70b56a9a277279ef36cca73979a8a402.ppt
- Количество слайдов: 42
 NMR Spectroscopy: n n Introduction – History of NMR Hardware and Software n n n Superconducting Cryo-Magnet Probe RF Console Computer + NMR Software + Printer/Plotter Solution NMR n n Sample Preparation Presentation of Data 1
NMR Spectroscopy: n n Introduction – History of NMR Hardware and Software n n n Superconducting Cryo-Magnet Probe RF Console Computer + NMR Software + Printer/Plotter Solution NMR n n Sample Preparation Presentation of Data 1
 Important NMR Milestones n n n n n 1938 - NMR in molecular beams Rabi (Columbia University) 1946 - NMR of Liquids and Solids Purcell, Torrey, Pound (Harvard) Bloch, Hansen, Packard (Cal. Tech) 1952 - First commercial NMR spectrometer 1962 - First Superconducting Magnet for NMR 1968 - First Pulse Fourier Transform NMR 1969 - First Concept of MRI Scanners 1971 - First 2 D NMR Experiment – COSY (Jean Jeener) 1985 - Protein Structures 2009 - First Gigahertz NMR Spectrometer 2
Important NMR Milestones n n n n n 1938 - NMR in molecular beams Rabi (Columbia University) 1946 - NMR of Liquids and Solids Purcell, Torrey, Pound (Harvard) Bloch, Hansen, Packard (Cal. Tech) 1952 - First commercial NMR spectrometer 1962 - First Superconducting Magnet for NMR 1968 - First Pulse Fourier Transform NMR 1969 - First Concept of MRI Scanners 1971 - First 2 D NMR Experiment – COSY (Jean Jeener) 1985 - Protein Structures 2009 - First Gigahertz NMR Spectrometer 2
 NMR Nobel Prize Winners n n n 1944 Isador Rabi 1952 Felix Bloch & Edwin Purcell 1991 Richard Ernst 2002 Kurt Wüthrich 2003 Paul Lauterbur & Sir Peter Mansfield 3 From: Bruker Spin. Report, Vol 153
NMR Nobel Prize Winners n n n 1944 Isador Rabi 1952 Felix Bloch & Edwin Purcell 1991 Richard Ernst 2002 Kurt Wüthrich 2003 Paul Lauterbur & Sir Peter Mansfield 3 From: Bruker Spin. Report, Vol 153
 Laukien Prize Winners n n n n 1999 Konstantin Pervushin, Roland Riek, Gerhard Wider, and Kurt Wüthrich; TROSY 2000 Lucio Frydman; Quadrupolar MQMAS 2001 Peter Boesiger, Klaas Prüßmann, Markus Weiger; Sensitivity-encoded magnetic resonance imaging 2002 Ad Bax, Aksel Bothner-By and James Prestegard; Residual dipolar couplings of weakly aligned molecules in solution 2003 Jacob Schaefer; REDOR Technique for Solid State NMR 2004 Lewis E. Kay, NMR of Biological Macromolecules 2005 Stephan Grzesiek, J couplings across hydrogen bonds 2006 Thomas Szyperski, Eriks Kupce, Ray Freeman, and Rafael Bruschweiler; Acceleration of Multi-dimensional NMR by novel procedures for scanning data space and efficiently processing results to obtain a conventional spectral representation 2007 Robert G. Griffin; High-field dynamic nuclear polarization (DNP) for sensitivity enhancement in solid-state MAS NMR 2008 Malcom H. Levitt; Optimized pulses and pulse sequences to enhance the power of liquid & solid state NMR 2009 Daniel P. Weitekamp; PASADENA and BOOMERANG significantly improve NMR force detection by circumventing the problems of inhomogeneous magnetic fields 2010 Paul T. Callahan; Contributions to the study of polymeric and heterogeneous materials by advanced NMR exchange, diffusion and relaxation techniques, and for his innovative q-space-diffusion-related developments that were relevant in the context of the development of diffusion-tensor imaging. 2011 Daniel Rugar, John Mamin, and John Sidles; Magnetic Resonance Force Microscopy (MRFM). 2012 Klaes Golman and Jan Henrik Ardenkjaer-Larsen: Dissolution-DNP NMR 4
