43831c50f837572f4bd8a0633c447069.ppt
- Количество слайдов: 71
 Non-Convulsive Status Epilepticus (NCSE): Our Experience at a Tertiary Care Center Brennen Bittel, DO Clinical Neurophysiology Fellow
Non-Convulsive Status Epilepticus (NCSE): Our Experience at a Tertiary Care Center Brennen Bittel, DO Clinical Neurophysiology Fellow
 Overview n Background information: q q q Epidemiology Clinical features Electrographic definition n q q q EDX pitfalls Treatment Pathology Outcomes n KU Data q 2009 -2013
Overview n Background information: q q q Epidemiology Clinical features Electrographic definition n q q q EDX pitfalls Treatment Pathology Outcomes n KU Data q 2009 -2013
 Incidence/prevalence n n SE* in emergency room or intensive care units ~ 150, 000/yr NCSE: q q n n n 25 % of all SE 1. 5 – 60/100, 000/yr 34% of all SE in a tertiary care center 27% of ICU pts w/ altered mental status 8% of pts in coma Celesia 1976, Tomson 1992, Drislane 2000, Towne 2000
Incidence/prevalence n n SE* in emergency room or intensive care units ~ 150, 000/yr NCSE: q q n n n 25 % of all SE 1. 5 – 60/100, 000/yr 34% of all SE in a tertiary care center 27% of ICU pts w/ altered mental status 8% of pts in coma Celesia 1976, Tomson 1992, Drislane 2000, Towne 2000
 Definition 1. Diminished level of consciousness, confusion 2. Epileptiform EEG (continuous or discrete) 3. Response to treatment? ?
Definition 1. Diminished level of consciousness, confusion 2. Epileptiform EEG (continuous or discrete) 3. Response to treatment? ?
 1. Change in mental status- Semiology n Ambulatory confused patients, mildly confused hospitalized patients n Lethargic and comatose patients in intensive care units
1. Change in mental status- Semiology n Ambulatory confused patients, mildly confused hospitalized patients n Lethargic and comatose patients in intensive care units
 Diminished Level of Consciousness, Confusion
Diminished Level of Consciousness, Confusion

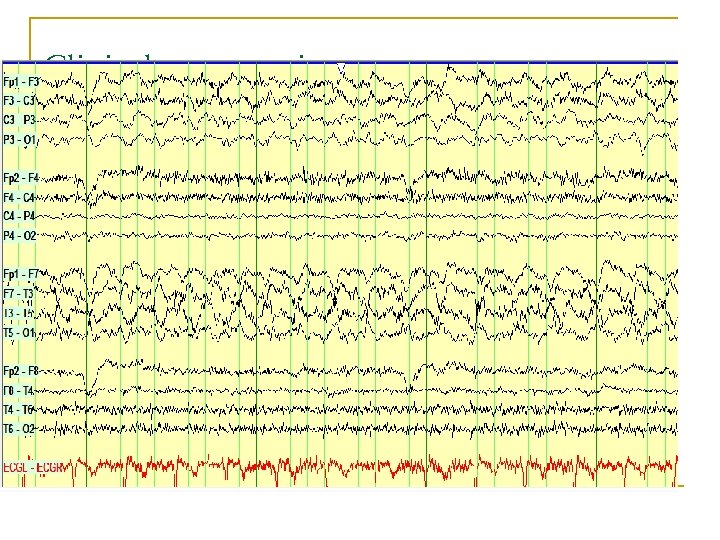 Clinical presentations
Clinical presentations

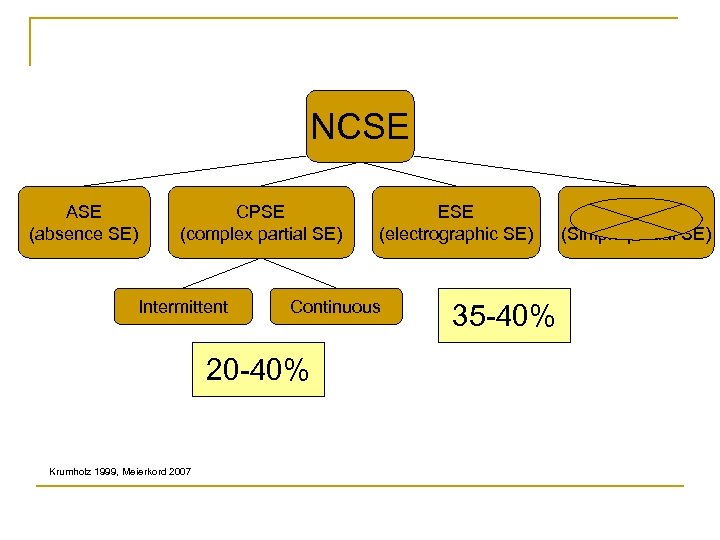 NCSE ASE (absence SE) CPSE (complex partial SE) Intermittent Continuous 20 -40% Krumholz 1999, Meierkord 2007 ESE (electrographic SE) 35 -40% SPSE (Simple partial SE)
NCSE ASE (absence SE) CPSE (complex partial SE) Intermittent Continuous 20 -40% Krumholz 1999, Meierkord 2007 ESE (electrographic SE) 35 -40% SPSE (Simple partial SE)
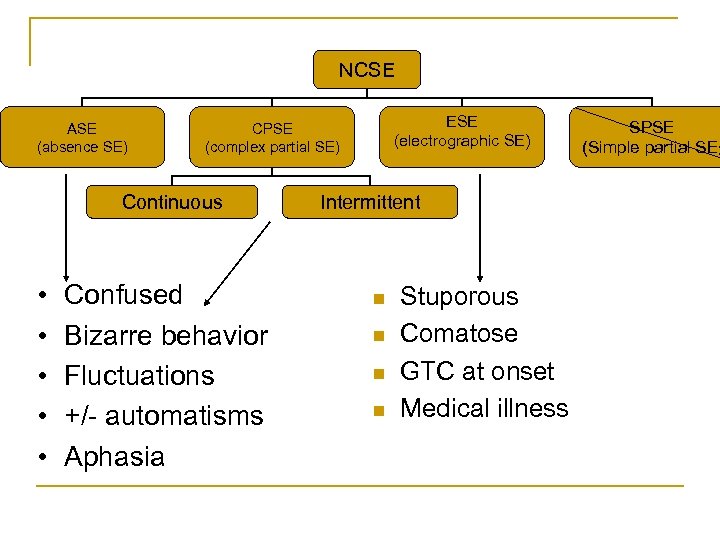 NCSE ASE (absence SE) Continuous • • • ESE (electrographic SE) CPSE (complex partial SE) Confused Bizarre behavior Fluctuations +/- automatisms Aphasia Intermittent n n Stuporous Comatose GTC at onset Medical illness SPSE (Simple partial SE)
NCSE ASE (absence SE) Continuous • • • ESE (electrographic SE) CPSE (complex partial SE) Confused Bizarre behavior Fluctuations +/- automatisms Aphasia Intermittent n n Stuporous Comatose GTC at onset Medical illness SPSE (Simple partial SE)
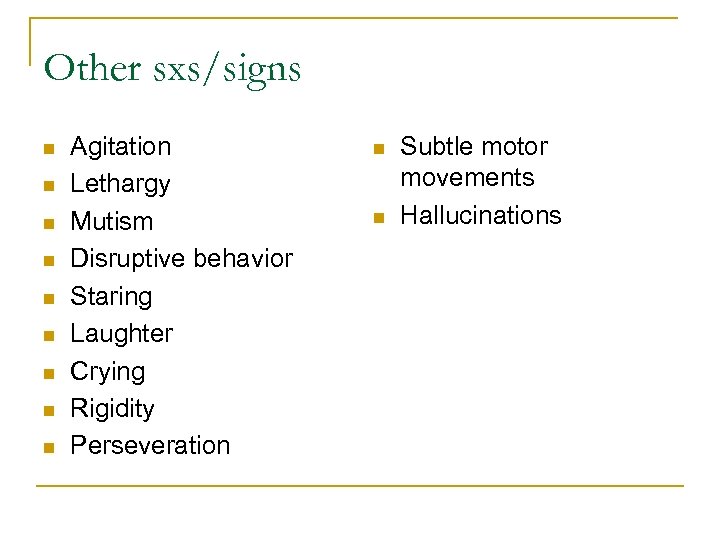 Other sxs/signs n n n n n Agitation Lethargy Mutism Disruptive behavior Staring Laughter Crying Rigidity Perseveration n n Subtle motor movements Hallucinations
Other sxs/signs n n n n n Agitation Lethargy Mutism Disruptive behavior Staring Laughter Crying Rigidity Perseveration n n Subtle motor movements Hallucinations
 DDx n n n Metabolic/toxic encephalopathy Complicated migraine/aura Prolonged post-ictal state Psychiatric disorders Substance abuse/withdrawal/intoxication q n n DTs TIA Transient global amnesia
