d83959952ba8a9d49624c16909d712a3.ppt
- Количество слайдов: 70
 Nuclear Physics and Radioactivity
Nuclear Physics and Radioactivity
 Online Introduction to Nuclear Physics • http: //www. sciencejoywagon. com/physicsz one/lesson/12 nuclear/intronuc. htm • Online lesson on nuclear decay http: //207. 10. 97. 102/chemzone/lessons/11 n uclear/nuclear. htm • Nuclear Fusion http: //ippex. pppl. gov/ippex/About_fusion/I NDEX. HTML
Online Introduction to Nuclear Physics • http: //www. sciencejoywagon. com/physicsz one/lesson/12 nuclear/intronuc. htm • Online lesson on nuclear decay http: //207. 10. 97. 102/chemzone/lessons/11 n uclear/nuclear. htm • Nuclear Fusion http: //ippex. pppl. gov/ippex/About_fusion/I NDEX. HTML
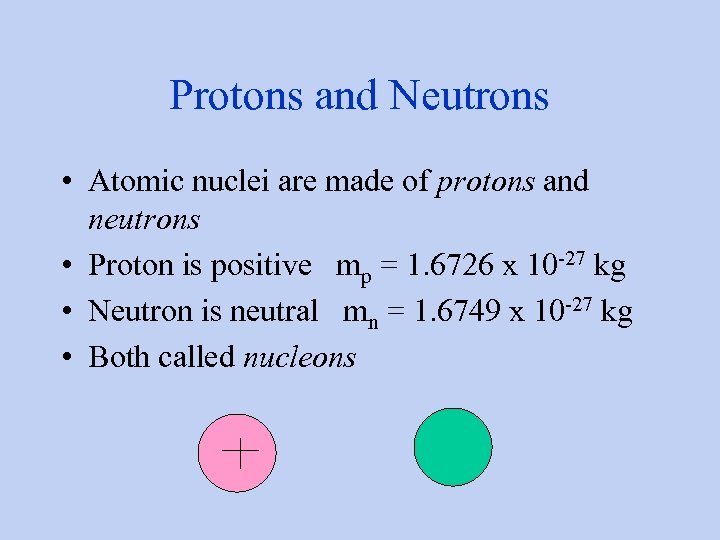 Protons and Neutrons • Atomic nuclei are made of protons and neutrons • Proton is positive mp = 1. 6726 x 10 -27 kg • Neutron is neutral mn = 1. 6749 x 10 -27 kg • Both called nucleons
Protons and Neutrons • Atomic nuclei are made of protons and neutrons • Proton is positive mp = 1. 6726 x 10 -27 kg • Neutron is neutral mn = 1. 6749 x 10 -27 kg • Both called nucleons
 Courtesy Lawrence Berkeley Laboratory
Courtesy Lawrence Berkeley Laboratory
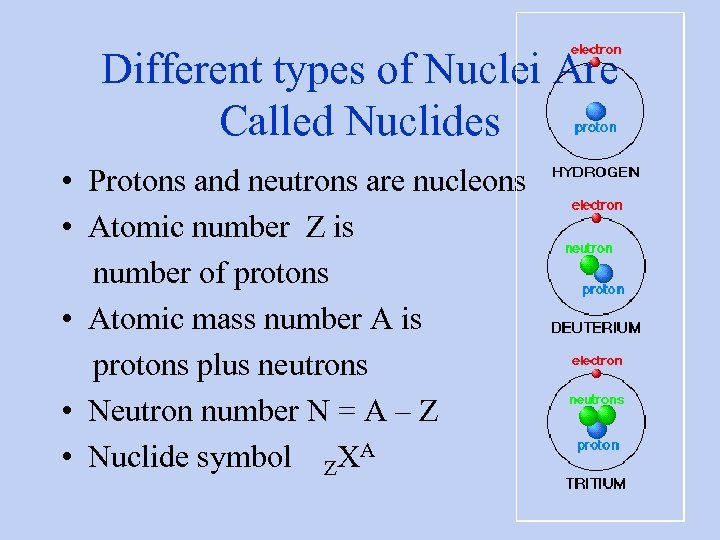 Different types of Nuclei Are Called Nuclides • Protons and neutrons are nucleons • Atomic number Z is number of protons • Atomic mass number A is protons plus neutrons • Neutron number N = A – Z • Nuclide symbol ZXA
Different types of Nuclei Are Called Nuclides • Protons and neutrons are nucleons • Atomic number Z is number of protons • Atomic mass number A is protons plus neutrons • Neutron number N = A – Z • Nuclide symbol ZXA
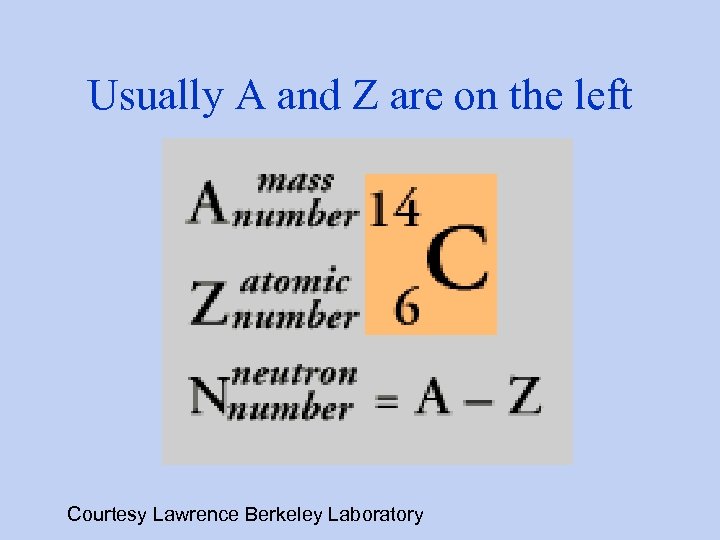 Usually A and Z are on the left Courtesy Lawrence Berkeley Laboratory
Usually A and Z are on the left Courtesy Lawrence Berkeley Laboratory
 What is 7 • • • 15 ? N Chemical element? Nitrogen 7 Atomic number? Atomic mass number? 15 Neutron number? 8 Pronounced? Nitrogen Fifteen
What is 7 • • • 15 ? N Chemical element? Nitrogen 7 Atomic number? Atomic mass number? 15 Neutron number? 8 Pronounced? Nitrogen Fifteen
 Properties • Atomic properties determined by number of electrons • Nuclei with certain atomic number but different neutron number are called isotopes • Most elements have many isotopes
Properties • Atomic properties determined by number of electrons • Nuclei with certain atomic number but different neutron number are called isotopes • Most elements have many isotopes
 Nuclear Masses C 12 has mass 12. 000000 u • 6 • Neutron 1. 008665 u • Proton 1. 007276 u • Neutral hydrogen atom 1. 007825 u • By E = mc 2 1 u = 1. 6605 x 10 -27 kg = 931. 5 Me. V/c 2 Try this yourself
Nuclear Masses C 12 has mass 12. 000000 u • 6 • Neutron 1. 008665 u • Proton 1. 007276 u • Neutral hydrogen atom 1. 007825 u • By E = mc 2 1 u = 1. 6605 x 10 -27 kg = 931. 5 Me. V/c 2 Try this yourself
 2 Rest Masses in Me. V/c • Electron 0. 51100 • Proton 938. 27 • Neutron 939. 57 H 1 atom 938. 78 • 1 • Is hydrogen more or less massive than proton and electron together? • How can you explain this?
