d459529dc9b04c767b2b77caa9b2717c.ppt
- Количество слайдов: 102

Nuclear Science Merit Badge Class 1
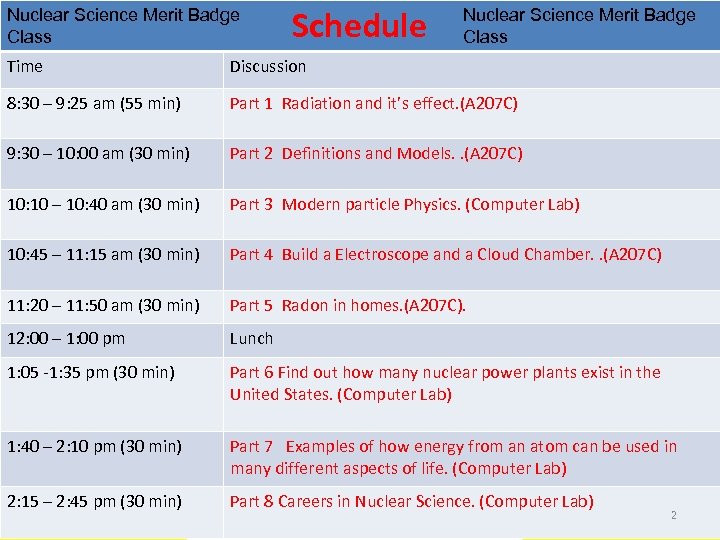
Nuclear Science Merit Badge Class Schedule Nuclear Science Merit Badge Class Time Discussion 8: 30 – 9: 25 am (55 min) Part 1 Radiation and it’s effect. (A 207 C) 9: 30 – 10: 00 am (30 min) Part 2 Definitions and Models. . (A 207 C) 10: 10 – 10: 40 am (30 min) Part 3 Modern particle Physics. (Computer Lab) 10: 45 – 11: 15 am (30 min) Part 4 Build a Electroscope and a Cloud Chamber. . (A 207 C) 11: 20 – 11: 50 am (30 min) Part 5 Radon in homes. (A 207 C). 12: 00 – 1: 00 pm Lunch 1: 05 -1: 35 pm (30 min) Part 6 Find out how many nuclear power plants exist in the United States. (Computer Lab) 1: 40 – 2: 10 pm (30 min) Part 7 Examples of how energy from an atom can be used in many different aspects of life. (Computer Lab) 2: 15 – 2: 45 pm (30 min) Part 8 Careers in Nuclear Science. (Computer Lab) 2

Nuclear Science Merit Badge “Nuclear science gives us a simple explanation of the natural world. The ultimate goal of nuclear science is to find out if there is one fundamental rule that explains how matter and forces interact. Earning the Nuclear Science Merit badge is a chance for Scouts to learn about this exciting field at the cutting edge of science today. ” 3

Part 1 Radiation and it’s effects. 4

1. Do the following: a. Tell what radiation is. b. Describe the hazards of radiation to humans, the environment, and wildlife. Explain the difference between radiation exposure and contamination. In your explanation, discuss the nature and magnitude of radiation risks to humans from nuclear power, medical radiation, and background radiation including radon. Explain the ALARA principle and measures required by law to minimize these risks. c. c. Describe the radiation hazard symbol and explain where it should be used. Tell why and how people must use radiation or radioactive materials carefully. 5

Radiation gives Superhuman Powers to The Hulk 6
Chernobyl 7

Radiation is • • • Plot device for fiction Scary Deadly Life saving Misunderstood Useful 8
Radiation is Energy • The energy is given off by unstable (radioactive) atoms and some machines. We will be focusing on ionizing radiation and its health effects. 9

Viewing of “Atom A Closer Look” 10

Nuclear Science Merit Badge Radiation Naturally 11

ALARA Principle and what is it? • ALARA" is an acronym for "As Low As Reasonably Achievable". 12

• How is ALARA used in the practice of radiation protection? – ALARA is a basic radiation protection concept or philosophy. – It is an application of the "Linear No Threshold Hypothesis, " which assumes that there is no "safe" dose of radiation. – Under this assumption, the probability for harmful biological effects increases with increased radiation dose, no matter how small. – Therefore, it is important to keep radiation doses to affected populations (for example, radiation workers, minors, visitors, students, members of the general public, etc. ) as low as is reasonably achievable. 13

• Where are ALARA principles utilized? – ALARA principles can be utilized in an infinite number of situations. • For example, the proper design of a nuclear facility depends on ALARA considerations (e. g. , can the addition of more shielding to an area be justified in terms of the lower doses it will achieve? ). • In addition, designing an x-ray facility for medical applications requires consideration of the amount of shielding needed to ensure that individuals located near the facility (e. g. , on the other side of the wall from the xray unit) do not receive any more dose than is really necessary during operation of the x-ray device. 14
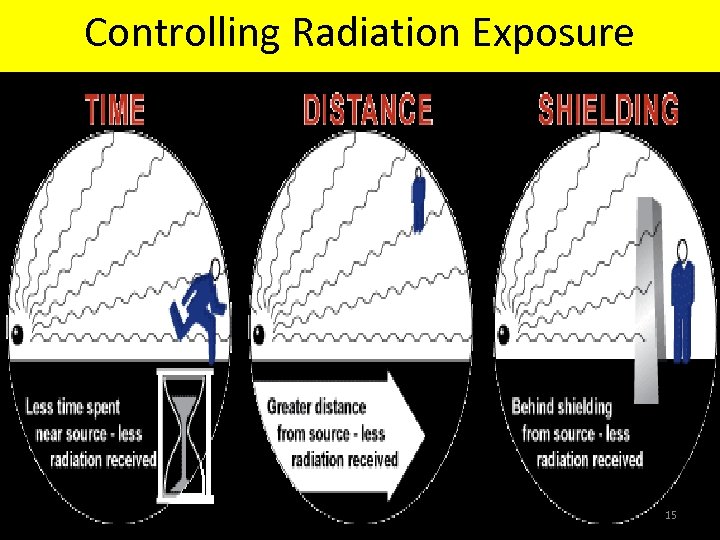
Controlling Radiation Exposure 15