DDx n n n Metabolic/toxic encephalopathy Complicated migraine/aura Prolonged post-ictal state Psychiatric disorders Substance abuse/withdrawal/intoxication q n n DTs TIA Transient global amnesia
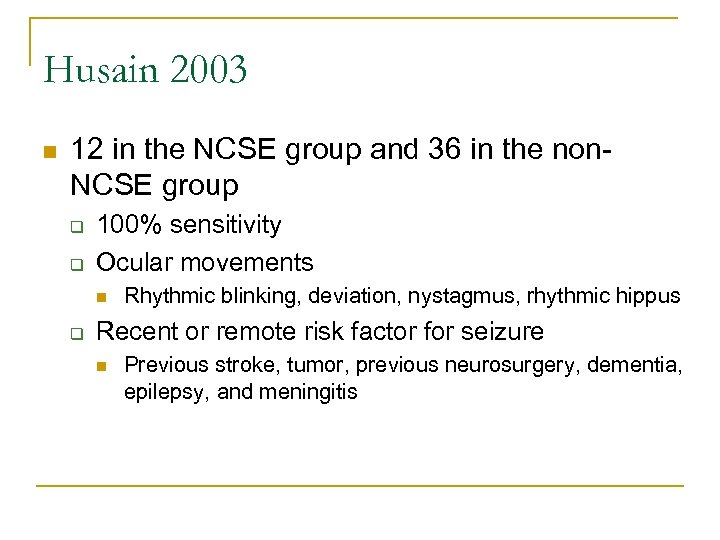 Husain 2003 n 12 in the NCSE group and 36 in the non. NCSE group q q 100% sensitivity Ocular movements n q Rhythmic blinking, deviation, nystagmus, rhythmic hippus Recent or remote risk factor for seizure n Previous stroke, tumor, previous neurosurgery, dementia, epilepsy, and meningitis
Husain 2003 n 12 in the NCSE group and 36 in the non. NCSE group q q 100% sensitivity Ocular movements n q Rhythmic blinking, deviation, nystagmus, rhythmic hippus Recent or remote risk factor for seizure n Previous stroke, tumor, previous neurosurgery, dementia, epilepsy, and meningitis
 Epileptiform EEG
Epileptiform EEG
 2. Epileptiform EEG n n Frequency Morphology Evolution Rhythmicity
2. Epileptiform EEG n n Frequency Morphology Evolution Rhythmicity
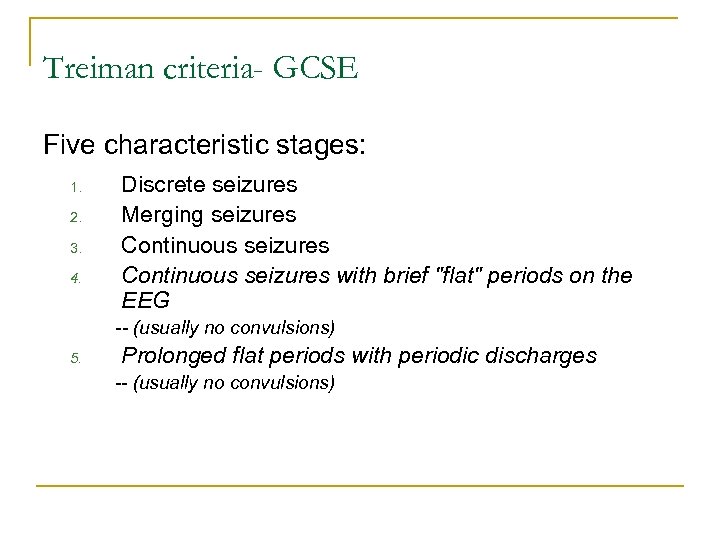 Treiman criteria- GCSE Five characteristic stages: 1. 2. 3. 4. Discrete seizures Merging seizures Continuous seizures with brief "flat" periods on the EEG -- (usually no convulsions) 5. Prolonged flat periods with periodic discharges -- (usually no convulsions)
Treiman criteria- GCSE Five characteristic stages: 1. 2. 3. 4. Discrete seizures Merging seizures Continuous seizures with brief "flat" periods on the EEG -- (usually no convulsions) 5. Prolonged flat periods with periodic discharges -- (usually no convulsions)
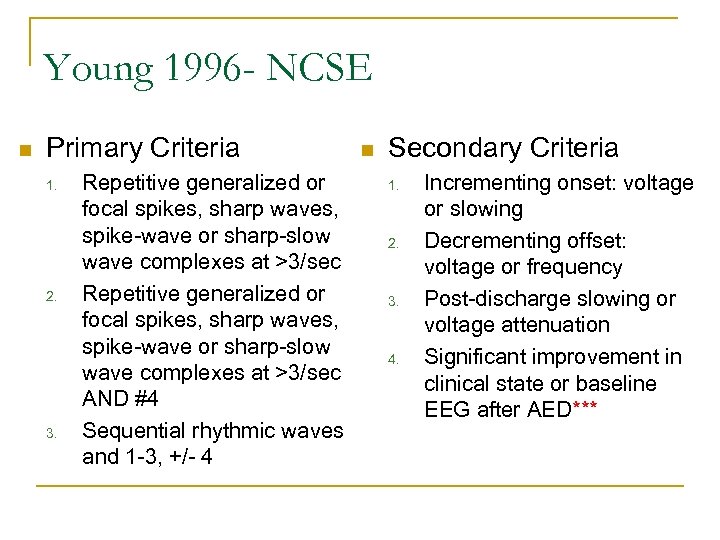 Young 1996 - NCSE n Primary Criteria 1. 2. 3. Repetitive generalized or focal spikes, sharp waves, spike-wave or sharp-slow wave complexes at >3/sec AND #4 Sequential rhythmic waves and 1 -3, +/- 4 n Secondary Criteria 1. 2. 3. 4. Incrementing onset: voltage or slowing Decrementing offset: voltage or frequency Post-discharge slowing or voltage attenuation Significant improvement in clinical state or baseline EEG after AED***
Young 1996 - NCSE n Primary Criteria 1. 2. 3. Repetitive generalized or focal spikes, sharp waves, spike-wave or sharp-slow wave complexes at >3/sec AND #4 Sequential rhythmic waves and 1 -3, +/- 4 n Secondary Criteria 1. 2. 3. 4. Incrementing onset: voltage or slowing Decrementing offset: voltage or frequency Post-discharge slowing or voltage attenuation Significant improvement in clinical state or baseline EEG after AED***
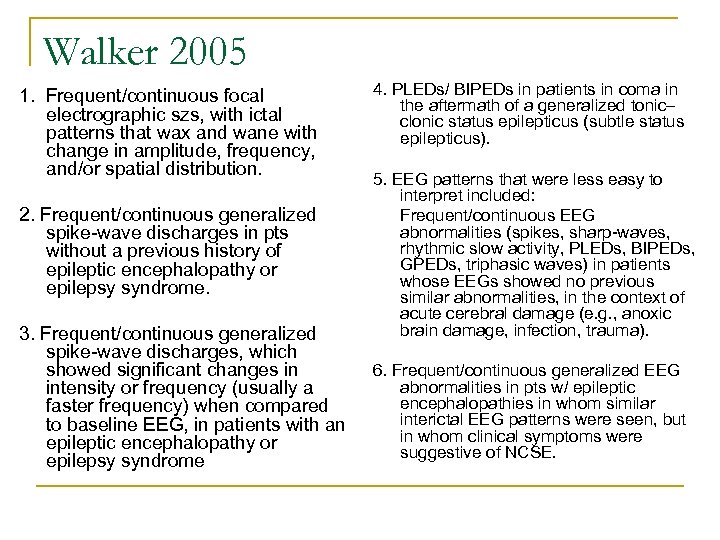 Walker 2005 1. Frequent/continuous focal electrographic szs, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution. 2. Frequent/continuous generalized spike-wave discharges in pts without a previous history of epileptic encephalopathy or epilepsy syndrome. 3. Frequent/continuous generalized spike-wave discharges, which showed significant changes in intensity or frequency (usually a faster frequency) when compared to baseline EEG, in patients with an epileptic encephalopathy or epilepsy syndrome 4. PLEDs/ BIPEDs in patients in coma in the aftermath of a generalized tonic– clonic status epilepticus (subtle status epilepticus). 5. EEG patterns that were less easy to interpret included: Frequent/continuous EEG abnormalities (spikes, sharp-waves, rhythmic slow activity, PLEDs, BIPEDs, GPEDs, triphasic waves) in patients whose EEGs showed no previous similar abnormalities, in the context of acute cerebral damage (e. g. , anoxic brain damage, infection, trauma). 6. Frequent/continuous generalized EEG abnormalities in pts w/ epileptic encephalopathies in whom similar interictal EEG patterns were seen, but in whom clinical symptoms were suggestive of NCSE.
