
Obesity Suzan M. Mansour PH. D.

Obesity is a significant risk factor for major diseases including Type II diabetes, coronary heart disease, hypertension and certain forms of cancer. Obesity arises when energy intake, principally stored as triglycerides, exceeds energy expenditure. Obesity is a complex trait influenced by diet, developmental stage, physical activity and genes.
Obese Syndrome Components n n n n Glucose intolerance Insulin resistance Dyslipidemia Type 2 diabetes Hypertenesion Elevated plasma leptin concentration Increased visceral adipose tissue Increased risk of CHD & some cancers
Genetic predisposition is a key contributing factor in obesity as demonstrated by familial aggregation, twin and adoption studies. Estimates for the genetic basis of phenotypic variations in obesity range from approximately 40 to 70%. This matches or exceeds the accepted genetic contribution to height. The idea that genetic loci alter body fat genetic loci content has been substantiated by identification of mutations that cause lowor high-fat phenotypes in rodents and humans.
There is convincing experimental evidence showing that the balance between energy intake (food consumption) and energy expenditure (basal metabolic rate, i. e. biochemical processes required to maintain cellular viability, physical activity and adaptive thermogenesis) is tightly regulated. A homeostatic network: maintains energy stores Thus, genes that encode the molecular components of this system may underlie obesity and related disorders. through a complex interplay between the feeding regulatory centers in the CNS, particularly in the hypothalamus and the regulated storage and mobilization of fat stores.
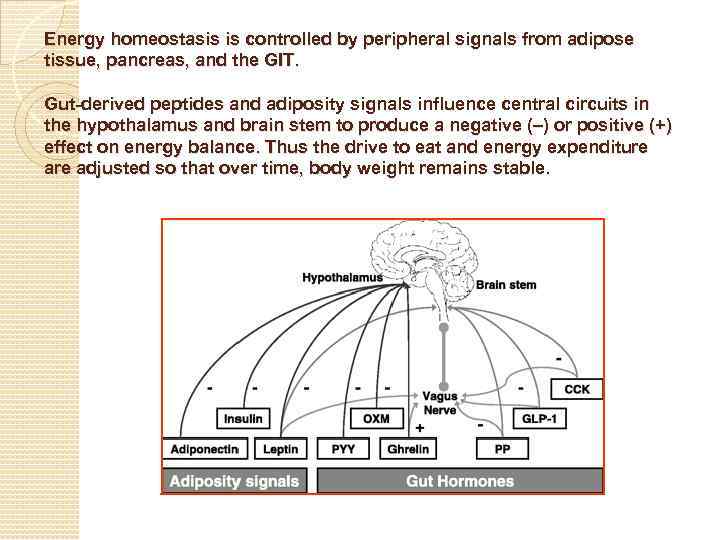
Energy homeostasis is controlled by peripheral signals from adipose tissue, pancreas, and the GIT. Gut-derived peptides and adiposity signals influence central circuits in the hypothalamus and brain stem to produce a negative (–) or positive (+) effect on energy balance. Thus the drive to eat and energy expenditure adjusted so that over time, body weight remains stable.

In mammals, white adipose tissue functions as the main depot for fuel storage. In the past decade, identification of protein signals secreted from this tissue has led to its recognition as a major endocrine organ. This was principally based on the discovery of leptin, a cytokine-like hormone secreted from white adipose tissue in proportion to fat mass. Activation of hypothalamic leptin receptors suppresses food intake and promotes energy expenditure pathways.

Insulin is another key afferent signal to the CNS that controls energy balance. Insulin is secreted from the endocrine pancreas in proportion to fat mass and exerts potent effects on peripheral nutrient storage. Similar to leptin, insulin causes long-term inhibitory effects on energy intake. There is cross talk between insulin and leptin signaling in a common set of hypothalamic neurons. Moreover, a series of neuropeptides (e. g. , the melanocortin system, neuropeptide Y) and neurotransmitters (e. g. , serotonin, dopamine and noradrenaline) function in the hypothalamus to coordinate behavioral, physiological and metabolic responses. Together, these responses maintain energy balance via both intake and expenditure pathways.
In addition to these long-term adiposity signals, short-term meal-related signals are transmitted to the CNS through afferent nerves or gut-secreted peptides (e. g. , cholecystokinin, ghrelin). Finally, neurons in the CNS also directly sense carbohydrate and fats.