Types of Radioactivity Six Common Types Alpha Decay Beta Decay Gamma Decay • Each type of radiation is ionizing • But different properties • affect the hazards they pose • the detection mechanism • shielding Fission Fusion Cosmic Rays 16
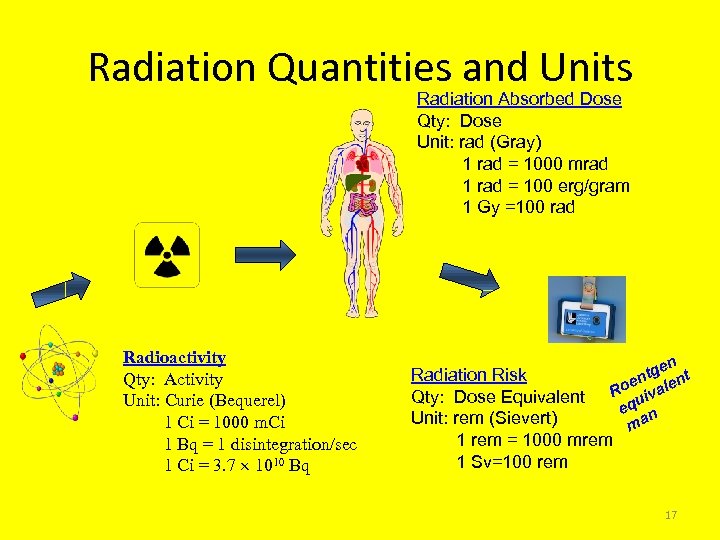
Radiation Quantities and Units Radiation Absorbed Dose Qty: Dose Unit: rad (Gray) 1 rad = 1000 mrad 1 rad = 100 erg/gram 1 Gy =100 rad Radioactivity Qty: Activity Unit: Curie (Bequerel) 1 Ci = 1000 m. Ci 1 Bq = 1 disintegration/sec 1 Ci = 3. 7 1010 Bq n tge Radiation Risk en alent Ro iv Qty: Dose Equivalent u eq n Unit: rem (Sievert) ma 1 rem = 1000 mrem 1 Sv=100 rem 17
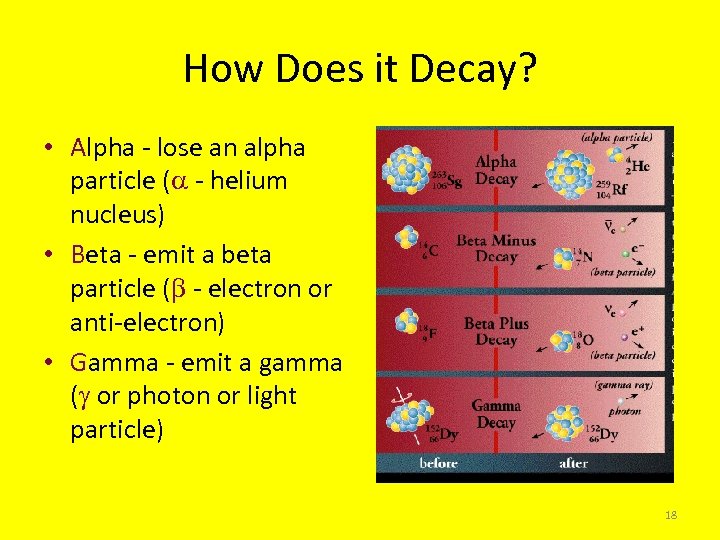
How Does it Decay? • Alpha - lose an alpha particle ( - helium nucleus) • Beta - emit a beta particle ( - electron or anti-electron) • Gamma - emit a gamma ( or photon or light particle) 18

Alpha Decay • Alpha particle or helium nucleus emitted • Nucleus changes mass by four units and charge by two units • Common for heavy elements • Changes chemical properties • Alpha particle easily stopped – 4 x nucleon mass – +2 Charge – Big 19
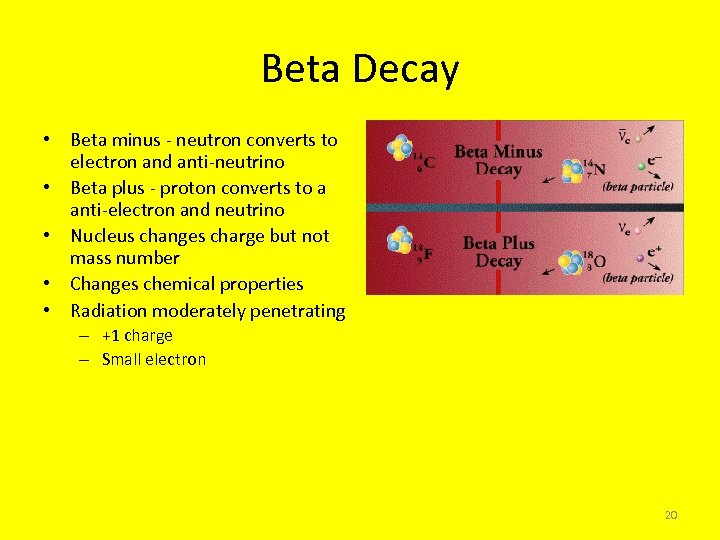
Beta Decay • Beta minus - neutron converts to electron and anti-neutrino • Beta plus - proton converts to a anti-electron and neutrino • Nucleus changes charge but not mass number • Changes chemical properties • Radiation moderately penetrating – +1 charge – Small electron 20
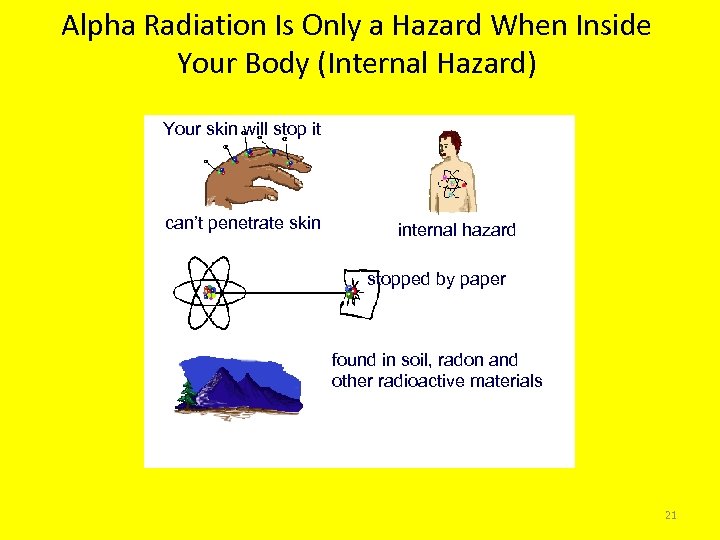
Alpha Radiation Is Only a Hazard When Inside Your Body (Internal Hazard) Your skin will stop it can’t penetrate skin internal hazard stopped by paper found in soil, radon and other radioactive materials 21
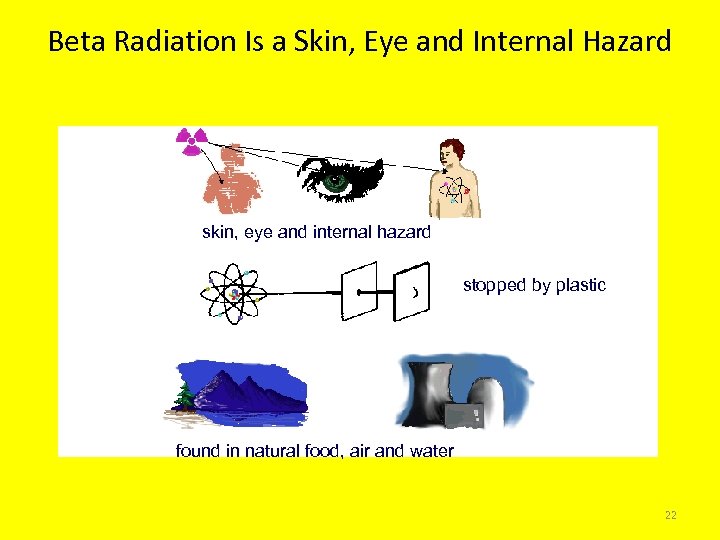
Beta Radiation Is a Skin, Eye and Internal Hazard skin, eye and internal hazard stopped by plastic found in natural food, air and water 22
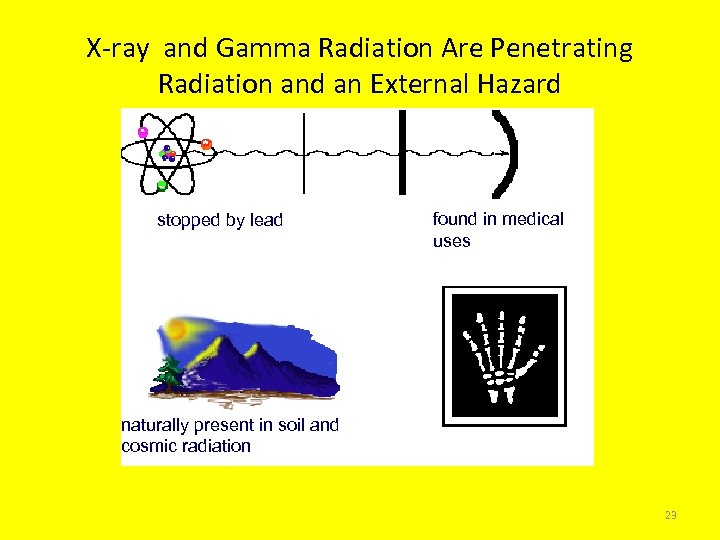
X-ray and Gamma Radiation Are Penetrating Radiation and an External Hazard stopped by lead found in medical uses naturally present in soil and cosmic radiation 23

