705dba013f42fa69a1350d3db47e0f16.ppt
- Количество слайдов: 73

Nuclear Physics

Shortcut to Atomic. Bomb. Test
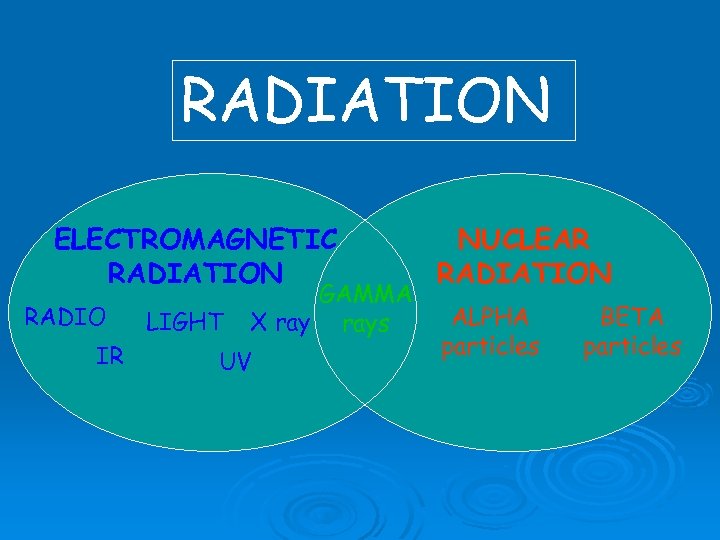
RADIATION ELECTROMAGNETIC RADIATION RADIO IR LIGHT GAMMA X rays UV NUCLEAR RADIATION ALPHA particles BETA particles

Using Nuclear Radiation
Cancer Therapy

Electricity Production
Nuclear Powered Pacemaker

Smoke Detectors

Sterilisation

Radiocarbon Dating
Radioactive Tracers
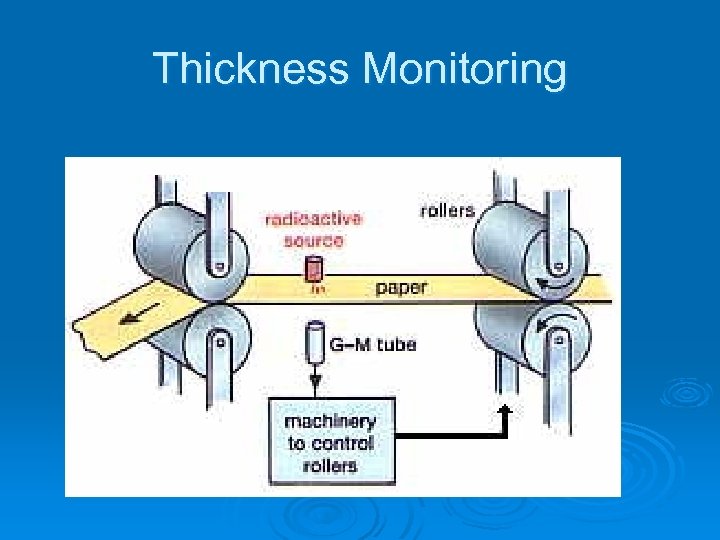
Thickness Monitoring

Understanding Atoms Ø Our understanding of what’s inside atoms has developed in the last 2000 years……. .

Democritus Everything is made of atoms
The Dalton Model In about 1810, James Dalton decided that matter was made of tiny, solid, spherical particles called atoms.
Ø He introduced the idea of atoms as elementary particles.
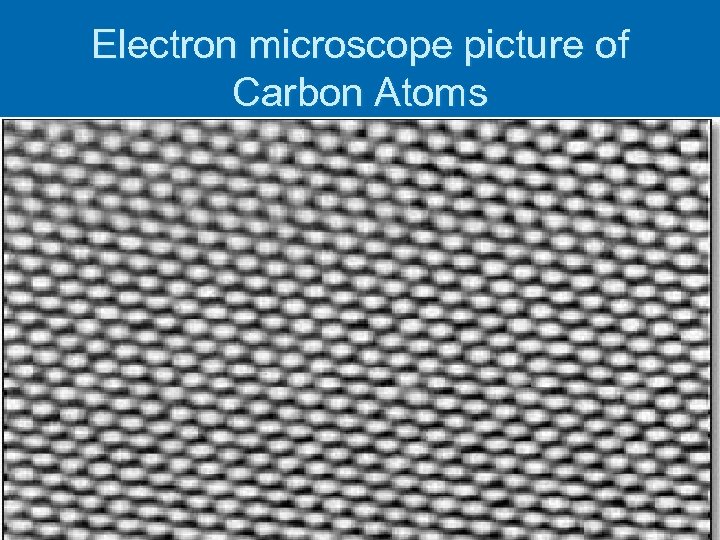
Electron microscope picture of Carbon Atoms