- Insulin: Down-regulating either insulin pathway components promotes fat accumulation in adults. In mammals, insulin signaling has both peripheral and central actions on fat homeostasis. Importantly, tissue-specific knockouts or reconstitution of the insulin receptor in mice has begun to reveal contributions of different tissues to glucose and fat homeostasis. For instance, neuronal insulin receptor knockout and muscle insulin receptor knockout mice are obese while fat cell insulin receptor knockout mice are lean and resistant to diet induced obesity.
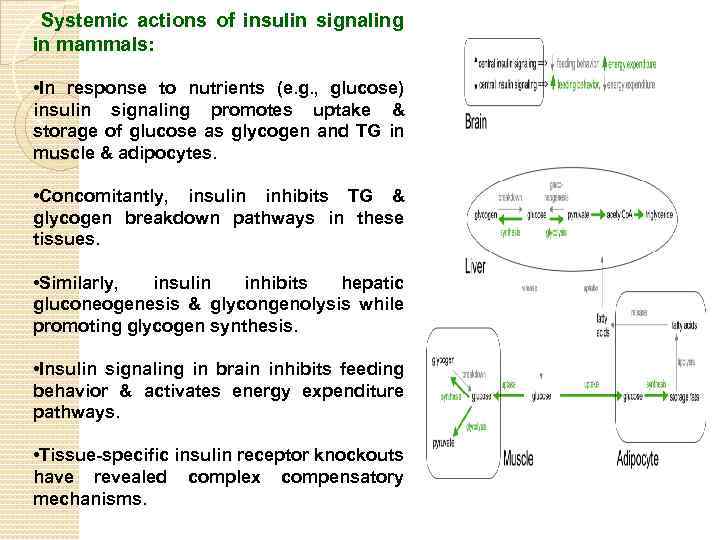
Systemic actions of insulin signaling in mammals: • In response to nutrients (e. g. , glucose) insulin signaling promotes uptake & storage of glucose as glycogen and TG in muscle & adipocytes. • Concomitantly, insulin inhibits TG & glycogen breakdown pathways in these tissues. • Similarly, insulin inhibits hepatic gluconeogenesis & glycongenolysis while promoting glycogen synthesis. • Insulin signaling in brain inhibits feeding behavior & activates energy expenditure pathways. • Tissue-specific insulin receptor knockouts have revealed complex compensatory mechanisms.

Leptin hormone: A peptide hormone which is coded for by the obesity gene. Influences the quantity of food consumed relative to the amount of energy expended. ◦ When leptin levels are high, appetite is reduced and energy expenditure is increased. Leptin has been found in gastric epithelium, placenta and adipose tissue ◦ Most abundant in white adipose tissue.

White Adipose Tissue (WAT) • Composed mainly of adipocytes (fat cells) – Store energy in the form of triglycerides in times of nutritional affluence – Release free fatty acids during nutritional deprivation • WAT mass is determined by the balance between energy intake and expenditure – This is influenced by genetic, neuroendocrine, and environmental factors • Under normal conditions this system is carefully regulated so that WAT mass remains constant and close to well defined ‘set point’ • Disruption of the steady state can lead to chronic decreases or increases in the quantity of WAT – Decreased amounts are associated with weight alterations during peroids of diet, malnutrition, eating disorders, etc – Increased amounts indicate obesity
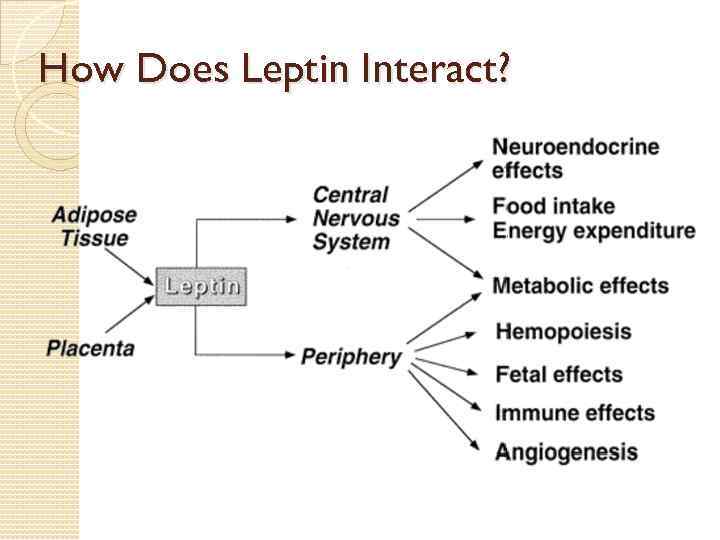
How Does Leptin Interact?