Laukien Prize Winners n n n n 1999 Konstantin Pervushin, Roland Riek, Gerhard Wider, and Kurt Wüthrich; TROSY 2000 Lucio Frydman; Quadrupolar MQMAS 2001 Peter Boesiger, Klaas Prüßmann, Markus Weiger; Sensitivity-encoded magnetic resonance imaging 2002 Ad Bax, Aksel Bothner-By and James Prestegard; Residual dipolar couplings of weakly aligned molecules in solution 2003 Jacob Schaefer; REDOR Technique for Solid State NMR 2004 Lewis E. Kay, NMR of Biological Macromolecules 2005 Stephan Grzesiek, J couplings across hydrogen bonds 2006 Thomas Szyperski, Eriks Kupce, Ray Freeman, and Rafael Bruschweiler; Acceleration of Multi-dimensional NMR by novel procedures for scanning data space and efficiently processing results to obtain a conventional spectral representation 2007 Robert G. Griffin; High-field dynamic nuclear polarization (DNP) for sensitivity enhancement in solid-state MAS NMR 2008 Malcom H. Levitt; Optimized pulses and pulse sequences to enhance the power of liquid & solid state NMR 2009 Daniel P. Weitekamp; PASADENA and BOOMERANG significantly improve NMR force detection by circumventing the problems of inhomogeneous magnetic fields 2010 Paul T. Callahan; Contributions to the study of polymeric and heterogeneous materials by advanced NMR exchange, diffusion and relaxation techniques, and for his innovative q-space-diffusion-related developments that were relevant in the context of the development of diffusion-tensor imaging. 2011 Daniel Rugar, John Mamin, and John Sidles; Magnetic Resonance Force Microscopy (MRFM). 2012 Klaes Golman and Jan Henrik Ardenkjaer-Larsen: Dissolution-DNP NMR 4
 Varian Prize Winners n n n n n 2012 2011 2010 2009 2008 2007 2006 2005 2004 2002 Ray Freeman and Weston A. Anderson Gareth Alun Morris, The University of Manchester, UK Martin Karplus, Harvard University, Cambridge, Massachusetts Albert W. Overhauser, Purdue University, West Lafayette, IN Alexander Pines, UC Berkeley, and Lawrence Berkeley National Laboratory Alfred G. Redfield, Brandeis University, Waltham, Massachusetts John S. Waugh, MIT, Cambridge, Massachusetts Nicolaas Bloembergen, University of Arizona, Tucson, Arizona Erwin L. Hahn, Professor Emeritus, University of California, Berkeley Jean Jeener, Universite Libre de Bruxelles, Belgium Nuclear Magnetic Double Resonance INEPT Karplus equations NOE & Dynamic Polarization Cross Polarization Spin Dynamics Average Hamiltonian Theory (AHT) Nuclear Magnetic Relaxation Spin Echoes Two-dimensional NMR 5