Thomson’s Model of the Atom In 1897, JJ Thomson discovered electrons, and suggested that the atom was a solid sphere of positive charge with electrons stuck in it like plums in a plum pudding.
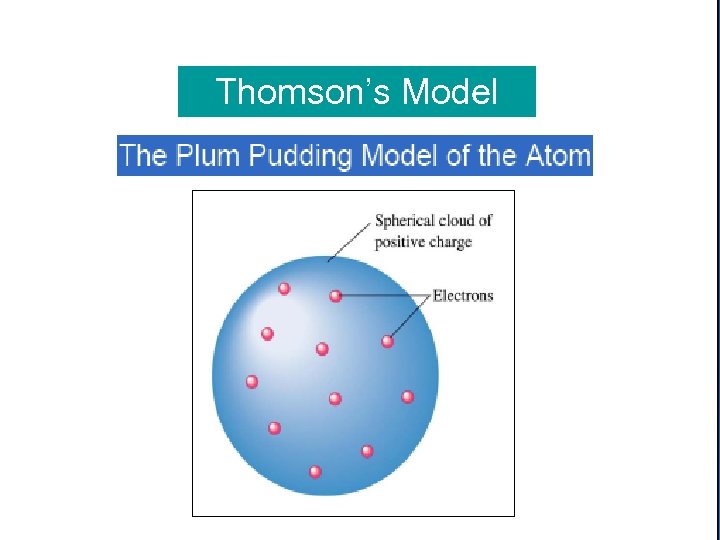
Copyright © Houghton Thomson’s Model Mifflin Company. All rights reserved. 2– 19 Rutherford's Experiment On α-Particle. Houghton Mifflin Company. of Bombardment Copyright © All rights reserved. 2– 19 Metal Foil Rutherford's Experiment On α-Particle Bombardment of Metal Foil

The Rutherford Model

Rutherford’s Experiment In 1909, Ernest Rutherford wanted to carry out an experiment to test Thomson’s Model. Ø We can’t see inside an atom with our eyes, but he wanted to “see” inside. Ø
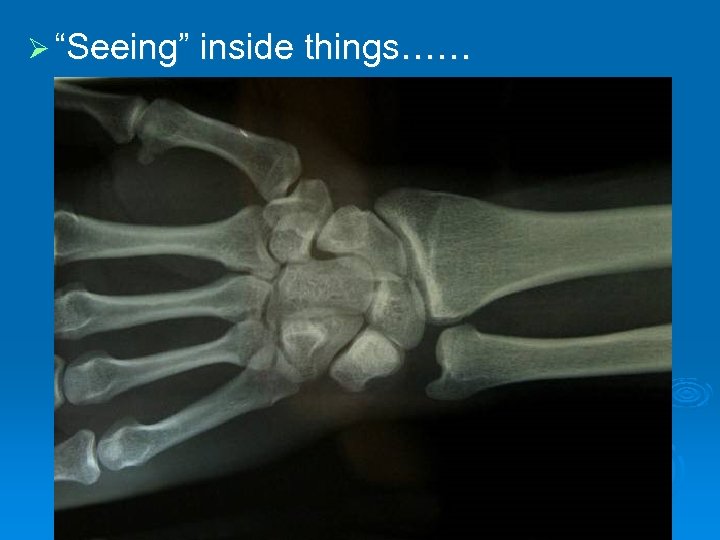
Ø “Seeing” inside things……
Ø “Seeing” inside things……
Ø How do we know what’s in the earth?

Ø Rutherford managed to “look inside” the atom by firing tiny particles called alpha particles at a thin gold foil to see how the alpha particles were deflected by the atoms of gold. Ø Alpha particles are the same as Helium nucleii, they are emitted from some radioactive atoms.
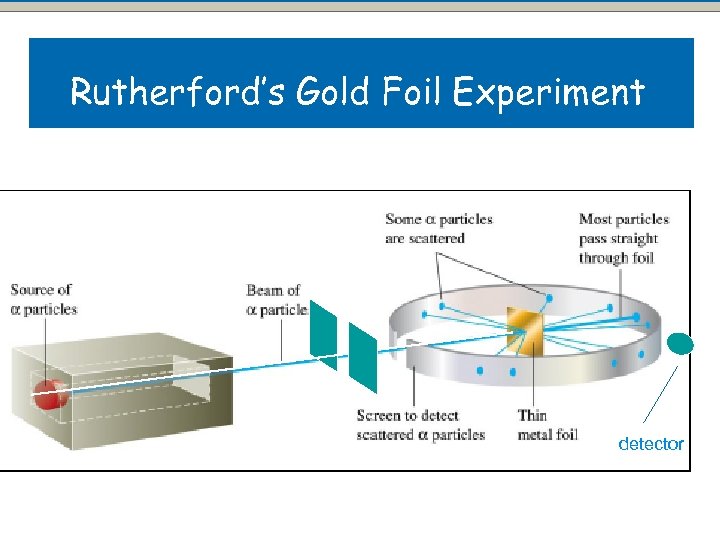
Rutherford’s Gold Foil Experiment detector