Regulating Food Intake and Energy Expenditure Leptin binds to its receptor which is expressed primarily in the brains hypothalamus region, which modulates food intake and energy expenditure. When low leptin levels are detected, the body is warned of limited energy supplies. If high leptin levels are detected, the hypothalamus senses the body as being overweight. ◦ This then trigger the body to eat less and expend more energy When energy intake and output are equal, leptin reflects the amount of triglyceride stored in the bodies adipose tissue.
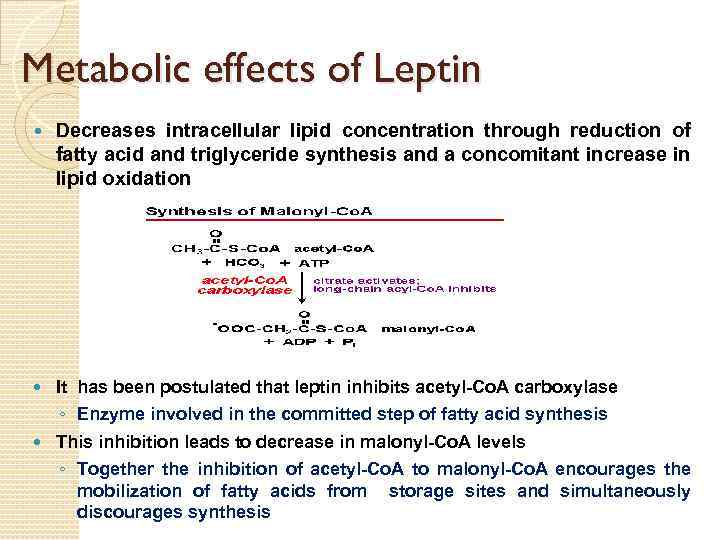
Metabolic effects of Leptin Decreases intracellular lipid concentration through reduction of fatty acid and triglyceride synthesis and a concomitant increase in lipid oxidation It has been postulated that leptin inhibits acetyl-Co. A carboxylase ◦ Enzyme involved in the committed step of fatty acid synthesis This inhibition leads to decrease in malonyl-Co. A levels ◦ Together the inhibition of acetyl-Co. A to malonyl-Co. A encourages the mobilization of fatty acids from storage sites and simultaneously discourages synthesis