Types of Exposure & Health Effects • Acute Dose - Deterministic – Large radiation dose in a short period of time – Large doses may result in observable health effects • Early: Nausea & vomiting • Hair loss, fatigue, & medical complications • Burns and wounds heal slowly – Examples: medical exposures and accidental exposure to sealed sources • Chronic Dose - Stochastic – – Radiation dose received over a long period of time Body more easily repairs damage from chronic doses Does not usually result in observable effects Inhalation Examples: Background Radiation and Internal Deposition 24
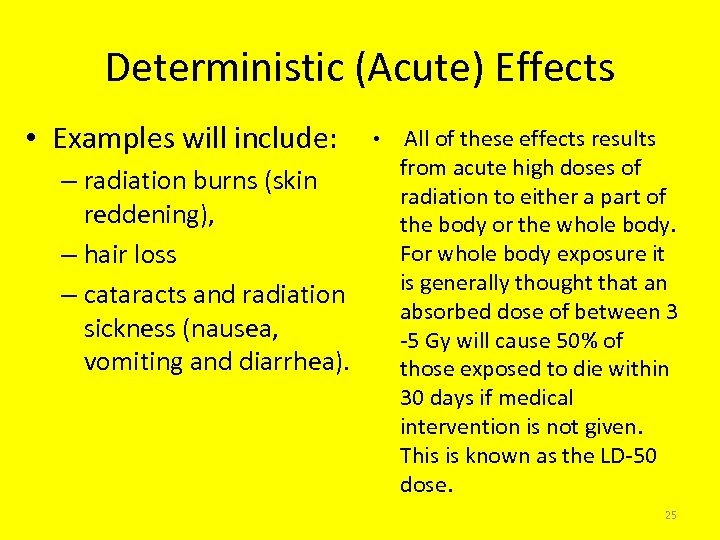
Deterministic (Acute) Effects • Examples will include: – radiation burns (skin reddening), – hair loss – cataracts and radiation sickness (nausea, vomiting and diarrhea). • All of these effects results from acute high doses of radiation to either a part of the body or the whole body. For whole body exposure it is generally thought that an absorbed dose of between 3 -5 Gy will cause 50% of those exposed to die within 30 days if medical intervention is not given. This is known as the LD-50 dose. 25
Stochastic (Chronic) Effects • Cancer • Leukemia • Genetic effects • Cataracts 26