What they expected to happen: GOLD FOIL

What did happen: GOLD FOIL

Ø This is what they expected to happen Thomson Model They expected to see small or no deflection of the alpha particles Ø This is what did happen Rutherford model
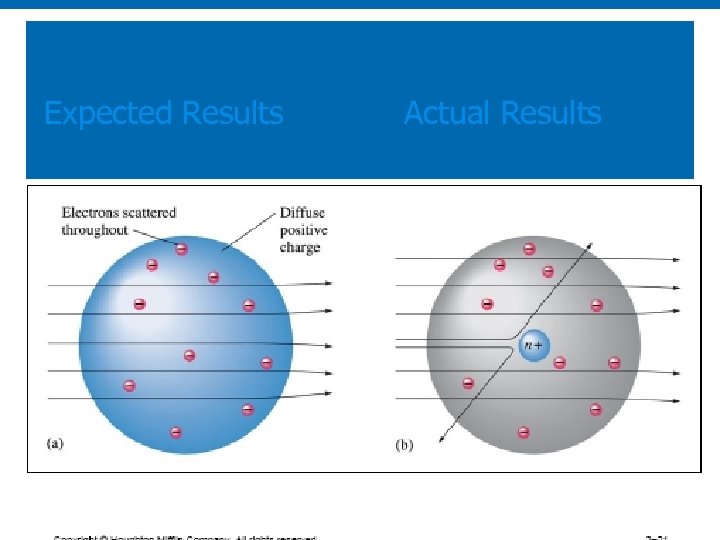
Expected Results Actual Results

Rutherford’s Experiment (1911) Results: Ø Most particles go straight through. Ø Some particles are deflected from straight path. A few even go backwards Interpretation: Ø Most of the atom is empty space. Ø nucleus must be positive, very dense, more massive than alpha particles. Ø Negative electrons orbit the nucleus, but are much lighter

Rutherford’s Atom
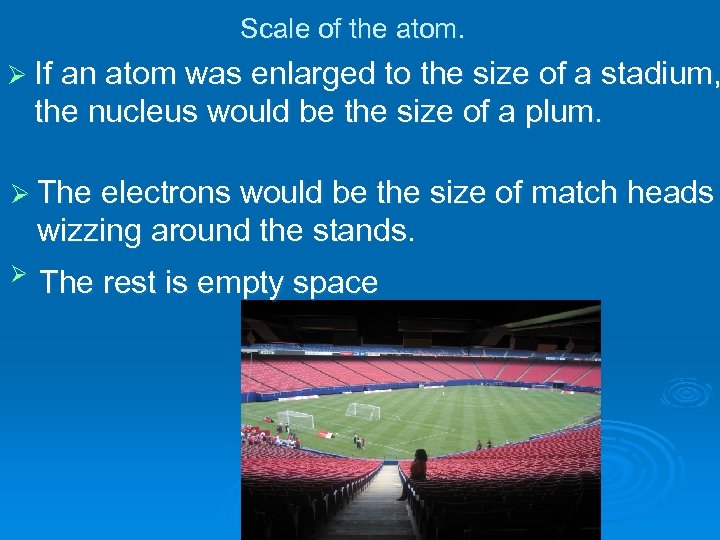
Scale of the atom. Ø If an atom was enlarged to the size of a stadium, the nucleus would be the size of a plum. Ø The electrons would be the size of match heads wizzing around the stands. Ø The rest is empty space

Size of the Atom Ø An atom is roughly 10 -10 m in diameter Ø This means a full stop is roughly ten million atoms across. Ø A small nucleus is roughly 10 -15 m in diameter Ø This is 1/100, 000 ths the diameter of the atom