2 Rest Masses in Me. V/c • Electron 0. 51100 • Proton 938. 27 • Neutron 939. 57 H 1 atom 938. 78 • 1 • Is hydrogen more or less massive than proton and electron together? • How can you explain this?
 Binding Energy • Energy holding the nucleus together • Stable Nucleus called a bound state • Mass of stable nucleus less than sum of masses of protons and neutrons in it • It takes energy to break it apart • Binding energy is negative
Binding Energy • Energy holding the nucleus together • Stable Nucleus called a bound state • Mass of stable nucleus less than sum of masses of protons and neutrons in it • It takes energy to break it apart • Binding energy is negative
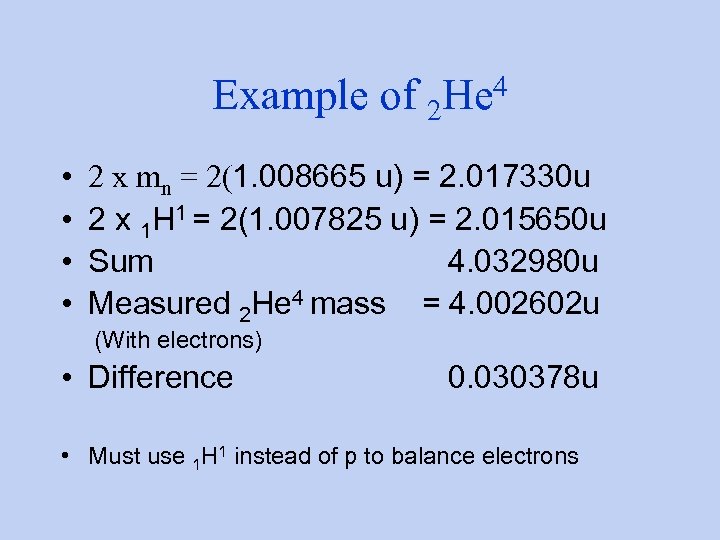 Example of 2 • • 4 He 2 x mn = 2(1. 008665 u) = 2. 017330 u 2 x 1 H 1 = 2(1. 007825 u) = 2. 015650 u Sum 4. 032980 u Measured 2 He 4 mass = 4. 002602 u (With electrons) • Difference 0. 030378 u • Must use 1 H 1 instead of p to balance electrons
Example of 2 • • 4 He 2 x mn = 2(1. 008665 u) = 2. 017330 u 2 x 1 H 1 = 2(1. 007825 u) = 2. 015650 u Sum 4. 032980 u Measured 2 He 4 mass = 4. 002602 u (With electrons) • Difference 0. 030378 u • Must use 1 H 1 instead of p to balance electrons
 2 4 continued He • 0. 030378 u x 931. 5 Me. V/c 2/u =28. 3 Me. V • Total binding energy of nucleus • Energy that must go into nucleus to split it into separate nucleons Comparison: binding energy of electron in hydrogen atom is 13. 6 e. V. What does that tell you?
2 4 continued He • 0. 030378 u x 931. 5 Me. V/c 2/u =28. 3 Me. V • Total binding energy of nucleus • Energy that must go into nucleus to split it into separate nucleons Comparison: binding energy of electron in hydrogen atom is 13. 6 e. V. What does that tell you?
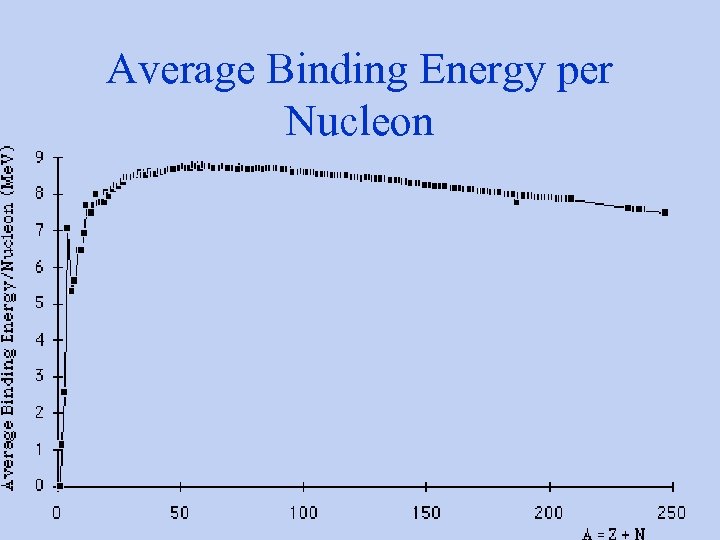 Average Binding Energy per Nucleon
Average Binding Energy per Nucleon
 Four Forces of Nature (in order of decreasing strength) • • Strong Electromagnetic Weak Gravity The strong force holds the nucleus together. It is very short range compared to electric and gravity
Four Forces of Nature (in order of decreasing strength) • • Strong Electromagnetic Weak Gravity The strong force holds the nucleus together. It is very short range compared to electric and gravity
 Radioactivity • Some nuclei change disintegrate into pieces whose total mass is less than mass of nucleus • Called radioactive decay • Discovered by Bequerel in 1896 (U) • Curies found Ra and Po Pitchblende sample
Radioactivity • Some nuclei change disintegrate into pieces whose total mass is less than mass of nucleus • Called radioactive decay • Discovered by Bequerel in 1896 (U) • Curies found Ra and Po Pitchblende sample
 Marie and Pierre Curie • She coined term “radioactivity” • Both won Nobel prize • Pierre killed crossing street • Marie gets his teaching Job at Sorbonne-first Woman to teach there in 650 Years. Later she dies of anemia.
Marie and Pierre Curie • She coined term “radioactivity” • Both won Nobel prize • Pierre killed crossing street • Marie gets his teaching Job at Sorbonne-first Woman to teach there in 650 Years. Later she dies of anemia.
 Three Kinds of Radioactivity • Alpha (a) – Positively charged – Least penetrating. Paper stops it • Beta (b) – Negatively charged – ½ cm Aluminum stops it • Gamma (g) – Uncharged, released as photons of light – Most penetrating. Thick lead may not stop it.
Three Kinds of Radioactivity • Alpha (a) – Positively charged – Least penetrating. Paper stops it • Beta (b) – Negatively charged – ½ cm Aluminum stops it • Gamma (g) – Uncharged, released as photons of light – Most penetrating. Thick lead may not stop it.
 Neutron Emission A fourth type of Radioactivity- emits no charged particles, just releases a neutron from the nucleus. By far the most damaging of any type, takes 3 ft of lead or a massive amount of concrete to stop it.
Neutron Emission A fourth type of Radioactivity- emits no charged particles, just releases a neutron from the nucleus. By far the most damaging of any type, takes 3 ft of lead or a massive amount of concrete to stop it.