Varian Prize Winners n n n n n 2012 2011 2010 2009 2008 2007 2006 2005 2004 2002 Ray Freeman and Weston A. Anderson Gareth Alun Morris, The University of Manchester, UK Martin Karplus, Harvard University, Cambridge, Massachusetts Albert W. Overhauser, Purdue University, West Lafayette, IN Alexander Pines, UC Berkeley, and Lawrence Berkeley National Laboratory Alfred G. Redfield, Brandeis University, Waltham, Massachusetts John S. Waugh, MIT, Cambridge, Massachusetts Nicolaas Bloembergen, University of Arizona, Tucson, Arizona Erwin L. Hahn, Professor Emeritus, University of California, Berkeley Jean Jeener, Universite Libre de Bruxelles, Belgium Nuclear Magnetic Double Resonance INEPT Karplus equations NOE & Dynamic Polarization Cross Polarization Spin Dynamics Average Hamiltonian Theory (AHT) Nuclear Magnetic Relaxation Spin Echoes Two-dimensional NMR 5
 NMR Spectroscopy n. NUCLEAR n. MAGNETIC n. RESONANCE 6
NMR Spectroscopy n. NUCLEAR n. MAGNETIC n. RESONANCE 6
 Superconducting Cryo-Magnet 7
Superconducting Cryo-Magnet 7
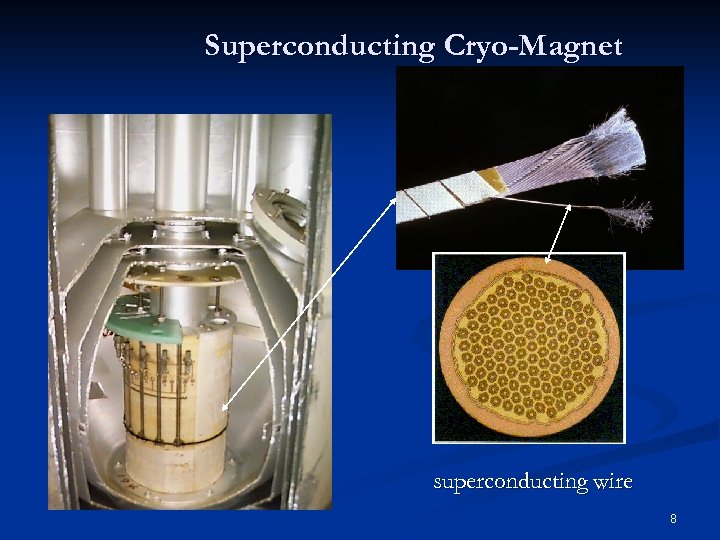 Superconducting Cryo-Magnet superconducting wire 8
Superconducting Cryo-Magnet superconducting wire 8
 NMR Magnet Safety n NMR magnets are always charged! n n n NMR magnets may interfere with medical devices (i. e. pacemakers, insulin pumps) NMR magnets will erase credit cards, ID cards, floppy disks, hard disks (some mp 3 players). NMR magnets and RF consoles may interfere with electronic and mechanical devices and may damage them (cell phones, pagers, watches, I-pods, etc. ) NMR magnets will attract ferromagnetic objects of any size (i. e. paper clips, coins, keys, pens, scissors, screw drivers, wrenches, metallic chairs, gas cylinders, etc. ) and spectrometer and people may sustain severe damage or injury, if handled carelessly. NMR magnets contain Cryogens (liquid Helium and Nitrogen) n n Cryogens can cause severe burns if handled improperly (use eye protection and gloves during refills). Cryogens evaporate and may cause asphyxiation if a lab is not properly ventilated. During a magnet quench up to 100 liters of liquid Helium are vaporized in a matter of minutes (2600 cu ft, 70, 000 liters gas) and may cause asphyxiation, even if the lab is well ventilated. If a magnet quenches, leave the lab immediately. Don’t panic, helium gas will rise to the ceiling and escape through cracks. During a refill the refill rubber tubing may shatter. Frozen rubber cuts like glass!! 9
NMR Magnet Safety n NMR magnets are always charged! n n n NMR magnets may interfere with medical devices (i. e. pacemakers, insulin pumps) NMR magnets will erase credit cards, ID cards, floppy disks, hard disks (some mp 3 players). NMR magnets and RF consoles may interfere with electronic and mechanical devices and may damage them (cell phones, pagers, watches, I-pods, etc. ) NMR magnets will attract ferromagnetic objects of any size (i. e. paper clips, coins, keys, pens, scissors, screw drivers, wrenches, metallic chairs, gas cylinders, etc. ) and spectrometer and people may sustain severe damage or injury, if handled carelessly. NMR magnets contain Cryogens (liquid Helium and Nitrogen) n n Cryogens can cause severe burns if handled improperly (use eye protection and gloves during refills). Cryogens evaporate and may cause asphyxiation if a lab is not properly ventilated. During a magnet quench up to 100 liters of liquid Helium are vaporized in a matter of minutes (2600 cu ft, 70, 000 liters gas) and may cause asphyxiation, even if the lab is well ventilated. If a magnet quenches, leave the lab immediately. Don’t panic, helium gas will rise to the ceiling and escape through cracks. During a refill the refill rubber tubing may shatter. Frozen rubber cuts like glass!! 9
