42595101bae72698ec8cfe12d0de7afd.ppt
- Количество слайдов: 45

NIDA Drug Abuse Treatment Clinical Trials Network The First Decade Betty Tai, Ph. D. February 3, 2010 Director Center for the Clinical Trials Network National Institute on Drug Abuse NIDA NATIONAL INSTITUTE ON DRUG ABUSE

Institute of Medicine, 1998 Bridging the Gap Research

CTN Premises • Addiction treatment services will be improved as evidence-based treatments are broadly implemented in community-based treatment programs, • Randomized, controlled clinical trials are the gold standard for generating evidence-based treatments, • Engage the providers in the research process will improve the acceptability/adoption of research results. B. Tai 2010 B. Tai 2009

Council Report 2/3/2010 ü Infrastructure ü Research utilization ü CTN next decade B. Tai 2010
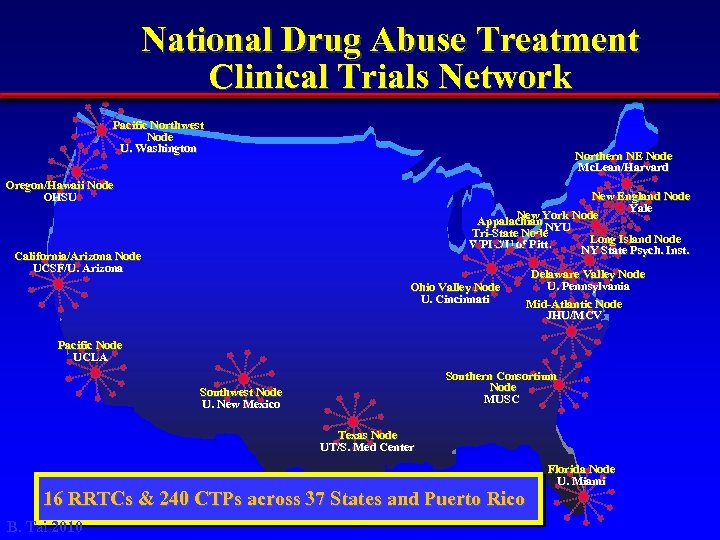
National Drug Abuse Treatment Clinical Trials Network Pacific Northwest Node U. Washington Northern NE Node Mc. Lean/Harvard Oregon/Hawaii Node OHSU New England Node Yale New York Node Appalachian NYU Tri-State Node Long Island Node WPIC/U of Pitt. NY State Psych. Inst. California/Arizona Node UCSF/U. Arizona Ohio Valley Node U. Cincinnati Delaware Valley Node U. Pennsylvania Mid-Atlantic Node JHU/MCV Pacific Node UCLA Southern Consortium Node MUSC Southwest Node U. New Mexico Texas Node UT/S. Med Center 16 RRTCs & 240 CTPs across 37 States and Puerto Rico B. Tai 2010 Florida Node U. Miami
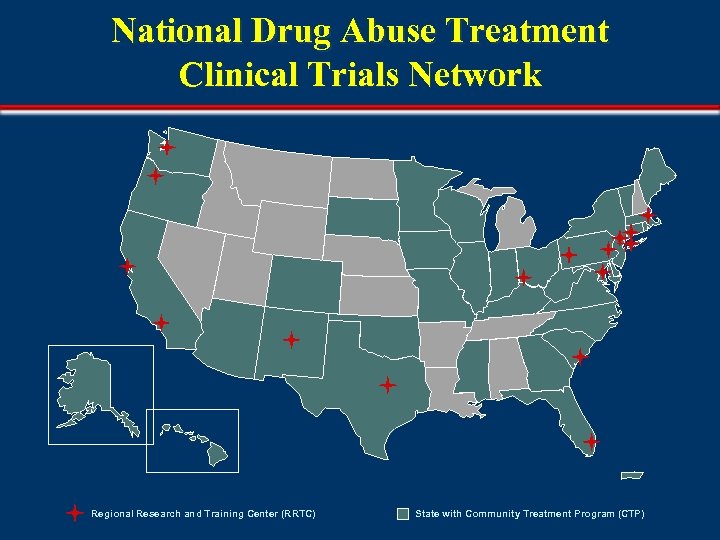
National Drug Abuse Treatment Clinical Trials Network Regional Research and Training Center (RRTC) State with Community Treatment Program (CTP)