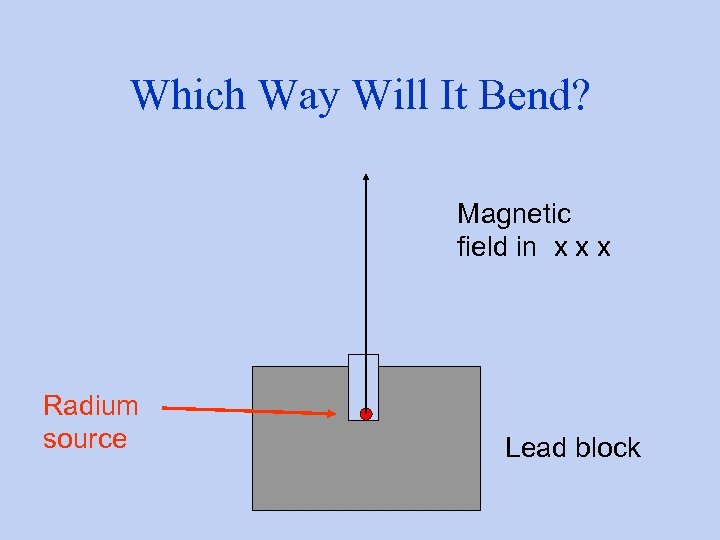 Which Way Will It Bend? Magnetic field in x x x Radium source Lead block
Which Way Will It Bend? Magnetic field in x x x Radium source Lead block
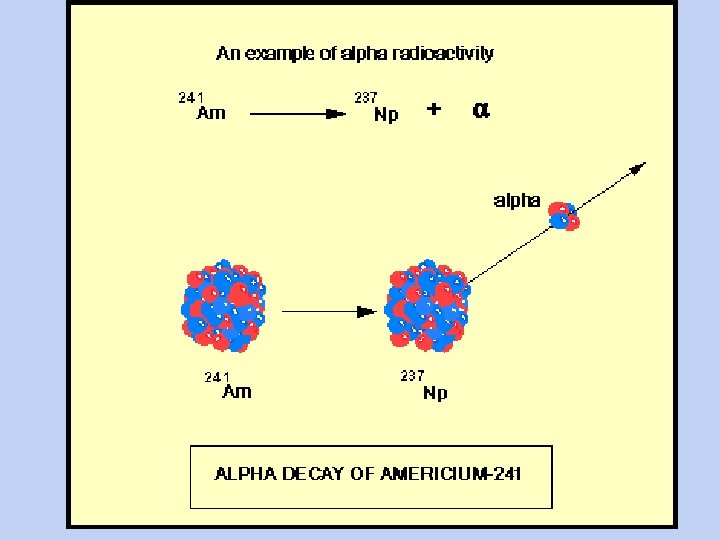
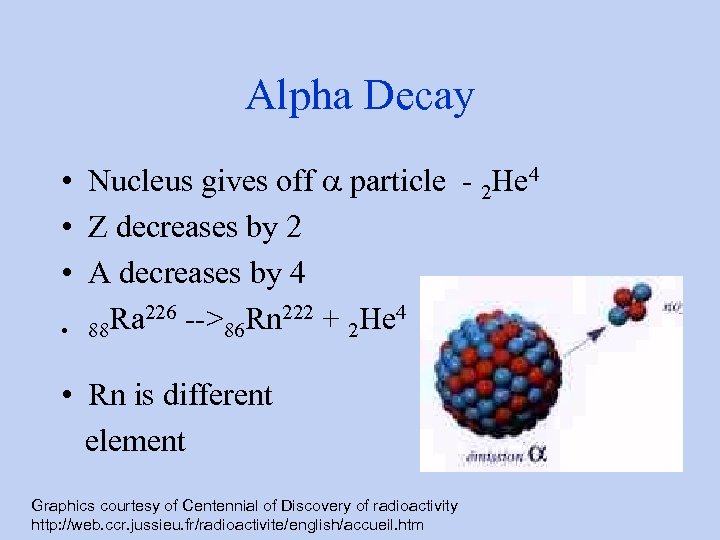 Alpha Decay • Nucleus gives off a particle - 2 He 4 • Z decreases by 2 • A decreases by 4 Ra 226 -->86 Rn 222 + 2 He 4 • 88 • Rn is different element Graphics courtesy of Centennial of Discovery of radioactivity http: //web. ccr. jussieu. fr/radioactivite/english/accueil. htm
Alpha Decay • Nucleus gives off a particle - 2 He 4 • Z decreases by 2 • A decreases by 4 Ra 226 -->86 Rn 222 + 2 He 4 • 88 • Rn is different element Graphics courtesy of Centennial of Discovery of radioactivity http: //web. ccr. jussieu. fr/radioactivite/english/accueil. htm
 Energy in a Decay Energy released is (Mp – Md – ma) c 2 (Mp – Md – ma) = mass defect Mp is mass of parent 88 Ra 226 Md is mass of daughter 86 Rn 222 Energy appears as KE of a particle and daughter (recoil energy) Compare the energy of the a particle with that of the recoiling daughter. • • • What is true about their momenta and directions?
Energy in a Decay Energy released is (Mp – Md – ma) c 2 (Mp – Md – ma) = mass defect Mp is mass of parent 88 Ra 226 Md is mass of daughter 86 Rn 222 Energy appears as KE of a particle and daughter (recoil energy) Compare the energy of the a particle with that of the recoiling daughter. • • • What is true about their momenta and directions?