Fundamental Forces Ø What are the only two Fundamental Forces you are familiar with? Ø Gravity Ø Electric
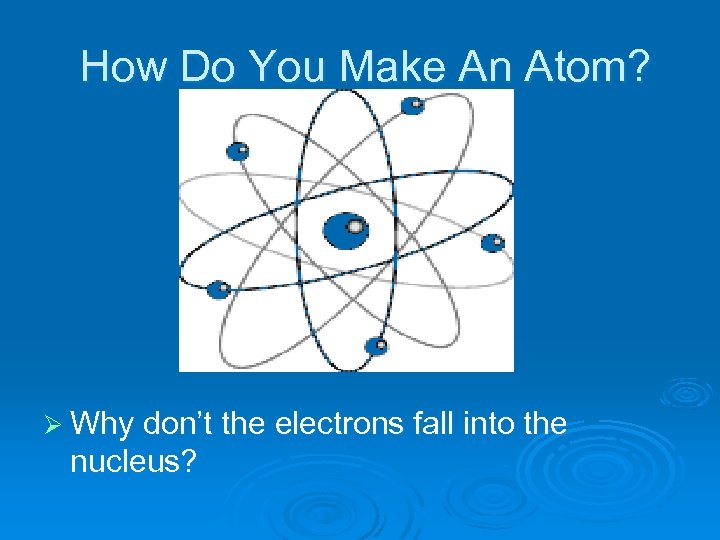
How Do You Make An Atom? Ø Why don’t the electrons fall into the nucleus?
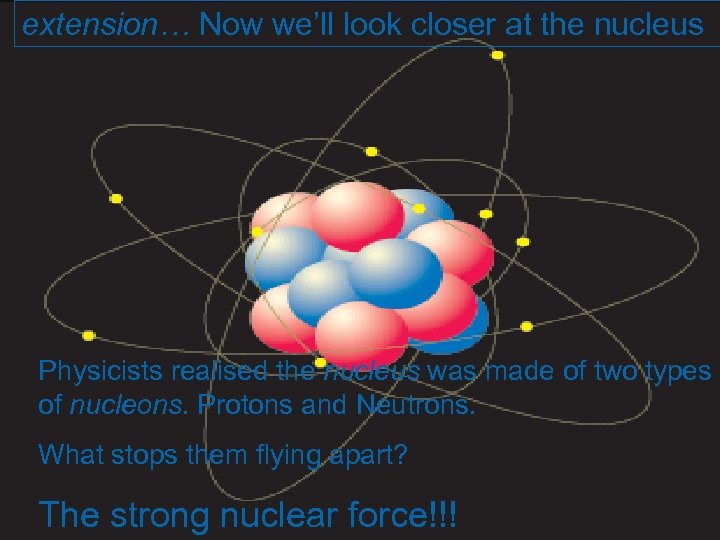
extension… Now we’ll look closer at the nucleus Physicists realised the nucleus was made of two types of nucleons. Protons and Neutrons. What stops them flying apart? The strong nuclear force!!!
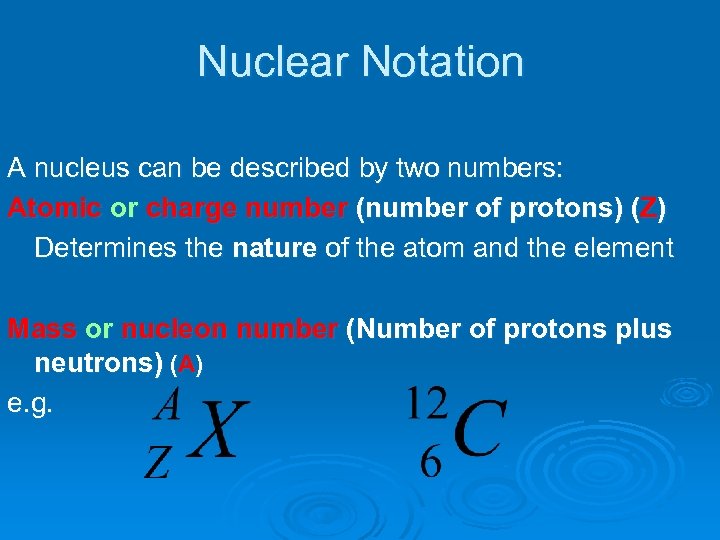
Nuclear Notation A nucleus can be described by two numbers: Atomic or charge number (number of protons) (Z) Determines the nature of the atom and the element Mass or nucleon number (Number of protons plus neutrons) (A) e. g.
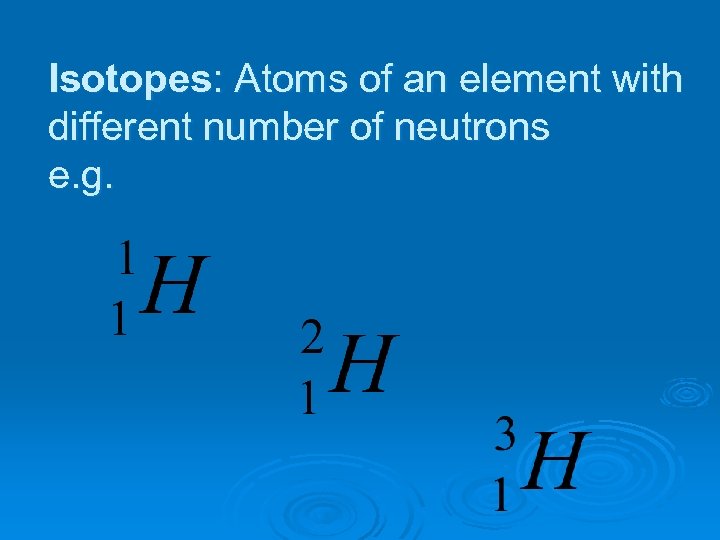
Isotopes: Atoms of an element with different number of neutrons e. g.

Radioactive Decay Ø Nuclei that have too much energy are unstable. Ø They become more stable by firing out some nuclear radiation Ø There are three types of radioactive decay……
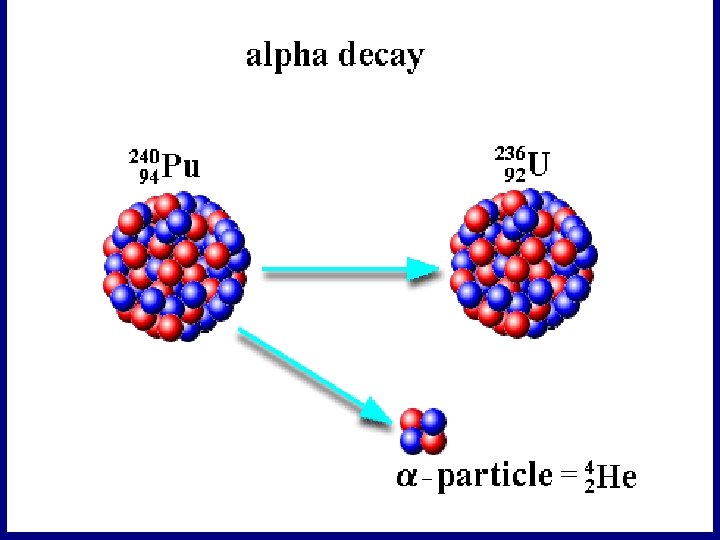