The Drug Abuse Treatment Community Hospitals Kaiser Permanente VA Hospitals Mayo Clinic Mercy Hospital RWJ Medical Center Rehab Centers Betty Ford Center Hazelden Foundation Caron Foundation “Drug free programs” Odyssey House Center for Drug-Free Living Walden House, Phoenix House Na’Nizhoozi Center Homeward Bound Social Model B. Tai 2010 Methadone Clinics Evergreen Treatment Services Addiction Research & Treatment Corp. Bi-Valley Medical Clinic Hartford Dispensary Behavioral Model Medical Model

CTN Coordinating Centers Organizational design of a multisite trial is as important to its success as is the experimental design. Curtis Meinert, Controlled Clinical Trials 1, 305 (1981) “Science and process are equally important to ensure success of large prospective studies Timothy Sprosen, UK Biobank NIH New Models for Large Prospective Studies symposium (1/22/2010) B. Tai 2010
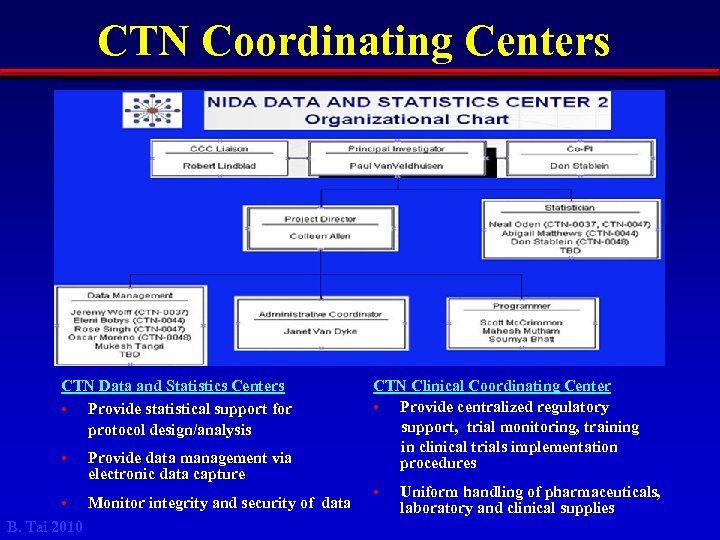
CTN Coordinating Centers CTN Data and Statistics Centers • Provide statistical support for protocol design/analysis • Provide data management via electronic data capture • Monitor integrity and security of data B. Tai 2010 CTN Clinical Coordinating Center • Provide centralized regulatory support, trial monitoring, training in clinical trials implementation procedures • Uniform handling of pharmaceuticals, laboratory and clinical supplies