 Conservation Laws in Nuclear Processes • • • Total energy is conserved Momentum is conserved Charge is conserved Angular momentum is conserved Number of nucleons (plus anti-nucleons) is conserved
Conservation Laws in Nuclear Processes • • • Total energy is conserved Momentum is conserved Charge is conserved Angular momentum is conserved Number of nucleons (plus anti-nucleons) is conserved
 You Find Out • What does Americium 241 decays into • Use your periodic table at back of text Answer 93 Np 237 Neptunium Application Am 241 is used in smoke detectors • 95
You Find Out • What does Americium 241 decays into • Use your periodic table at back of text Answer 93 Np 237 Neptunium Application Am 241 is used in smoke detectors • 95
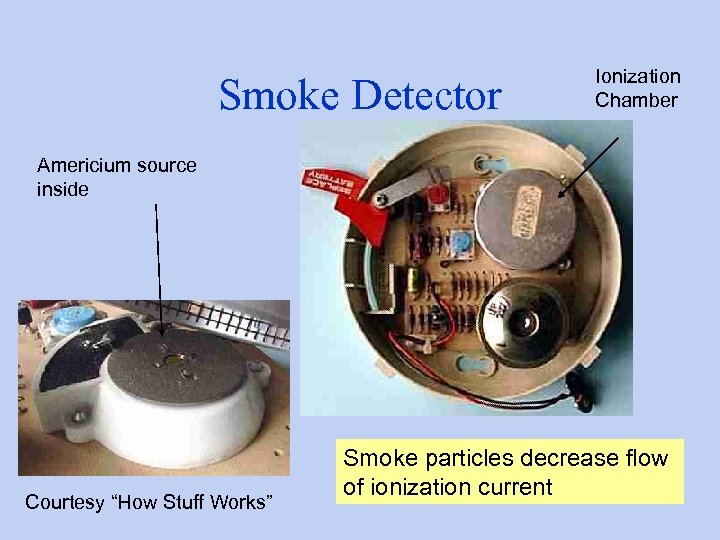 Smoke Detector Ionization Chamber Americium source inside Courtesy “How Stuff Works” Smoke particles decrease flow of ionization current
Smoke Detector Ionization Chamber Americium source inside Courtesy “How Stuff Works” Smoke particles decrease flow of ionization current
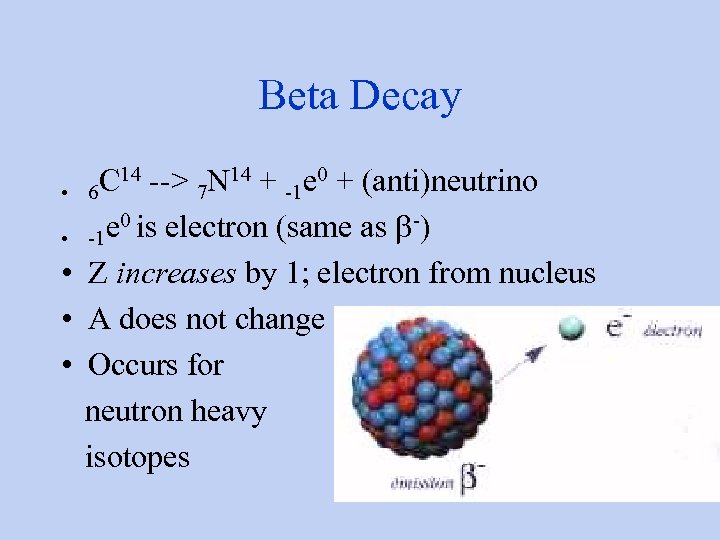 Beta Decay C 14 --> 7 N 14 + -1 e 0 + (anti)neutrino • 6 e 0 is electron (same as b-) • -1 • Z increases by 1; electron from nucleus • A does not change • Occurs for neutron heavy isotopes
Beta Decay C 14 --> 7 N 14 + -1 e 0 + (anti)neutrino • 6 e 0 is electron (same as b-) • -1 • Z increases by 1; electron from nucleus • A does not change • Occurs for neutron heavy isotopes
 Wolfgang Pauli What is a Neutrino? • Massless*, neutral particle that travels with the speed of light (hypothesized by Pauli in 1930) • Incredibly penetrating - passes through Earth • Required to be emitted in beta decay in order that momentum and energy be conserved(beta energies are not unique) • Observed in 1956 by Reines and Cowan • Symbol is n(nu) with bar over it - antineutrino *There is some evidence that the neutrino has a tiny non-zero mass
Wolfgang Pauli What is a Neutrino? • Massless*, neutral particle that travels with the speed of light (hypothesized by Pauli in 1930) • Incredibly penetrating - passes through Earth • Required to be emitted in beta decay in order that momentum and energy be conserved(beta energies are not unique) • Observed in 1956 by Reines and Cowan • Symbol is n(nu) with bar over it - antineutrino *There is some evidence that the neutrino has a tiny non-zero mass
 Positron (Beta+) Decay Ne 19 --> 9 F 19 + e+ + n • 10 • e+ is positron(anti-electron) • Z of nucleus decreases by 1 • A does not change • Occurs for neutron light isotopes
Positron (Beta+) Decay Ne 19 --> 9 F 19 + e+ + n • 10 • e+ is positron(anti-electron) • Z of nucleus decreases by 1 • A does not change • Occurs for neutron light isotopes
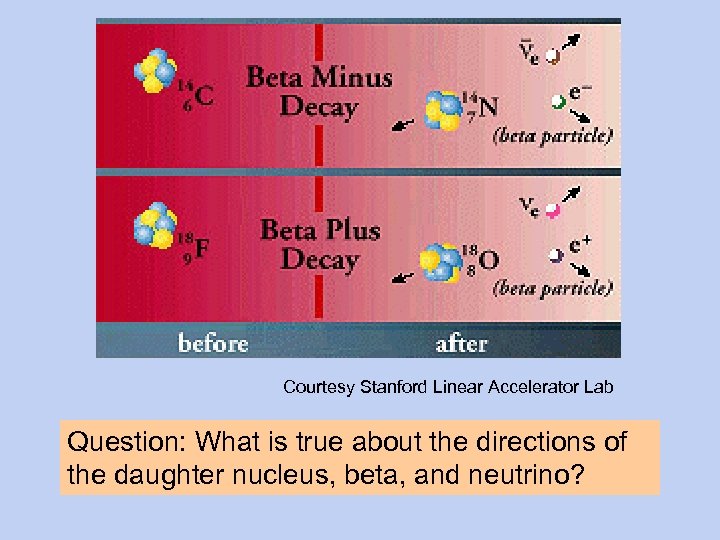 Courtesy Stanford Linear Accelerator Lab Question: What is true about the directions of the daughter nucleus, beta, and neutrino?
Courtesy Stanford Linear Accelerator Lab Question: What is true about the directions of the daughter nucleus, beta, and neutrino?
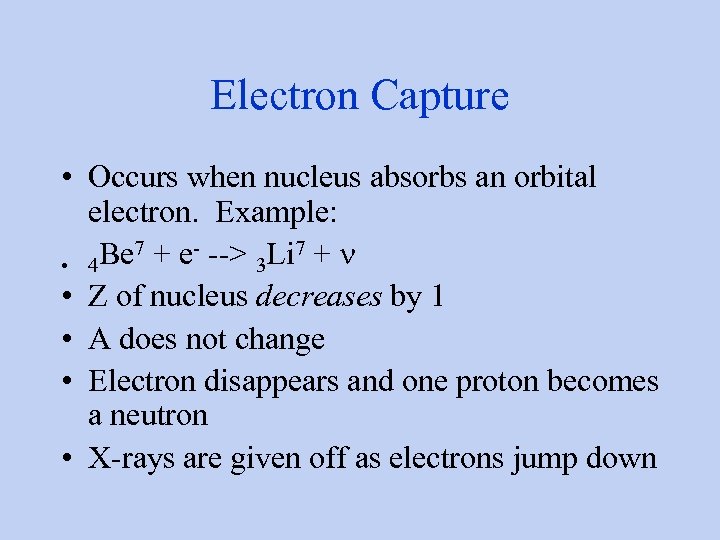 Electron Capture • Occurs when nucleus absorbs an orbital electron. Example: Be 7 + e- --> 3 Li 7 + n • 4 • Z of nucleus decreases by 1 • A does not change • Electron disappears and one proton becomes a neutron • X-rays are given off as electrons jump down
Electron Capture • Occurs when nucleus absorbs an orbital electron. Example: Be 7 + e- --> 3 Li 7 + n • 4 • Z of nucleus decreases by 1 • A does not change • Electron disappears and one proton becomes a neutron • X-rays are given off as electrons jump down
 Fermi’s Theory • Explained beta decay and EC in terms of a new “weak” force • Fermi was last “double threat physicist; great theorist and experimenter.
Fermi’s Theory • Explained beta decay and EC in terms of a new “weak” force • Fermi was last “double threat physicist; great theorist and experimenter.