 NMR Magnet Safety n NMR magnets are always charged! n NMR magnets contain Cryogens (liquid Helium and Nitrogen) 10
NMR Magnet Safety n NMR magnets are always charged! n NMR magnets contain Cryogens (liquid Helium and Nitrogen) 10
 NMR Console with Computer 11
NMR Console with Computer 11
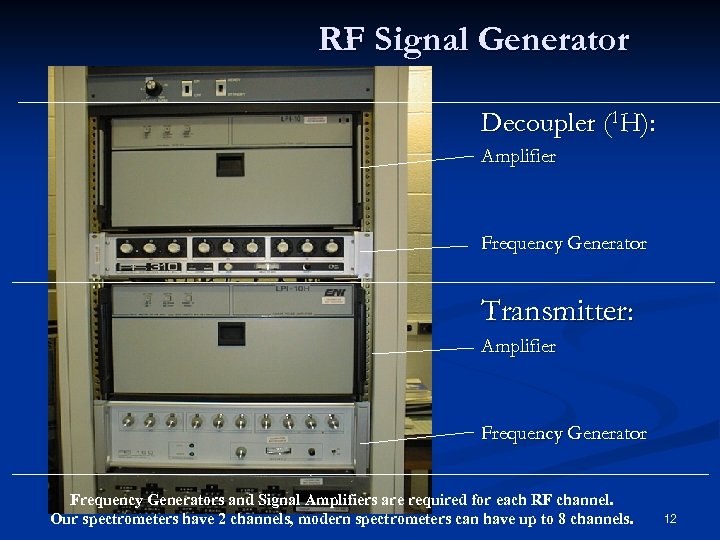 RF Signal Generator Decoupler (1 H): Amplifier Frequency Generator Transmitter: Amplifier Frequency Generators and Signal Amplifiers are required for each RF channel. Our spectrometers have 2 channels, modern spectrometers can have up to 8 channels. 12
RF Signal Generator Decoupler (1 H): Amplifier Frequency Generator Transmitter: Amplifier Frequency Generators and Signal Amplifiers are required for each RF channel. Our spectrometers have 2 channels, modern spectrometers can have up to 8 channels. 12
 Magic angle (54. 7°) NMR Probes Solids Liquids 13
Magic angle (54. 7°) NMR Probes Solids Liquids 13
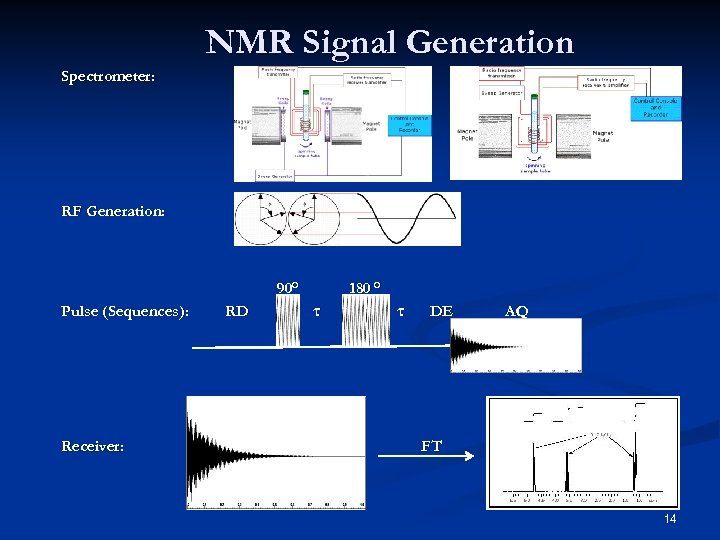 NMR Signal Generation Spectrometer: RF Generation: 90° Pulse (Sequences): Receiver: RD 180 ° τ τ DE AQ FT 14
NMR Signal Generation Spectrometer: RF Generation: 90° Pulse (Sequences): Receiver: RD 180 ° τ τ DE AQ FT 14
 NMR Samples Types of NMR sample holders n. Sample preparation n. Spectrum quality n n 15
NMR Samples Types of NMR sample holders n. Sample preparation n. Spectrum quality n n 15
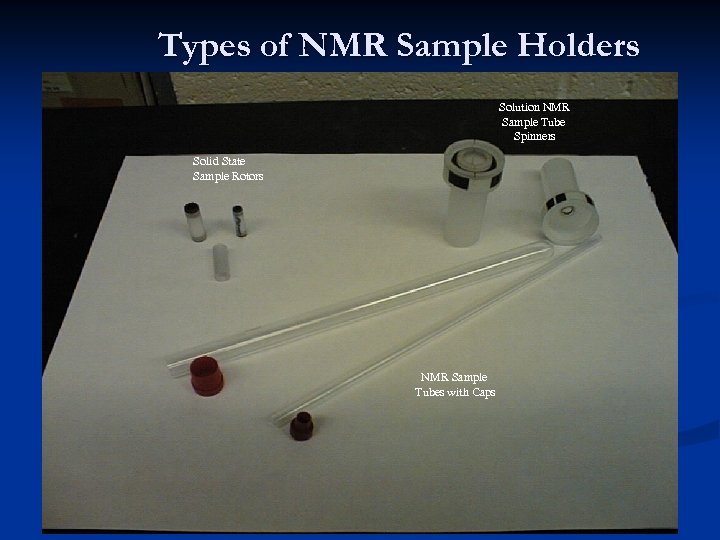 Types of NMR Sample Holders Solution NMR Sample Tube Spinners Solid State Sample Rotors NMR Sample Tubes with Caps 16
Types of NMR Sample Holders Solution NMR Sample Tube Spinners Solid State Sample Rotors NMR Sample Tubes with Caps 16
 NMR Sample Preparation Tubes and Caps: n NMR tubes are a standard length (7 and 9 inch). When chipped (and reduced in length) they should not be reused as an unbalanced tube will not spin. n Always clean the tubes thoroughly after use. First use the solvent you were using to recover your previous sample, then rinse several times with acetone and finally dry the sample tube laying flat on a layer of kimwipes or placed upside-down on a kimwipe in a beaker or Erlenmeyer flask. Choose the container so the tubes stand vertically. Don’t heat the tubes above 50 °C, as the glass might warp. Always store unused, clean tubes uncapped and laying on a flat surface. n Tube caps are disposable and replacements can be easily obtained in bags of 100 ($5) or 1000 ($40 at www. wilmad. com). Degassing Samples: n NMR spectra recorded using degassed solvents usually benefit from reduced halfheight line-width and thus better S/N. (O 2 gas is paramagnetic!) n There are several ways of degassing your sample: n the best is the freeze-pump-thaw technique, n placing the sample in a ultrasonic bath works moderately well, n bubbling nitrogen through or over the sample less well. 17
