f2c585e3ce336f3b215be07ac070da5c.ppt
- Количество слайдов: 46

Nucleic Acid Testing vs Infectivity Michael P. Busch, M. D. , Ph. D. Blood Centers of the Pacific Blood Systems, Inc. EPFA, Lisbon Portugal May, 2001
NAT vs Infectivity n Overview of stages of infections and importance of understanding stage-specific g. Eq: infectivity ratios (concentration vs volume infused) n Review of NA dynamics (pre/peri/post-SC) for HIV, HCV and HBV n Review infectivity data for each stage n Need for future studies n animal transmission models n lookback studies (NAT/SC donors; recipient cases)

Stages of TTVIs and infectivity • • • Pre-ramp-up viremia Ramp-up viremia Plateau phase / peak viremia Peri-SC viremia Post-SC viremia – Persistent carrier (viral load set point) – “Resolved” infection? • Immunosilent carrier • Transient Viremia w/o SC
Stages of TTVIs and infectivity • Pre-ramp-up Viremia – Low-level, intermittent “blips” of RNA/DNA detected prior to quantifiable ramp-up phase – Innoculum vs focal replication that seeds dissemination vs abortive replication – Can this occur transiently w/o subsequent ramp-up/SC? – Is this viremia infectious? – Kinetic, viral sequencing & infectivity studies in progress
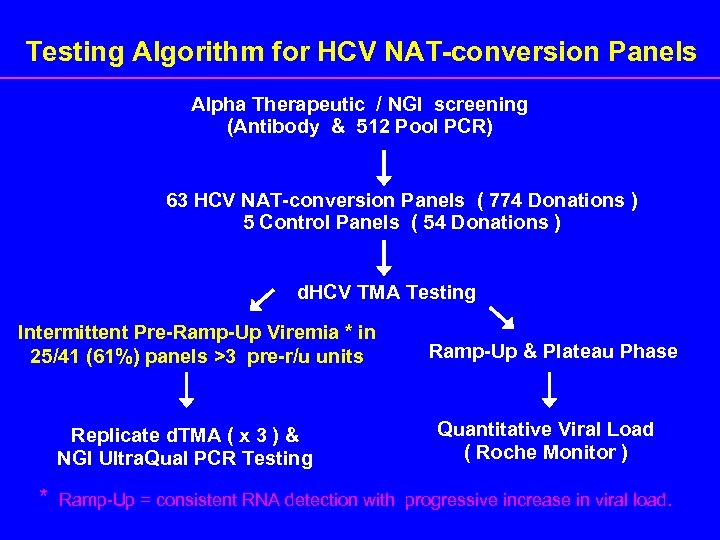
Testing Algorithm for HCV NAT-conversion Panels Alpha Therapeutic / NGI screening (Antibody & 512 Pool PCR) 63 HCV NAT-conversion Panels ( 774 Donations ) 5 Control Panels ( 54 Donations ) d. HCV TMA Testing Intermittent Pre-Ramp-Up Viremia * in 25/41 (61%) panels >3 pre-r/u units Replicate d. TMA ( x 3 ) & NGI Ultra. Qual PCR Testing * Ramp-Up & Plateau Phase Quantitative Viral Load ( Roche Monitor ) Ramp-Up = consistent RNA detection with progressive increase in viral load.
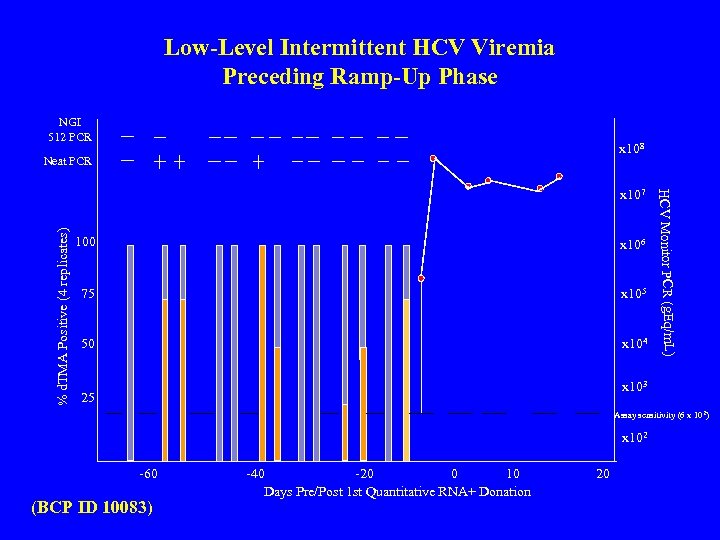
Low-Level Intermittent HCV Viremia Preceding Ramp-Up Phase NGI 512 PCR x 108 Neat PCR % d. TMA Positive (4 replicates) 100 x 106 75 x 105 50 x 104 HCV Monitor PCR (g. Eq/m. L) x 107 x 103 25 Assay sensitivity (6 x 102) x 102 -60 (BCP ID 10083) -40 -20 0 10 Days Pre/Post 1 st Quantitative RNA+ Donation 20