Center for the Clinical Trials Network B. Tai 2010

Council Report 2/3/2010 ü Infrastructure ü Research utilization ü CTN next decade B. Tai 2010
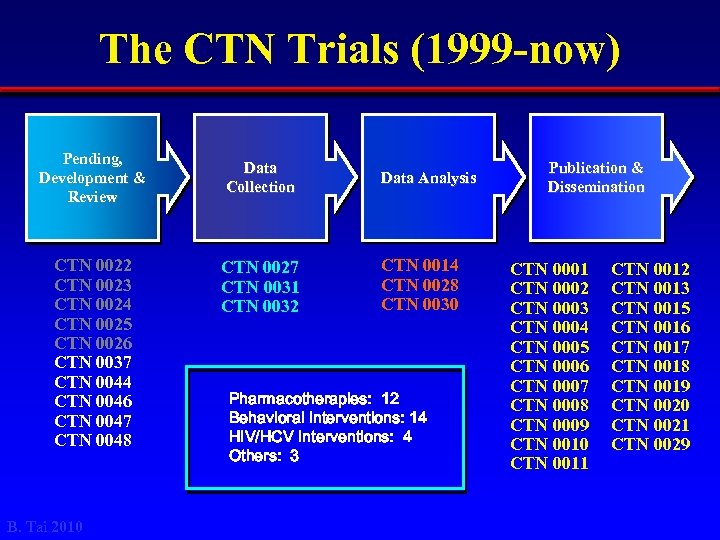
The CTN Trials (1999 -now) Pending, Development & Review CTN 0022 CTN 0023 CTN 0024 CTN 0025 CTN 0026 CTN 0037 CTN 0044 CTN 0046 CTN 0047 CTN 0048 B. Tai 2010 Data Collection CTN 0027 CTN 0031 CTN 0032 Data Analysis CTN 0014 CTN 0028 CTN 0030 Pharmacotherapies: 12 Behavioral Interventions: 14 HIV/HCV Interventions: 4 Others: 3 Publication & Dissemination CTN 0001 CTN 0002 CTN 0003 CTN 0004 CTN 0005 CTN 0006 CTN 0007 CTN 0008 CTN 0009 CTN 0010 CTN 0011 CTN 0012 CTN 0013 CTN 0015 CTN 0016 CTN 0017 CTN 0018 CTN 0019 CTN 0020 CTN 0021 CTN 0029
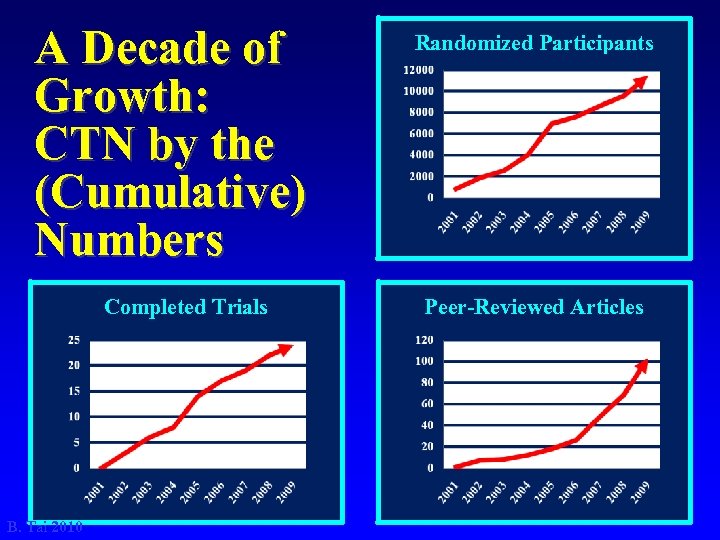
A Decade of Growth: CTN by the (Cumulative) Numbers Completed Trials B. Tai 2010 Randomized Participants Peer-Reviewed Articles

CTN Studies in Development • STRIDE (CTN 0037): • • • – Stimulant Reduction Intervention Using Dosed Exercise Web-delivery of Evidence-Based, Psychosocial Treatment for Substance Use Disorders (CTN 0044) S-CAST (CTN 0046) – Smoking Cessation and Stimulant Treatment SMART-ED (CTN 0047) – Screening, Motivational Assessment, Referral and Treatment in Emergency Departments • CURB (CTN 0048) – Cocaine Use Reduction with Buprenorphine B. Tai 2009

Highlighting CTN Pharmacological Therapy Trials • Buprenorphine (Suboxone) – CTN 0001, 0002 Bup/Nx vs Clonidine in detox – CTN 0027 Bup/Nx vs Methadone in maintenance – CTN 0010 Bup/Nx for adolescents tapering – CTN 0030 Bup/Nx for prescription opiate dependence • Osmotic-Release Methylphenidate (Concerta) – CTN 0029 Adult smokers – CTN 0028 Adolescents Substance users • Nicotine replacement in drug treatment – CTN 0009 – CTN 0029 B. Tai 2010