Possible Reasons For Increased Leptin In Obese Individuals Differences in the fat production rate of leptin ◦ Some obese people may make leptin at greater rate to compensate for faulty signaling process or action. Resistance to leptin at its site of action ◦ If resistance is partial, not complete, more leptin may be required for action. A combination of both could influence eating behaviors and energy use to cause obesity. All these possibilities indicate that obese individuals are in a state of perceived starvation.
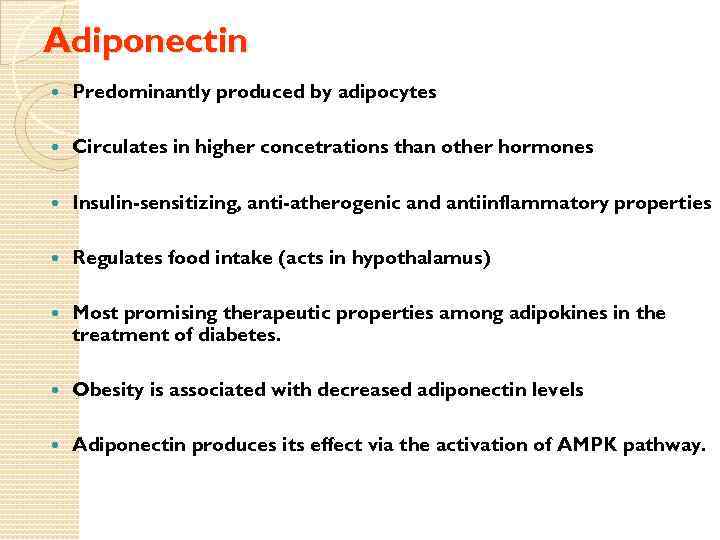
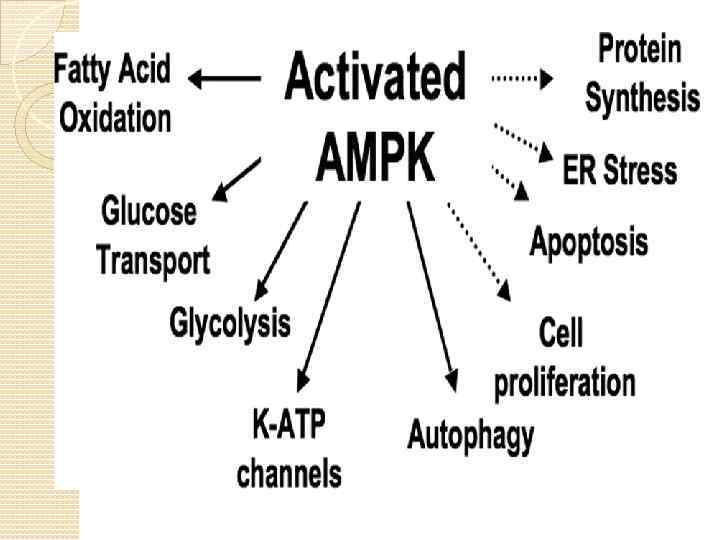
Adiponectin Predominantly produced by adipocytes Circulates in higher concetrations than other hormones Insulin-sensitizing, anti-atherogenic and antiinflammatory properties Regulates food intake (acts in hypothalamus) Most promising therapeutic properties among adipokines in the treatment of diabetes. Obesity is associated with decreased adiponectin levels Adiponectin produces its effect via the activation of AMPK pathway.
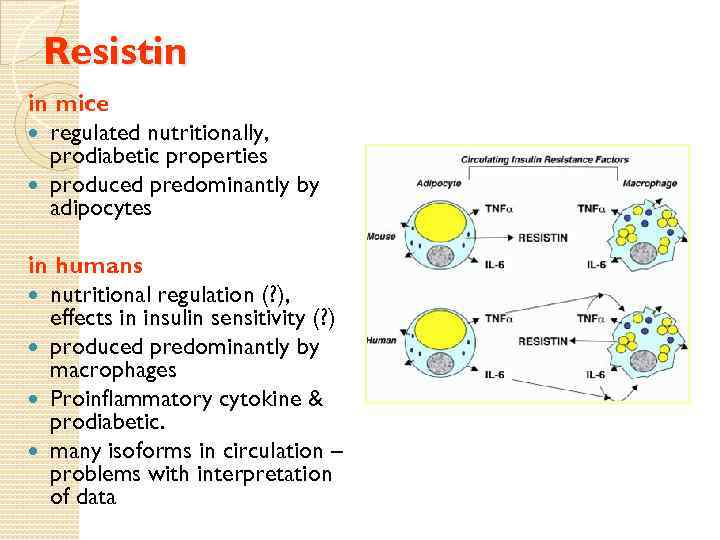
Resistin in mice regulated nutritionally, prodiabetic properties produced predominantly by adipocytes in humans nutritional regulation (? ), effects in insulin sensitivity (? ) produced predominantly by macrophages Proinflammatory cytokine & prodiabetic. many isoforms in circulation – problems with interpretation of data
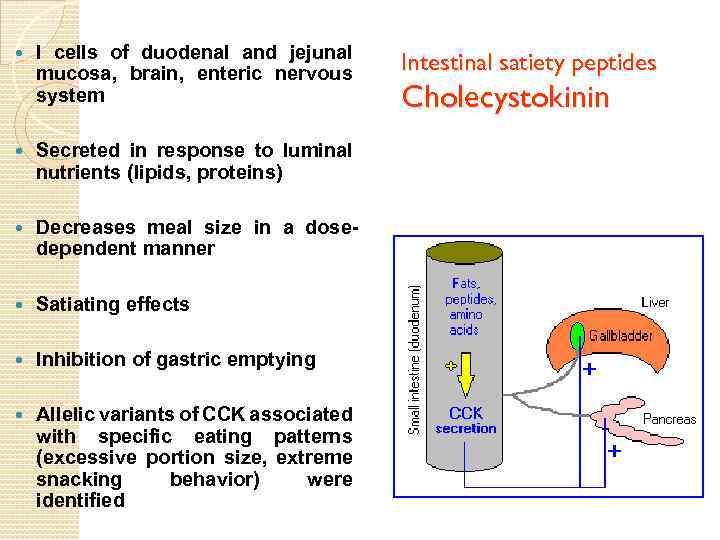
I cells of duodenal and jejunal mucosa, brain, enteric nervous system Secreted in response to luminal nutrients (lipids, proteins) Decreases meal size in a dosedependent manner Satiating effects Inhibition of gastric emptying Allelic variants of CCK associated with specific eating patterns (excessive portion size, extreme snacking behavior) were identified Intestinal satiety peptides Cholecystokinin