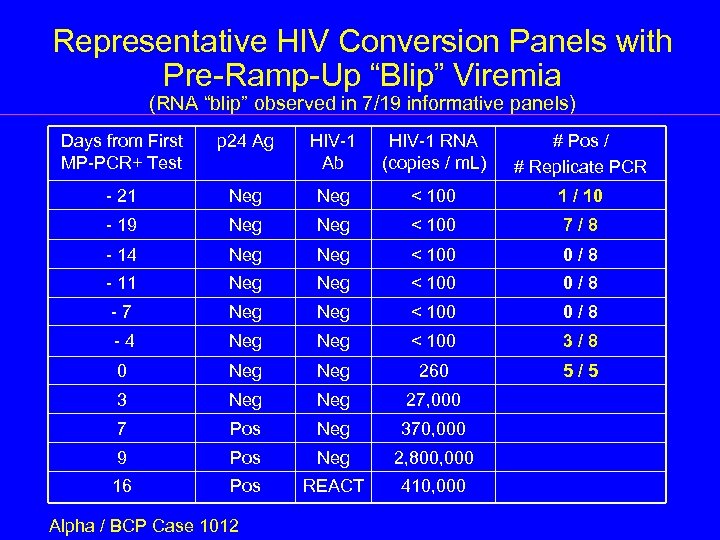
Representative HIV Conversion Panels with Pre-Ramp-Up “Blip” Viremia (RNA “blip” observed in 7/19 informative panels) Days from First MP-PCR+ Test p 24 Ag HIV-1 Ab HIV-1 RNA (copies / m. L) # Pos / # Replicate PCR - 21 Neg < 100 1 / 10 - 19 Neg < 100 7/8 - 14 Neg < 100 0/8 - 11 Neg < 100 0/8 -7 Neg < 100 0/8 -4 Neg < 100 3/8 0 Neg 260 5/5 3 Neg 27, 000 7 Pos Neg 370, 000 9 Pos Neg 2, 800, 000 16 Pos REACT 410, 000 Alpha / BCP Case 1012
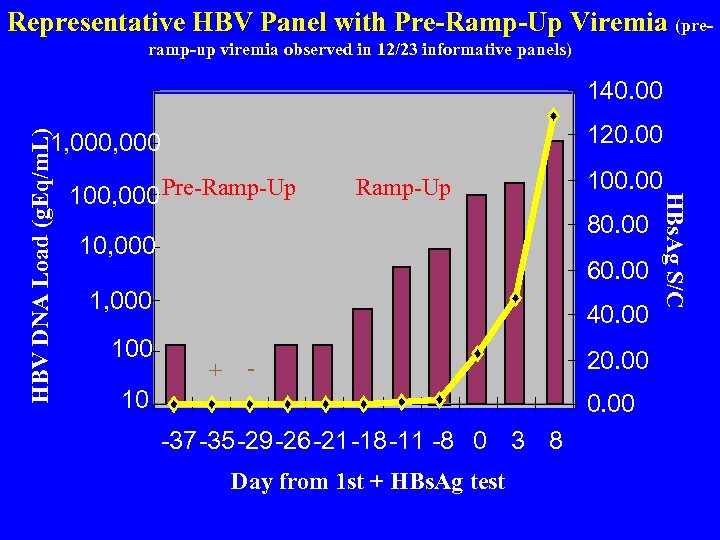
Representative HBV Panel with Pre-Ramp-Up Viremia (preramp-up viremia observed in 12/23 informative panels) 140. 00 120. 00 100, 000 Pre-Ramp-Up 80. 00 10, 000 60. 00 1, 000 100. 00 40. 00 + - 10 20. 00 -37 -35 -29 -26 -21 -18 -11 -8 0 3 8 Day from 1 st + HBs. Ag test HBs. Ag S/C HBV DNA Load (g. Eq/m. L) 1, 000
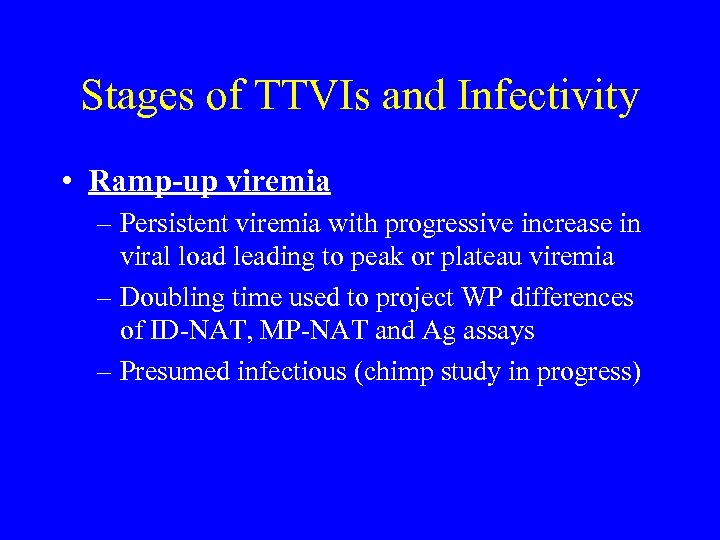
Stages of TTVIs and Infectivity • Ramp-up viremia – Persistent viremia with progressive increase in viral load leading to peak or plateau viremia – Doubling time used to project WP differences of ID-NAT, MP-NAT and Ag assays – Presumed infectious (chimp study in progress)
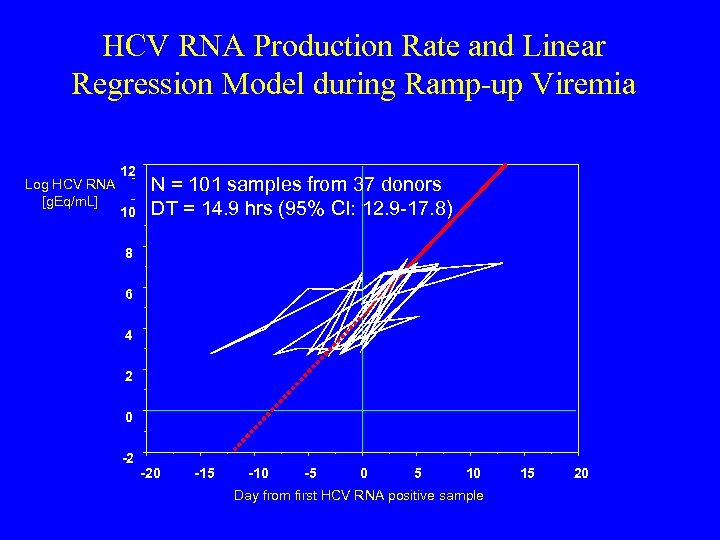
HCV RNA Production Rate and Linear Regression Model during Ramp-up Viremia Log HCV RNA [g. Eq/m. L] 12 10 N = 101 samples from 37 donors DT = 14. 9 hrs (95% CI: 12. 9 -17. 8) 8 6 4 2 0 -2 -20 -15 -10 -5 0 5 10 Day from first HCV RNA positive sample 15 20
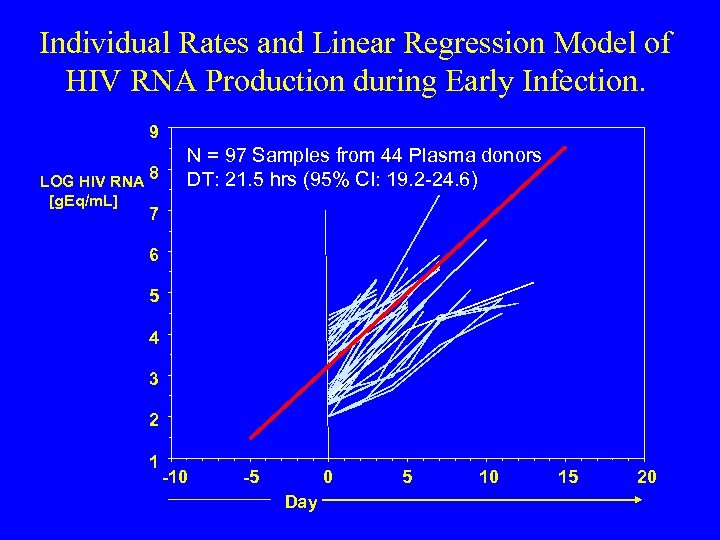
Individual Rates and Linear Regression Model of HIV RNA Production during Early Infection. 9 LOG HIV RNA 8 [g. Eq/m. L] N = 97 Samples from 44 Plasma donors DT: 21. 5 hrs (95% CI: 19. 2 -24. 6) 7 6 5 4 3 2 1 -10 -5 0 Day 5 10 15 20
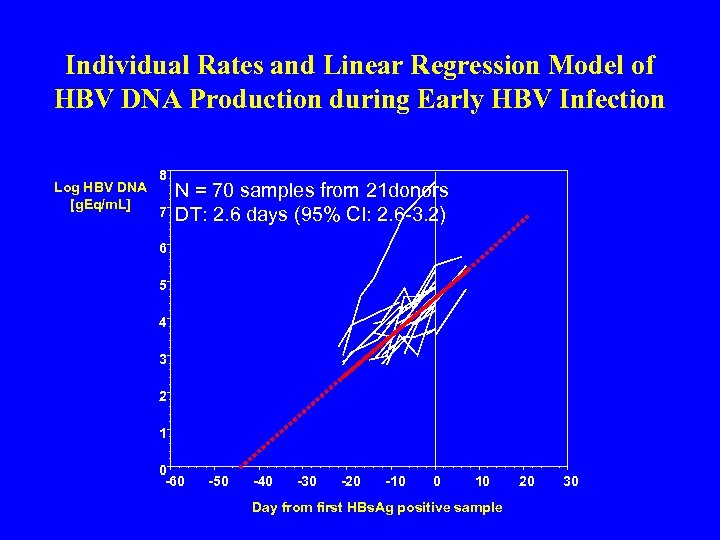