Walker 2005 1. Frequent/continuous focal electrographic szs, with ictal patterns that wax and wane with change in amplitude, frequency, and/or spatial distribution. 2. Frequent/continuous generalized spike-wave discharges in pts without a previous history of epileptic encephalopathy or epilepsy syndrome. 3. Frequent/continuous generalized spike-wave discharges, which showed significant changes in intensity or frequency (usually a faster frequency) when compared to baseline EEG, in patients with an epileptic encephalopathy or epilepsy syndrome 4. PLEDs/ BIPEDs in patients in coma in the aftermath of a generalized tonic– clonic status epilepticus (subtle status epilepticus). 5. EEG patterns that were less easy to interpret included: Frequent/continuous EEG abnormalities (spikes, sharp-waves, rhythmic slow activity, PLEDs, BIPEDs, GPEDs, triphasic waves) in patients whose EEGs showed no previous similar abnormalities, in the context of acute cerebral damage (e. g. , anoxic brain damage, infection, trauma). 6. Frequent/continuous generalized EEG abnormalities in pts w/ epileptic encephalopathies in whom similar interictal EEG patterns were seen, but in whom clinical symptoms were suggestive of NCSE.
 EEG Diagnosis n Inevitably subjective
EEG Diagnosis n Inevitably subjective
 Which tracing shows NCSE?
Which tracing shows NCSE?
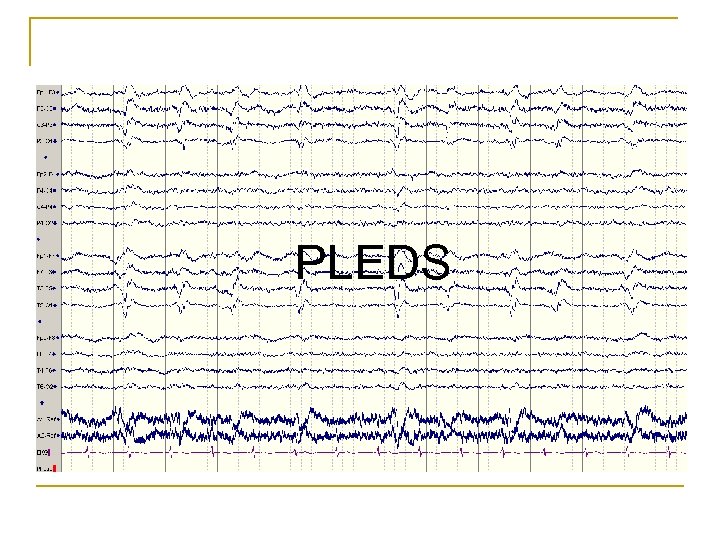 PLEDS
PLEDS
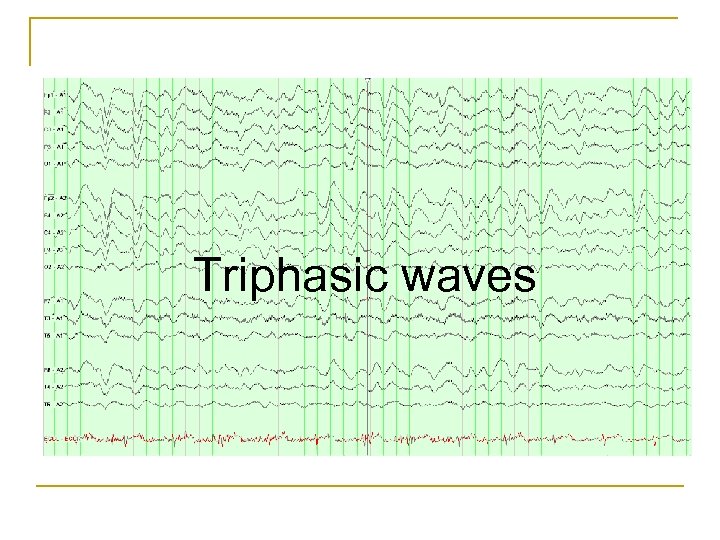 Triphasic waves
Triphasic waves
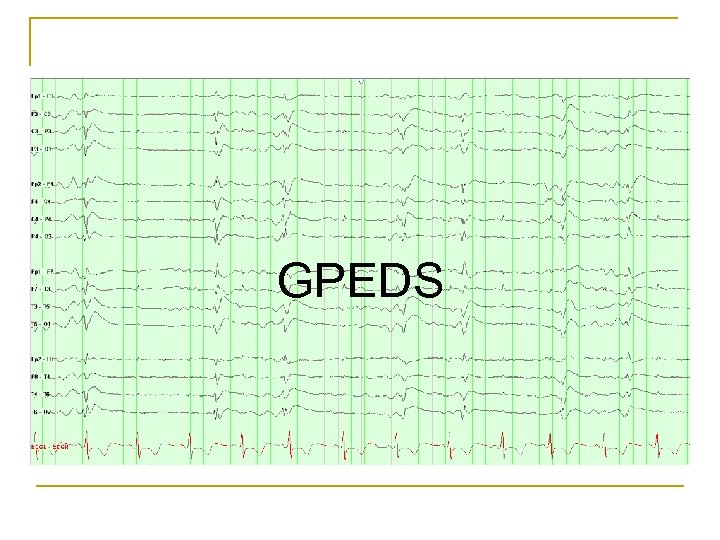 GPEDS
GPEDS
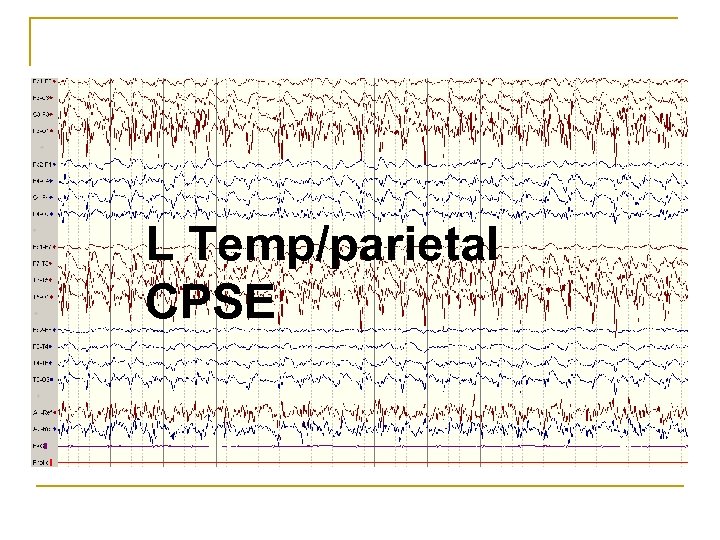 L Temp/parietal CPSE
L Temp/parietal CPSE
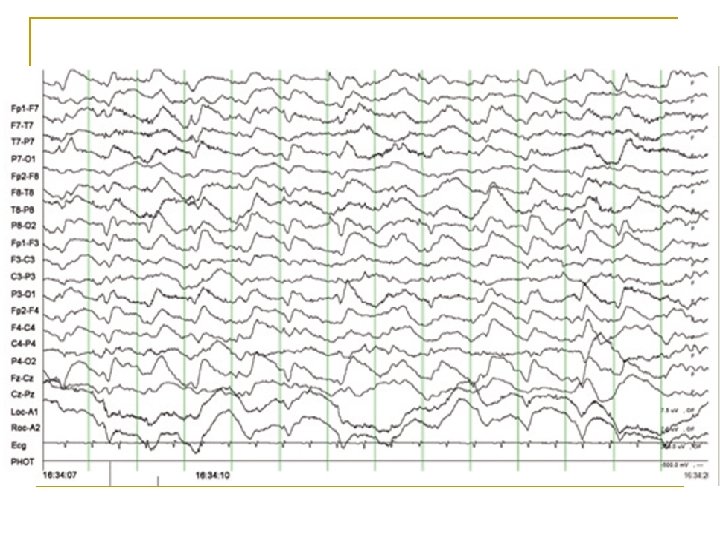
 Diagnostic pitfalls n n PLEDs, Bi. PLEDs, GPEDs, SIRPIDs Encephalopathy Status myoclonus CJD
Diagnostic pitfalls n n PLEDs, Bi. PLEDs, GPEDs, SIRPIDs Encephalopathy Status myoclonus CJD
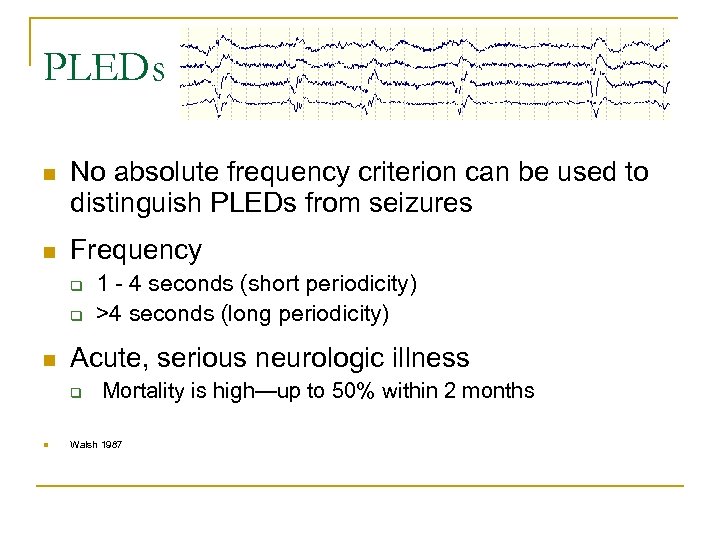 PLEDs n No absolute frequency criterion can be used to distinguish PLEDs from seizures n Frequency q q n Acute, serious neurologic illness q n 1 - 4 seconds (short periodicity) >4 seconds (long periodicity) Mortality is high—up to 50% within 2 months Walsh 1987
PLEDs n No absolute frequency criterion can be used to distinguish PLEDs from seizures n Frequency q q n Acute, serious neurologic illness q n 1 - 4 seconds (short periodicity) >4 seconds (long periodicity) Mortality is high—up to 50% within 2 months Walsh 1987
 PLEDs n Associated with: • • • Stroke (the most common cause in many reports) Tumors Infections- Viral (acute and chronic) Metabolic disturbances Head injury SDH Anoxia Brain abscess Congenital lesions Tuberous sclerosis Multiple sclerosis Creutzfeld–Jakob disease
PLEDs n Associated with: • • • Stroke (the most common cause in many reports) Tumors Infections- Viral (acute and chronic) Metabolic disturbances Head injury SDH Anoxia Brain abscess Congenital lesions Tuberous sclerosis Multiple sclerosis Creutzfeld–Jakob disease
 PLEDs n 80 -90% of pts had recent clinical seizures q n 66% had some form of SE Risk for more seizures q Half patients without prior epilepsy developed subsequent epilepsy n Most PLEDs will resolve after days to weeks n Part of an ictal-interictal spectrum n Snodgrass 1989, Kaplan 2007, Chong 2005, Walsh 1987
PLEDs n 80 -90% of pts had recent clinical seizures q n 66% had some form of SE Risk for more seizures q Half patients without prior epilepsy developed subsequent epilepsy n Most PLEDs will resolve after days to weeks n Part of an ictal-interictal spectrum n Snodgrass 1989, Kaplan 2007, Chong 2005, Walsh 1987
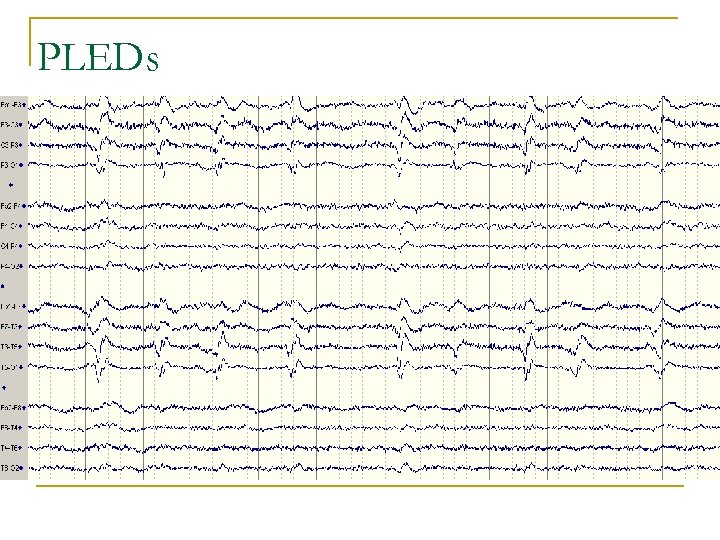 PLEDs
PLEDs
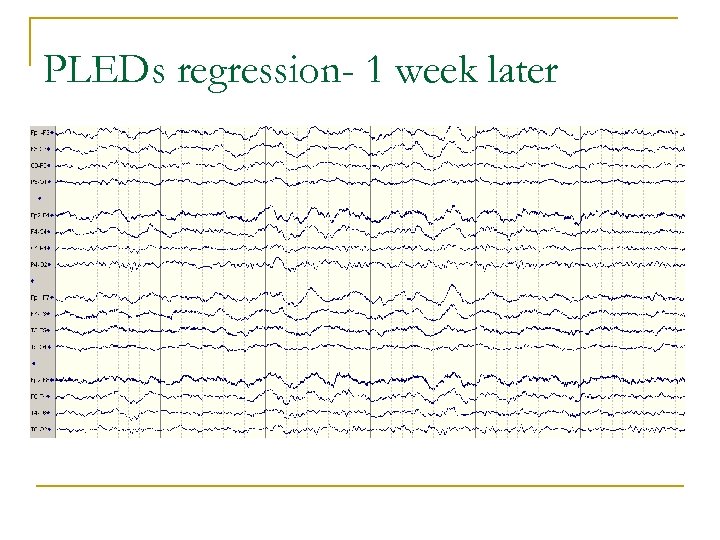 PLEDs regression- 1 week later
PLEDs regression- 1 week later
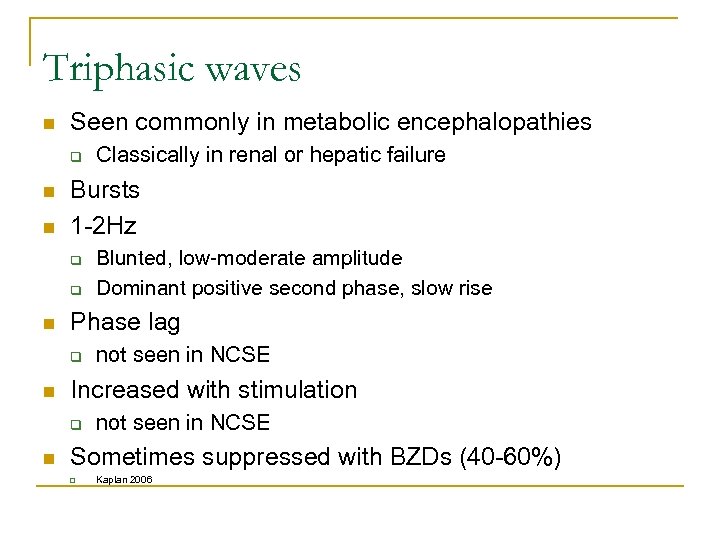 Triphasic waves n Seen commonly in metabolic encephalopathies q n n Bursts 1 -2 Hz q q n not seen in NCSE Increased with stimulation q n Blunted, low-moderate amplitude Dominant positive second phase, slow rise Phase lag q n Classically in renal or hepatic failure not seen in NCSE Sometimes suppressed with BZDs (40 -60%) q Kaplan 2006