Alpha Decay Write the equation for the alpha decay of Radium to Radon

Beta Decay beta decay is when a nucleus fires out an electron…
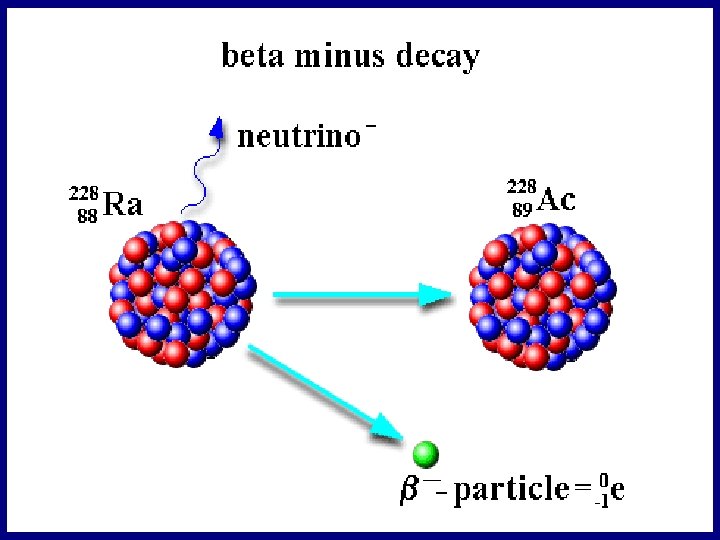
Beta Decay
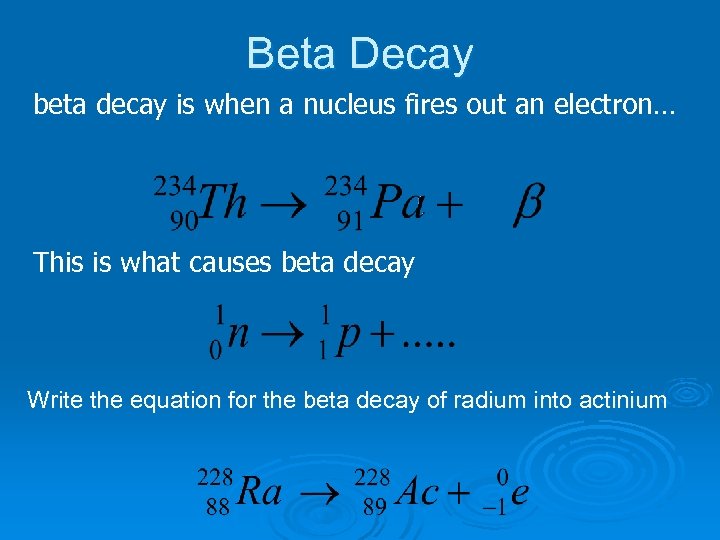
Beta Decay beta decay is when a nucleus fires out an electron… This is what causes beta decay Write the equation for the beta decay of radium into actinium
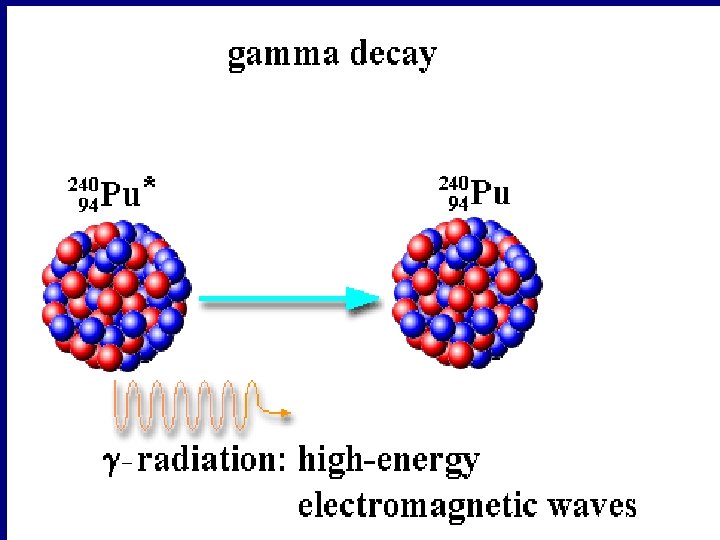
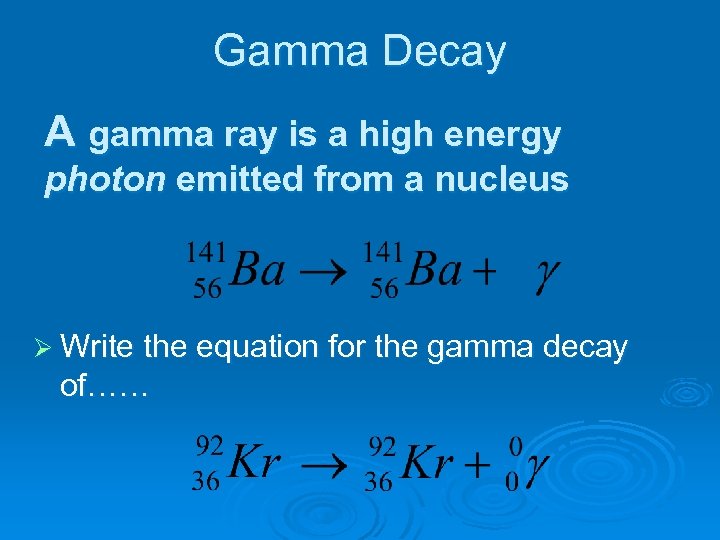
Gamma Decay A gamma ray is a high energy photon emitted from a nucleus Ø Write the equation for the gamma decay of……