Individual Rates and Linear Regression Model of HBV DNA Production during Early HBV Infection Log HBV DNA [g. Eq/m. L] 8 7 N = 70 samples from 21 donors DT: 2. 6 days (95% CI: 2. 6 -3. 2) 6 5 4 3 2 1 0 -60 -50 -40 -30 -20 -10 0 10 Day from first HBs. Ag positive sample 20 30
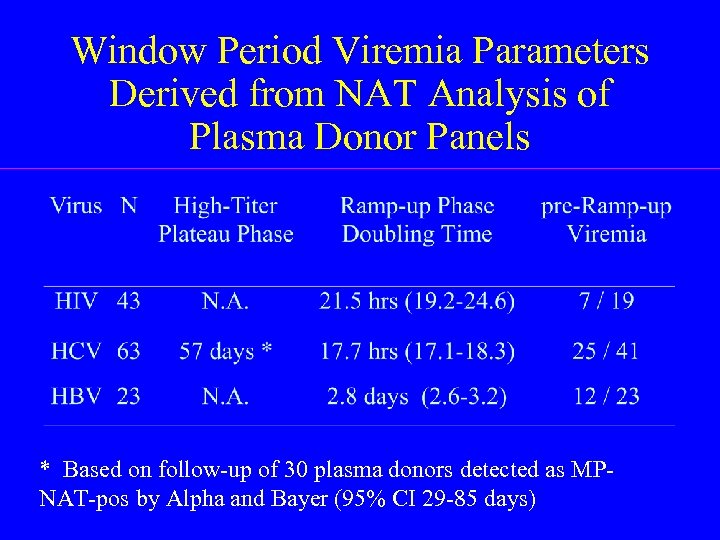
Window Period Viremia Parameters Derived from NAT Analysis of Plasma Donor Panels * Based on follow-up of 30 plasma donors detected as MPNAT-pos by Alpha and Bayer (95% CI 29 -85 days)
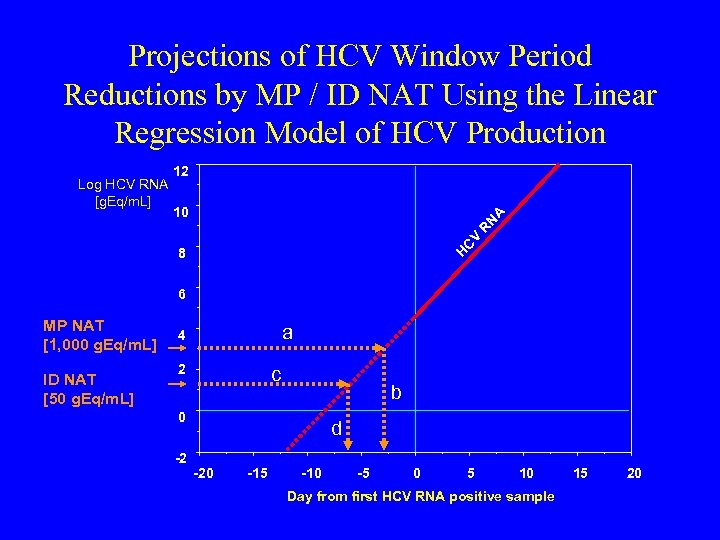
Projections of HCV Window Period Reductions by MP / ID NAT Using the Linear Regression Model of HCV Production N A 10 C V R Log HCV RNA [g. Eq/m. L] 12 H 8 6 MP NAT [1, 000 g. Eq/m. L] ID NAT [50 g. Eq/m. L] a 4 2 c b 0 -2 d -20 -15 -10 -5 0 5 10 Day from first HCV RNA positive sample 15 20
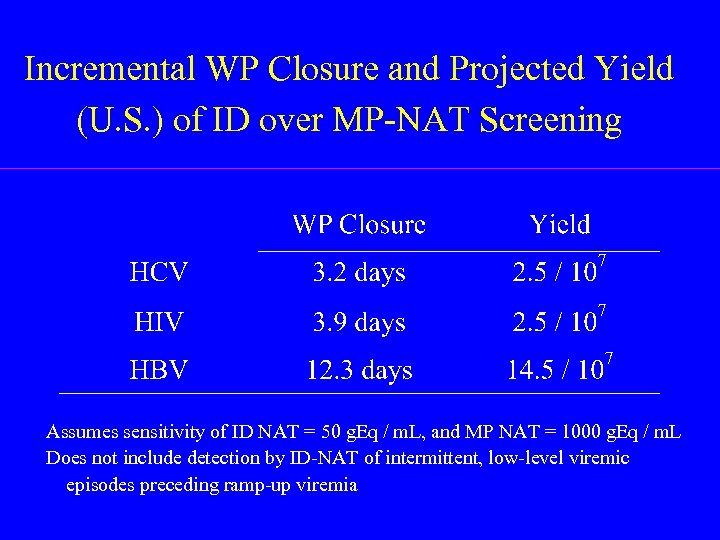
Incremental WP Closure and Projected Yield (U. S. ) of ID over MP-NAT Screening Assumes sensitivity of ID NAT = 50 g. Eq / m. L, and MP NAT = 1000 g. Eq / m. L Does not include detection by ID-NAT of intermittent, low-level viremic episodes preceding ramp-up viremia
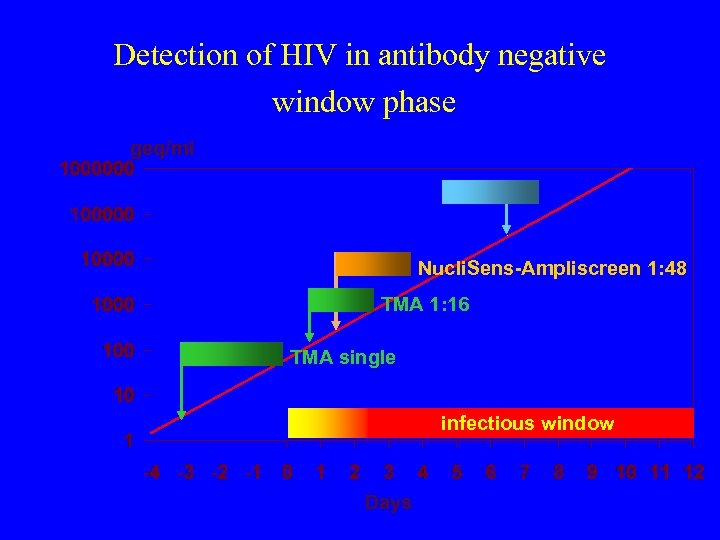
Detection of HIV in antibody negative window phase geq/ml Nucli. Sens-Ampliscreen 1: 48 TMA 1: 16 TMA single infectious window Days
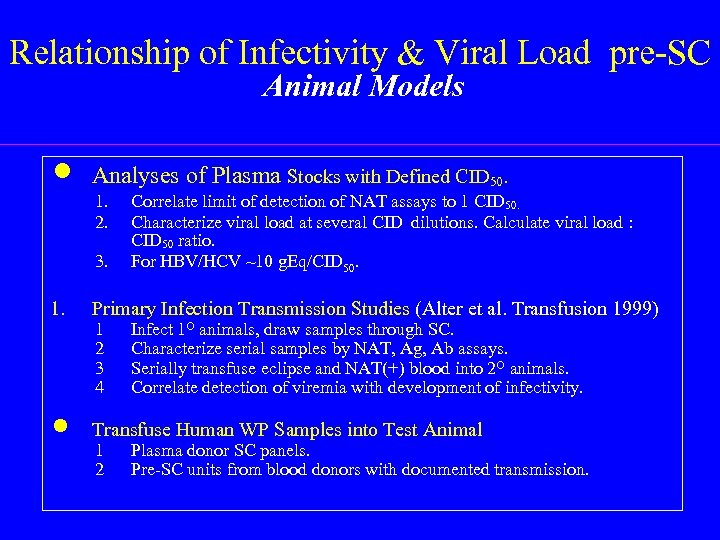
Relationship of Infectivity & Viral Load pre-SC Animal Models n Analyses of Plasma Stocks with Defined CID 50. 1. 2. 3. Correlate limit of detection of NAT assays to 1 CID 50. Characterize viral load at several CID dilutions. Calculate viral load : CID 50 ratio. For HBV/HCV ~10 g. Eq/CID 50. 1. Primary Infection Transmission Studies (Alter et al. Transfusion 1999) n Transfuse Human WP Samples into Test Animal 1 2 3 4 1 2 Infect 1 O animals, draw samples through SC. Characterize serial samples by NAT, Ag, Ab assays. Serially transfuse eclipse and NAT(+) blood into 2 O animals. Correlate detection of viremia with development of infectivity. Plasma donor SC panels. Pre-SC units from blood donors with documented transmission.