Triphasic waves n Seen commonly in metabolic encephalopathies q n n Bursts 1 -2 Hz q q n not seen in NCSE Increased with stimulation q n Blunted, low-moderate amplitude Dominant positive second phase, slow rise Phase lag q n Classically in renal or hepatic failure not seen in NCSE Sometimes suppressed with BZDs (40 -60%) q Kaplan 2006
 Encephalopathies w/Epileptic Features n Reversible q q Usually no hx of epilepsy Medication related n n n n Irreversible q q Post-anoxic Creutzfeld-Jacob BZD withdrawal Cephalosporin Abx Ifosfamide Baclofen Psychotropics Rhythmic, semirhythmic delta Drislane 2000 n Importance of c-VEEG q Look for subtle clinical changes a/w rhythmicity
Encephalopathies w/Epileptic Features n Reversible q q Usually no hx of epilepsy Medication related n n n n Irreversible q q Post-anoxic Creutzfeld-Jacob BZD withdrawal Cephalosporin Abx Ifosfamide Baclofen Psychotropics Rhythmic, semirhythmic delta Drislane 2000 n Importance of c-VEEG q Look for subtle clinical changes a/w rhythmicity
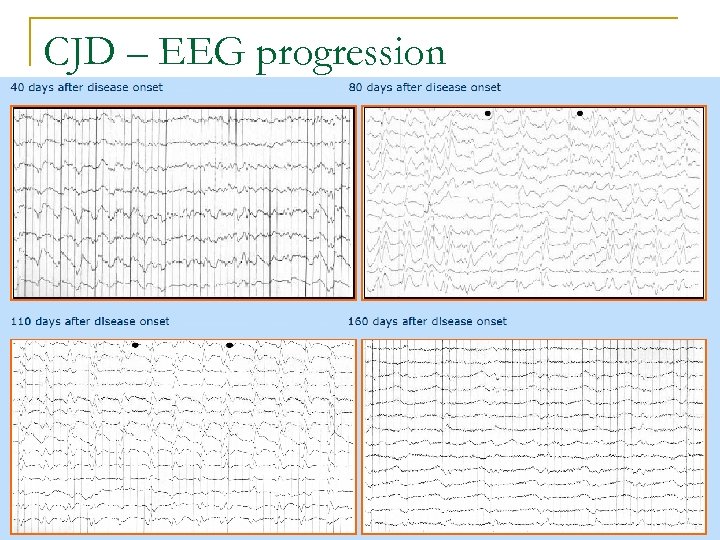 CJD – EEG progression
CJD – EEG progression
 Patients at risk 1. Following seizures or GCSE -- Up to 50% in NCSE after convulsions cease 2. 3. 4. 5. AMS with subtle motor signs AMS in epileptic w/ acute medical illness Post-stroke pt faring worse or recovery halted Elderly pt with AMS (post BZD withdrawal) De. Lorenzo 1998, Drislane 2000
Patients at risk 1. Following seizures or GCSE -- Up to 50% in NCSE after convulsions cease 2. 3. 4. 5. AMS with subtle motor signs AMS in epileptic w/ acute medical illness Post-stroke pt faring worse or recovery halted Elderly pt with AMS (post BZD withdrawal) De. Lorenzo 1998, Drislane 2000
 Risk factors n Mental status changes q q q q ICH SAH Large vessel CVA Meningoencephalitis CHI/TBI Tumor Post-surgical Drislane 2000
Risk factors n Mental status changes q q q q ICH SAH Large vessel CVA Meningoencephalitis CHI/TBI Tumor Post-surgical Drislane 2000
 3. Treatment Response n Treatment response less often considered diagnostic q Clinical response may be delayed hours to days q Shneker 2003
3. Treatment Response n Treatment response less often considered diagnostic q Clinical response may be delayed hours to days q Shneker 2003
 Treatment n CPSE q q q n BZDs IV AEDs Usually recurs ESE q q q 60% respond to initial BZD (clinical delay) 15% resistant to BZD Require IV AEDs n +/- Anesthesia n Granner 1994, Shneker 2003
Treatment n CPSE q q q n BZDs IV AEDs Usually recurs ESE q q q 60% respond to initial BZD (clinical delay) 15% resistant to BZD Require IV AEDs n +/- Anesthesia n Granner 1994, Shneker 2003
 Anesthesia- Claassen 2002 n 193 pts w/ refractory SE q Tx with midazolam vs propofol vs pentobarbitol n Midazolam q q n Pentobarbitol q q n n Increased breakthrough seizures Less hypotension Lowest treatment failure/recurrence More hypotension Refractory NCSE- more common with propofol and midazolam No standardized treatment regimen for use of anesthesia in SE
Anesthesia- Claassen 2002 n 193 pts w/ refractory SE q Tx with midazolam vs propofol vs pentobarbitol n Midazolam q q n Pentobarbitol q q n n Increased breakthrough seizures Less hypotension Lowest treatment failure/recurrence More hypotension Refractory NCSE- more common with propofol and midazolam No standardized treatment regimen for use of anesthesia in SE
 Anesthesia n No consensus on NCSE q More harm than good? n n n q Hypotension Sepsis/line infection DVT Ultimate effect on brain? n Outcomes…
Anesthesia n No consensus on NCSE q More harm than good? n n n q Hypotension Sepsis/line infection DVT Ultimate effect on brain? n Outcomes…
 Pathologic changes n Animal models q q q Induced GCSE, up to 5 hours, in baboons Hippocampal volume loss n ↑ with frequent, prolonged seizures n ↓ if paralytic used to abolish convulsions q Hyperpyrexia, hypotension, hypoxia, acidosis, and hypoglycemia Changes in high-frequency (10 Hz) vs low frequency (1 Hz) discharges n Bertram 1990
Pathologic changes n Animal models q q q Induced GCSE, up to 5 hours, in baboons Hippocampal volume loss n ↑ with frequent, prolonged seizures n ↓ if paralytic used to abolish convulsions q Hyperpyrexia, hypotension, hypoxia, acidosis, and hypoglycemia Changes in high-frequency (10 Hz) vs low frequency (1 Hz) discharges n Bertram 1990
