further co-payments (e. g. prescription fees) Ø Reference price systems Warsaw 29 October 2007 9
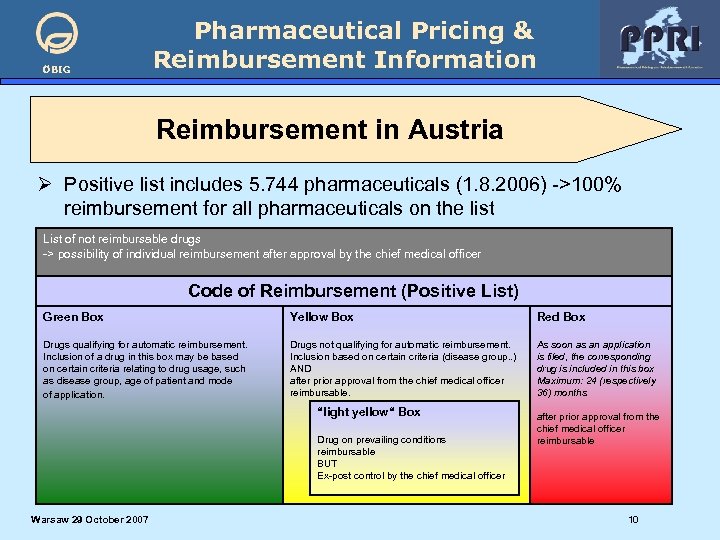
ÖBIG Pharmaceutical Pricing & Reimbursement Information Reimbursement in Austria Ø Positive list includes 5. 744 pharmaceuticals (1. 8. 2006) ->100% reimbursement for all pharmaceuticals on the list List of not reimbursable drugs -> possibility of individual reimbursement after approval by the chief medical officer Code of Reimbursement (Positive List) Green Box Yellow Box Red Box Drugs qualifying for automatic reimbursement. Inclusion of a drug in this box may be based on certain criteria relating to drug usage, such as disease group, age of patient and mode of application. Drugs not qualifying for automatic reimbursement. Inclusion based on certain criteria (disease group. . ) AND after prior approval from the chief medical officer reimbursable. As soon as an application is filed, the corresponding drug is included in this box Maximum: 24 (respectively 36) months “light yellow“ Box Drug on prevailing conditions reimbursable BUT Ex-post control by the chief medical officer Warsaw 29 October 2007 after prior approval from the chief medical officer reimbursable 10
Pharmaceutical Pricing & Reimbursement Information ÖBIG Reimbursement in Austria Ø Criteria Ø Product-specific criteria (Pharmacological evaluation, medical -therapeutic evaluation, health-economic evaluation) Ø Pricing procedure Ø External price refercening: EU average price Ø Updating of the list twice per year (1 January and 1 July) Ø Individual reimbursement Ø In case were no treatment alternative is available Ø Ex-ante or ex-post approval is necessary Ø On average 45. 000 prescriptions per month Warsaw 29 October 2007 11
cb7a0394d82b6eb294564d78c53b7719.ppt