Biological effects of radiation to humans. 1. Type of radiation involved. -All kinds of ionizing radiation can produce health effects. 2. Size of dose received. -The higher the dose of radiation received, the higher the likelihood of health effects. 3. Rate the dose is received. 4. Part of the body exposed. 5. The age of the individual. 6. Biological differences 27
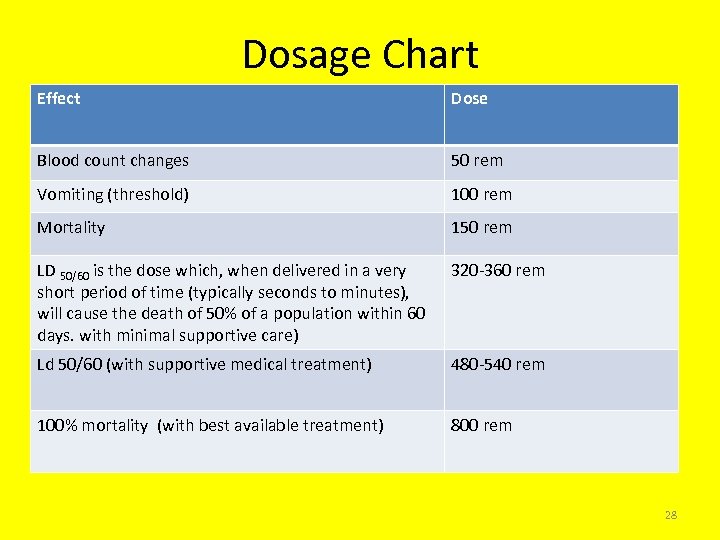
Dosage Chart Effect Dose Blood count changes 50 rem Vomiting (threshold) 100 rem Mortality 150 rem LD 50/60 is the dose which, when delivered in a very short period of time (typically seconds to minutes), will cause the death of 50% of a population within 60 days. with minimal supportive care) 320 -360 rem Ld 50/60 (with supportive medical treatment) 480 -540 rem 100% mortality (with best available treatment) 800 rem 28
29
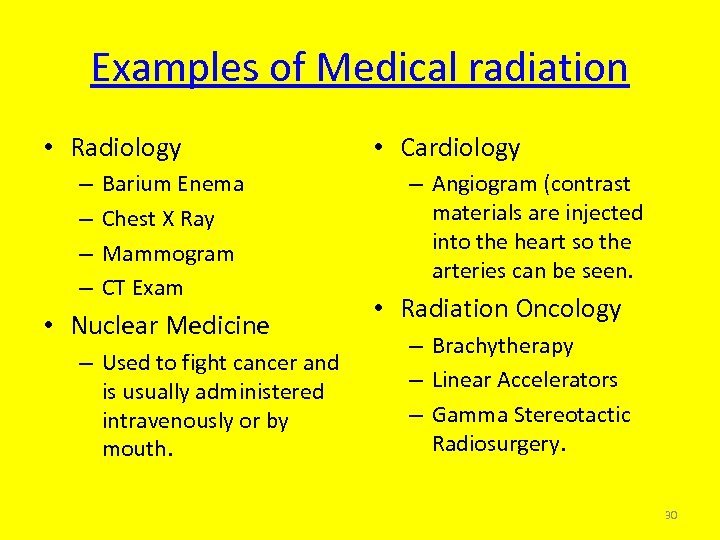
Examples of Medical radiation • Radiology – – Barium Enema Chest X Ray Mammogram CT Exam • Nuclear Medicine – Used to fight cancer and is usually administered intravenously or by mouth. • Cardiology – Angiogram (contrast materials are injected into the heart so the arteries can be seen. • Radiation Oncology – Brachytherapy – Linear Accelerators – Gamma Stereotactic Radiosurgery. 30

Background radiation • This radiation is constantly present in the environment and comes from a variety of sources. – Food and water – Space – Radon gas – Self-luminous dials and signs – Global radioactive contamination due to historical nuclear weapons testing. 31

Background radiation cont. • Global radioactive contamination due to historical nuclear weapons testing • Nuclear power station or nuclear fuel reprocessing accidents • Normal operation of facilities used for nuclear power and scientific research • Emissions from burning fossil fuels, such as coal fired power plants • Emissions from nuclear medicine facilities and patients 32

Radiation Hazard Symbol The symbol is placed on a placard with the word CAUTION or DANGER or GRAVE DANGER centered about it. Under the symbol is the information addressing the types of hazards. Examples are: Radiation Area High Radiation Area Airborne Radioactivity Area Contaminated Area Radioactive Materials Area 60° R 1. 5 R 5 R 33
Other examples of Radiation symbols United Nations Symbol 34

Part 2 35
• Do the following: a. Tell the meaning of the following: atom, nucleus, proton, neutron, electron, quark, isotope; alpha particle, beta particle, gamma ray, X-ray; ionization, radioactivity, and radioisotope. b. Choose an element from the periodic table. Construct 3 -D models for the atoms of three isotopes of this element, showing neutrons, protons, and electrons. – Use three models to explain the difference between atomic number and mass number and the difference between the quark structure of a neutron and a proton. 36
Terms and Definitions • Atom Basic component of matter. An atom is the smallest part of an element having all the chemical properties of that element. An atom consists of a nucleus (that contains protons and neutrons) and surrounding electrons. • Nucleus The central part of an atom that contains protons and neutrons. The number of protons uniquely defines the chemical element. • Proton One of three basic particles in an atom. Protons are located in the atom nucleus, have a positive 37 electrical charge, and each has mass about equal to a

Terms and Definitions • Neutron One of three basic particles in all atoms except hydrogen. Neutrons are located in the atom nucleus, are electrically neutral, and each has mass about equal to a proton. • Electron One of three basic particles in an atom. The electron has a negative electrical charge, orbits the atom nucleus, and has very little mass compared to the nucleus. • Quark basic building block of protons, neutrons, other baryons, and mesons. 38

Terms and Definitions • Isotope: atomic nuclei having same number of protons but different numbers of neutrons. • Alpha particle: positively- charged particles consisting of two protons and two neutrons emitted by radioactive materials. • Beta particle: high speed electron emitted by a radioactive nucleus in beta decay. • Gamma particle: high energy photon emitted by a radioactive nucleus. 39

Terms and Definitions • X ray: high- energy photons; high- frequency, short-wavelength electromagnetic waves. • Ionizing radiation particles or waves that can remove electrons from atoms, molecules, or atoms in a solid. • Radioactivity Spontaneous emission of radiation from the unstable nucleus of an atom. • Radioactive isotope Element that emits ionizing radiation when it decays. Radioactive isotopes are commonly used in science, industry, and medicine. 40

Definitions • Background radiation: Radiation arising from natural sources always present in the environment, including solar and cosmic radiation from outer space and naturally radioactive elements in the atmosphere, the ground, building materials, and the human body. • Contamination: Act of making a substance impure, radioactive, or unclean. 41

Definitions • Becquerel (Bq): Measure of the rate of decay of a radioactive substance. One Bq is 1 disintegration per second. The human body has thousands of disintegrations from the presence of potassium-40. • Curie (Ci): Unit of measure of the rate of decay of a radioactive material. One Curie is the radioactive intensity of one gram of radium--37 billion disintegrations per second. • Half-life: Time for a radioactive substance to lose half of its activity due to radioactive decay. At the end of one half-life, 50% of the original radioactive material has decayed. 42