 Pathologic changes n Human autopsy studies q q GCSE > epilepsy w/o SE > normal Synergistic damage n Increase in excitatory neurotransmitters Metabolic changes (lactate, pyruvate) n Earnest 1992, Kruhmholz 1995 n
Pathologic changes n Human autopsy studies q q GCSE > epilepsy w/o SE > normal Synergistic damage n Increase in excitatory neurotransmitters Metabolic changes (lactate, pyruvate) n Earnest 1992, Kruhmholz 1995 n
 Outcomes: Mortality n Vary highly based on the underlying etiology of the condition q q q n Brain tumors (30 -40%) Acute stroke (35%) Epilepsy (3%) Duration of seizures q 43 ICU pts in NCSE on VEEG n n <10 h = death in 10% >20 h = death in 85% n Age > 60 y n Rarely fatal in isolation q Young 1996, Meierkord 2007, Towne 1994
Outcomes: Mortality n Vary highly based on the underlying etiology of the condition q q q n Brain tumors (30 -40%) Acute stroke (35%) Epilepsy (3%) Duration of seizures q 43 ICU pts in NCSE on VEEG n n <10 h = death in 10% >20 h = death in 85% n Age > 60 y n Rarely fatal in isolation q Young 1996, Meierkord 2007, Towne 1994
 Outcomes: Morbidity n CPSE q No difference between continuous and intermittent electrographic sz activity n n n Return to baseline cognitive status (n=20) Cognitive decline, memory issues (n=10) ESE q q Determined by primary etiology Tend to have poorer prognosis n Drislane 1999, Cockerell 1994, Krumholz 1995
Outcomes: Morbidity n CPSE q No difference between continuous and intermittent electrographic sz activity n n n Return to baseline cognitive status (n=20) Cognitive decline, memory issues (n=10) ESE q q Determined by primary etiology Tend to have poorer prognosis n Drislane 1999, Cockerell 1994, Krumholz 1995
 Outcomes: MICU vs NICU n 168 visits over 3 yrs q 27% NICU n n n More pts w/ stroke More CPSE Avg age: 59 Alert/somnolent pts Fewer pts intubated, more tracheostomized Varelas 2013 q 73% MICU n n n More toxic/metabolic enceph More GCSE Avg age: 51 Obtunded/comatose pts Higher APACHE 2 scores
Outcomes: MICU vs NICU n 168 visits over 3 yrs q 27% NICU n n n More pts w/ stroke More CPSE Avg age: 59 Alert/somnolent pts Fewer pts intubated, more tracheostomized Varelas 2013 q 73% MICU n n n More toxic/metabolic enceph More GCSE Avg age: 51 Obtunded/comatose pts Higher APACHE 2 scores
 MICU vs NICU n No difference in outcomes q q n Length of ICU/hospital stay Functional status at discharge (m. RS) Limitations: q q Smaller NICU population Neuro illness with longer recovery period?
MICU vs NICU n No difference in outcomes q q n Length of ICU/hospital stay Functional status at discharge (m. RS) Limitations: q q Smaller NICU population Neuro illness with longer recovery period?
 KU Data
KU Data
 KU Cohort n Objective: q Review and describe non-convulsive status epilepticus (NCSE) cases n n Etiology Co-morbidities Medical treatment Clinical outcomes
KU Cohort n Objective: q Review and describe non-convulsive status epilepticus (NCSE) cases n n Etiology Co-morbidities Medical treatment Clinical outcomes
 KU Cohort n Methods: q Medical records reviewed from Jan 2009 -2013 n n n q ICD 9 for status epilepticus, at discharge CPT code for video-EEG monitoring ICU room charge during hospital stay Patients selected based on the following inclusion criteria: n n n Age: 10 - 110 years of age Diagnosis made utilizing routine or continuous video electroencephalogram Patients with hypoxic-ischemic brain injury were excluded
KU Cohort n Methods: q Medical records reviewed from Jan 2009 -2013 n n n q ICD 9 for status epilepticus, at discharge CPT code for video-EEG monitoring ICU room charge during hospital stay Patients selected based on the following inclusion criteria: n n n Age: 10 - 110 years of age Diagnosis made utilizing routine or continuous video electroencephalogram Patients with hypoxic-ischemic brain injury were excluded
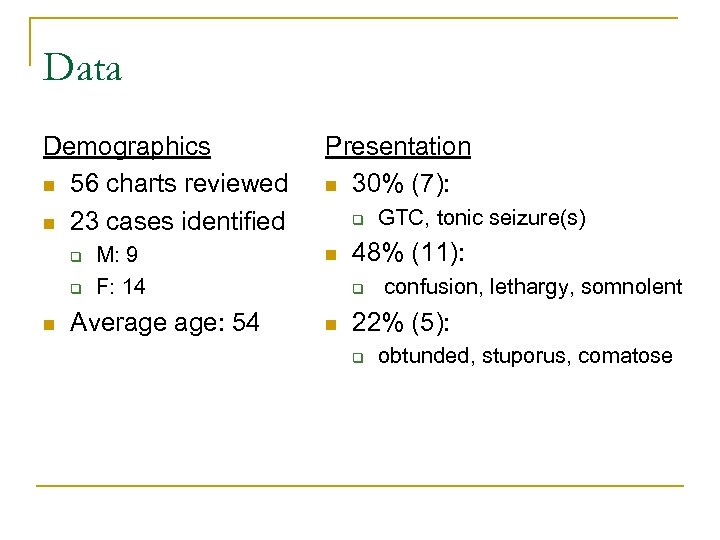 Data Demographics n 56 charts reviewed n 23 cases identified q q n M: 9 F: 14 Average age: 54 Presentation n 30% (7): q n 48% (11): q n GTC, tonic seizure(s) confusion, lethargy, somnolent 22% (5): q obtunded, stuporus, comatose
Data Demographics n 56 charts reviewed n 23 cases identified q q n M: 9 F: 14 Average age: 54 Presentation n 30% (7): q n 48% (11): q n GTC, tonic seizure(s) confusion, lethargy, somnolent 22% (5): q obtunded, stuporus, comatose