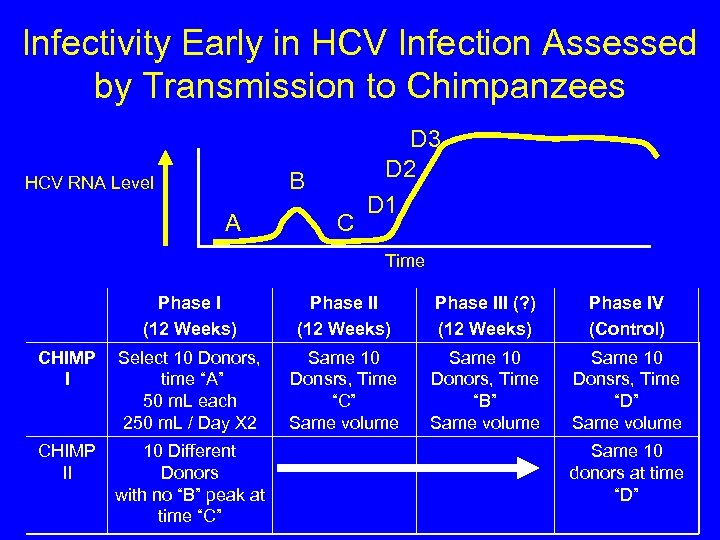
Infectivity Early in HCV Infection Assessed by Transmission to Chimpanzees B HCV RNA Level A C D 3 D 2 D 1 Time Phase I (12 Weeks) Phase III (? ) (12 Weeks) Phase IV (Control) CHIMP I Select 10 Donors, time “A” 50 m. L each 250 m. L / Day X 2 Same 10 Donsrs, Time “C” Same volume Same 10 Donors, Time “B” Same volume Same 10 Donsrs, Time “D” Same volume CHIMP II 10 Different Donors with no “B” peak at time “C” Same 10 donors at time “D”

Relationship of Infectivity & Viral Load pre-SC Human Transmission Data 1. Investigate Recipients of Prior Donations by NAT / Ab SC Donors (Donor directed lookback) n n n Model Duration of "Infectious WP" Based on Rate of Recipient Infection Relative to ID Intervals Petersen, et al. Transfusion 1993; 33: 552 -7 For Cases with Documented Transmission and Available Plasma, Characterize Viral Load and Detection by MP/SD NAT Tests Sch ttler, et al. Lancet 2000 Robbins, et al. JAMA 2000 Roth, et al. submitted Transfuse human infectious plasma with low viral load into animals to validate sensitivity of the models
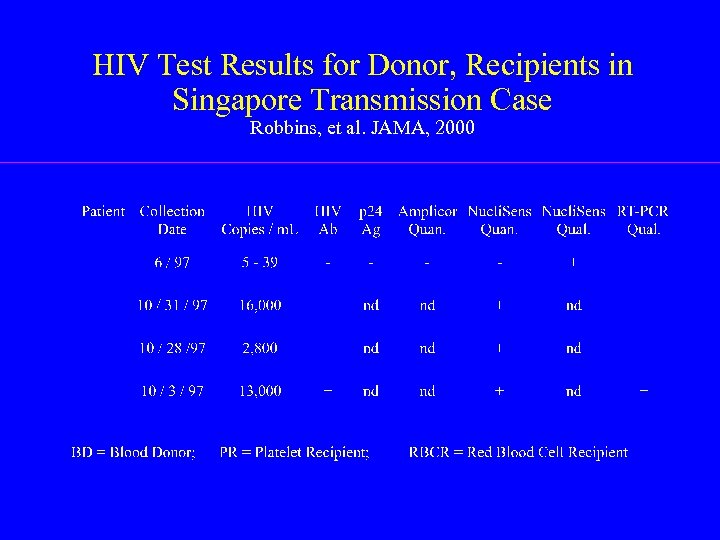
HIV Test Results for Donor, Recipients in Singapore Transmission Case Robbins, et al. JAMA, 2000
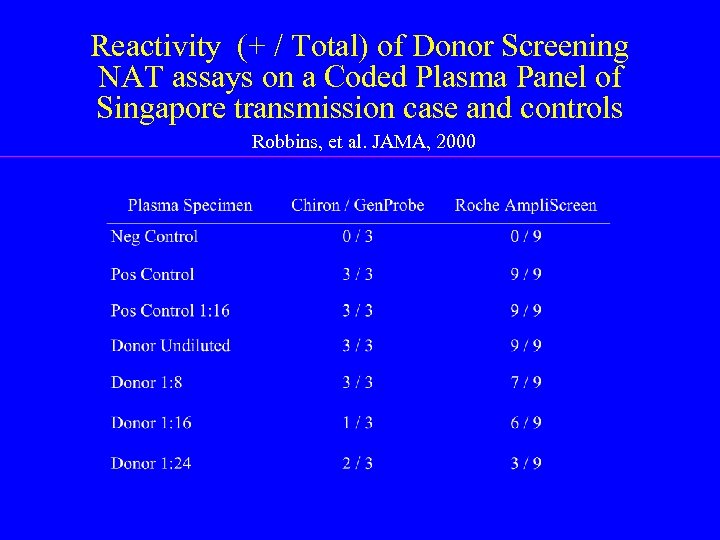
Reactivity (+ / Total) of Donor Screening NAT assays on a Coded Plasma Panel of Singapore transmission case and controls Robbins, et al. JAMA, 2000
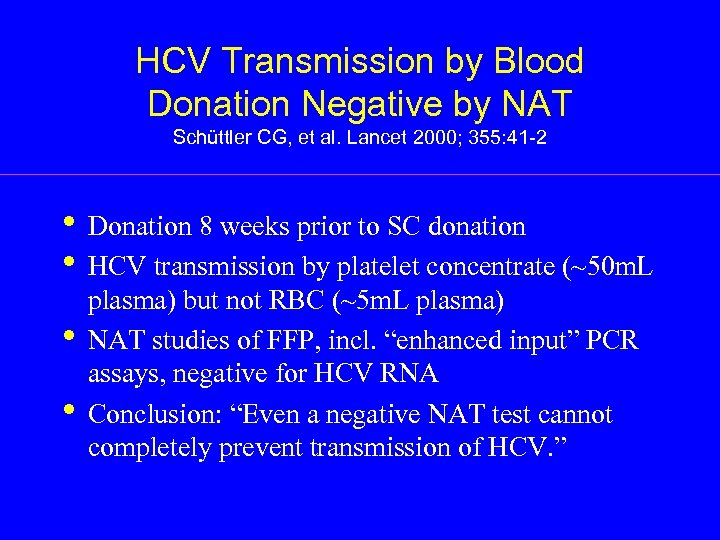
HCV Transmission by Blood Donation Negative by NAT Schüttler CG, et al. Lancet 2000; 355: 41 -2 • Donation 8 weeks prior to SC donation • HCV transmission by platelet concentrate (~50 m. L • • plasma) but not RBC (~5 m. L plasma) NAT studies of FFP, incl. “enhanced input” PCR assays, negative for HCV RNA Conclusion: “Even a negative NAT test cannot completely prevent transmission of HCV. ”
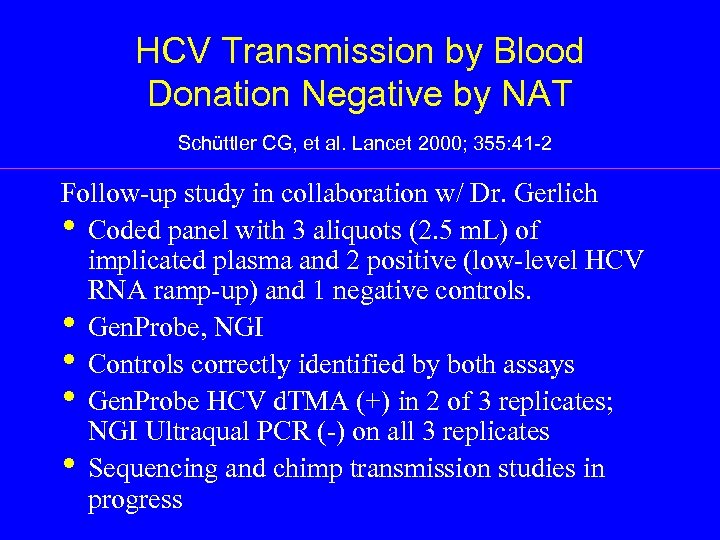