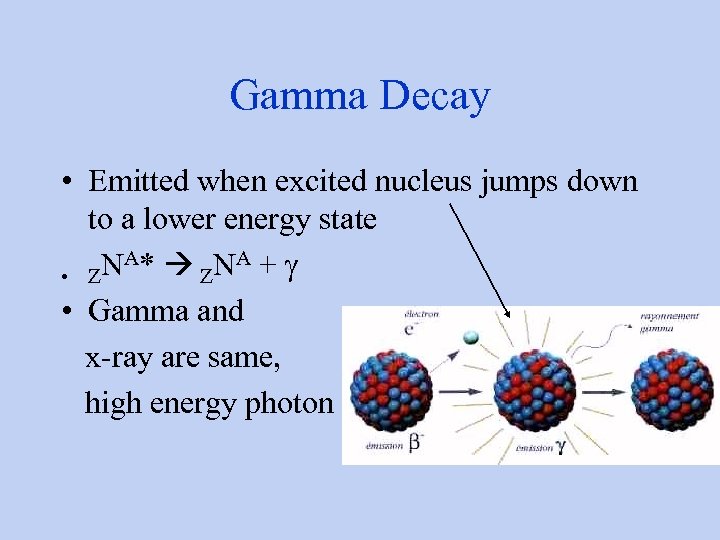 Gamma Decay • Emitted when excited nucleus jumps down to a lower energy state NA* ZNA + g • Z • Gamma and x-ray are same, high energy photon
Gamma Decay • Emitted when excited nucleus jumps down to a lower energy state NA* ZNA + g • Z • Gamma and x-ray are same, high energy photon
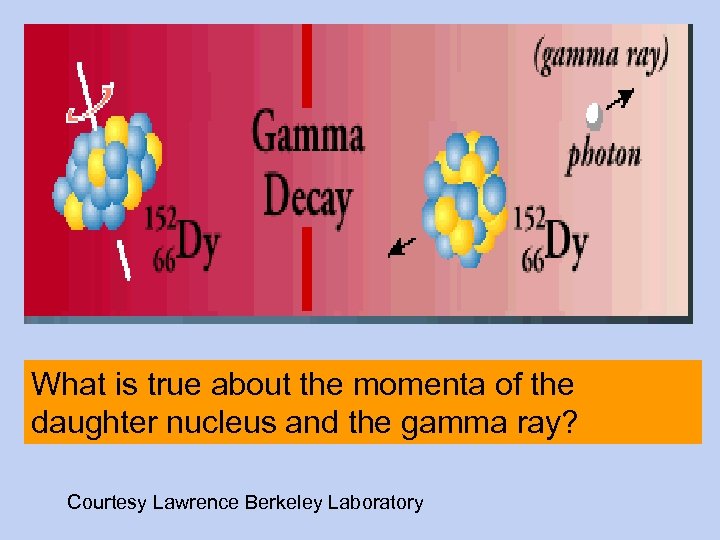 What is true about the momenta of the daughter nucleus and the gamma ray? Courtesy Lawrence Berkeley Laboratory
What is true about the momenta of the daughter nucleus and the gamma ray? Courtesy Lawrence Berkeley Laboratory
 Review • There are stable nuclides (isotopes) and unstable (radioactive ones) • Stable means mass of pieces is more than that of whole nucleus. • Unstable means opposite • MOST isotopes are NOT stable; they undergo one form of decay or another
Review • There are stable nuclides (isotopes) and unstable (radioactive ones) • Stable means mass of pieces is more than that of whole nucleus. • Unstable means opposite • MOST isotopes are NOT stable; they undergo one form of decay or another
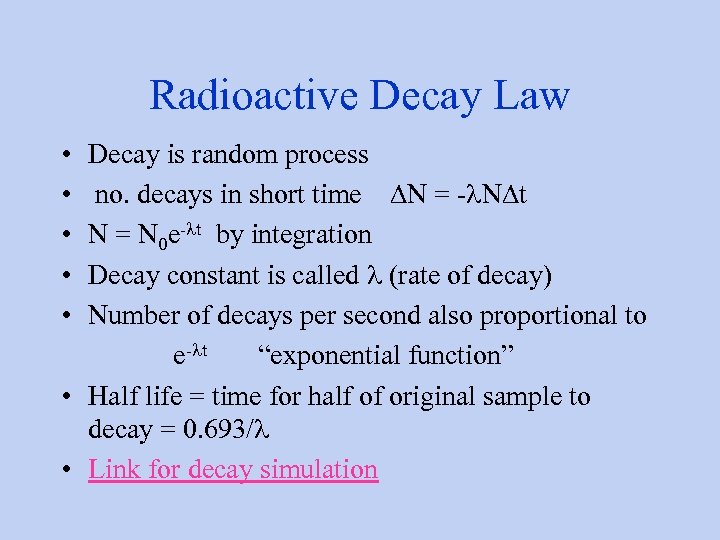 Radioactive Decay Law • Decay is random process • no. decays in short time DN = -l. NDt • N = N 0 e-lt by integration • Decay constant is called l (rate of decay) • Number of decays per second also proportional to e-lt “exponential function” • Half life = time for half of original sample to decay = 0. 693/l • Link for decay simulation
Radioactive Decay Law • Decay is random process • no. decays in short time DN = -l. NDt • N = N 0 e-lt by integration • Decay constant is called l (rate of decay) • Number of decays per second also proportional to e-lt “exponential function” • Half life = time for half of original sample to decay = 0. 693/l • Link for decay simulation
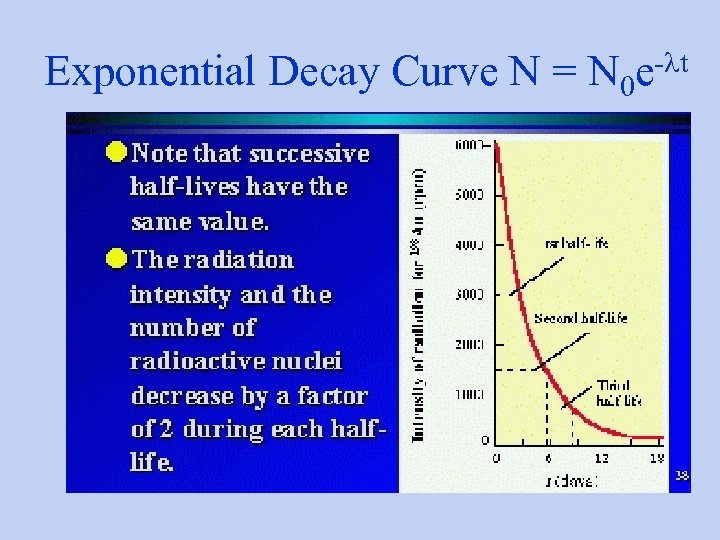 Exponential Decay Curve N = N 0 -lt e
Exponential Decay Curve N = N 0 -lt e
 Question • A sample contains about 1000 nuclei of a certain radioisotope. The half life is four minutes. About how many nuclei will remain after 16 minutes? • Hint: make a table Answer: about 62 nuclei
Question • A sample contains about 1000 nuclei of a certain radioisotope. The half life is four minutes. About how many nuclei will remain after 16 minutes? • Hint: make a table Answer: about 62 nuclei
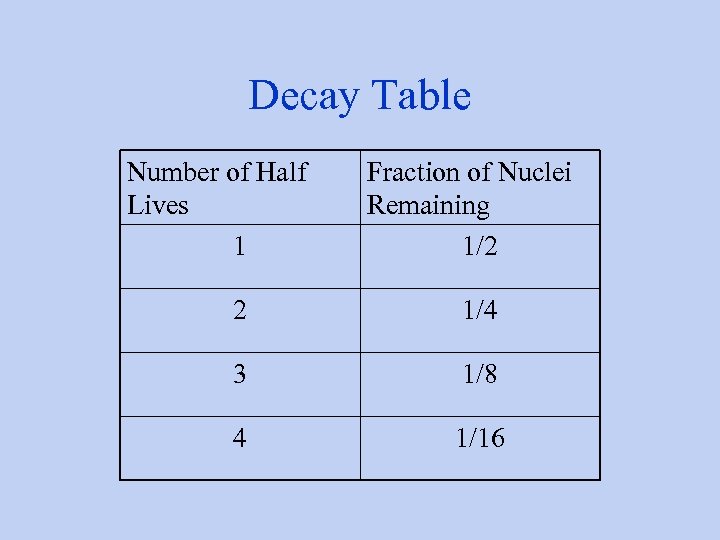 Decay Table Number of Half Lives 1 Fraction of Nuclei Remaining 1/2 2 1/4 3 1/8 4 1/16
Decay Table Number of Half Lives 1 Fraction of Nuclei Remaining 1/2 2 1/4 3 1/8 4 1/16
 Randomness of Decay • No way to tell which nucleus will decay when • Actual number that decay varies around a most probable number • Uncertainty is proportional to
Randomness of Decay • No way to tell which nucleus will decay when • Actual number that decay varies around a most probable number • Uncertainty is proportional to