Intestinal satiety peptides Glucagon-like peptide 1 L cells of distal small intestine and colon Biphasic stimulation by ingested nutrients (lipids, carbohydrates) It is a potent antihyperglyceamic hormone. GLP-1 inhibits gastric secretion and motility. This delays and protracts carbohydrate absorption and contributes to a satiating effect. inhibits food intake and reduces body weight Modulator of the stress response related to taste aversion Potent insulinotropic and glucagonostatic hormone (improves insulin release, attenuates glucagon release, improves glucose disposal) It decreases food intake by increasing satiety in brain.
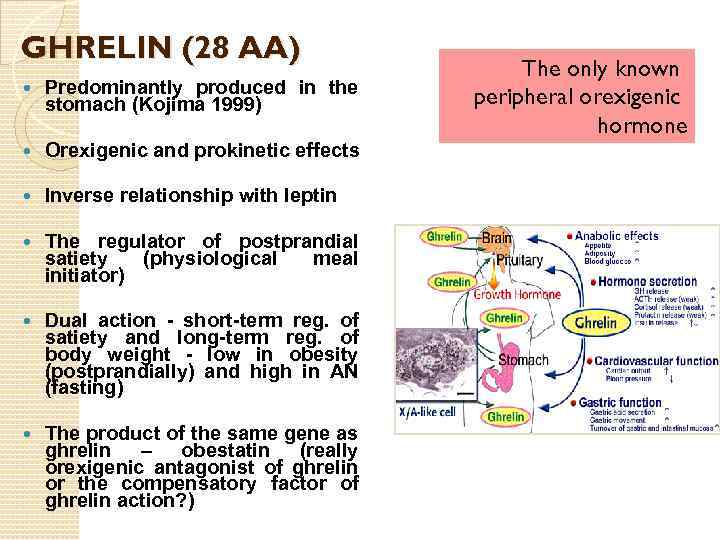
GHRELIN (28 AA) Predominantly produced in the stomach (Kojima 1999) Orexigenic and prokinetic effects Inverse relationship with leptin The regulator of postprandial satiety (physiological meal initiator) Dual action - short-term reg. of satiety and long-term reg. of body weight - low in obesity (postprandially) and high in AN (fasting) The product of the same gene as ghrelin – obestatin (really orexigenic antagonist of ghrelin or the compensatory factor of ghrelin action? ) The only known peripheral orexigenic hormone

Assessment and Classification of Overweight and Obesity Although accurate methods to assess body fat exist, the measurement of body fat by these techniques is expensive and is often not readily available to most clinicians. Two surrogate measures are important to assess body fat: ◦ Body mass index (BMI) ◦ Waist circumference
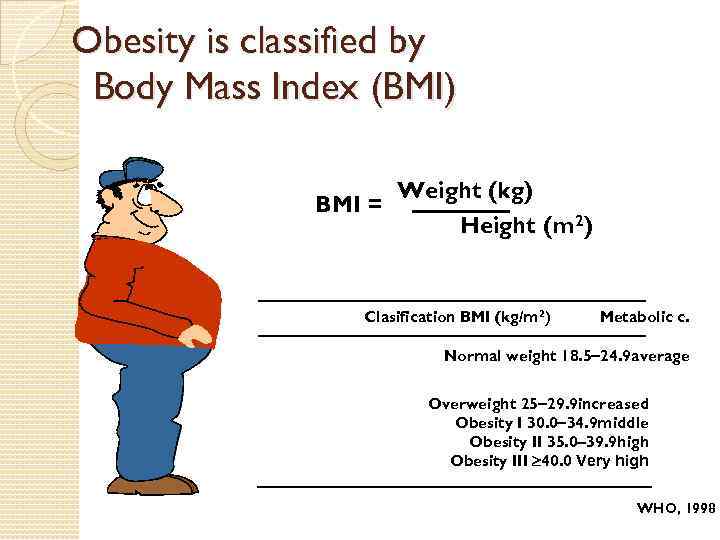
Obesity is classified by Body Mass Index (BMI) Weight (kg) BMI = Height (m 2) Clasification BMI (kg/m 2) Metabolic c. Normal weight 18. 5 24. 9 average Overweight 25 29. 9 increased Obesity I 30. 0 34. 9 middle Obesity II 35. 0 39. 9 high Obesity III 40. 0 Very high WHO, 1998

Waist circumference is a helping indicator of visceral fat – this fat is the most metabolically active and thus the most harmful Women Men cm >88 cm = highly increased risk 1 >80 cm = increased risk 1 >102 cm = highly increased risk 1 >94 cm = increased risk 1 1 Lean MEJ, et al. Lancet; 1998: 351: 853– 6

Although waist circumference and BMI are interrelated, waist circumference provides an independent prediction of risk over and above that of BMI. The waist circumference measurement is particularly useful in patients who are categorized as normal or overweight in terms of BMI. For individuals with a BMI ≥ 35, waist circumference adds little to the predictive power of the disease risk. A high waist circumference is associated with an increased risk for type-2 diabetes, dyslipidemia, hypertension, and CVD in patients with a BMI between 25 and 34. 9 kg/m.