Definitions • Nuclear energy : Energy, usually in the form of heat or electricity, produced by the process of nuclear fission within a nuclear reactor. The coolant that removes the heat from the nuclear reactor is normally used to boil water, and the resultant steam drives steam turbines that rotate electrical generators. Nuclear energy is also produced when two nuclei fuse. • Nuclear Reactor: Any of several devices in which a chain reaction is initiated and controlled, with the resulting heat typically used for power generation and the neutrons and fission products used for military, experimental, and medical purposes. Also called atomic reactor. 43

Definitions • Particle accelerator: A device, such as a cyclotron or linear accelerator, that accelerates charged subatomic particles or nuclei to high energies. Also called atom smasher. • Rad: Basic unit of absorbed dose of ionizing radiation. • Gray: unit of absorbed dose of ionizing radiation. • Radiation: Particles and electromagnetic rays (waves) emitted from the center of an atom during radioactive disintegration. 44

Definitions • Radon: Heavy, natural, radioactive gas formed by the radioactive decay of radium, a decay product of uranium. Its atomic number is 86 and its atomic weight is 222. It’s symbol is Rn. • rem: (Roentgen equivalent man), a unit used in radiation protection to measure the amount of damage to human tissue from a dose of ionizing radiation. An average American receives about 0. 360 rems of radiation per year. • Sievert : Unit that measures the effect of radiation on the body. "Sievert" replaces the old unit "REM" (Radiation Equivalent Man), a calculated number based on dose and the body organ (e. g. a dose on your eye would give a different number from the same dose on the liver). 1 REM = 10 milli. Sieverts (m. Sv). 45
Computer Lab • Construct a 3 -D model for atoms. – Choose 3 elements and explain the difference between them. 46
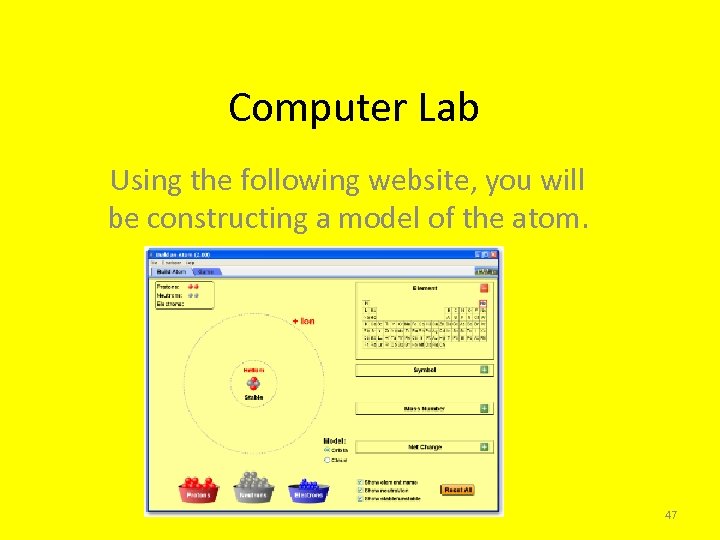
Computer Lab Using the following website, you will be constructing a model of the atom. 47

Part 3 48

Discuss modern particle physics with your counselor: a. Name three particle accelerators and describe several experiments that each accelerator performs. b. then discuss modern particle physics with your counselor: 49
Types of particle accelerators • Cyclotrons • Synchrotrons – These all used single – a type of particle beams with fixed targets. accelerator similar to a They tended to have very betatron but having an briefly-run, inexpensive, electric field of fixed and unnamed experiments frequency with electrons • Fixed-target accelerators but not with protons as – More modern accelerators well as a changing that were also run in fixed magnetic field. target mode 50

Types of particle accelerators • Electron-positron colliders – LEP collides together bunches of electrons with bunches of positrons, as they travel in opposite directions round a ring 27 km in circumference, at velocities close to the speed of light. When the bunches of particles meet, some electrons and positrons annihilate, creating, for a fraction of a second, bursts of high energy which echo the state of the early Universe, but are quite harmless. 51

Types of Particle accelerators • Hadron colliders – Two beams of subatomic particles called 'hadrons' – either protons or lead ions – will travel in opposite directions inside the circular accelerator, gaining energy with every lap. Physicists will use the LHC to recreate the conditions just after the Big Bang, by colliding the two beams head-on at very high energy 52
• Ion colliders (ALICE) • Electron-proton colliders – What happens to matter when it is heated to 100, 000 times the temperature at the centre of the Sun ? – Why do protons and neutrons weigh 100 times more than the quarks they are made of ? – Can the quarks inside the protons and neutrons be freed ? 53

Six Individuals important to the field of atomic energy 1. Abert Einstein 2. Marie Sklodowski Curie 3. Ernest Rutherford 4. Neils Bohr 5. Enrico Fermi 6. Robert Oppenheimer 7. Glenn Seaborg 54

Marie Curie (1867 -1891) Movie clip 1. She discovered the mysterious element radium. 2. It opened the door to deep changes in the way scientists think about matter and energy. 3. She also led the way to a new era for medical knowledge and the treatment of diseases. 55
Ernest Rutherford 1871 -1937 • Known as the father of nuclear physics. • Mostly known for discovering the correct structure of atoms. 56

Albert Einstein (1879 -1955) 57
Albert Einstein (1879 -1955) • 1905, produced theory of relativity (E=mc 2) • This resulted in the shocking conclusion that time depends on the observer. • When moving at high speeds – Effective mass increases – Time slows – Length shrinks • only the speed of light remains the same. 58

Albert Einstein (1879 -1955) • Experimenters have carried extremely accurate atomic clocks on high-speed jets on around-the-world journeys. And when they compared these clocks to the extremely accurate clocks they left at home, the traveling clock had indeed gone slower and lost time. But by very little. 59
Albert Einstein (1879 -1955) • received the 1921 Nobel Prize for work in mathematical physics & stating the law of the Photoelectric Effect – Einstein proposed that under certain circumstances light can be considered as consisting of particles – Also hypothesized that the energy carried by any light particle, called a photon, is proportional to the frequency of the radiation 60
Niels Bohr (1885 -1962) • Danish physicist who made fundamental contributions to understanding atomic structure and quantum mechanics. 61

Enrico Fermi (1901 -1954) • An Italian physicist most noted for his work on the development of the first nuclear reactor. • He is regarded as one of the leading scientists of the 20 h century, 62

Robert Oppenheimer (1904 -1967) • An American theoretical physicist and professor of physics at the University of California, Berkeley. • He is best known for his role as the scientific director of the Manhattan Project. • The Manhattan Project is the code name for the US government's secret project that was established before World War II and culminated in the development of the nuclear bomb 63