 Data n 35% (8): Automatism, subtle motor mvts q q q n Head turning Subtle limb, facial, tongue movements Eyelid flutter 22% (5): eye deviation
Data n 35% (8): Automatism, subtle motor mvts q q q n Head turning Subtle limb, facial, tongue movements Eyelid flutter 22% (5): eye deviation
 Data n CPSE (74%) q q q LOS: 19. 2 d ICU: 11. 1 d VEEG: 6. 1 d # AEDs: 2. 6 Anesthesia: 4. 6 d n ESE (13%) q q q LOS: 45. 7 d ICU: 20. 7 d VEEG: 8 d # AEDs: 3 Anesthesia: 7. 5 d
Data n CPSE (74%) q q q LOS: 19. 2 d ICU: 11. 1 d VEEG: 6. 1 d # AEDs: 2. 6 Anesthesia: 4. 6 d n ESE (13%) q q q LOS: 45. 7 d ICU: 20. 7 d VEEG: 8 d # AEDs: 3 Anesthesia: 7. 5 d
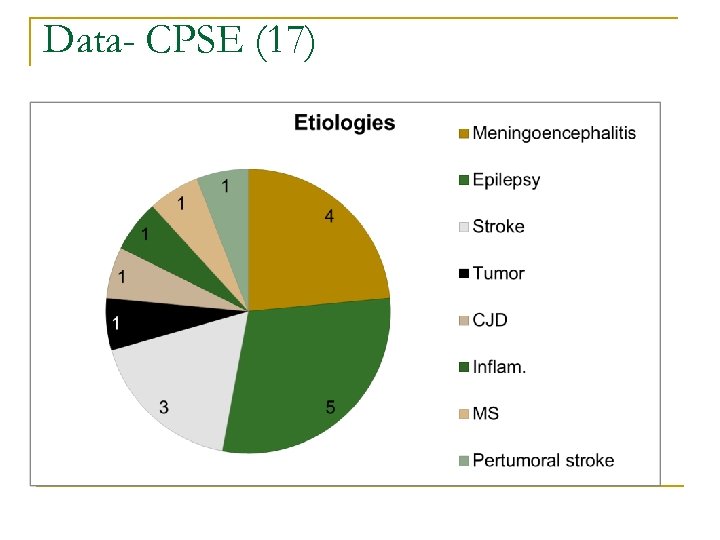 Data- CPSE (17)
Data- CPSE (17)
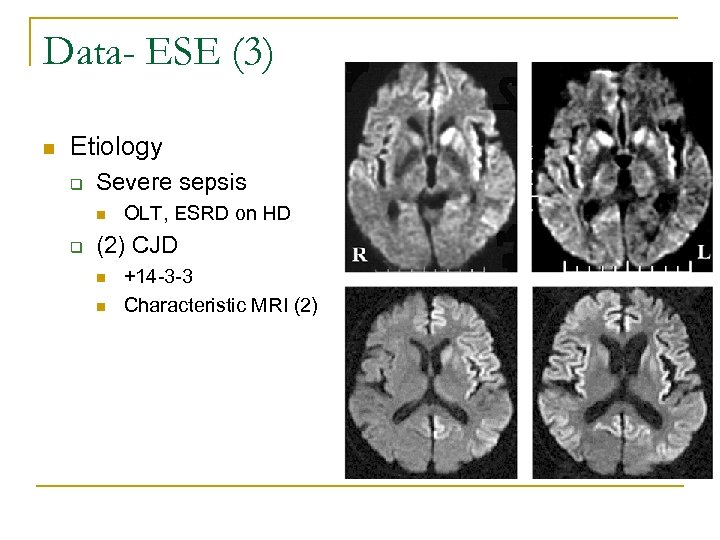 Data- ESE (3) n Etiology q Severe sepsis n q OLT, ESRD on HD (2) CJD n n +14 -3 -3 Characteristic MRI (2)
Data- ESE (3) n Etiology q Severe sepsis n q OLT, ESRD on HD (2) CJD n n +14 -3 -3 Characteristic MRI (2)
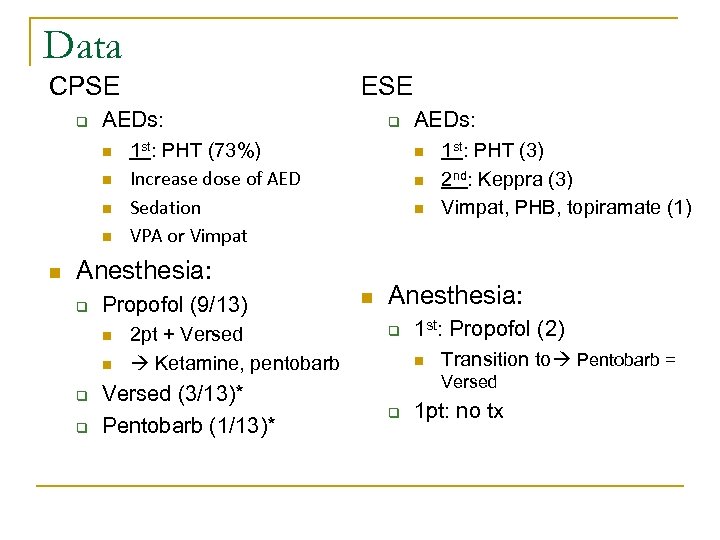 Data CPSE q AEDs: n n n ESE q 1 st: PHT (73%) Increase dose of AED Sedation VPA or Vimpat n n n Anesthesia: q Propofol (9/13) n n q q 2 pt + Versed Ketamine, pentobarb Versed (3/13)* Pentobarb (1/13)* AEDs: n 1 st: PHT (3) 2 nd: Keppra (3) Vimpat, PHB, topiramate (1) Anesthesia: q 1 st: Propofol (2) n Transition to Pentobarb = Versed q 1 pt: no tx
Data CPSE q AEDs: n n n ESE q 1 st: PHT (73%) Increase dose of AED Sedation VPA or Vimpat n n n Anesthesia: q Propofol (9/13) n n q q 2 pt + Versed Ketamine, pentobarb Versed (3/13)* Pentobarb (1/13)* AEDs: n 1 st: PHT (3) 2 nd: Keppra (3) Vimpat, PHB, topiramate (1) Anesthesia: q 1 st: Propofol (2) n Transition to Pentobarb = Versed q 1 pt: no tx
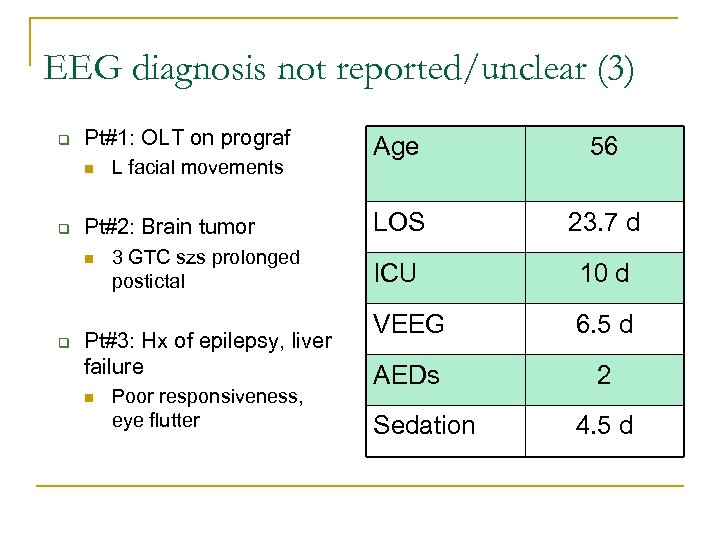 EEG diagnosis not reported/unclear (3) q Pt#1: OLT on prograf n q Pt#2: Brain tumor n q L facial movements 3 GTC szs prolonged postictal Pt#3: Hx of epilepsy, liver failure n Poor responsiveness, eye flutter Age 56 LOS 23. 7 d ICU 10 d VEEG 6. 5 d AEDs 2 Sedation 4. 5 d
EEG diagnosis not reported/unclear (3) q Pt#1: OLT on prograf n q Pt#2: Brain tumor n q L facial movements 3 GTC szs prolonged postictal Pt#3: Hx of epilepsy, liver failure n Poor responsiveness, eye flutter Age 56 LOS 23. 7 d ICU 10 d VEEG 6. 5 d AEDs 2 Sedation 4. 5 d
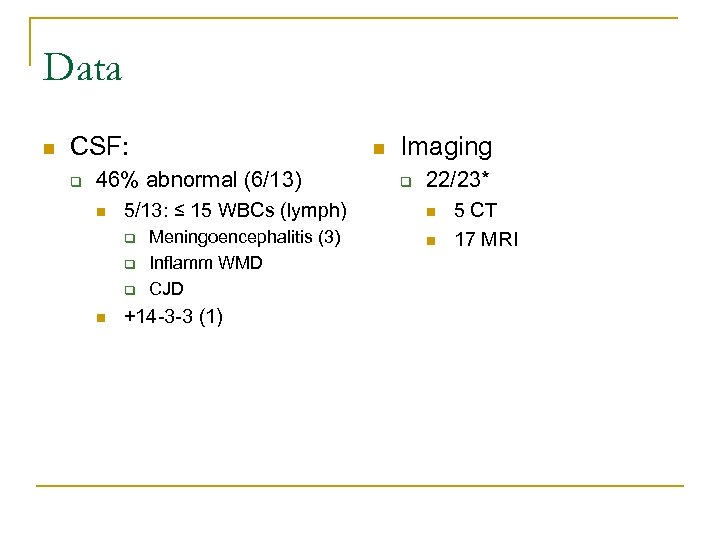 Data n CSF: q n 46% abnormal (6/13) n 5/13: ≤ 15 WBCs (lymph) q q q n Meningoencephalitis (3) Inflamm WMD CJD +14 -3 -3 (1) Imaging q 22/23* n n 5 CT 17 MRI
Data n CSF: q n 46% abnormal (6/13) n 5/13: ≤ 15 WBCs (lymph) q q q n Meningoencephalitis (3) Inflamm WMD CJD +14 -3 -3 (1) Imaging q 22/23* n n 5 CT 17 MRI
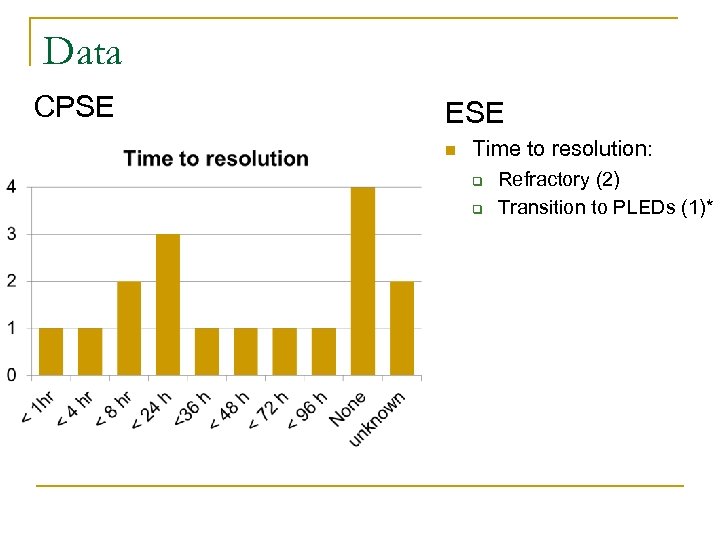 Data CPSE ESE n Time to resolution: q q Refractory (2) Transition to PLEDs (1)*
Data CPSE ESE n Time to resolution: q q Refractory (2) Transition to PLEDs (1)*
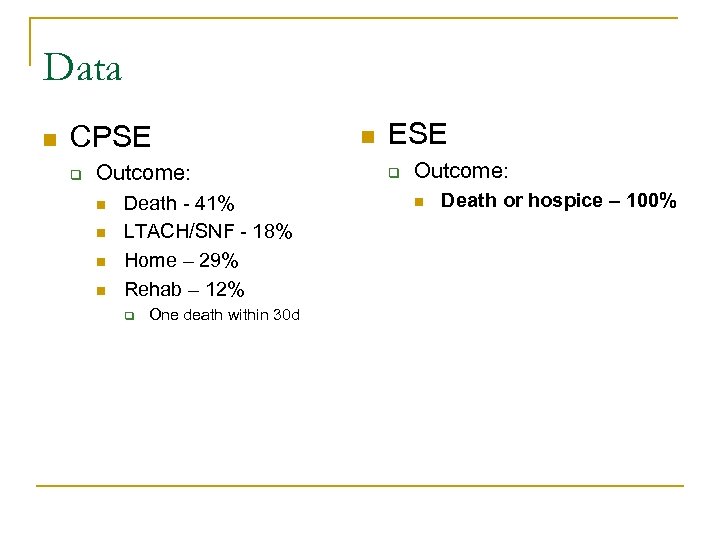 Data n CPSE q Outcome: n n Death - 41% LTACH/SNF - 18% Home – 29% Rehab – 12% q One death within 30 d n ESE q Outcome: n Death or hospice – 100%
Data n CPSE q Outcome: n n Death - 41% LTACH/SNF - 18% Home – 29% Rehab – 12% q One death within 30 d n ESE q Outcome: n Death or hospice – 100%
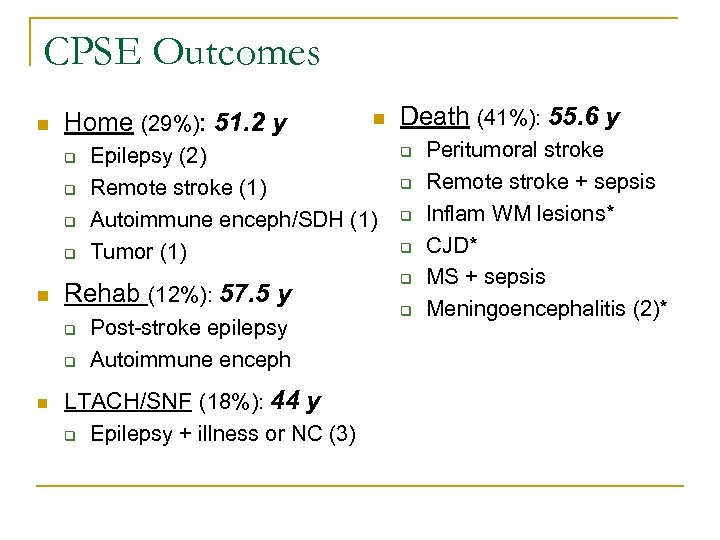 CPSE Outcomes n Home (29%): 51. 2 y q q n q n Epilepsy (2) Remote stroke (1) Autoimmune enceph/SDH (1) Tumor (1) Rehab (12%): 57. 5 y q n Post-stroke epilepsy Autoimmune enceph LTACH/SNF (18%): 44 y q Epilepsy + illness or NC (3) Death (41%): 55. 6 y q q q Peritumoral stroke Remote stroke + sepsis Inflam WM lesions* CJD* MS + sepsis Meningoencephalitis (2)*
CPSE Outcomes n Home (29%): 51. 2 y q q n q n Epilepsy (2) Remote stroke (1) Autoimmune enceph/SDH (1) Tumor (1) Rehab (12%): 57. 5 y q n Post-stroke epilepsy Autoimmune enceph LTACH/SNF (18%): 44 y q Epilepsy + illness or NC (3) Death (41%): 55. 6 y q q q Peritumoral stroke Remote stroke + sepsis Inflam WM lesions* CJD* MS + sepsis Meningoencephalitis (2)*
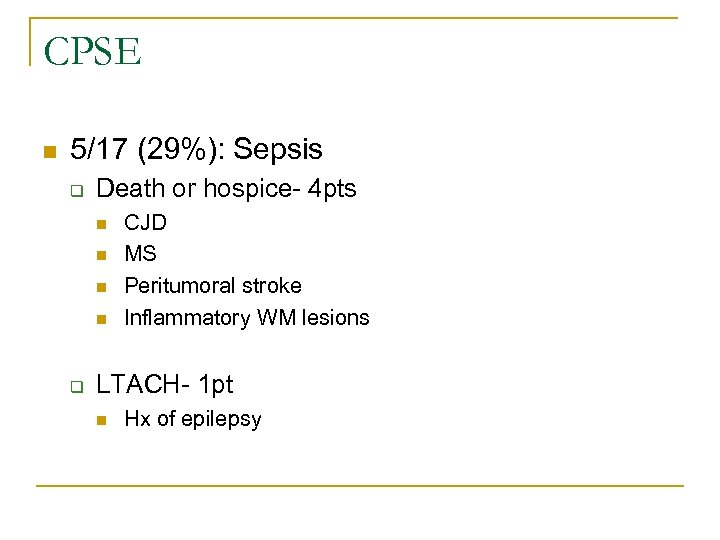 CPSE n 5/17 (29%): Sepsis q Death or hospice- 4 pts n n q CJD MS Peritumoral stroke Inflammatory WM lesions LTACH- 1 pt n Hx of epilepsy