Men are at increased relative risk if they have a waist circumference greater than 102 cm, while women are at an increased relative risk if they have a waist circumference greater than 88 cm. In overweight and obese persons weight loss is recommended to accomplish the following: ◦ Lower elevated blood pressure in those with high blood pressure. ◦ Lower elevated blood glucose levels in those with type-2 diabetes. ◦ Lower elevated levels of total cholesterol, LDLcholesterol, and triglycerides, and raise low levels of HDL-cholesterol in those with dyslipidemia.
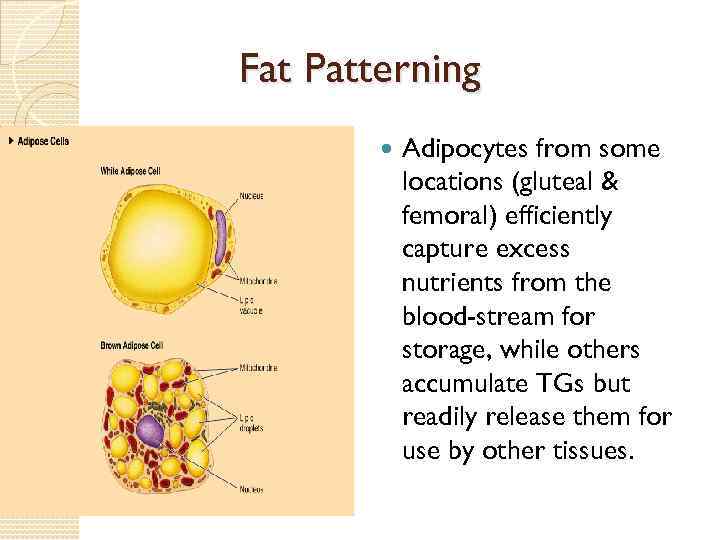
Fat Patterning Adipocytes from some locations (gluteal & femoral) efficiently capture excess nutrients from the blood-stream for storage, while others accumulate TGs but readily release them for use by other tissues.
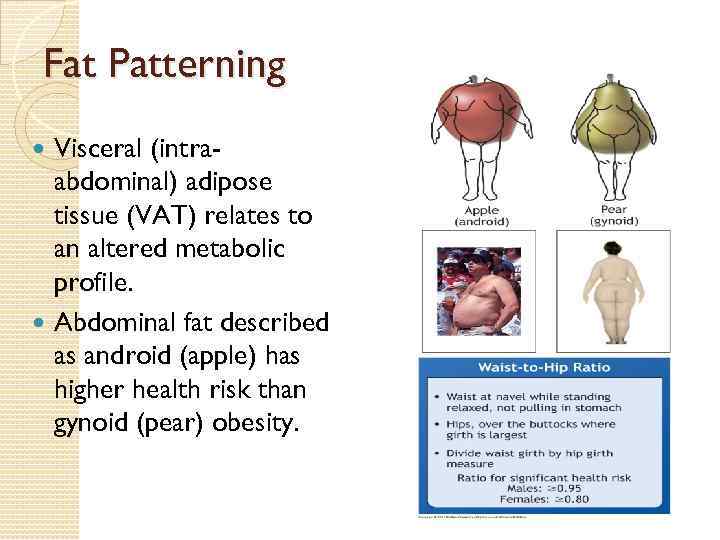
Fat Patterning Visceral (intraabdominal) adipose tissue (VAT) relates to an altered metabolic profile. Abdominal fat described as android (apple) has higher health risk than gynoid (pear) obesity.
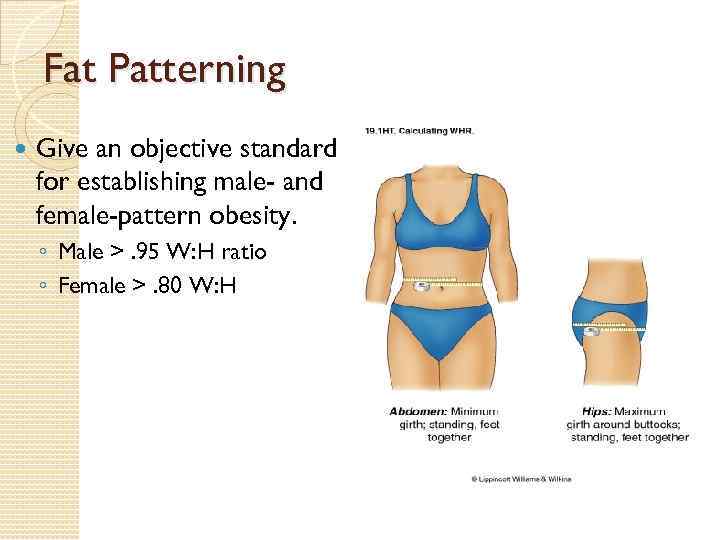
Fat Patterning Give an objective standard for establishing male- and female-pattern obesity. ◦ Male >. 95 W: H ratio ◦ Female >. 80 W: H