HCV Transmission by Blood Donation Negative by NAT Schüttler CG, et al. Lancet 2000; 355: 41 -2 Follow-up study in collaboration w/ Dr. Gerlich • Coded panel with 3 aliquots (2. 5 m. L) of implicated plasma and 2 positive (low-level HCV RNA ramp-up) and 1 negative controls. • Gen. Probe, NGI • Controls correctly identified by both assays • Gen. Probe HCV d. TMA (+) in 2 of 3 replicates; NGI Ultraqual PCR (-) on all 3 replicates • Sequencing and chimp transmission studies in progress
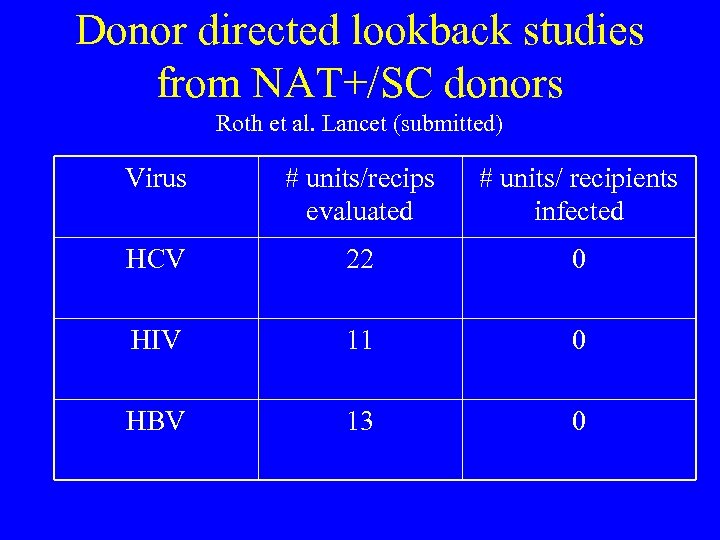
Donor directed lookback studies from NAT+/SC donors Roth et al. Lancet (submitted) Virus # units/recips evaluated # units/ recipients infected HCV 22 0 HIV 11 0 HBV 13 0
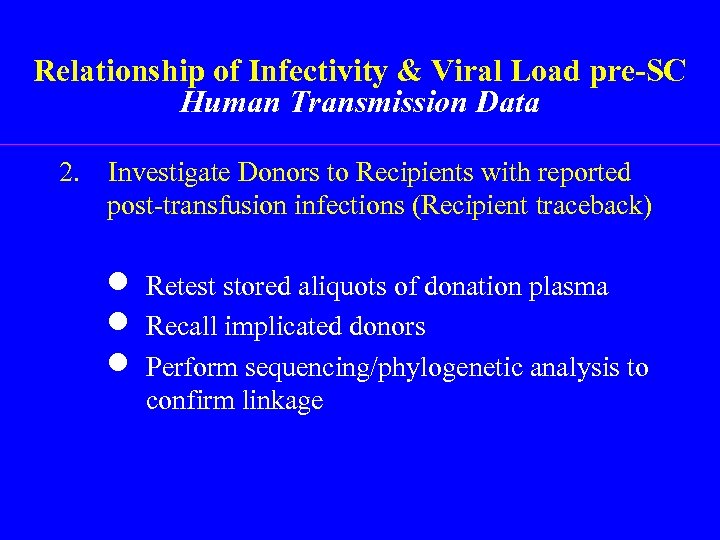
Relationship of Infectivity & Viral Load pre-SC Human Transmission Data 2. Investigate Donors to Recipients with reported post-transfusion infections (Recipient traceback) n Retest stored aliquots of donation plasma n Recall implicated donors n Perform sequencing/phylogenetic analysis to confirm linkage
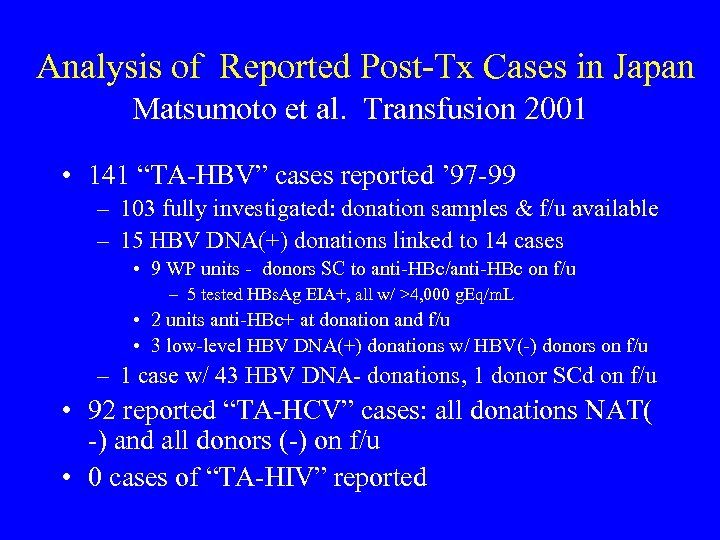
Analysis of Reported Post-Tx Cases in Japan Matsumoto et al. Transfusion 2001 • 141 “TA-HBV” cases reported ’ 97 -99 – 103 fully investigated: donation samples & f/u available – 15 HBV DNA(+) donations linked to 14 cases • 9 WP units - donors SC to anti-HBc/anti-HBc on f/u – 5 tested HBs. Ag EIA+, all w/ >4, 000 g. Eq/m. L • 2 units anti-HBc+ at donation and f/u • 3 low-level HBV DNA(+) donations w/ HBV(-) donors on f/u – 1 case w/ 43 HBV DNA- donations, 1 donor SCd on f/u • 92 reported “TA-HCV” cases: all donations NAT( -) and all donors (-) on f/u • 0 cases of “TA-HIV” reported
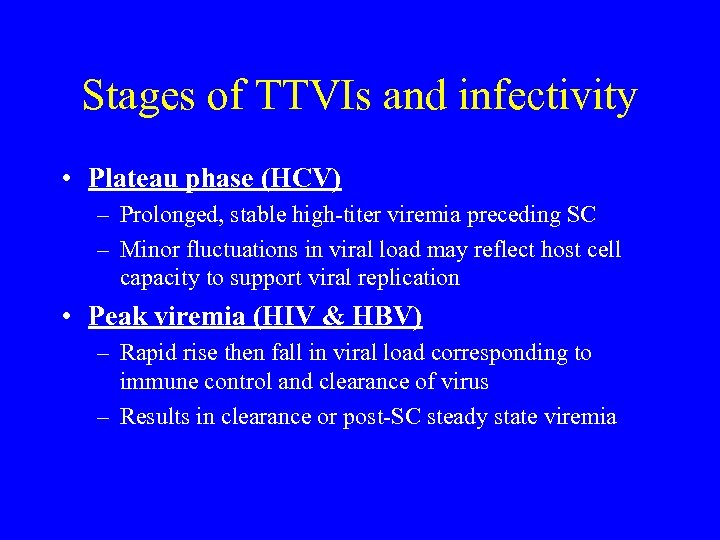
Stages of TTVIs and infectivity • Plateau phase (HCV) – Prolonged, stable high-titer viremia preceding SC – Minor fluctuations in viral load may reflect host cell capacity to support viral replication • Peak viremia (HIV & HBV) – Rapid rise then fall in viral load corresponding to immune control and clearance of virus – Results in clearance or post-SC steady state viremia
Stages of TTVIs and infectivity • Peri-SC phase – cellular and humoral immune response results in down-modulation of viral production and, in some cases, clearance – Smooth decline in viral load to clearance or steady state viremia in most cases – Some cases evidence marked fluctuation in viremia including intermittent neg-pos ID NAT results