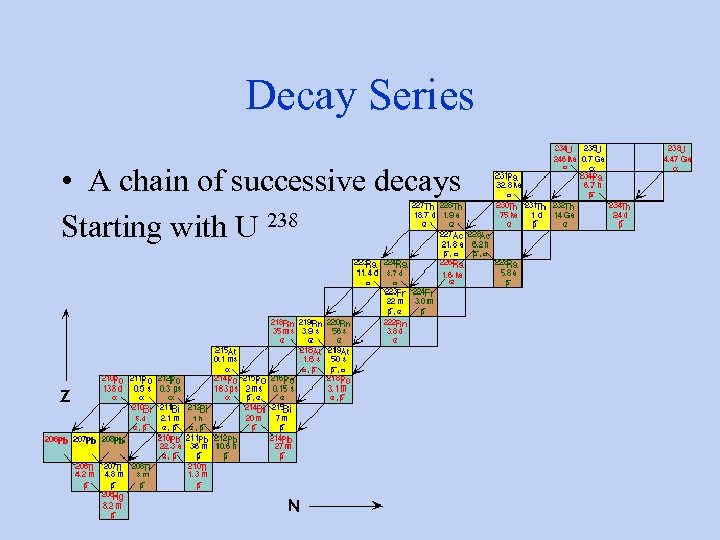 Decay Series • A chain of successive decays Starting with U 238
Decay Series • A chain of successive decays Starting with U 238
 Radioactive Dating • n + 7 N 14 6 C 14 + p provides continual supply of carbon 14 at about rate of decay C 14 --> 7 N 14 + -1 e 0 + antineutrino • 6 • When organism dies no more supply so ratio of carbon 14 to 12 decreases – with 5730 yr half life • Useful for dating objects up to 60, 000 years old
Radioactive Dating • n + 7 N 14 6 C 14 + p provides continual supply of carbon 14 at about rate of decay C 14 --> 7 N 14 + -1 e 0 + antineutrino • 6 • When organism dies no more supply so ratio of carbon 14 to 12 decreases – with 5730 yr half life • Useful for dating objects up to 60, 000 years old
 Nuclear Reactions • Transformation of one element into another is called transmutation. • Sought unsuccessfully by Alchemists • Usually happens in collision • Rutherford(1919) discovered in 2 He 4 + 7 N 14 8 O 17 + 1 H 1
Nuclear Reactions • Transformation of one element into another is called transmutation. • Sought unsuccessfully by Alchemists • Usually happens in collision • Rutherford(1919) discovered in 2 He 4 + 7 N 14 8 O 17 + 1 H 1
 Conservation Laws in Nuclear Reactions • • • Momentum Energy Charge Nucleon(Baryon) Number – heavy particles Lepton Number – light particles
Conservation Laws in Nuclear Reactions • • • Momentum Energy Charge Nucleon(Baryon) Number – heavy particles Lepton Number – light particles
 Example: slow neutron reaction n 1 + 5 B 10 3 Li 7 + ? • 0 • Answer 2 He 4 which is also called an • Alpha particle • Challenge: Given speed of helium atom 9. 30 x 106 m/s find the – Velocity and KE of the lithium atom – Hint: what is initial momentum of the system?
Example: slow neutron reaction n 1 + 5 B 10 3 Li 7 + ? • 0 • Answer 2 He 4 which is also called an • Alpha particle • Challenge: Given speed of helium atom 9. 30 x 106 m/s find the – Velocity and KE of the lithium atom – Hint: what is initial momentum of the system?
 Nuclear Fission and Fusion • In fission a large nucleus breaks apart releasing energy • In fusion light nuclei merge to form a heavier nucleus and energy is released.
Nuclear Fission and Fusion • In fission a large nucleus breaks apart releasing energy • In fusion light nuclei merge to form a heavier nucleus and energy is released.
 Nuclear Fission • Uranium nucleus absorbs neutron and splits in two • Easier to do with 92 U 235 than common U 238 92 • Discovered Germany 1938 • Dangerous time
Nuclear Fission • Uranium nucleus absorbs neutron and splits in two • Easier to do with 92 U 235 than common U 238 92 • Discovered Germany 1938 • Dangerous time
 Courtesy students at Illinois Math and Science Academy
Courtesy students at Illinois Math and Science Academy
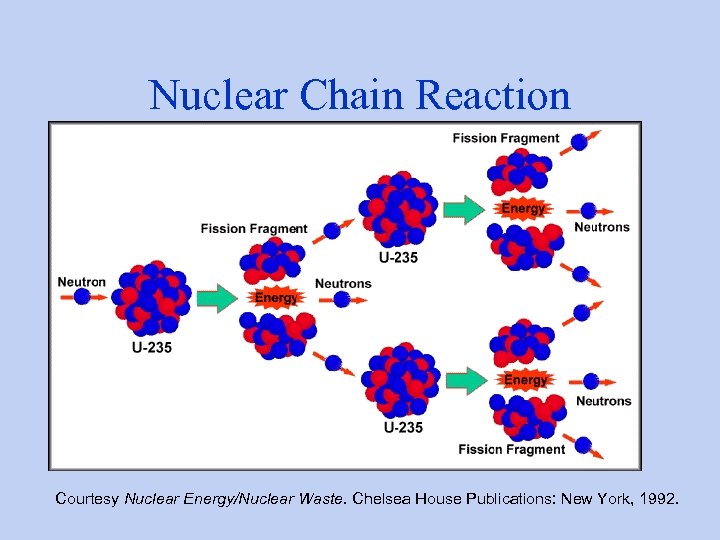 Nuclear Chain Reaction Courtesy Nuclear Energy/Nuclear Waste. Chelsea House Publications: New York, 1992.