CTN Pharmacotherapy Trials CTN 0010 Context: Detox only • Increased heroin and pharmaceutical opioid use among 12 th graders • Usual Tx for Opioid addicted youth: Detox and counseling Bup/NX 12 weeks • First RCT study of continued agonist Tx in this young population B. Tai 2010

Highlighting CTN Behavioral Therapy Trials • MI or CM: Lower cost motivational incentives • MI/MET: Motivational interviewing & enhancement – Adults, pregnant women, Spanish speakers • Seeking Safety: trauma counseling for women • BSFT: Brief Strategic Family Therapy – Adolescent • STAGE-12: Twelve Step Facilitation Therapy • Reducing HIV/HCV risk behavior – Injection drug use, sex risks for women & men B. Tai 2009

MET for Spanish-Speaking Substance Users CTN 0021 B. Tai 2010

Study Challenges and Opportunities CTN 0021 • Recruited 436 representative population of US • • Hispanics across 5 CTP sites Bilingual investigators/therapists/RAs Translation of 70% of project materials – Consent forms – CRFs – Assessment instruments – Intervention manual Bilingual Training and fidelity monitoring Shovel ready project for Spanish language international collaboration B. Tai 2010

CTN 0032: HIV Rapid Testing and Counseling in Drug Treatment Recruitmen What is the more What is the t and Enrollment effective testing more effective strategy to ensure testing strategy Brief First participant randomized: 01/05/2009 Baseline they get HIV participant Assessment 12/31/2009 to reduce their Last completed tested and receive risk behaviors? Random their results? Total Participants Assignment Randomized: 1281 Offer Rapid Testing with RESPECT Counseling Offer Rapid Testing with Minimal Counseling Client post-intervention data collection B. Tai 2010 - Offer Referral for Testing in Community
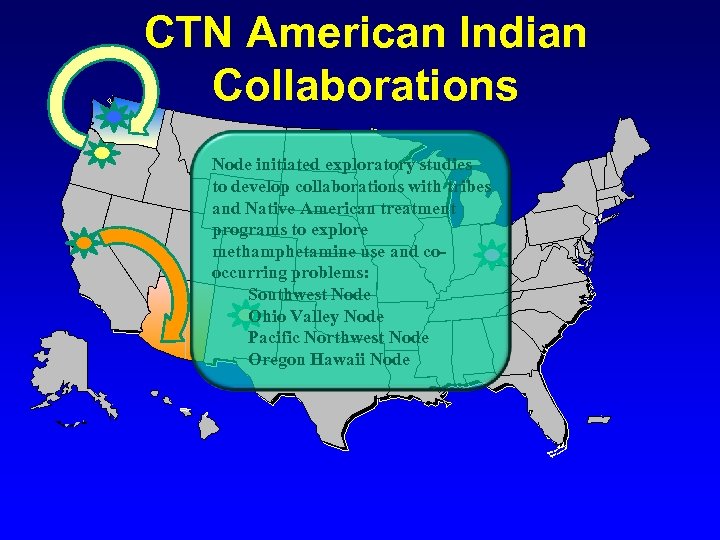
CTN American Indian Collaborations Node initiated exploratory studies to develop collaborations with tribes and Native American treatment programs to explore methamphetamine use and cooccurring problems: Southwest Node Ohio Valley Node Pacific Northwest Node Oregon Hawaii Node