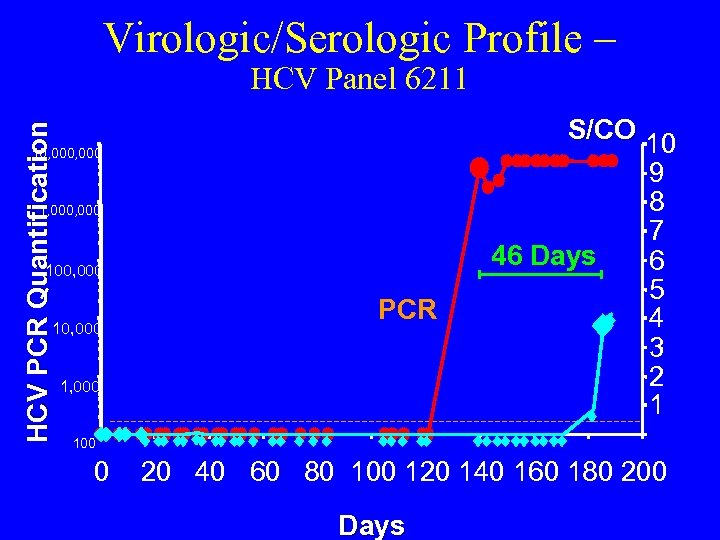
Virologic/Serologic Profile – HCV PCR Quantification HCV Panel 6211 S/CO 10 10, 000 1, 000 46 Days 100, 000 10, 000 PCR 1, 000 9 8 7 6 5 4 3 2 1 100 0 20 40 60 80 100 120 140 160 180 200 Days
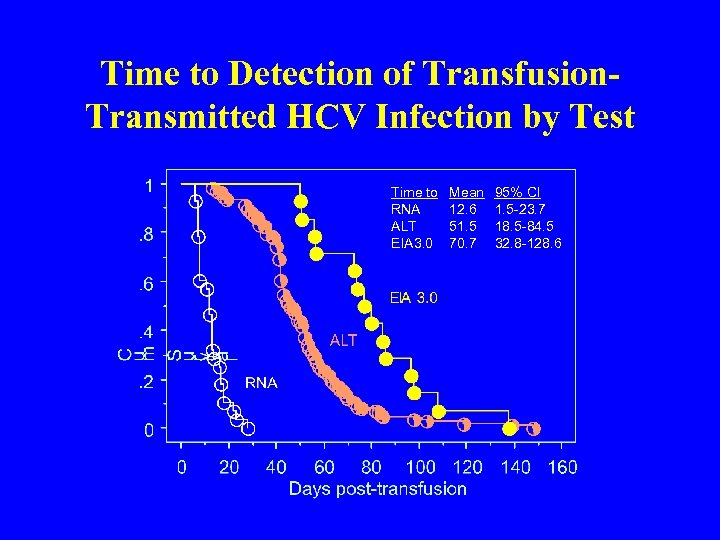
Time to Detection of Transfusion. Transmitted HCV Infection by Test Time to RNA ALT EIA 3. 0 Mean 12. 6 51. 5 70. 7 95% CI 1. 5 -23. 7 18. 5 -84. 5 32. 8 -128. 6
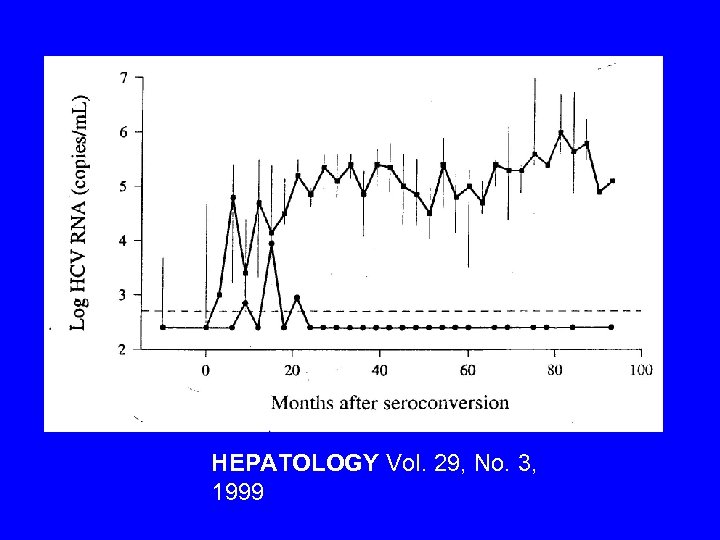
HEPATOLOGY Vol. 29, No. 3, 1999
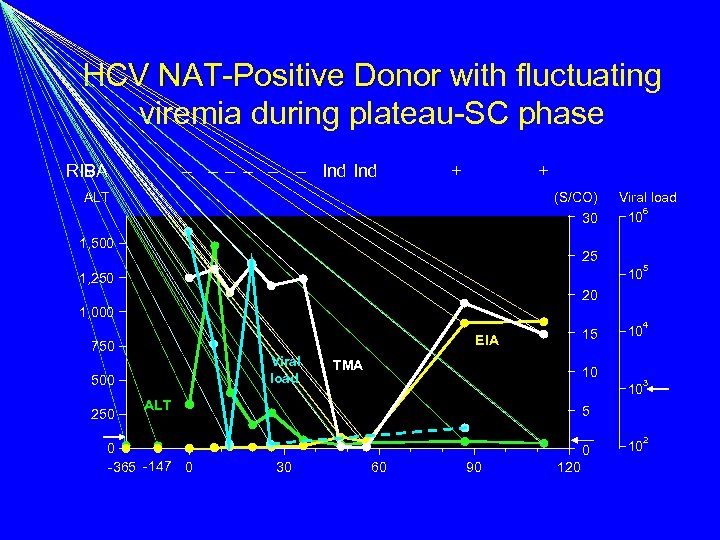
HCV NAT-Positive Donor with fluctuating viremia during plateau-SC phase RIBA – – – Ind + + ALT (S/CO) 30 1, 500 25 Viral load 6 10 5 10 1, 250 20 1, 000 EIA 750 Viral load 500 250 15 TMA 10 3 10 ALT 0 -365 -147 0 4 10 5 0 30 60 90 120 2 10
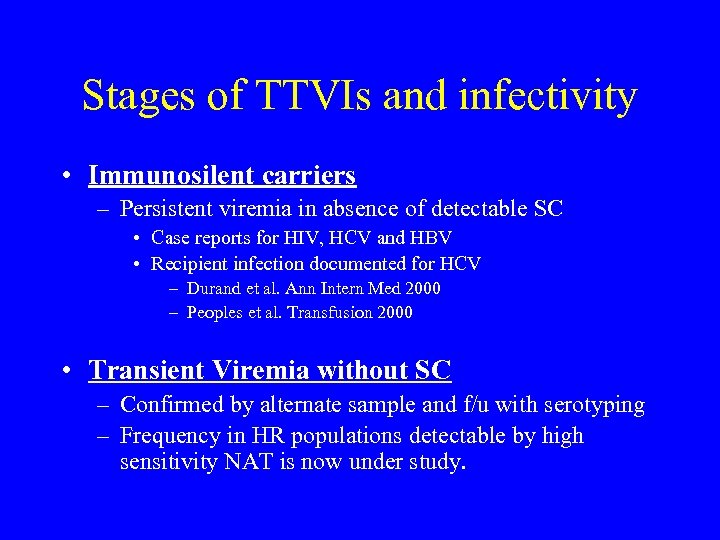
Stages of TTVIs and infectivity • Immunosilent carriers – Persistent viremia in absence of detectable SC • Case reports for HIV, HCV and HBV • Recipient infection documented for HCV – Durand et al. Ann Intern Med 2000 – Peoples et al. Transfusion 2000 • Transient Viremia without SC – Confirmed by alternate sample and f/u with serotyping – Frequency in HR populations detectable by high sensitivity NAT is now under study.
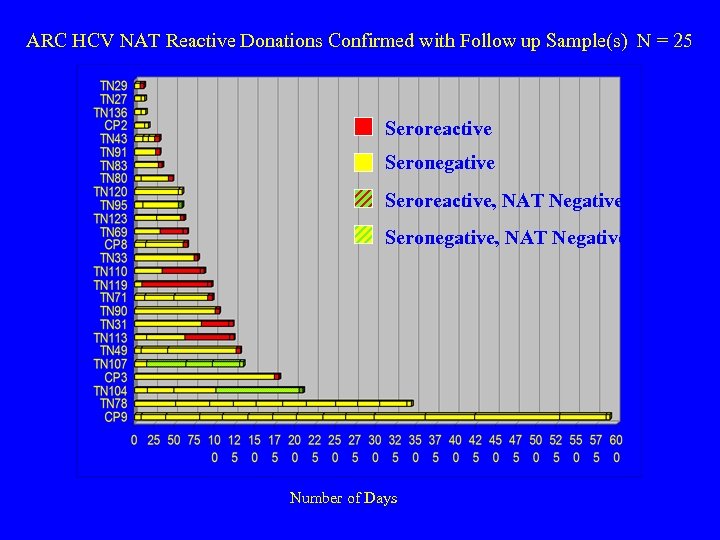
ARC HCV NAT Reactive Donations Confirmed with Follow up Sample(s) N = 25 Seroreactive Seronegative Seroreactive, NAT Negative Seronegative, NAT Negative Number of Days
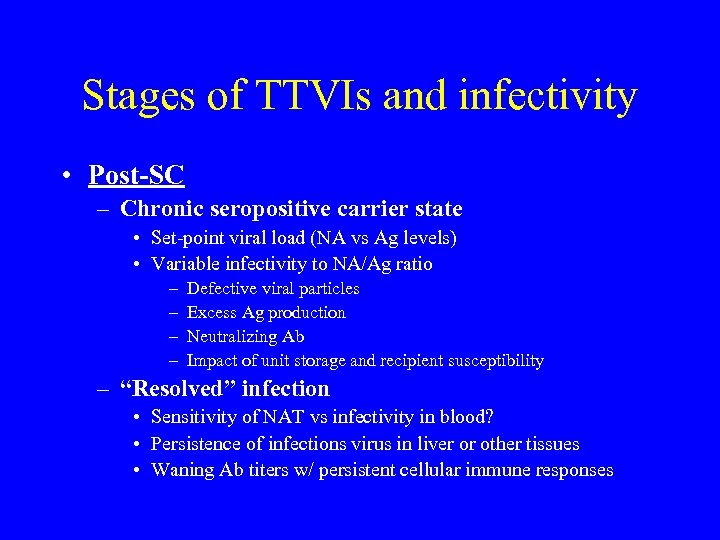
Stages of TTVIs and infectivity • Post-SC – Chronic seropositive carrier state • Set-point viral load (NA vs Ag levels) • Variable infectivity to NA/Ag ratio – – Defective viral particles Excess Ag production Neutralizing Ab Impact of unit storage and recipient susceptibility – “Resolved” infection • Sensitivity of NAT vs infectivity in blood? • Persistence of infections virus in liver or other tissues • Waning Ab titers w/ persistent cellular immune responses