Nuclear Chain Reaction Courtesy Nuclear Energy/Nuclear Waste. Chelsea House Publications: New York, 1992.
 Above All, Fission Produces Heat
Above All, Fission Produces Heat
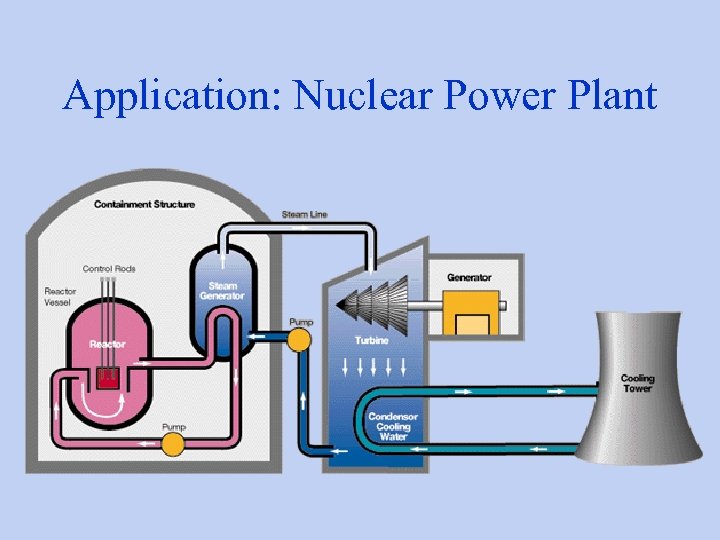 Application: Nuclear Power Plant
Application: Nuclear Power Plant
 How Control Rods Moderate Reaction http: //www. npp. hu/mukodes/anim/sta 1 -e. htm
How Control Rods Moderate Reaction http: //www. npp. hu/mukodes/anim/sta 1 -e. htm
 Diablo Canyon Nuclear Plant – PG&E Power Output 1100 MW each Domes are 215 feet high Courtesy Jim Zim
Diablo Canyon Nuclear Plant – PG&E Power Output 1100 MW each Domes are 215 feet high Courtesy Jim Zim
 Ranch Seco Nuclear Plant Near Sacramento • Shut down in 1989 • De-commissioning still underway • Planned completion 2011
Ranch Seco Nuclear Plant Near Sacramento • Shut down in 1989 • De-commissioning still underway • Planned completion 2011
 Three Mile Island Nuclear Plant • • Partial meltdown, March 28, 1979 50% of reactor core destroyed or melted Hydrogen bubble forms inside containment Metropolitan Edison lies about radiation release • Situation stabilized without injuries
Three Mile Island Nuclear Plant • • Partial meltdown, March 28, 1979 50% of reactor core destroyed or melted Hydrogen bubble forms inside containment Metropolitan Edison lies about radiation release • Situation stabilized without injuries
 Meltdown Scene Chernobyl Nuclear Plant Unit 4 Operating Power 3. 2 GW Thermal, 1 GW electrical Estimated number of radiation victims = 3. 2 million 400 times more radioactivity was released than in the explosion of the Hiroshima Atomic Bomb
Meltdown Scene Chernobyl Nuclear Plant Unit 4 Operating Power 3. 2 GW Thermal, 1 GW electrical Estimated number of radiation victims = 3. 2 million 400 times more radioactivity was released than in the explosion of the Hiroshima Atomic Bomb
 2001 Power Crisis Strikes California • Nuclear Power plant proposed for Alameda Point, Alameda • What do you think?
2001 Power Crisis Strikes California • Nuclear Power plant proposed for Alameda Point, Alameda • What do you think?
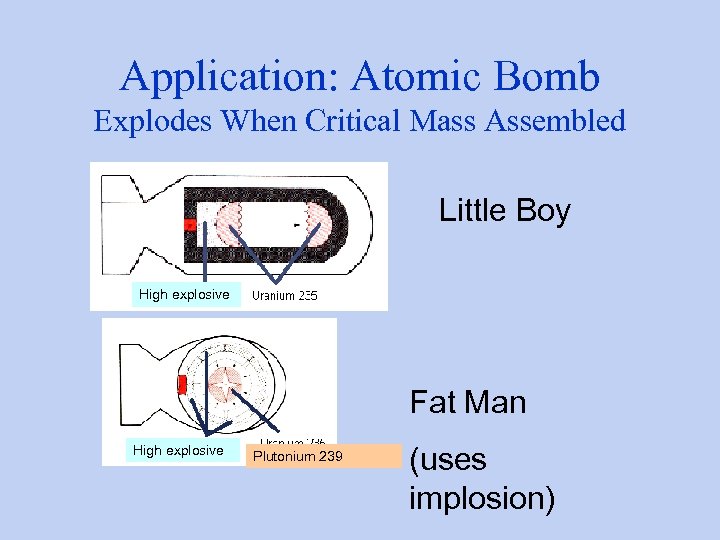 Application: Atomic Bomb Explodes When Critical Mass Assembled Little Boy High explosive Fat Man High explosive Plutonium 239 (uses implosion)
Application: Atomic Bomb Explodes When Critical Mass Assembled Little Boy High explosive Fat Man High explosive Plutonium 239 (uses implosion)

 Fission Bombs • Destructive Force about 20, 000 tons of TNT • 1945: Hiroshima and Nagasaki destroyed • 100, 000+ civilians killed
Fission Bombs • Destructive Force about 20, 000 tons of TNT • 1945: Hiroshima and Nagasaki destroyed • 100, 000+ civilians killed
 Nuclear Fusion • Light nuclei come together (fuse) to form heavier nucleus • Mass of product greater than sum of pieces • Large energy release • Powers the Sun • Used to make H-bombs – “thermonuclear bombs”