NMR Sample Preparation Tubes and Caps: n NMR tubes are a standard length (7 and 9 inch). When chipped (and reduced in length) they should not be reused as an unbalanced tube will not spin. n Always clean the tubes thoroughly after use. First use the solvent you were using to recover your previous sample, then rinse several times with acetone and finally dry the sample tube laying flat on a layer of kimwipes or placed upside-down on a kimwipe in a beaker or Erlenmeyer flask. Choose the container so the tubes stand vertically. Don’t heat the tubes above 50 °C, as the glass might warp. Always store unused, clean tubes uncapped and laying on a flat surface. n Tube caps are disposable and replacements can be easily obtained in bags of 100 ($5) or 1000 ($40 at www. wilmad. com). Degassing Samples: n NMR spectra recorded using degassed solvents usually benefit from reduced halfheight line-width and thus better S/N. (O 2 gas is paramagnetic!) n There are several ways of degassing your sample: n the best is the freeze-pump-thaw technique, n placing the sample in a ultrasonic bath works moderately well, n bubbling nitrogen through or over the sample less well. 17
 NMR Sample Preparation Quantity: n For proton NMR spectra of small organic compounds (up to MW=500) anything between 1 and 20 mg of sample will be fine. Concentrated solutions can be viscous and may result in broad signals. Very dilute samples could be masked by impurities and solvent peaks. n Carbon-13 is present at approximately 1. 1 % natural abundance. It is intrinsically less sensitive than protons (approx. six thousand times). Please provide as much sample as possible, 50 - 100 mg (or more) is fine. Preparing two samples - one dilute sample for proton NMR and one concentrated sample for carbon NMR is a useful, but unnecessary practice. n Solvent height (volume) should be uniform, 5 cm or 2 inches equal 0. 5 ml. The ends of the sample distort the field homogeneity, shimming on each sample corrects this effect and takes just a minute or so. However, vastly different solvent heights (volumes) prevent complete correction and require many minutes shimming to achieve acceptable homogeneity. n Samples prepared with too much solvent waste both time and money, and provide poorer S/N. However, If you have limited amounts of sample (less than 1 mg), using less solvent is permissible. Minimum height: 1 cm, however, this requires special positioning of the sample tube and very intensive shimming. 18
NMR Sample Preparation Quantity: n For proton NMR spectra of small organic compounds (up to MW=500) anything between 1 and 20 mg of sample will be fine. Concentrated solutions can be viscous and may result in broad signals. Very dilute samples could be masked by impurities and solvent peaks. n Carbon-13 is present at approximately 1. 1 % natural abundance. It is intrinsically less sensitive than protons (approx. six thousand times). Please provide as much sample as possible, 50 - 100 mg (or more) is fine. Preparing two samples - one dilute sample for proton NMR and one concentrated sample for carbon NMR is a useful, but unnecessary practice. n Solvent height (volume) should be uniform, 5 cm or 2 inches equal 0. 