CTN Ancillary Studies • Cost analysis (NIDA DESPR: Services Research Branch) – CTN 0004, 0005, 0006, 0007, 0010, 0032, 0046 • Genetics – (NIDA Genetics Consortium) – CTN 0027 • Training methods (NIDA DCNBR: Behavioral & Integrative Treatment Research Branch) – CTN 0014 • Psychometric study (NIAAA) – CTN 0031 • Brain Imaging study (NIDA DCNBR: Clinical Neuroscience Branch) – CTN 0030 B. Tai 2010
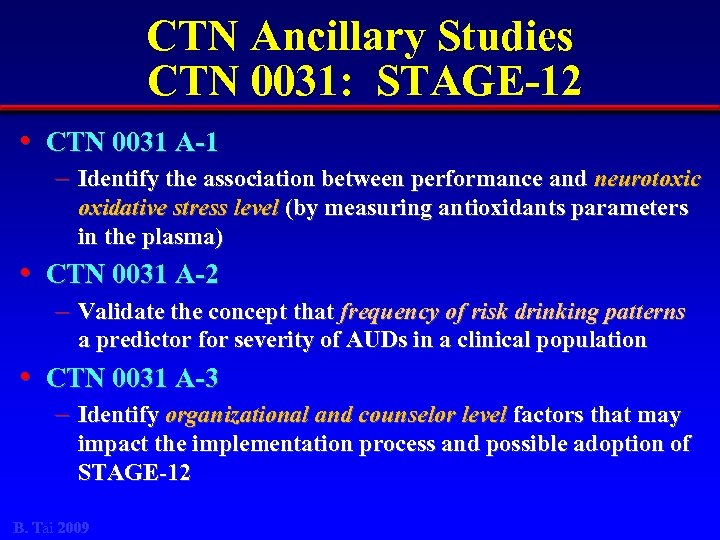
CTN Ancillary Studies CTN 0031: STAGE-12 • CTN 0031 A-1 – Identify the association between performance and neurotoxic oxidative stress level (by measuring antioxidants parameters in the plasma) • CTN 0031 A-2 – Validate the concept that frequency of risk drinking patterns a predictor for severity of AUDs in a clinical population • CTN 0031 A-3 – Identify organizational and counselor level factors that may impact the implementation process and possible adoption of STAGE-12 B. Tai 2009

CTN Secondary Analysis Studies • Scientific Rationale – Secondary clinically useful information – E. G. Who drops out when • • – Mediators and moderators and their interactions at baseline Scientist Rationale – – – Investigators (especially junior) benefit A platform for independent research support Training opportunities (e. g. , K Awards) Economic Rationale – Inexpensive methods to address important questions – More precisely define future research steps – Shorten measures to save future costs B. Tai 2010

Do Treatment Improvements in PTSD Severity Affect Substance Use Outcomes? A Secondary Analysis From a Randomized Clinical Trial in NIDA’s Clinical Trials Network Denise A. Hien et al. American Journal of Psychiatry January 2010, Vol. 167, No. 1, 95 -101 B. Tai 2010
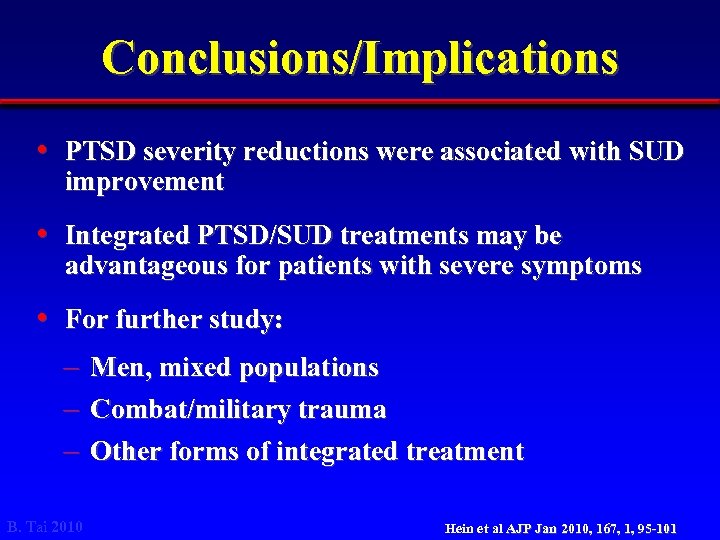
Conclusions/Implications • PTSD severity reductions were associated with SUD improvement • Integrated PTSD/SUD treatments may be advantageous for patients with severe symptoms • For further study: – Men, mixed populations – Combat/military trauma – Other forms of integrated treatment B. Tai 2010 Hein et al AJP Jan 2010, 167, 1, 95 -101