CPSE n 5/17 (29%): Sepsis q Death or hospice- 4 pts n n q CJD MS Peritumoral stroke Inflammatory WM lesions LTACH- 1 pt n Hx of epilepsy
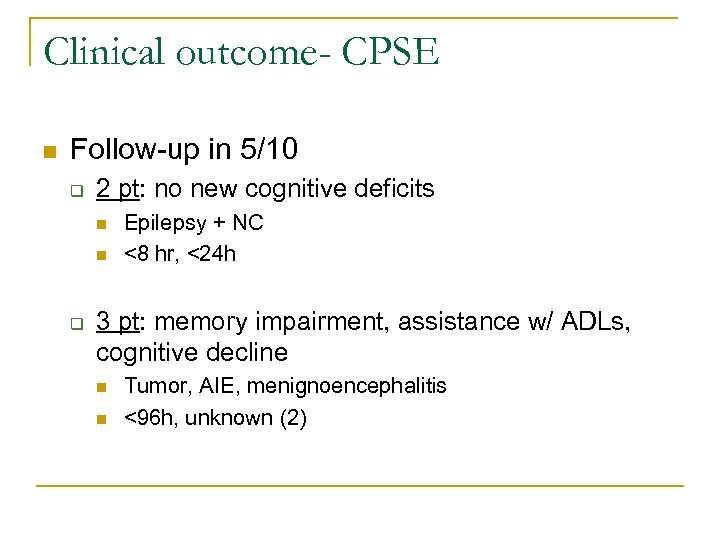 Clinical outcome- CPSE n Follow-up in 5/10 q 2 pt: no new cognitive deficits n n q Epilepsy + NC <8 hr, <24 h 3 pt: memory impairment, assistance w/ ADLs, cognitive decline n n Tumor, AIE, menignoencephalitis <96 h, unknown (2)
Clinical outcome- CPSE n Follow-up in 5/10 q 2 pt: no new cognitive deficits n n q Epilepsy + NC <8 hr, <24 h 3 pt: memory impairment, assistance w/ ADLs, cognitive decline n n Tumor, AIE, menignoencephalitis <96 h, unknown (2)
 Limitations n Limited number of patients q n Majority from 2012, only 3 from 2009, 1 from 2010 Inclusion of patients with CJD q q 100% mortality Encephalopathy with epileptic features n Documentation, access to archived studies n Lack of clinical follow-up information n No cases of NCSE in acute stroke
Limitations n Limited number of patients q n Majority from 2012, only 3 from 2009, 1 from 2010 Inclusion of patients with CJD q q 100% mortality Encephalopathy with epileptic features n Documentation, access to archived studies n Lack of clinical follow-up information n No cases of NCSE in acute stroke
 Conclusions n Outcomes worse is ESE n Worse if underlying dx is CJD n Underlying epilepsy portends better outcome n Longer duration of uncontrolled NCSE adverse cognitive impact n Pt’s treated with Versed as initial agent, worse outcomes (2/3) death n Outcomes worse when pt diagnosed with sepsis
Conclusions n Outcomes worse is ESE n Worse if underlying dx is CJD n Underlying epilepsy portends better outcome n Longer duration of uncontrolled NCSE adverse cognitive impact n Pt’s treated with Versed as initial agent, worse outcomes (2/3) death n Outcomes worse when pt diagnosed with sepsis
 Thanks n n n Nancy Hammond, MD Utku Uysal, MD Ivan Osorio, MD William Nowack, MD Rhonda Reliford
Thanks n n n Nancy Hammond, MD Utku Uysal, MD Ivan Osorio, MD William Nowack, MD Rhonda Reliford
 References n n n Celesia CG. Modern concepts of status epilepticus. JAMA 1976: 235: 1771 -4. Tomson T, Svanbog, E, Wedlund J. E. Nonconvulsive status epilepticus: high incidence of complex partial status. Epilepsia. 1986; 27: 276 -85. Drislane F. Presentation, Evaluation, and Treatment of Nonconvulsive Status Epilepticus. Epilepsy and Behavior. 2000; 1: 301 -314. Towne AR. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000; 54(2): 340 -4. Krumholz A. Epidemiology and evidence for morbidity of nonconvulsive status epilepticus. J Clin Neurophysiology. 1999; 16(4): 314 -22. Meierkord H. The risk of epilepsy after status epilepticus in children and adults. Epilepsia. 2007; 48 suppl 8: 94 -5. Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: Usefulness of clinical features in selecting patients for urgent EEG. J. Neurol Neurosurg Psychiatry. 2003 Feb; 74(2): 189 -91. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: An investigation of variables associated with mortality. Neurology. 1996 Jul; 47(1): 83 -9. Treiman DM, Walton NY, Kendrick C. A progressive sequence of electrographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990; 5: 49 -60. Walker M. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord. 2005 Sep; 7(3): 253 -96. Walsh JM, Brenner RP. Periodic lateralized epileptiform discharges: long-term outcome in adults. Epilepsia 1987; 28: 533– 6.
References n n n Celesia CG. Modern concepts of status epilepticus. JAMA 1976: 235: 1771 -4. Tomson T, Svanbog, E, Wedlund J. E. Nonconvulsive status epilepticus: high incidence of complex partial status. Epilepsia. 1986; 27: 276 -85. Drislane F. Presentation, Evaluation, and Treatment of Nonconvulsive Status Epilepticus. Epilepsy and Behavior. 2000; 1: 301 -314. Towne AR. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000; 54(2): 340 -4. Krumholz A. Epidemiology and evidence for morbidity of nonconvulsive status epilepticus. J Clin Neurophysiology. 1999; 16(4): 314 -22. Meierkord H. The risk of epilepsy after status epilepticus in children and adults. Epilepsia. 2007; 48 suppl 8: 94 -5. Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: Usefulness of clinical features in selecting patients for urgent EEG. J. Neurol Neurosurg Psychiatry. 