Nuclear Fusion • Light nuclei come together (fuse) to form heavier nucleus • Mass of product greater than sum of pieces • Large energy release • Powers the Sun • Used to make H-bombs – “thermonuclear bombs”
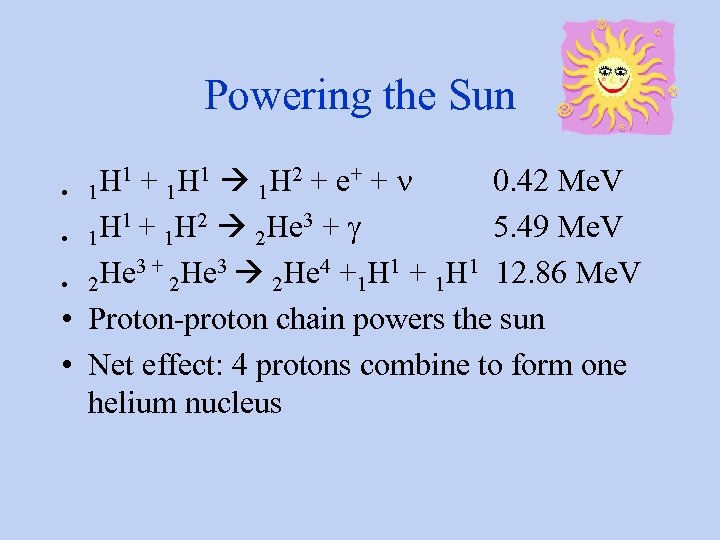 Powering the Sun H 1 + 1 H 1 1 H 2 + e+ + n 0. 42 Me. V • 1 H 1 + 1 H 2 2 He 3 + g 5. 49 Me. V • 1 He 3 + 2 He 3 2 He 4 +1 H 1 + 1 H 1 12. 86 Me. V • 2 • Proton-proton chain powers the sun • Net effect: 4 protons combine to form one helium nucleus
Powering the Sun H 1 + 1 H 1 1 H 2 + e+ + n 0. 42 Me. V • 1 H 1 + 1 H 2 2 He 3 + g 5. 49 Me. V • 1 He 3 + 2 He 3 2 He 4 +1 H 1 + 1 H 1 12. 86 Me. V • 2 • Proton-proton chain powers the sun • Net effect: 4 protons combine to form one helium nucleus
 Condition for Fusion • Product needs more binding energy than reactants • Reactants must be heated to millions of degrees to get close enough for nuclear reaction to be possible(very hot plasma) • Overcome coulomb repulsion • Nuclear forces very short range
Condition for Fusion • Product needs more binding energy than reactants • Reactants must be heated to millions of degrees to get close enough for nuclear reaction to be possible(very hot plasma) • Overcome coulomb repulsion • Nuclear forces very short range
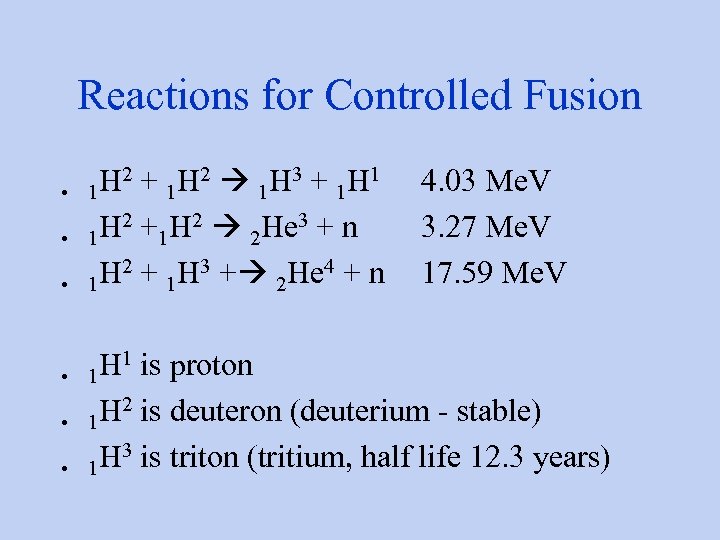 Reactions for Controlled Fusion • • • H 2 + 1 H 2 1 H 3 + 1 H 1 4. 03 Me. V 1 H 2 +1 H 2 2 He 3 + n 3. 27 Me. V 1 H 2 + 1 H 3 + 2 He 4 + n 17. 59 Me. V 1 H 1 is proton 1 H 2 is deuteron (deuterium - stable) 1 H 3 is triton (tritium, half life 12. 3 years) 1
Reactions for Controlled Fusion • • • H 2 + 1 H 2 1 H 3 + 1 H 1 4. 03 Me. V 1 H 2 +1 H 2 2 He 3 + n 3. 27 Me. V 1 H 2 + 1 H 3 + 2 He 4 + n 17. 59 Me. V 1 H 1 is proton 1 H 2 is deuteron (deuterium - stable) 1 H 3 is triton (tritium, half life 12. 3 years) 1
 Question • How can you recognize a fusion reaction? Makes lighter elements into heavier ones Releases energy
Question • How can you recognize a fusion reaction? Makes lighter elements into heavier ones Releases energy
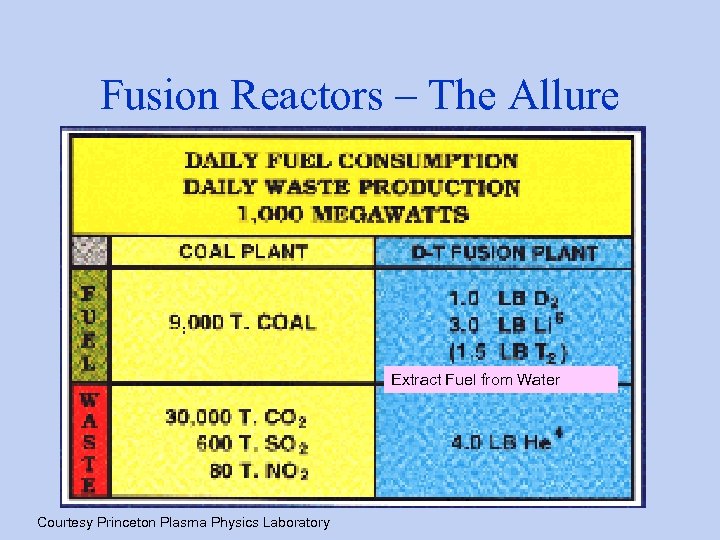 Fusion Reactors – The Allure Extract Fuel from Water Courtesy Princeton Plasma Physics Laboratory
Fusion Reactors – The Allure Extract Fuel from Water Courtesy Princeton Plasma Physics Laboratory
 Fusion Reactors – The Challenge • Need to create conditions at center of a star • Need to contain bulk amounts of plasma at temps above 20 million degrees • Need to get more energy out than you put in • Need to demonstrate on commercial scale
Fusion Reactors – The Challenge • Need to create conditions at center of a star • Need to contain bulk amounts of plasma at temps above 20 million degrees • Need to get more energy out than you put in • Need to demonstrate on commercial scale
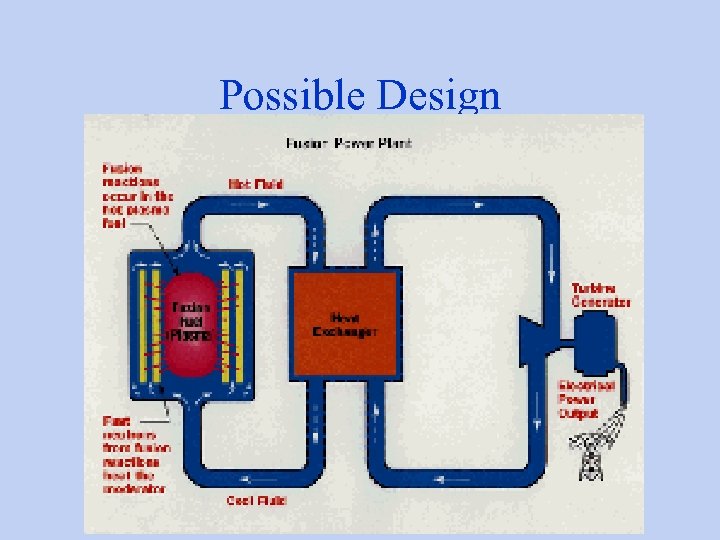 Possible Design
Possible Design
 Tokamak – Magnetic Confinement in a Hollow Doughnut (Torus) Courtesy Princeton Plasma Physics Laboratory
Tokamak – Magnetic Confinement in a Hollow Doughnut (Torus) Courtesy Princeton Plasma Physics Laboratory