Ionisation Ø When alpha particles collide with atoms, they can knock electrons off. Ø This will produce a positive ion and a free electron.
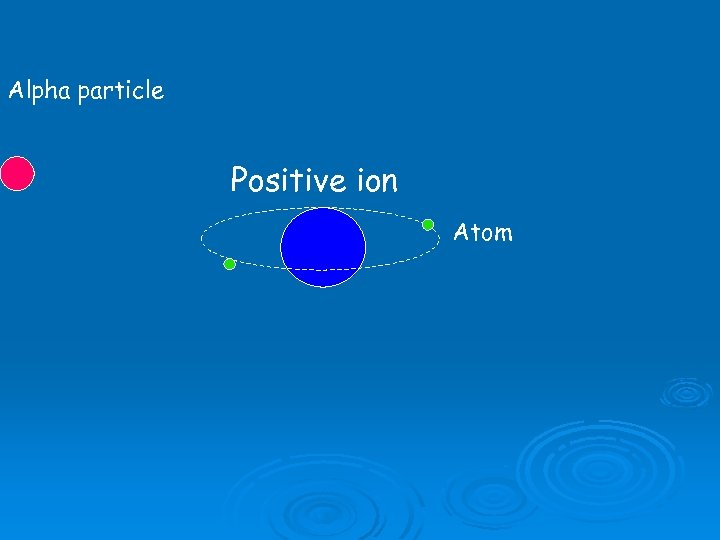
Alpha particle Positive ion Atom
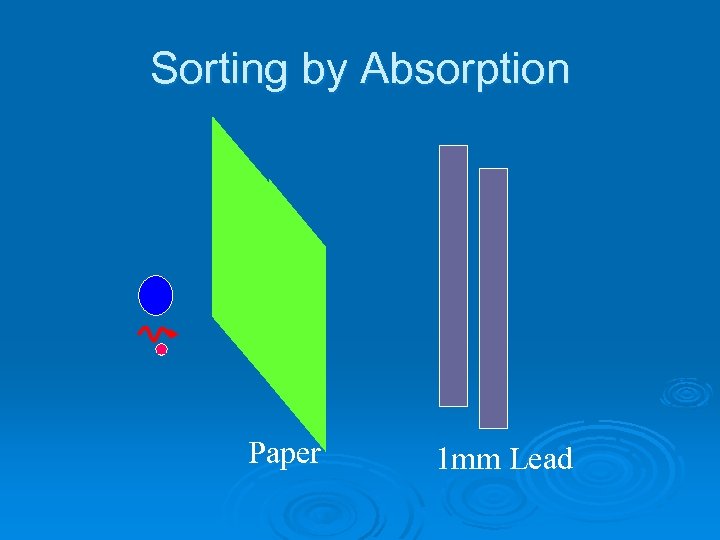
Sorting by Absorption Paper 1 mm Lead

Sorting with a Magnetic Field Ø Identify each type of radiation

Half Life The half life of an isotope is the time taken for half of a sample to decay into another isotope. OR, the time taken for the activity of an isotope to halve. The shorter the half life, the less stable it is. e. g. Uranium 238: 4, 500 MY Radon 218 0. 04 s link to half life 1

Radioactivity and Probability Ø Radioactivity is all about chance. Ø You can’t say when a certain nucleus will decay, but it might have a 1 in 10 chance of decaying in the next 5 seconds. Ø For the example above: 1000 nuclei 100 ……. . 100 nuclei 10 ……. . decays in 5 s

Ø So the rate of decay depends on: l l The number of nuclei remaining The half life Ø This means there are more decays when there are more nuclei. Ø A shorter half life means a greater rate of decay

Ø e. g Beryllium 11 decays to Boron 11 with a half life of 14 s So If you have 16 g of Beryllium 11 now, after 14 s you will have 8 g etc. Ø link to half life 2 Time(s) Amount of Be 0 14 28 42 56 16 g 8 g 4 g 2 g 1 g
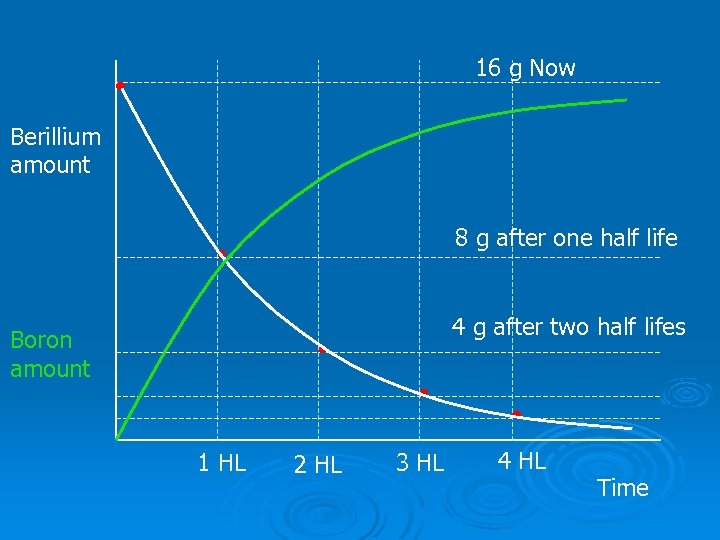
16 g Now Berillium amount 8 g after one half life 4 g after two half lifes Boron amount 1 HL 2 HL 3 HL 4 HL Time

Ø Note that the total mass of the sample is about the same, because as the Beryllium decays, it doesn’t disappear, it changes into Boron. The Boron is still there. Ø The mass is slightly less because the lump of beryllium is emitting …. . beta particles (electrons)

Sample Question. Ø A radioactive isotope has a half life of 3 years. Ø A 5 g sample of the isotope produces 30 decays per sec. Ø What will the decay rate of a 1 g sample be in 9 years time?