2003 Feb; 74(2): 189 -91. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: An investigation of variables associated with mortality. Neurology. 1996 Jul; 47(1): 83 -9. Treiman DM, Walton NY, Kendrick C. A progressive sequence of electrographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990; 5: 49 -60. Walker M. Nonconvulsive status epilepticus: Epilepsy research foundation workshop reports. Epileptic Disord. 2005 Sep; 7(3): 253 -96. Walsh JM, Brenner RP. Periodic lateralized epileptiform discharges: long-term outcome in adults. Epilepsia 1987; 28: 533– 6.
 References n n n n n Snodgrass SM, Tsuburaya K, Ajmone-Marsan C. Clinical significance of periodic lateralized epileptiform discharges: Relationship with status epilepticus. J Clin Neurophysiol. 1989 Apr; 6(2): 159 -72. Kaplan PW. EEG criteria for nonconvulsive status epilepticus. Epilepsia. 2007; 48 Suppl 8: 39 -41. Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005 Apr; 22(2): 79 -91. Kaplan PW. EEG monitoring in the intensive care unit. Am J Electroneurodiagnostic Technol. 2006 Jun; 46(2): 81 -97. De. Lorenzo RJ, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998 Aug; 39(8): 833 -40. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003 Oct 28; 61(8): 1066 -73. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia. 1994 Jan-Feb; 35(1): 42 -7. Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002 Feb; 43(2): 146 -53. Lothman EW, et al. Recurrent spontaneous hippocampal seizures in the rat as a chronic sequela to limbic status epilepticus. Epilepsy Res. 1990 Jul; 6(2): 110 -8.
References n n n n n Snodgrass SM, Tsuburaya K, Ajmone-Marsan C. Clinical significance of periodic lateralized epileptiform discharges: Relationship with status epilepticus. J Clin Neurophysiol. 1989 Apr; 6(2): 159 -72. Kaplan PW. EEG criteria for nonconvulsive status epilepticus. Epilepsia. 2007; 48 Suppl 8: 39 -41. Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005 Apr; 22(2): 79 -91. Kaplan PW. EEG monitoring in the intensive care unit. Am J Electroneurodiagnostic Technol. 2006 Jun; 46(2): 81 -97. De. Lorenzo RJ, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998 Aug; 39(8): 833 -40. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003 Oct 28; 61(8): 1066 -73. Granner MA, Lee SI. Nonconvulsive status epilepticus: EEG analysis in a large series. Epilepsia. 1994 Jan-Feb; 35(1): 42 -7. Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002 Feb; 43(2): 146 -53. Lothman EW, et al. Recurrent spontaneous hippocampal seizures in the rat as a chronic sequela to limbic status epilepticus. Epilepsy Res. 1990 Jul; 6(2): 110 -8.
 References n n n Earnest MP, Thomas GE, Eden RA, Hossack KF. The sudden unexplained death syndrome in epilepsy: demographic, clinical, and postmortem features. Epilepsia. 1992 Mar -Apr; 33(2): 310 -6. Krumholz A. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology. 1995 Aug; 45(8): 1499 -504. Drislane FW. Evidence against permanent neurologic damage from nonconvulsive status epilepticus. J Clin Neurophysiol. 1999 Jul; 16(4): 323 -31 Cockerell OC, Walker MC, Sander JW, Shorvon SD. Complex partial status epilepticus: a recurrent problem. J Neurol Neurosurg Psychiatry. 1994 Jul; 57(7): 835 -7. Varelas PN, et al. Emergent EEG: indications and diagnostic yield. Neurology. 2003 Sep 9; 61(5): 702 -4.
References n n n Earnest MP, Thomas GE, Eden RA, Hossack KF. The sudden unexplained death syndrome in epilepsy: demographic, clinical, and postmortem features. Epilepsia. 1992 Mar -Apr; 33(2): 310 -6. Krumholz A. Complex partial status epilepticus accompanied by serious morbidity and mortality. Neurology. 1995 Aug; 45(8): 1499 -504. Drislane FW. Evidence against permanent neurologic damage from nonconvulsive status epilepticus. J Clin Neurophysiol. 1999 Jul; 16(4): 323 -31 Cockerell OC, Walker MC, Sander JW, Shorvon SD. Complex partial status epilepticus: a recurrent problem. J Neurol Neurosurg Psychiatry. 1994 Jul; 57(7): 835 -7. Varelas PN, et al. Emergent EEG: indications and diagnostic yield. Neurology. 2003 Sep 9; 61(5): 702 -4.
 Thank you Questions? Comments?
Thank you Questions? Comments?