5 ml. The ends of the sample distort the field homogeneity, shimming on each sample corrects this effect and takes just a minute or so. However, vastly different solvent heights (volumes) prevent complete correction and require many minutes shimming to achieve acceptable homogeneity. n Samples prepared with too much solvent waste both time and money, and provide poorer S/N. However, If you have limited amounts of sample (less than 1 mg), using less solvent is permissible. Minimum height: 1 cm, however, this requires special positioning of the sample tube and very intensive shimming. 18
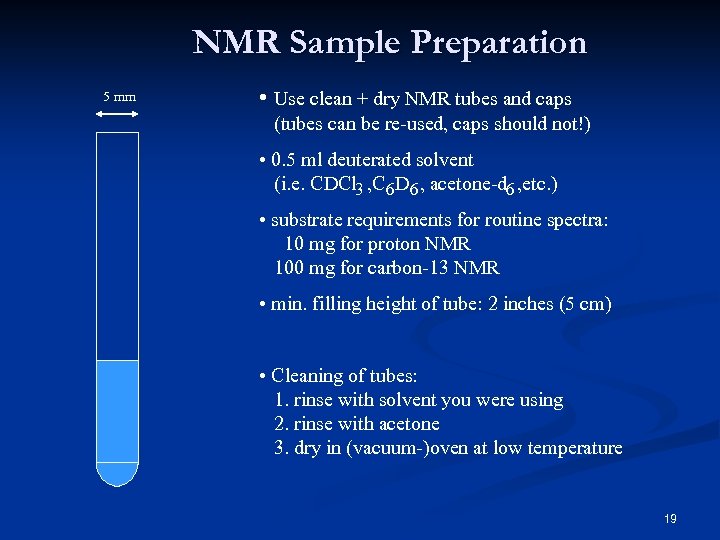 NMR Sample Preparation 5 mm • Use clean + dry NMR tubes and caps (tubes can be re-used, caps should not!) • 0. 5 ml deuterated solvent (i. e. CDCl 3 , C 6 D 6 , acetone-d 6 , etc. ) • substrate requirements for routine spectra: 10 mg for proton NMR 100 mg for carbon-13 NMR • min. filling height of tube: 2 inches (5 cm) • Cleaning of tubes: 1. rinse with solvent you were using 2. rinse with acetone 3. dry in (vacuum-)oven at low temperature 19
NMR Sample Preparation 5 mm • Use clean + dry NMR tubes and caps (tubes can be re-used, caps should not!) • 0. 5 ml deuterated solvent (i. e. CDCl 3 , C 6 D 6 , acetone-d 6 , etc. ) • substrate requirements for routine spectra: 10 mg for proton NMR 100 mg for carbon-13 NMR • min. filling height of tube: 2 inches (5 cm) • Cleaning of tubes: 1. rinse with solvent you were using 2. rinse with acetone 3. dry in (vacuum-)oven at low temperature 19
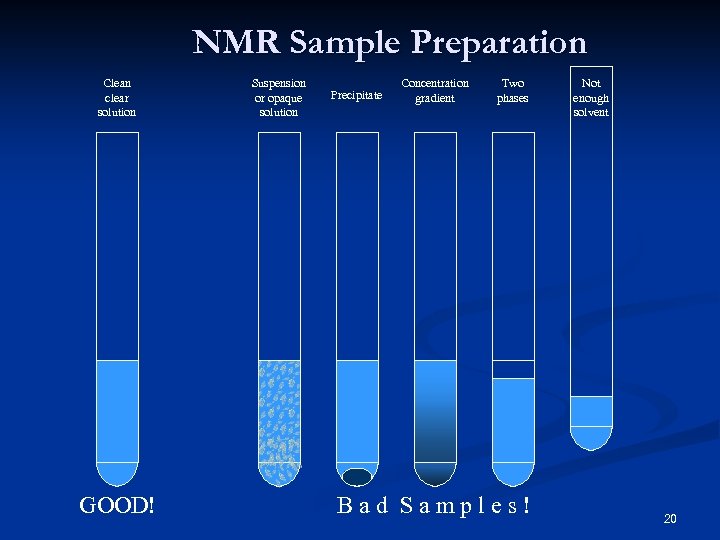 NMR Sample Preparation Clean clear solution GOOD! Suspension or opaque solution Precipitate Concentration gradient Two phases Bad Samples! Not enough solvent 20
NMR Sample Preparation Clean clear solution GOOD! Suspension or opaque solution Precipitate Concentration gradient Two phases Bad Samples! Not enough solvent 20
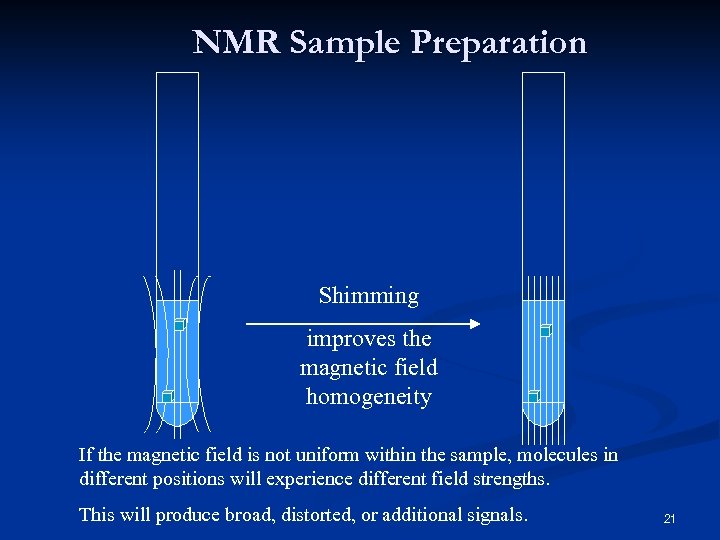 NMR Sample Preparation Shimming improves the magnetic field homogeneity If the magnetic field is not uniform within the sample, molecules in different positions will experience different field strengths. This will produce broad, distorted, or additional signals. 21
NMR Sample Preparation Shimming improves the magnetic field homogeneity If the magnetic field is not uniform within the sample, molecules in different positions will experience different field strengths. This will produce broad, distorted, or additional signals. 21
 Good and bad NMR Spectra … are the result of: Homogeneity of magnetic field n. Sample preparation n. Choice of solvent n. Data acquisition parameters n. Processing procedures n 22
Good and bad NMR Spectra … are the result of: Homogeneity of magnetic field n. Sample preparation n. Choice of solvent n. Data acquisition parameters n. Processing procedures n 22
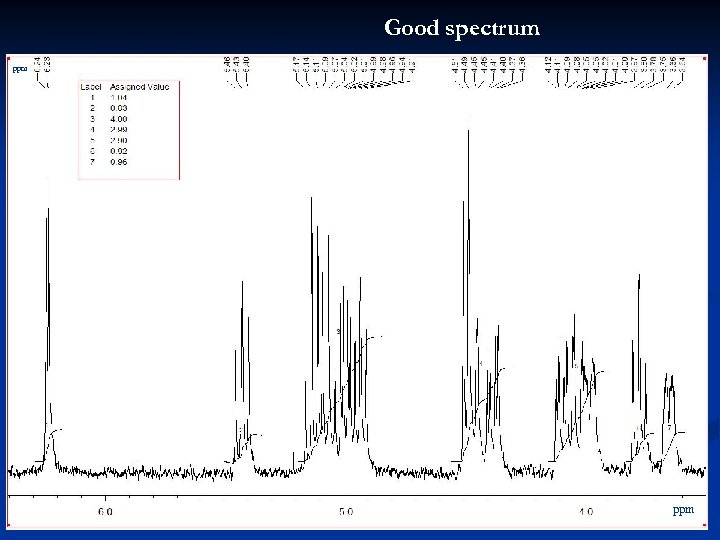 Good spectrum ppm 23
Good spectrum ppm 23
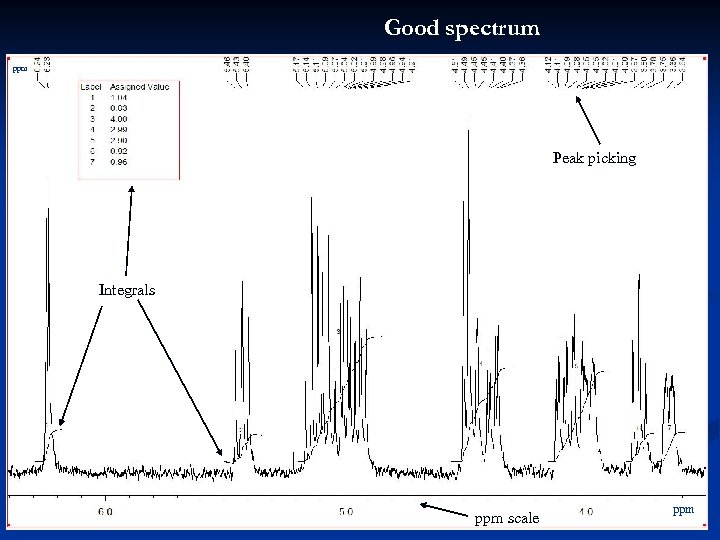 Good spectrum ppm Peak picking Integrals ppm scale ppm 24
Good spectrum ppm Peak picking Integrals ppm scale ppm 24
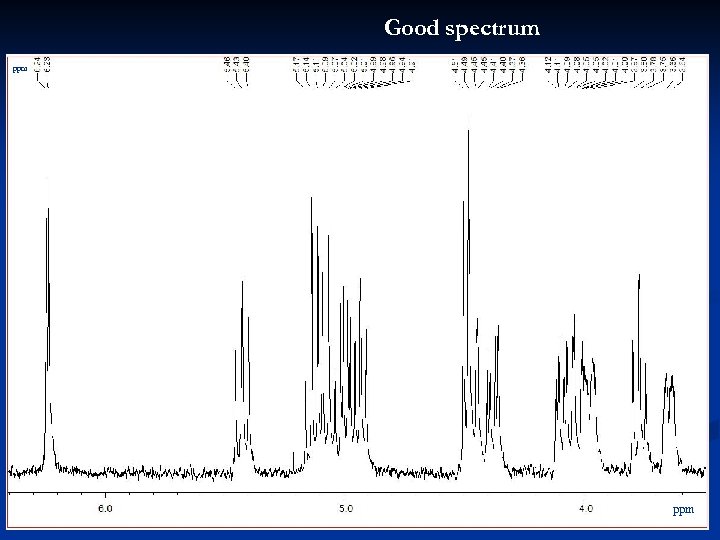 Good spectrum ppm 25
Good spectrum ppm 25
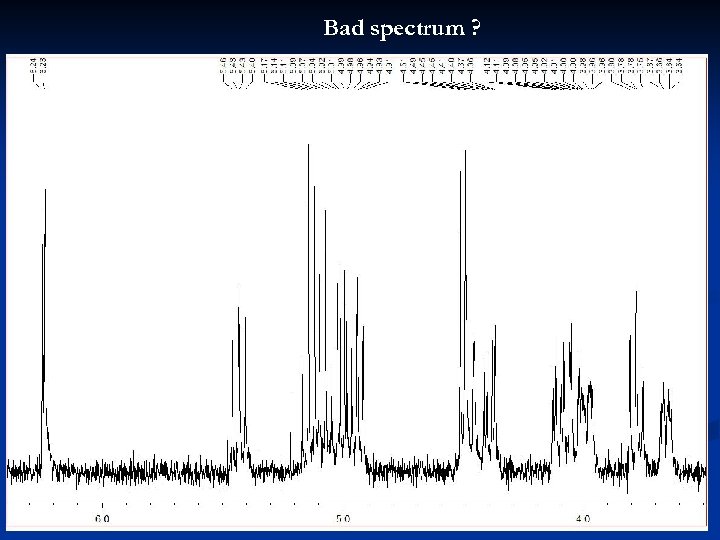 Bad spectrum ? 26
Bad spectrum ? 26