Glenn Seaborg (1912 -1999) 64

Glenn Seaborg • Co-discovered Plutonium and 9 other elements • Element 106 - Seaborgium named after him – Only living person to have element named after him • Identified more than 100 isotopes • Figured out how transuranium elements fit in the periodic table • Identified medical isotopes - saved his mother’s life with discovery of Iodine-131 65

Part 4 66

Do TWO of the following; then discuss with your counselor the different kinds of radiation and how they can be used: a. Build an electroscope. Show it works. Place a radiation source inside and explain the effect it causes. b. Make a cloud chamber. Show it can be used to see the tracks caused by radiation. Explain what is happening. 67
Electroscope • An electroscope is a device that is used to demonstrate properties of static electricity. • The electroscope demonstrates the repulsive force that is exerted between two nearby objects with the same electric charge. 68
Cloud Chamber • The cloud chamber, also known as the Wilson chamber, is used for detecting particles of ionizing radiation. • In its most basic form, a cloud chamber is a sealed environment containing a supersaturated vapor of water or alcohol. • When an alpha or beta particle interacts with the mixture, it ionizes it. • The resulting ions act as condensation nuclei, around which a mist will form (because the mixture is on the point of condensation). The high energies of alpha and beta particles mean that a trail is left, due to many ions being produced along the path of the charged particle. 69

Part 5 70

Part 5 Do ONE of the following; then discuss with your counselor the principles of radiation safety: b. Describe how radon is detected in homes. Discuss the steps taken for the long-term and short-term test methods, tell how to interpret the results, and explain when each type of test should be used. Explain the health concern related to radon gas and tell what steps can be taken to reduce radon in buildings. 71

Radon A Science Issue 72

What is Radon? • Radon is an indoor air pollutant. • Radon is a colorless, odorless radioactive gas that comes from naturally occurring uranium in the soil. • The only way to tell how much radon a home has is to TEST. 73

Surgeon General’s Warning • “Indoor radon is the second-leading cause of lung cancer in the United States and breathing it over prolonged periods can present a significant health risk to families all over the country. ” 74

Test! • The only way to know the radon level in a building is to test. • Basement, crawl space, slab on grade or foundation combinations can have a radon problem. 75
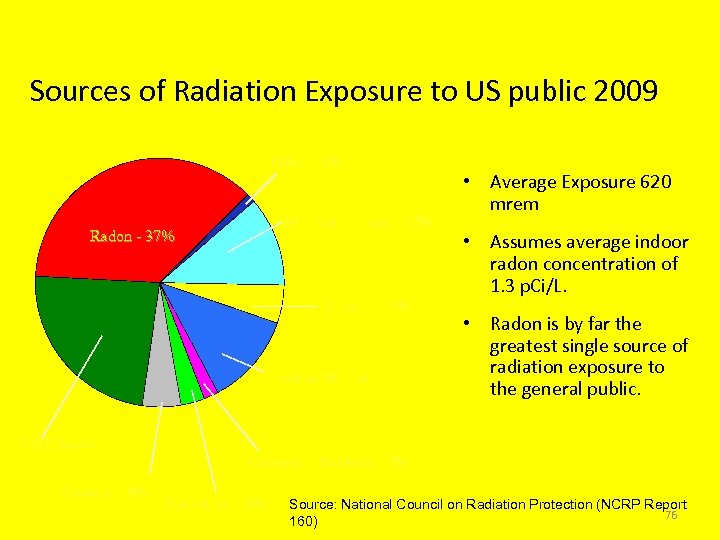
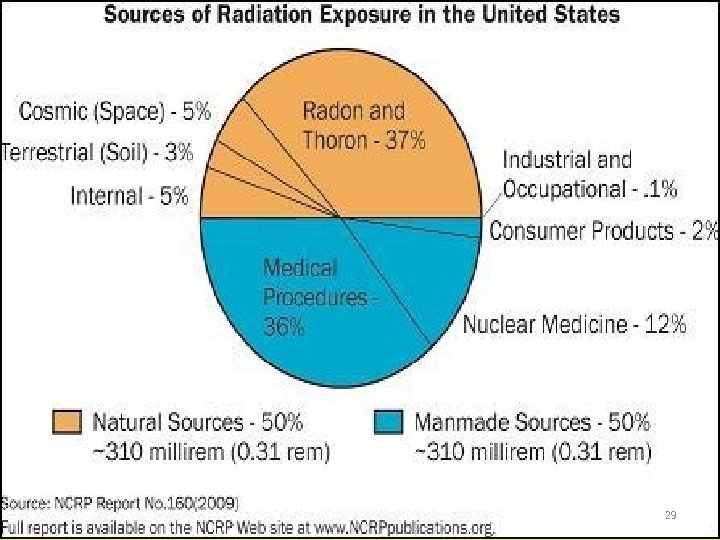
Sources of Radiation Exposure to US public 2009 Other - 1% Medical X-Rays - 12% Radon - 37% Internal - 5% Nuclear Medicine – 12% CAT Scans - 24% Cosmic - 5% • Average Exposure 620 mrem • Assumes average indoor radon concentration of 1. 3 p. Ci/L. • Radon is by far the greatest single source of radiation exposure to the general public. Consumer Products - 2% Terrestrial - 3% Source: National Council on Radiation Protection (NCRP Report 76 160)

Current Name • Radon has been known by its current name since 1923. • It was named after the element radium. The suffix “on” was used as with all other inert gases. 77

Radon Action Level • The USEPA set an action level for indoor radon concentration of 4. 0 picocuries of radon per liter of air (p. Ci/L). • USEPA selected 4. 0 p. Ci/L because of the technological and economical bases. • Risk at 4. 0 p. Ci/L about seven (7) people out of a thousand could get lung cancer. * *A Citizen’s Guide to Radon (2005). 78
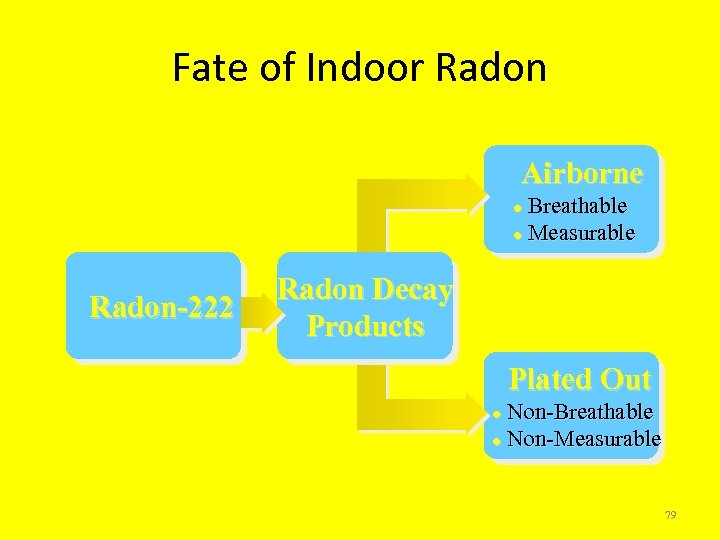
Fate of Indoor Radon Airborne Breathable l Measurable l Radon-222 Radon Decay Products Plated Out Non-Breathable l Non-Measurable l 79