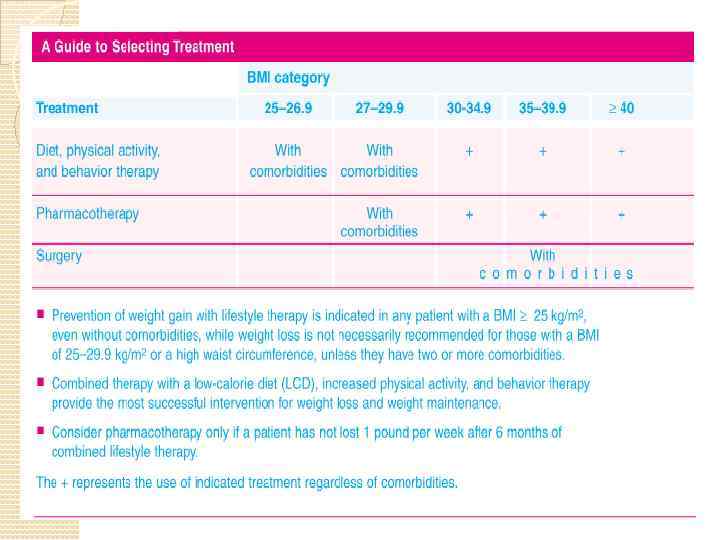
Weight Management Techniques Effective weight control involves multiple techniques and strategies including dietary therapy, physical activity, behavior therapy, pharmacotherapy, and surgery as well as combinations of these strategies. Relevant treatment strategies can also be used to foster longterm weight control and prevention of weight gain. Some strategies such as modifying dietary intake and physical activity can also impact on obesity-related comorbidities or risk factors. Since the diet recommended is a low calorie Step-1 diet, it not only modifies calorie intake but also reduces saturated fat, total fat, and cholesterol intake in order to help lower high blood cholesterol levels. The diet also includes the current recommendations for sodium, calcium and fiber intakes.

Increased physical activity is not only important for weight loss and weight loss maintenance but also impacts on other comorbidities and risk factors such as high blood pressure, and high blood cholesterol levels. Reducing body weight in overweight and obese patients not only helps reduce the risk of these comorbidities from developing but also helps in their management.

Pharmacotherapy of obesity Two classes of drugs are used in treating obesity: anorexiants (phentermine and diethylpropion) and lipase inhibitors (orlistat). Phentermine and diethylpropion are indicated for short-term management of obesity. Orlistat has been approved for up to 4 years of use.

A. Anorexiants (appetite suppressants): Phentermine exerts its pharmacologic action by increasing release of norepinephrine and dopamine from the nerve terminals and by inhibiting reuptake of these neurotransmitters, thereby increasing levels of neurotransmitters in the brain. Diethylpropion has norepinephrine. similar effects on

Pharmacokinetics: Primary route of excretion is via the kidney. Diethylpropion is rapidly absorbed and undergoes extensive first-pass metabolism. Many of the metabolites are active. Diethylpropion and its metabolites are excreted mainly via the kidney. The half-life of the metabolites is 4 to 8 hours.

Adverse effects: All of the appetite suppressants are Schedule IV controlled agents due to potential for dependence or abuse. Dry mouth, headache, common problems. insomnia, and constipation are Heart rate and blood pressure may be increased with these agents and, therefore, should be avoided in patients with a history of hypertension, CVD, arrhythmias, congestive heart failure, or stroke. In addition, phentermine has been associated with heart valve disorders and pulmonary hypertension. Concomitant use of appetite suppressants and monoamine oxidase inhibitors should be avoided.