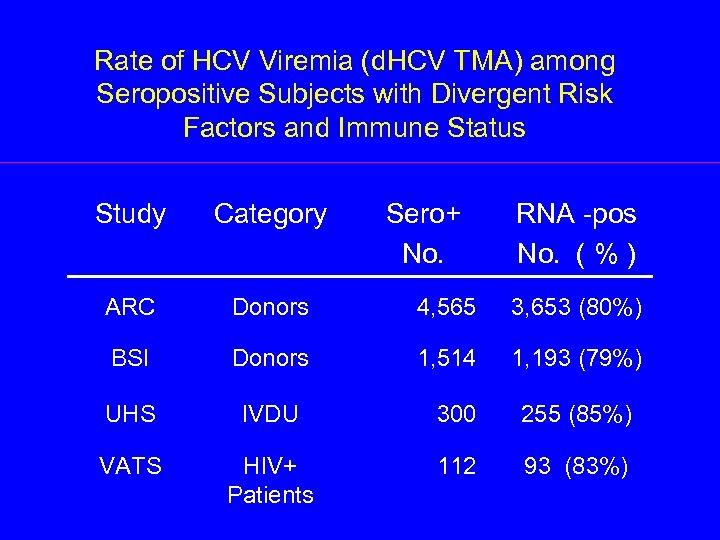
Rate of HCV Viremia (d. HCV TMA) among Seropositive Subjects with Divergent Risk Factors and Immune Status Study Category Sero+ No. RNA -pos No. ( % ) ARC Donors 4, 565 3, 653 (80%) BSI Donors 1, 514 1, 193 (79%) UHS IVDU 300 255 (85%) VATS HIV+ Patients 112 93 (83%)
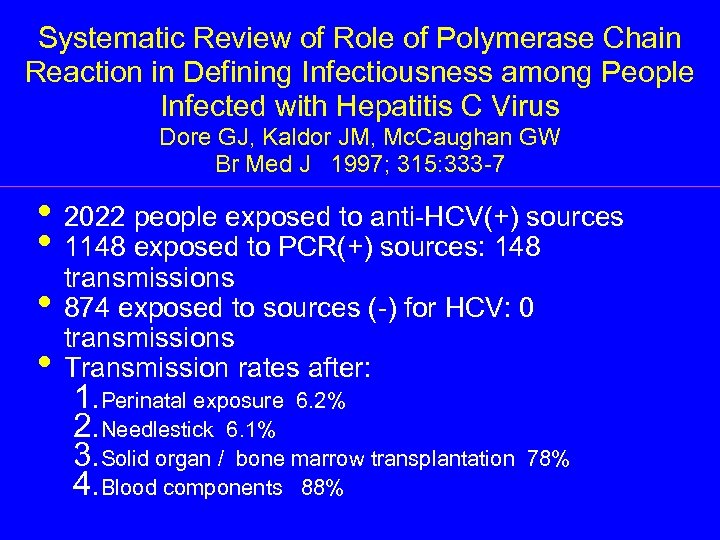
Systematic Review of Role of Polymerase Chain Reaction in Defining Infectiousness among People Infected with Hepatitis C Virus Dore GJ, Kaldor JM, Mc. Caughan GW Br Med J 1997; 315: 333 -7 • 2022 people exposed to anti-HCV(+) sources • 1148 exposed to PCR(+) sources: 148 transmissions • 874 exposed to sources (-) for HCV: 0 transmissions • Transmission rates after: 1. Perinatal exposure 6. 2% 2. Needlestick 6. 1% 3. Solid organ / bone marrow transplantation 4. Blood components 88% 78%
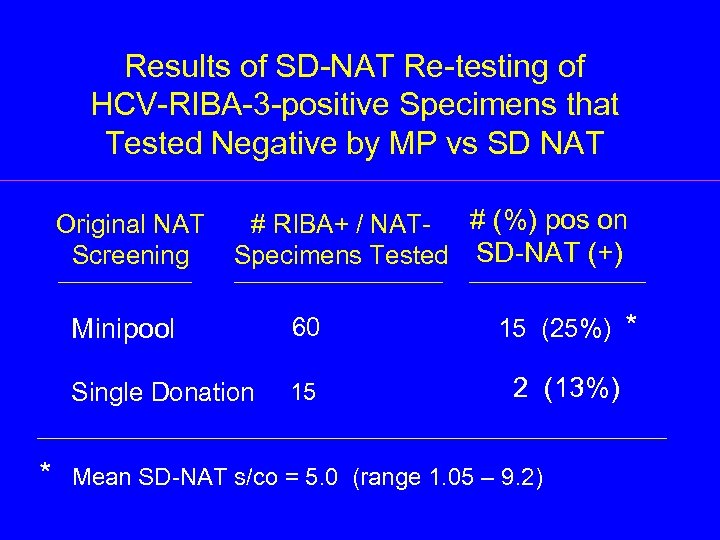
Results of SD-NAT Re-testing of HCV-RIBA-3 -positive Specimens that Tested Negative by MP vs SD NAT Original NAT Screening # RIBA+ / NAT- # (%) pos on Specimens Tested SD-NAT (+) Minipool Single Donation * 60 15 15 (25%) 2 (13%) Mean SD-NAT s/co = 5. 0 (range 1. 05 – 9. 2) *
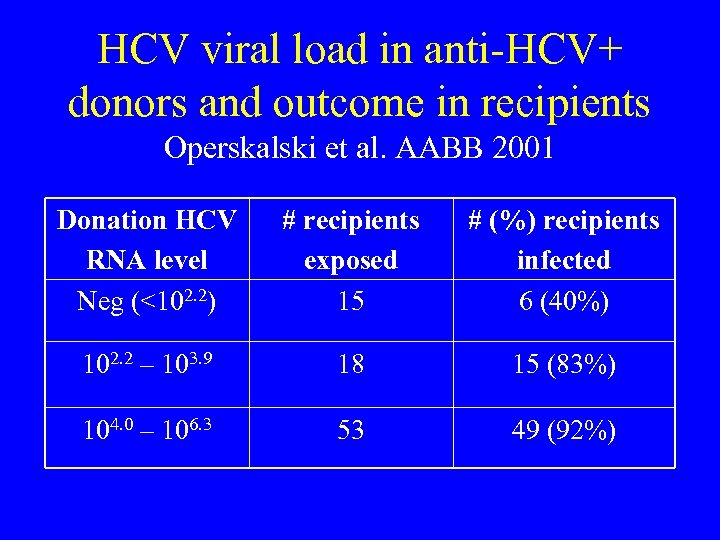
HCV viral load in anti-HCV+ donors and outcome in recipients Operskalski et al. AABB 2001 Donation HCV RNA level Neg (<102. 2) # recipients exposed 15 # (%) recipients infected 6 (40%) 102. 2 – 103. 9 18 15 (83%) 104. 0 – 106. 3 53 49 (92%)
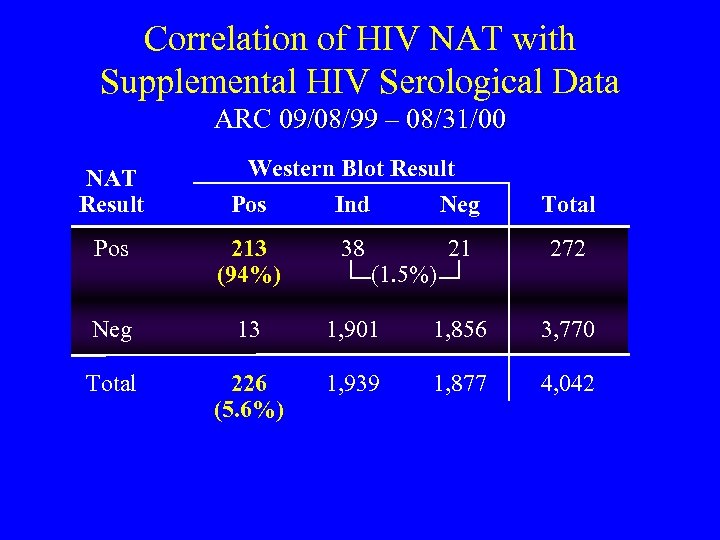
Correlation of HIV NAT with Supplemental HIV Serological Data ARC 09/08/99 – 08/31/00 NAT Result Western Blot Result Pos Ind Neg Total Pos 213 (94%) 38 21 272 Neg 13 1, 901 1, 856 3, 770 Total 226 (5. 6%) 1, 939 1, 877 4, 042 (1. 5%)
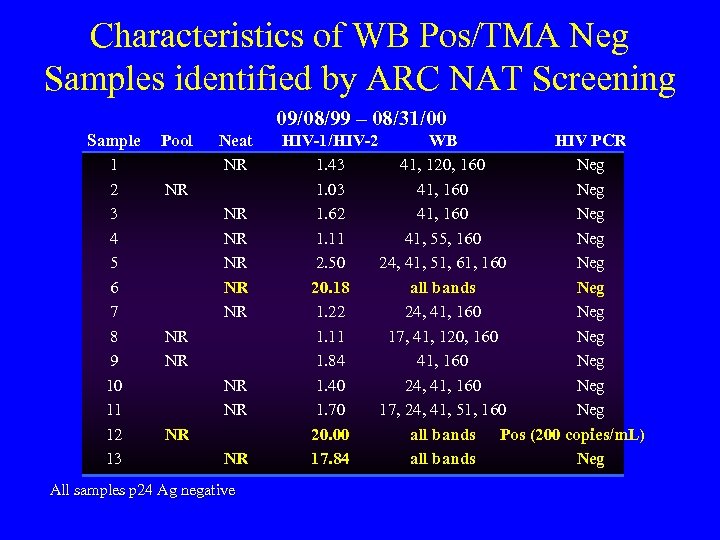
Characteristics of WB Pos/TMA Neg Samples identified by ARC NAT Screening 09/08/99 – 08/31/00 Sample 1 2 3 4 5 6 7 8 9 10 11 12 13 Pool Neat NR NR NR NR All samples p 24 Ag negative HIV-1/HIV-2 WB HIV PCR 1. 43 41, 120, 160 Neg 1. 03 41, 160 Neg 1. 62 41, 160 Neg 1. 11 41, 55, 160 Neg 2. 50 24, 41, 51, 61, 160 Neg 20. 18 all bands Neg 1. 22 24, 41, 160 Neg 1. 11 17, 41, 120, 160 Neg 1. 84 41, 160 Neg 1. 40 24, 41, 160 Neg 1. 70 17, 24, 41, 51, 160 Neg 20. 00 all bands Pos (200 copies/m. L) 17. 84 all bands Neg