Carbon Dating This image shows the Shroud of Turin. It was supposedly the cloth that Christ was buried in. Is it real or a medieval fake? ? ?

Ø In the Atmosphere, cosmic rays hit Nitrogen 14 changing it to Carbon 14. Ø The Carbon 14 decays with a half life of 6300 years. Ø So a small fraction of CO 2 molecules contain Carbon 14. This is taken in by plants, and hence animals. Ø When the organism dies, The Carbon 12 stays the same, the Carbon 14 decays. Ø By measuring the ratio of C 14 to C 12, the time since it was alive can be calculated
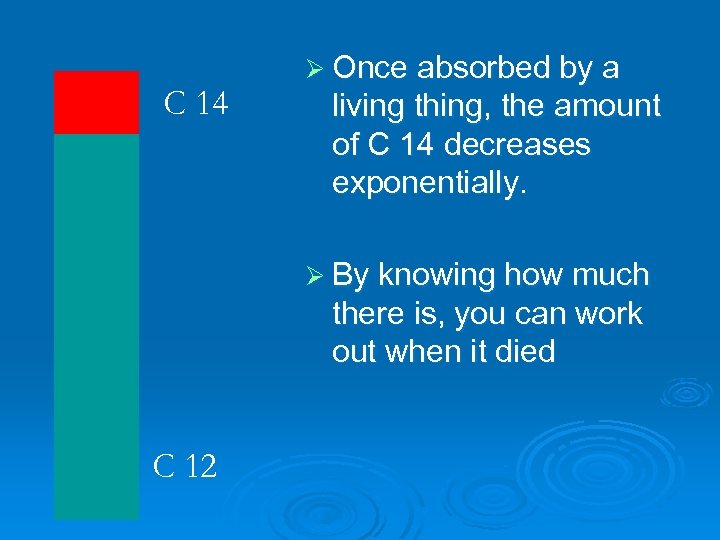
C 14 Ø Once absorbed by a living thing, the amount of C 14 decreases exponentially. Ø By knowing how much there is, you can work out when it died C 12

Ø Back to the shroud. The ratio of C 14 to C 12 showed it was about 800 years old!!! Ø Final question. A wooden axe handle has a ratio of C 14 to C 12 that is 1/8 th the ratio for new wood. How old is it?

NCEA type questions Ø Describe the Dalton model of the atom Ø Explain the evidence for the Thomson model Ø Explain the evidence for the Rutherford model

Ø (a) Cobalt-60 undergoes radioactive decay. . Ø Show the decay of cobalt-60 ( ) results in nickel-60 ( ).

Ø (a) A smoke detector contains radioactive americium 241 which emits radiation. Complete the following equation to identify the radiation emitted. Ø Explain why the radiation given out by the americium is unlikely to do any harm to the people living inside the house.

Ø The alpha particles ionise atoms in the air. Explain what this means.
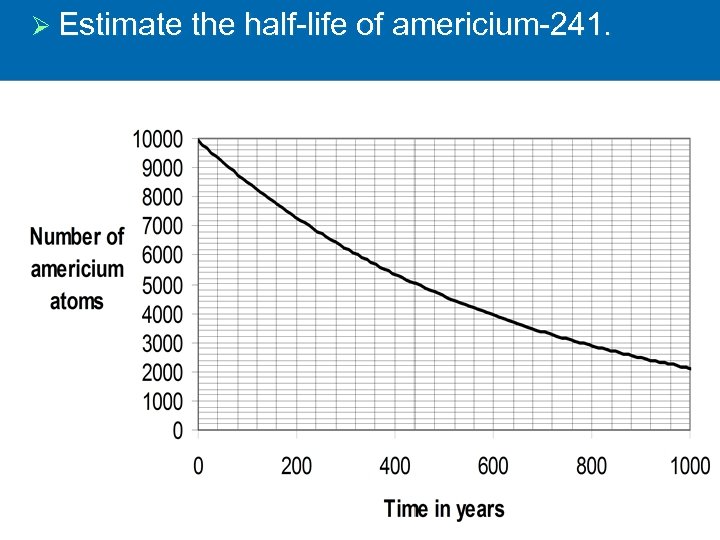
Ø Estimate the half-life of americium-241.