Radon Decay Product Characteristics • Source of cell damage in lungs through release of alpha and beta particles (radiation) • Short-lived decay products most significant • Have static charges • Chemically reactive • Solid particles • Heavy Metals 80

Radon Exposure • Radon and Radon Decay Products (RDPs) are breathed in and the Radon is exhaled. • Because they are solid particles, RDPs remain in lung tissue and are trapped in the bronchial epithelium and emit alpha particles which strike individual lung cells and may cause physical and/or chemical damage to DNA. 81

How did radon originate in Ohio? • Glaciers from Canada deposited uranium in the soil. • Radon results from the uranium deposits. 82
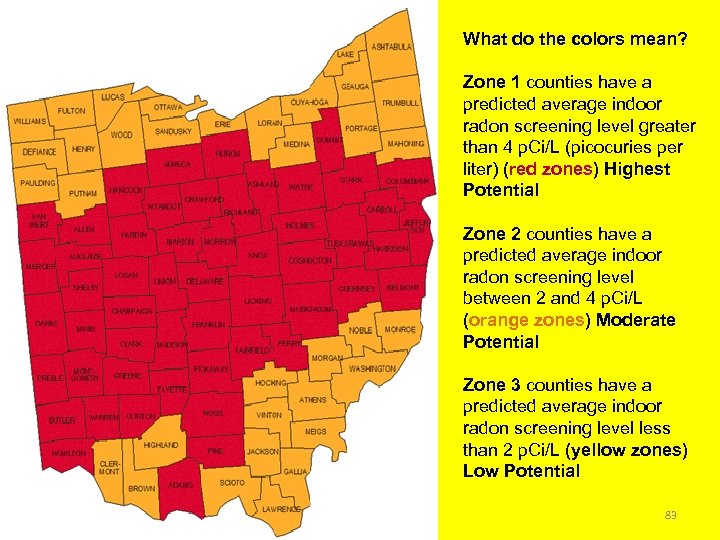
What do the colors mean? Zone 1 counties have a predicted average indoor radon screening level greater than 4 p. Ci/L (picocuries per liter) (red zones) Highest Potential Zone 2 counties have a predicted average indoor radon screening level between 2 and 4 p. Ci/L (orange zones) Moderate Potential Zone 3 counties have a predicted average indoor radon screening level less than 2 p. Ci/L (yellow zones) Low Potential 83
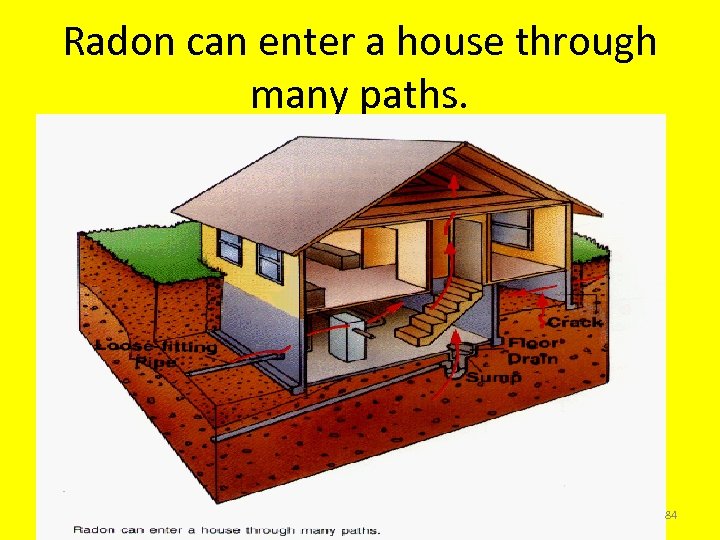
Radon can enter a house through many paths. 84
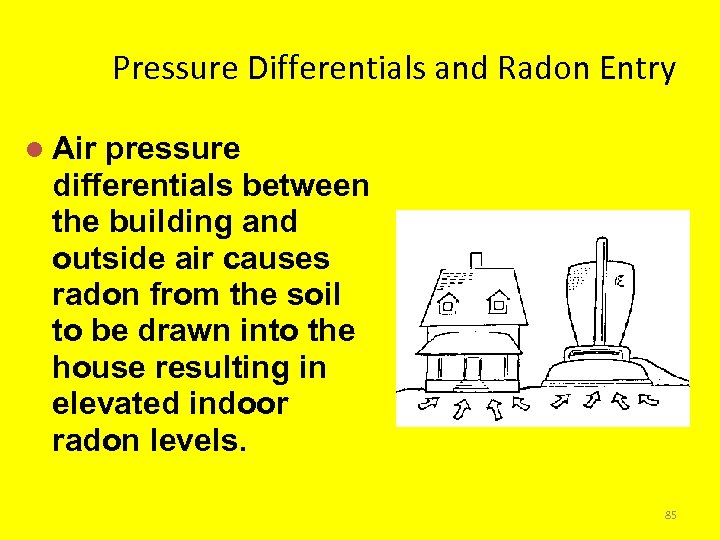
Pressure Differentials and Radon Entry l Air pressure differentials between the building and outside air causes radon from the soil to be drawn into the house resulting in elevated indoor radon levels. 85
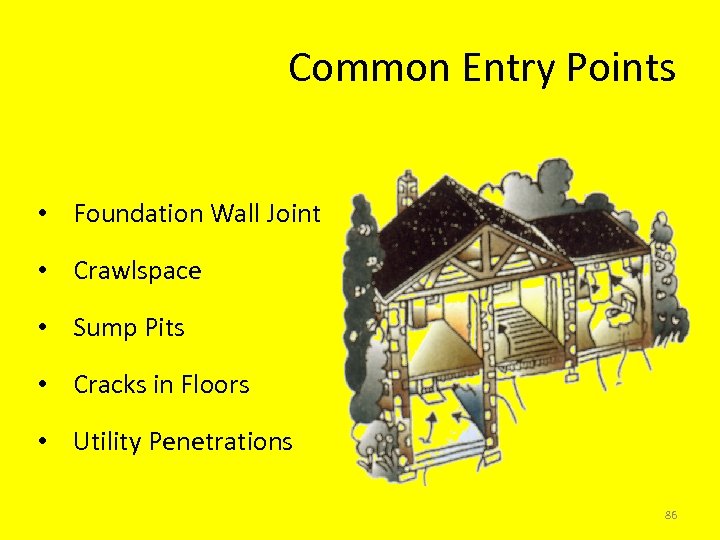
Common Entry Points • Foundation Wall Joint • Crawlspace • Sump Pits • Cracks in Floors • Utility Penetrations 86