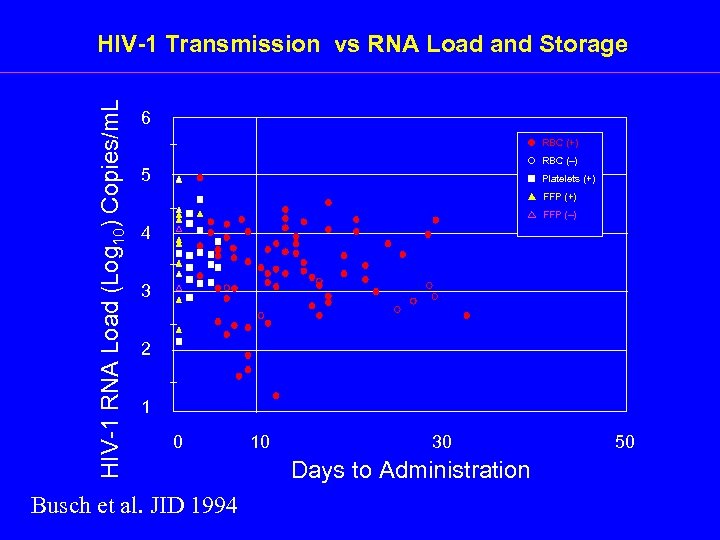
HIV-1 RNA Load (Log 10) Copies/m. L HIV-1 Transmission vs RNA Load and Storage 6 RBC (+) RBC (–) 5 Platelets (+) FFP (–) 4 3 2 1 0 Busch et al. JID 1994 10 30 Days to Administration 50
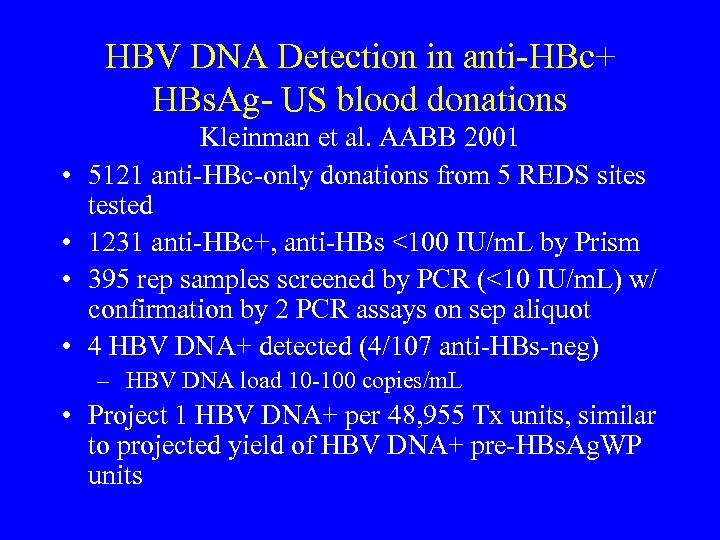
HBV DNA Detection in anti-HBc+ HBs. Ag- US blood donations • • Kleinman et al. AABB 2001 5121 anti-HBc-only donations from 5 REDS sites tested 1231 anti-HBc+, anti-HBs <100 IU/m. L by Prism 395 rep samples screened by PCR (<10 IU/m. L) w/ confirmation by 2 PCR assays on sep aliquot 4 HBV DNA+ detected (4/107 anti-HBs-neg) – HBV DNA load 10 -100 copies/m. L • Project 1 HBV DNA+ per 48, 955 Tx units, similar to projected yield of HBV DNA+ pre-HBs. Ag. WP units
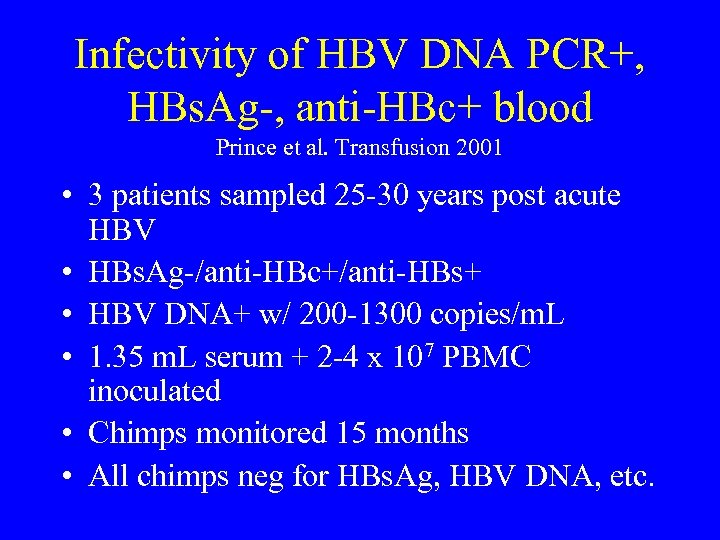
Infectivity of HBV DNA PCR+, HBs. Ag-, anti-HBc+ blood Prince et al. Transfusion 2001 • 3 patients sampled 25 -30 years post acute HBV • HBs. Ag-/anti-HBc+/anti-HBs+ • HBV DNA+ w/ 200 -1300 copies/m. L • 1. 35 m. L serum + 2 -4 x 107 PBMC inoculated • Chimps monitored 15 months • All chimps neg for HBs. Ag, HBV DNA, etc.
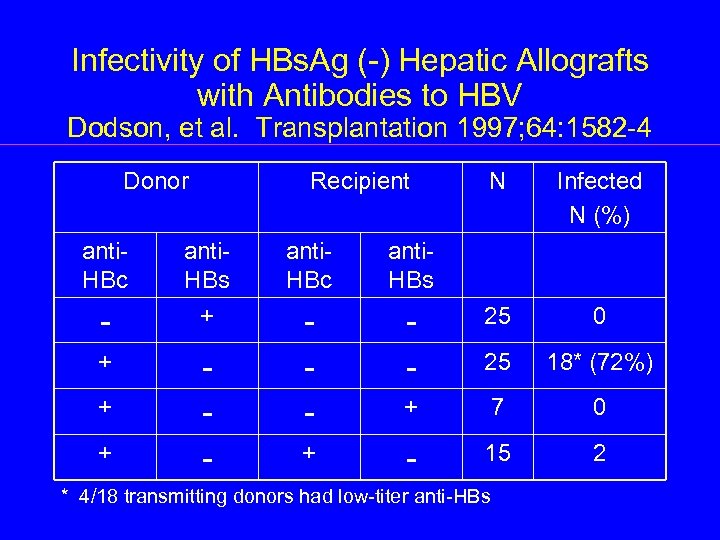
Infectivity of HBs. Ag (-) Hepatic Allografts with Antibodies to HBV Dodson, et al. Transplantation 1997; 64: 1582 -4 Donor Recipient N Infected N (%) anti. HBc anti. HBs - + 0 - - 25 + - 25 18* (72%) + 7 0 + - 15 2 + + * 4/18 transmitting donors had low-titer anti-HBs

Conclusions n Relationship between infectious units and RNA/DNA levels varies by stage of infection n During primary (pre-SC) infection, 1 -10 g. Eq/m. L plasma appears able to transmit infection n Ab+/NAT- donations must be presumed infectious (can’t d/c serology or reenter donors) n Need for future studies at international level 1. animal transmission model systems 2. donor lookback studies (prior units from NAT+ and Ab SC donors) 3. investigation of donors in TA-recipient infections
f2c585e3ce336f3b215be07ac070da5c.ppt