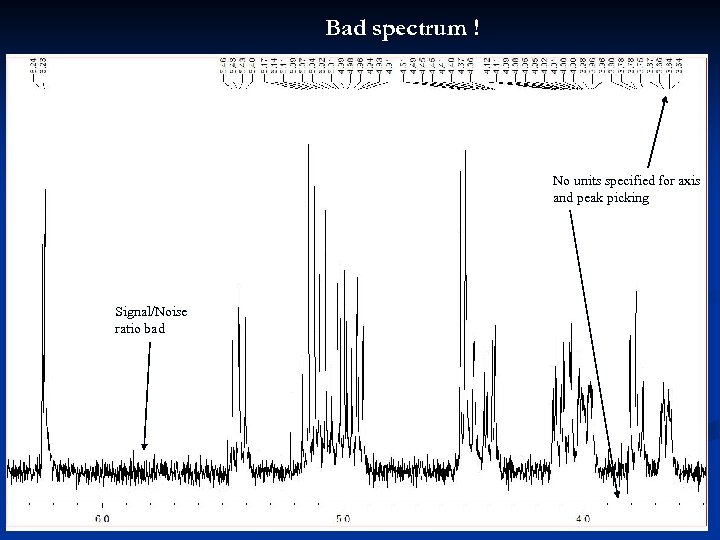 Bad spectrum ! No units specified for axis and peak picking Signal/Noise ratio bad 27
Bad spectrum ! No units specified for axis and peak picking Signal/Noise ratio bad 27
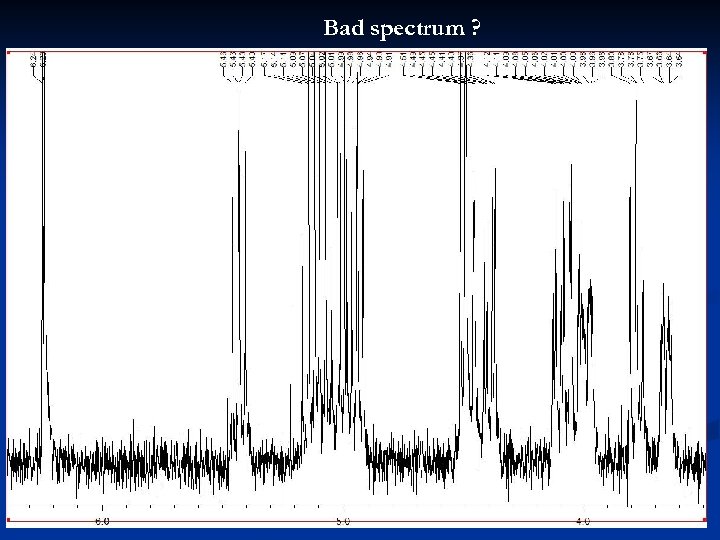 Bad spectrum ? 28
Bad spectrum ? 28
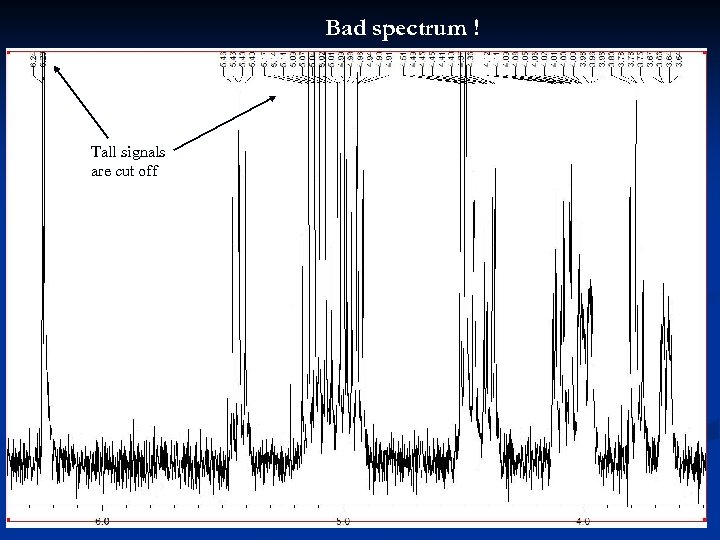 Bad spectrum ! Tall signals are cut off 29
Bad spectrum ! Tall signals are cut off 29
 Bad spectrum ? 30
Bad spectrum ? 30
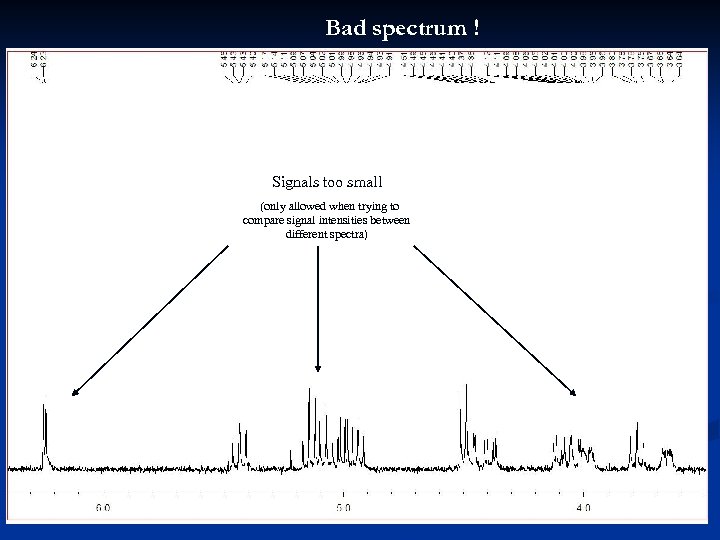 Bad spectrum ! Signals too small (only allowed when trying to compare signal intensities between different spectra) 31
Bad spectrum ! Signals too small (only allowed when trying to compare signal intensities between different spectra) 31
 Bad spectrum ? 32
Bad spectrum ? 32
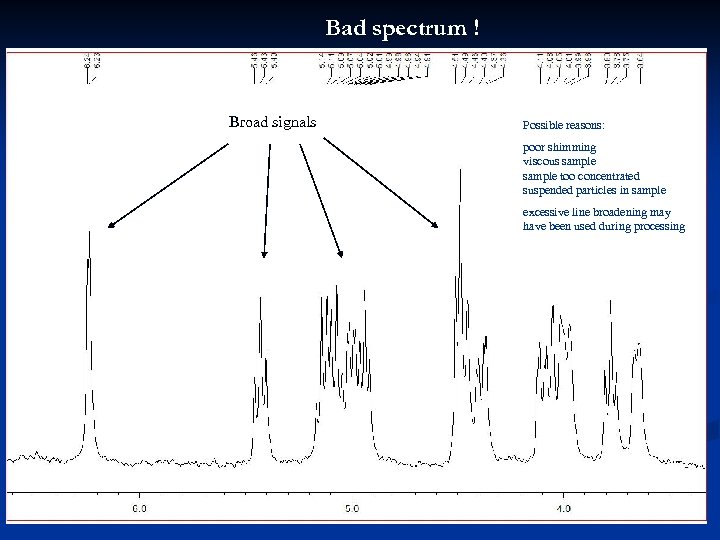 Bad spectrum ! Broad signals Possible reasons: poor shimming viscous sample too concentrated suspended particles in sample excessive line broadening may have been used during processing 33
Bad spectrum ! Broad signals Possible reasons: poor shimming viscous sample too concentrated suspended particles in sample excessive line broadening may have been used during processing 33
 Bad spectrum ? 34
Bad spectrum ? 34
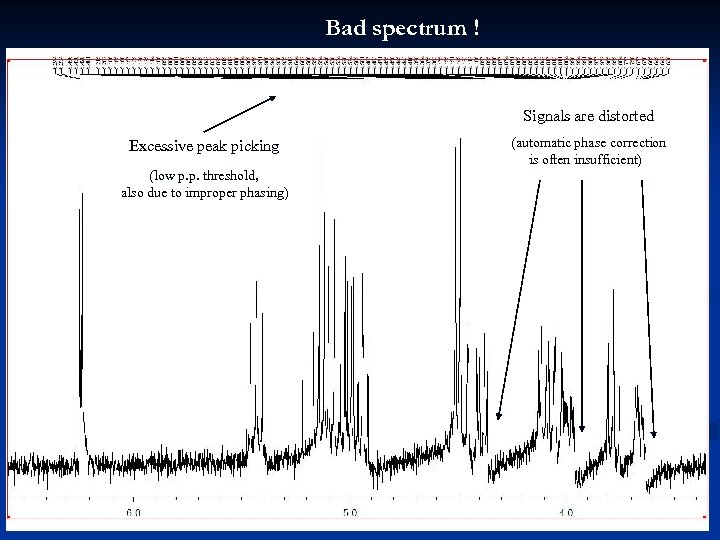 Bad spectrum ! Signals are distorted Excessive peak picking (automatic phase correction is often insufficient) (low p. p. threshold, also due to improper phasing) 35
Bad spectrum ! Signals are distorted Excessive peak picking (automatic phase correction is often insufficient) (low p. p. threshold, also due to improper phasing) 35
 Bad spectrum ? 36
Bad spectrum ? 36
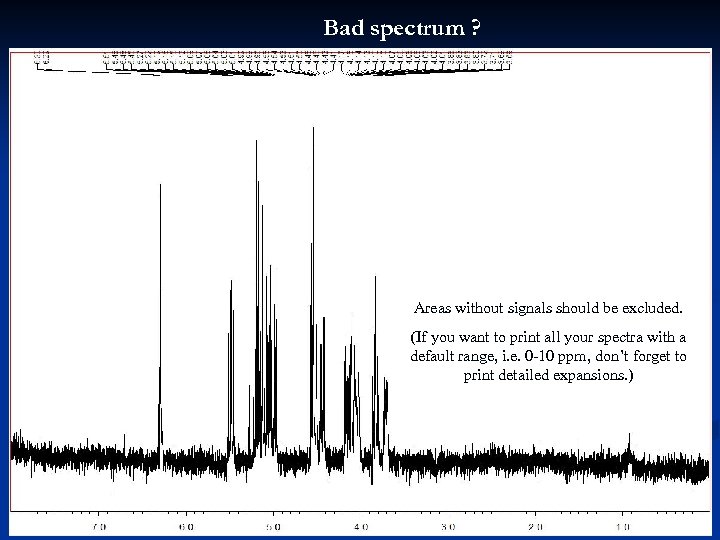 Bad spectrum ? Areas without signals should be excluded. (If you want to print all your spectra with a default range, i. e. 0 -10 ppm, don’t forget to print detailed expansions. ) 37
Bad spectrum ? Areas without signals should be excluded. (If you want to print all your spectra with a default range, i. e. 0 -10 ppm, don’t forget to print detailed expansions. ) 37
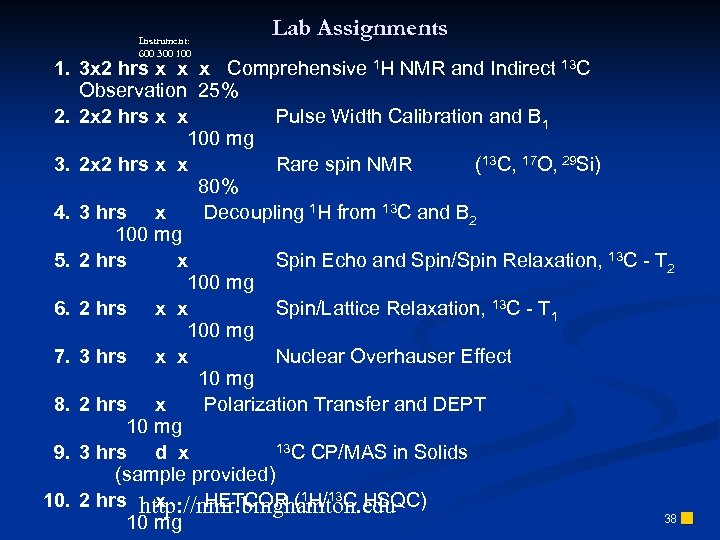 Instrument: 600 300 100 Lab Assignments 1. 3 x 2 hrs x x x Comprehensive 1 H NMR and Indirect 13 C Observation 25% 2. 2 x 2 hrs x x Pulse Width Calibration and B 1 100 mg 3. 2 x 2 hrs x x Rare spin NMR (13 C, 17 O, 29 Si) 80% 4. 3 hrs x Decoupling 1 H from 13 C and B 2 100 mg 5. 2 hrs x Spin Echo and Spin/Spin Relaxation, 13 C - T 2 100 mg 6. 2 hrs x x Spin/Lattice Relaxation, 13 C - T 1 100 mg 7. 3 hrs x x Nuclear Overhauser Effect 10 mg 8. 2 hrs x Polarization Transfer and DEPT 10 mg 13 C CP/MAS in Solids 9. 3 hrs d x (sample provided) 10. 2 hrs http: //nmr. binghamton. edu x HETCOR (1 H/13 C HSQC) 38 n 10 mg
Instrument: 600 300 100 Lab Assignments 1. 3 x 2 hrs x x x Comprehensive 1 H NMR and Indirect 13 C Observation 25% 2. 2 x 2 hrs x x Pulse Width Calibration and B 1 100 mg 3. 2 x 2 hrs x x Rare spin NMR (13 C, 17 O, 29 Si) 80% 4. 3 hrs x Decoupling 1 H from 13 C and B 2 100 mg 5. 2 hrs x Spin Echo and Spin/Spin Relaxation, 13 C - T 2 100 mg 6. 2 hrs x x Spin/Lattice Relaxation, 13 C - T 1 100 mg 7. 3 hrs x x Nuclear Overhauser Effect 10 mg 8. 2 hrs x Polarization Transfer and DEPT 10 mg 13 C CP/MAS in Solids 9. 3 hrs d x (sample provided) 10. 2 hrs http: //nmr. binghamton. edu x HETCOR (1 H/13 C HSQC) 38 n 10 mg