Who can test? • The occupant of a dwelling may test their own home. Test kits are available from hardware and department stores or directly from laboratories listed on the Ohio Department of Health Radon • website http: //www. radon. com/sub/oh/ 87
Test the Footprint • Footprint means each foundation type in direct contact with soil or other material. • Short-term or long-term measurements shall be made in each lowest structural area suitable for occupancy. For example, a split-level building with a basement, a slab-ongrade room and a room over crawlspace shall have measurements made in each of the foundation types: the basement, a slab-on-grade room and a room over the crawlspace. 88

Detector Placement is Crucial • Place in an area where the detector will not be disturbed. • At least 3 feet from doors or windows to the outside. • Out of the direct flow of air from a ventilation duct. • At least 1 foot from exterior walls. • 20 inches to 6 feet from the floor. • At least 4 inches away from other objects horizontally or vertically above the detector. • At least 4 feet from heat, fireplaces and furnaces, out of direct sunlight, etc. 89

Rooms to Test • Measurements shall be made in rooms that can be regularly occupied by individuals, such as family rooms, living rooms, dens, playrooms and bedrooms. 90

If Tests Are Above 4. 0 p. Ci/L • Ohio provides a list of Professional Radon Mitigators trained to reduce radon levels. • Professional Radon Mitigators and Technicians must meet specific requirements to obtain a license with Ohio. 91

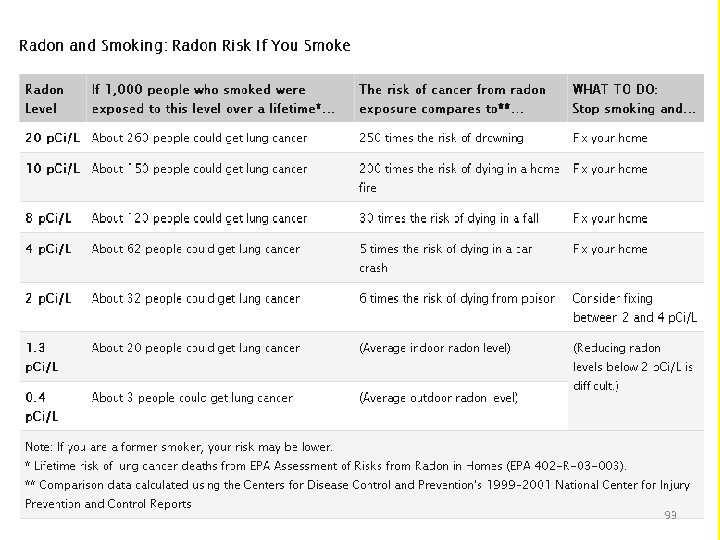
Interpreting Radon Test Results • The average indoor radon level is estimated to be about 1. 3 p. Ci/L; roughly 0. 4 p. Ci/L of radon is normally found in the outside air. • A radon level below 4 p. Ci/L still poses a risk. Consider fixing when the radon level is between 2 and 4 p. Ci/L. 92
93
• Mitigation Systems Reduce Radon Collecting radon prior to by: its entry into the building and discharging it above the highest eave. • Modifying building pressure differentials. 94

Active Soil Depressurization • Active Soil Depressurization uses a fan to draw radon from beneath the house. • All radon mitigation systems shall be designed to reduce a radon concentration in each area within the footprint of the building as low as reasonably achievable (ALARA). • Crawl spaces must be included in a radon reduction plan. 95
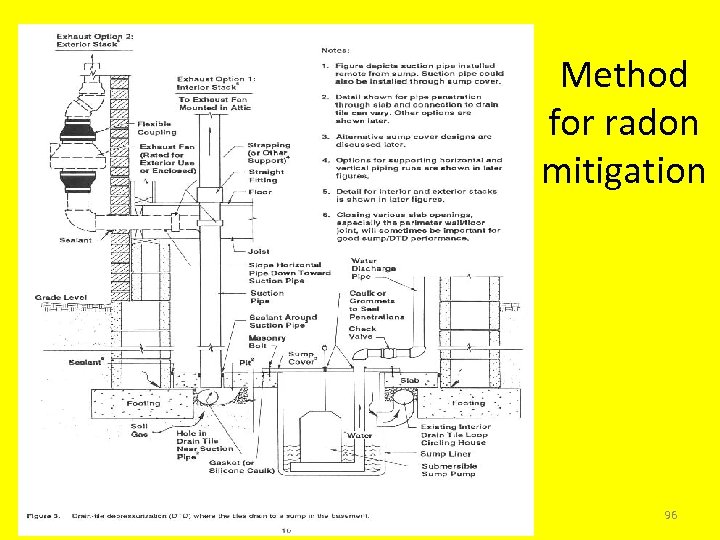
Method for radon mitigation 96

Part 6, 7, and 8 will be done in the Computer lab. 97

Part 6 Do the following; then discuss with your counselor how nuclear energy is used to produce electricity: c. Find out how many nuclear power plants exist in the United States. Locate the one nearest your home. Find out what percentage of electricity in the United States is generated by nuclear power plants, by coal, and by gas. 98

Part 7 99

• Give an example of each of the following in relation to how energy from an atom can be used: – nuclear medicine – environmental applications – industrial applications – space exploration, and radiation therapy. • For each example, explain the application and its significance to nuclear science. 100

Part 8 101

Part 8 • Find out about three career opportunities in nuclear science that interest you. – Pick one and find out the education, training, and experience required for this profession and discuss this with your counselor. – Tell why this profession interests you. 102
d459529dc9b04c767b2b77caa9b2717c.ppt