Sibutramine: Inhibits the re-uptake of noradrenaline and serotonin. It reduces appetite and is used as an adjunct to diet for up to one year. Blood pressure and pulse should be monitored. Contraindications include: major psychiatric illness, ischaemic heart disease, dysrrythmias, hyperthyroidism and pregnancy. Side effects include: dry mouth, nausea, abnormal taste, constipation, myalgia, palpitations, alopecia, seizures and bleeding disorders.

Rimonabant: Was approved in Europe in 2006. It is an oral selective cannabinoid CB 1 receptor antagonist which is used as an adjunct to diet to achieve weight loss. Rimonabant is contraindicated in (and may cause) depression. Adverse effects include: nausea, vomiting, diarrhoea, mood changes, anxiety, impaired memory, dizziness and sleep disorders. It is highly protein bound and metabolized by hepatic CYP 3 A 4. The half life is six to nine days in those with normal BMI, but approximately 16 days in obese patients.

Belviq (lorcaserin hydrochloride) Approved by FDA in 2012. Control appetite by activation of 5 -HT. Not used in diabetes-2 patients → low blood sugar. Side effects: headache, dizziness and fatigue.

Qsymia (phentermine & topiramate SR) Side effects: birth defects, increased HR, glaucoma, suicidal thoughts, diabetes-2 → low blood sugar. Exact mechanism is unknown but its effect may be due to: ◦ Blockage of voltage-dep. Sodium channels (↑GABA activity). . ? ? ?

B. Lipase inhibitor Orlistat is the first drug in a class of antiobesity drugs known as lipase inhibitors. Orlistat is a pentanoic acid ester that inhibits gastric and pancreatic lipases, thus decreasing the breakdown of dietary fat into smaller molecules that can be absorbed. Fat absorption is decreased by about 30 percent. The loss of calories is the main cause of weight loss, but adverse gastrointestinal effects associated with the drug may also contribute to a decreased intake of food.

Orlistat is administered three times daily with meals. The most common adverse effects associated with orlistat are gastrointestinal symptoms, such as oily spotting, flatulence with discharge, fecal urgency, and increased defecation. There have been rare reports of liver injury in people taking orlistat. Orlistat is contraindicated in patients with chronic malabsorption syndrome or cholestasis. It interferes with the absorption of fat-soluble vitamins and βcarotene. Thus, patients should be advised to take a multivitamin supplement that contains vitamins A, D, E, and K, and also βcarotene. The vitamin supplement should not be taken within 2 hours of orlistat. Orlistat can also interfere with the absorption of other medications, such as amiodarone and levothyroxine. The dose of levothyroxine should be separated from orlistat by at least 4 hours.

Bulk agents Substances such as methylcellulose and guar gum act as bulking agents in the diet and are ineffective at producing weight loss. A high-fibre diet may help weight loss, provided that total caloric intake is reduced.

Miscellaneous Diuretics cause a transient loss of weight through fluid loss, and their use for such an effect is to be deplored. Thyroxine has been used to increase the basal metabolic rate and reduce weight in euthyroid obese patients. This is both dangerous and irrational.

Anorexia Nervosa (AN) Severe psychiatric disorder of unclear etiology associated with significant morbidity and mortality (Hsu 1996) Prevalence 0. 3% of young girls, mortalty of 6%/decade (Dardeness 2007) Irrational fear of becoming fat even if patient is of normal or usually underweight Phobic response to food, abnormal eating behavior, hyperactivity, weakness, muscle aches, sleep disturbances, GIT complications, mood disturances, alterations of wide variety of hormonal and metabolic systems Combination of cultural-social, psychological, biological factors

Diagnostic criteria for AN Refusal to maintain body weight at or above a minimal normal weight for age and height (e. g. , weight loss leading to maintenance of body weight less than 85% of that expected; or failure to make expected weight gain during period of growth, leading to body weight less than 85% of that expected). Intense fear of gaining weight or becoming fat, even thought underweight. Disturbance in the way in which one´s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or denial of the seriousness of the current low body weight. In postmenarcheal females, amenorrhea, i. e. , the absence of at least three consecutive menstrual cycles. American Psychiatric Association Diagnostic and Statistical Manual of Disorders, DSM -Q, American Psychiatric Association, Washington, DC, 1994.