Ø (b) Radon-212 ( ) is a radioactive gas. Show that when radon-212 undergoes alpha decay, polonium is formed. Ø Radon-212 decays with a half-life of 24 minutes. If you start with 96 mg of radon-212, find the approximate mass of polonium-208 two hours later. Why is the actual mass less than your calculation?
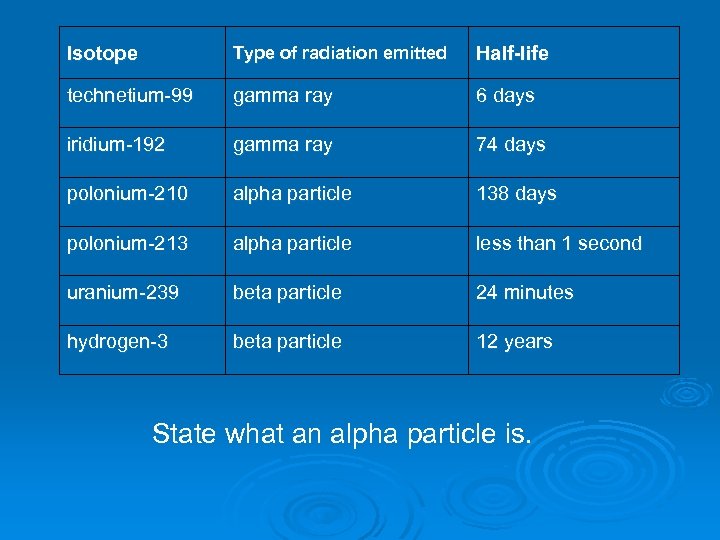
Isotope Type of radiation emitted Half-life technetium-99 gamma ray 6 days iridium-192 gamma ray 74 days polonium-210 alpha particle 138 days polonium-213 alpha particle less than 1 second uranium-239 beta particle 24 minutes hydrogen-3 beta particle 12 years State what an alpha particle is.

Isotope Type of radiation emitted Half-life technetium-99 gamma ray 6 days iridium-192 gamma ray 74 days polonium-210 alpha particle 138 days polonium-213 alpha particle less than 1 second uranium-239 beta particle 24 minutes hydrogen-3 beta particle 12 years Two isotopes of polonium are given in the table. How do the nuclei of these two isotopes differ?
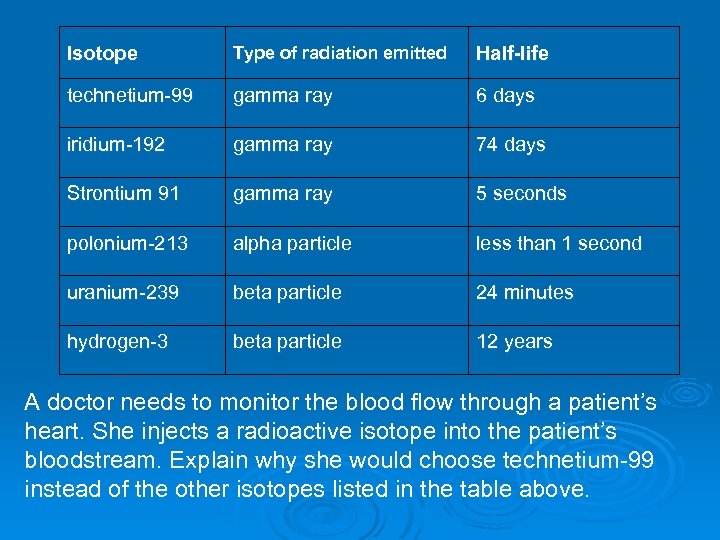
Isotope Type of radiation emitted Half-life technetium-99 gamma ray 6 days iridium-192 gamma ray 74 days Strontium 91 gamma ray 5 seconds polonium-213 alpha particle less than 1 second uranium-239 beta particle 24 minutes hydrogen-3 beta particle 12 years A doctor needs to monitor the blood flow through a patient’s heart. She injects a radioactive isotope into the patient’s bloodstream. Explain why she would choose technetium-99 instead of the other isotopes listed in the table above.
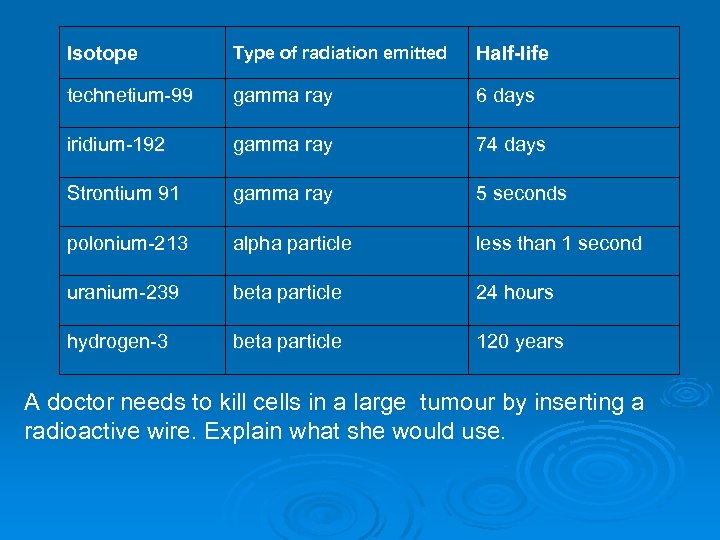
Isotope Type of radiation emitted Half-life technetium-99 gamma ray 6 days iridium-192 gamma ray 74 days Strontium 91 gamma ray 5 seconds polonium-213 alpha particle less than 1 second uranium-239 beta particle 24 hours hydrogen-3 beta particle 120 years A doctor needs to kill cells in a large tumour by inserting a radioactive wire. Explain what she would use.
705dba013f42fa69a1350d3db47e0f16.ppt