Providing Implications for Practice B. Tai 2010 Back, SE Am J Psychiatry 167: 1, January 2010
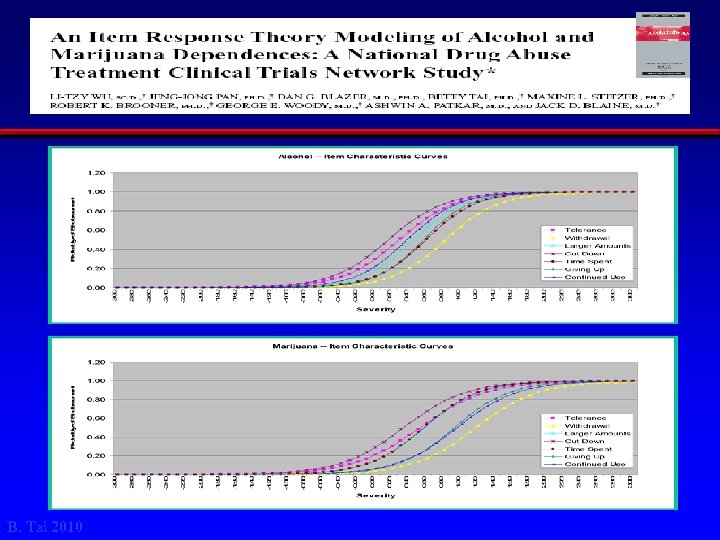
Figure 1 B. Tai 2010

Council Report 2/3/2010 ü Infrastructure ü Research utilization ü CTN next decade B. Tai 2010

Research Utilization Process through which research findings are put in use in the form of programs and policies • Difficult • Slow • Not an action that begins when research is completed • Rather a two way concurrent process Everett Rogers, Diffusion of Innovations, 2003

CTN Research Utilization Activities • Led by CTN Research Utilization Committee • Coordinate intra-CTN and intra-Node activities • Support and monitor the CTN dissemination library utilization • Serve as point of contact for – NIDA Blending Conference – SAMHSA ATTC Program • Major regional workshops in 2009 – June 2009 Pacific Region (Portland, Oregon) – October 2009 Appalachian Tri-state (Pittsburgh, Pa) – November 2009 Southern Consortium (Charlotte, SC) B. Tai 2010

The CTN Dissemination Library http: //ctndisseminationlibrary. org • • • Housed and maintained by Washington Node Inaugural year 2003 Library usage statistics YEAR Documents requested Publications added 2009 11, 592 344 39 2008 10, 489 77 36 2007 8, 644 19 33 2006 4, 459 17 18 2005 B. Tai 2010 Visitors 1, 003 NA 2

NIDA-SAMHSA Blending Products www. attcnetwork. org/explore/priorityareas/science/blendinginitiative/index. asp Buprenorphin Short-Term Treatment e Planning Opioid MATRS: Treatment: A Withdrawal Utilizing the Training for Using Multi. Buprenorphine Addiction Severity Index disciplinary (ASI) to Make Addiction Required Data Professionals Collection Useful B. Tai 2010 Motivational Interviewing Assessment : Supervisory Tools for Enhancing Proficiency (MIA-STEP) Promoting Awareness of Motivational Incentives (PAMI)

CTN Data Sharing http: //www. ctndatashare. org CTN 0001, 0002, 0003, 0004, 0005, 0006, 0007, 0008, 0009, 0011, 0012, 0013, 0016, 0017, 0018, 0019, 0021, 0029 • CDISC standardized • HIPAA Compliant • CTN Public data/documents include: – – B. Tai 2010 Data sets (SAS and ASCII) Defined file (aka data dictionary) Annotated Case Report Forms Study protocol and reference to study publication of primary outcomes