 In all assignments you are expected to report on the following information: n n n n n Signal-to-noise ratio Chemical shifts and method of reference in relation to structure J coupling (homo and heteronuclear) in relation to structure Peak intensity and area in relation to concentration and structure/dynamics/relaxation Effect of B 0 / lab magnetic field strength, Larmor frequency and gyromagnetic ratio Effect of B 0 inhomogeneity Pulse width and tip angle Magnitude of B 1 and B 2 fields / rf field strengths used Effect of B 1 and B 2 inhomogeneity Effects of O 1 and O 2 / frequency offsets Effects of sample spinning and location of spinning sidebands Method of decoupling if used as well as effectiveness Solvent and temperature used Pulse sequence and instrument control programming All critical instrument or data processing parameters Vector and spin diagrams, as needed. Note any experimental or instrumental anomalies The spectrometer manual to be used in this course can be downloaded from: http: //www. chem. binghamton. edu/staff/schulte/CHEM 585 f/Chem 585 f-Bruker. doc 39
In all assignments you are expected to report on the following information: n n n n n Signal-to-noise ratio Chemical shifts and method of reference in relation to structure J coupling (homo and heteronuclear) in relation to structure Peak intensity and area in relation to concentration and structure/dynamics/relaxation Effect of B 0 / lab magnetic field strength, Larmor frequency and gyromagnetic ratio Effect of B 0 inhomogeneity Pulse width and tip angle Magnitude of B 1 and B 2 fields / rf field strengths used Effect of B 1 and B 2 inhomogeneity Effects of O 1 and O 2 / frequency offsets Effects of sample spinning and location of spinning sidebands Method of decoupling if used as well as effectiveness Solvent and temperature used Pulse sequence and instrument control programming All critical instrument or data processing parameters Vector and spin diagrams, as needed. Note any experimental or instrumental anomalies The spectrometer manual to be used in this course can be downloaded from: http: //www. chem. binghamton. edu/staff/schulte/CHEM 585 f/Chem 585 f-Bruker. doc 39
 40
40
 41
41
 A letter in the New Scientist of 17 April 1999, signed by Terry Mc. Stea, Whitburn, Tyne and Wear: Your article on MRI (This Week, 3 April, p 7) reminded me of a story, probably untrue, related by a doctor friend. MRI used to be known in hospitals as nuclear magnetic resonance, or NMR. Unfortunately, patients who arrived at the hospital asking for "an NMR" often received a treatment that they were not expecting. Hence the change to MRI. 42
A letter in the New Scientist of 17 April 1999, signed by Terry Mc. Stea, Whitburn, Tyne and Wear: Your article on MRI (This Week, 3 April, p 7) reminded me of a story, probably untrue, related by a doctor friend. MRI used to be known in hospitals as nuclear magnetic resonance, or NMR. Unfortunately, patients who arrived at the hospital asking for "an NMR" often received a treatment that they were not expecting. Hence the change to MRI. 42