International Outreach: NIDA CTN INVEST Fellows • • • Create an international network of scientists CTN serves as the training platform Senior non-U. S. scientists and health administrators – 2007 (1), 2008 (3), 2009 (2) and 2010 (3) – Export CTN technology, identified future opportunities for collaboration and funding http: //international. drugabuse. gov/research/fellowships_invest. CTN. html

Council Report 2/3/2010 ü Infrastructure ü Research utilization ü CTN next decade B. Tai 2010

CTN RFA 2009 • • • B. Tai 2010 Published – June 2009 Proposals – November 2009 Review – February 2010 Council – May 2010 Award – September 2010 Stay Tuned…

Future of the CTN Mission/Goals • Bring drug abuse treatment into mainstream medical practice – Engage providers from medical settings • Flexible research strategy/portfolio – Seize new scientific opportunities – Address emerging public health concerns • Training platform for clinical workforce • Facilitate research utilization B. Tai 2010
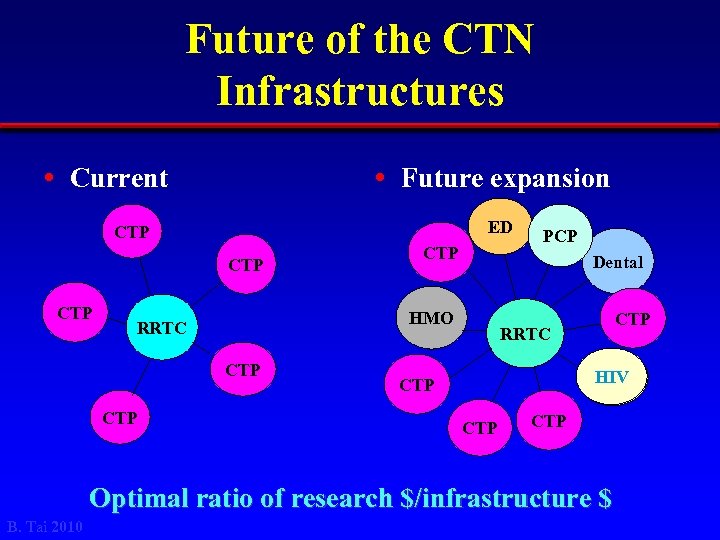
Future of the CTN Infrastructures • Current • Future expansion ED CTP CTP Dental HMO RRTC CTP CTP PCP CTP RRTC HIV CTP CTP Optimal ratio of research $/infrastructure $ B. Tai 2010

Future of the CTN Challenges and Opportunities • Information Technology – Novel approaches to data collection, processing & storage – EHR /EMR & real time EDC – Data standardization, access, reporting – Data privacy & confidentiality • Approaches to accommodate physiologic and genetic information B. Tai 2010 B. Tai 2009

Future of the CTN Challenges and Opportunities • New statistical tools and techniques – adaptive design – simulation and modeling – large database mining • Innovative trial/study designs – large, simpler and longer trials – pragmatic trials , comparative effectiveness trials – subgroup analysis • Chronic Disease management model – treatment algorithm for continuing care B. Tai 2010 B. Tai 2009

Future of the CTN Ask a different research question For a Chronic disease like addiction, what is the first line treatment? Is there a next best treatment sequence at steps 2, 3, and 4? What sequence is best for whom? What is the long-term outcome after steps 1, 2, 3, and 4? Research questions that impact practice • “What” and “for whom” questions • “How” questions – How to best practice based on efficacy and side effects, • Decision questions – How much time, training, and cost is entailed in the new treatment? Is the clinical benefit worth the cost? Is there a cost offset? B. Tai 2010

Future CTN Premises • Addiction treatment services will be improved as evidence-based treatments are broadly implemented in community-based treatment programs, • Randomized, controlled clinical trials are the gold standard for generating evidence-based treatments, • Engage the providers in the research process will improve the acceptability/adoption of research results. • Address research questions that impact practice. B. Tai 2010 B. Tai 2009

www. nida. nih. gov/CTN/Index. htm B. Tai 2009

Q&A B. Tai 2010
42595101bae72698ec8cfe12d0de7afd.ppt