8ef6a1e02927e7e03f66e16c3877c3de.ppt
- Количество слайдов: 27

NHS Research Scotland - Overview - Feasibility - R&D permissions Dr Alison Walker National Coordinator, NRS Permissions CC Commercial presentation
NHS Research Scotland (NRS) • Collaboration – CSO – 14 NHS Boards in Scotland • Funding – CSO and Scottish Enterprise • Function – coordinate R&D processes and systems ‘. . agree and implement national policy to deliver greater efficiency in the NHS R&D and Research Ethics function’ More efficient and effective service for Pharma CSO – Chief Scientist office
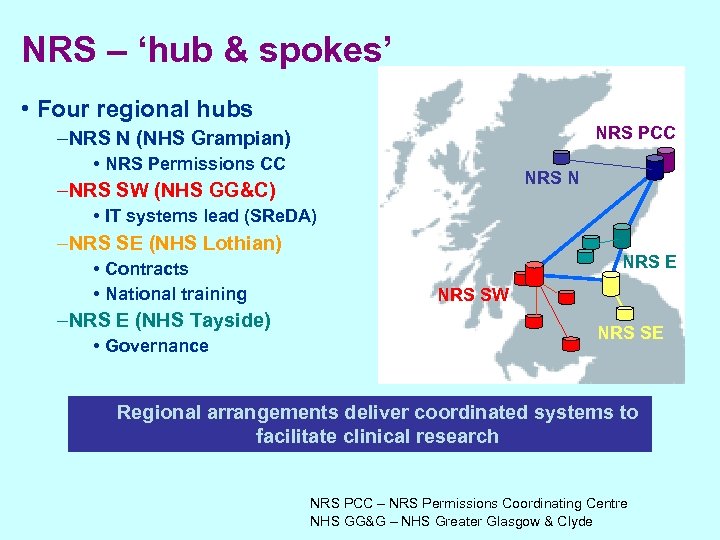
NRS – ‘hub & spokes’ • Four regional hubs NRS PCC –NRS N (NHS Grampian) • NRS Permissions CC NRS N –NRS SW (NHS GG&C) • IT systems lead (SRe. DA) –NRS SE (NHS Lothian) • Contracts • National training NRS E NRS SW –NRS E (NHS Tayside) • Governance NRS W NRS SE Regional arrangements deliver coordinated systems to facilitate clinical research NRS PCC – NRS Permissions Coordinating Centre NHS GG&G – NHS Greater Glasgow & Clyde
NRS Permissions CC • Dedicated administrative team • Coordination – Feasibility – Master CDAs – R&D permissions process for multicentre 1 studies • Single point of contact • Contact point for rest of UK – link with other coordinated systems: - eg. CSP Unit, NISCHR PCU Streamlined process to obtain R&D permission for multicentre research in Scotland 1 Multicentre = > 1 Health Board/Trust in UK CDAs – Confidential Disclosure Agreements

NRS Permissions CC – the team • Director – Professor David Reid • National Co-ordinator – Dr Alison Walker • Senior Administrators – Pamela Shand – Karen Innes • Administrator – Lindsay Grant

Processes managed by NRS Permissions CC • Feasibility in Scotland • Coordination of R&D permissions process • Amendments • Addition of new sites

Feasibility in Scotland • No formal ‘adoption’ process in Scotland • Feasibility request/questionnaire/CDA sent to Permissions CC • Permissions CC forwards study information to: • R&D commercial managers/facilitators (agreed Health Boards) • Scottish Clinical Specialty Lead (if applicable) • Scottish Topic-Specific Research Network Managers • Investigators approached/supported to participate • 2 week deadline for response • Permissions CC actively chases / feeds back to Industry • Companies are put directly in touch with interested parties to take discussions forward • At least involve NHS R&D commercial managers

Scotland: therapeutic areas of expertise • Cardiovascular Disease Dermatology • Gastrointestinal Disease Infectious Disease • Inflammation/Immunology Metabolic Disease/Diabetes • Neuroscience Oncology • Opthalmology Mental Health • Respiratory Disease Stroke • Tissue Research Women’s Health • Extensive imaging infrastructure and latest biomedical NMR imaging techniques • Scottish Clinical Specialty Lead represented on UKCRN/NIHR Specialty Groups • Topic-Specific Research Networks eg. Diabetes, Mental Health, Dementia, Cancer, Stroke, Medicines for Children, Primary Care

When to use NRS Permissions CC • More than one Board/Trust within UK – Regardless of adoption in England – Regardless of use of CSP, etc. • Only time NRS Permissions CC is not used is for SINGLE SITE IN UK studies
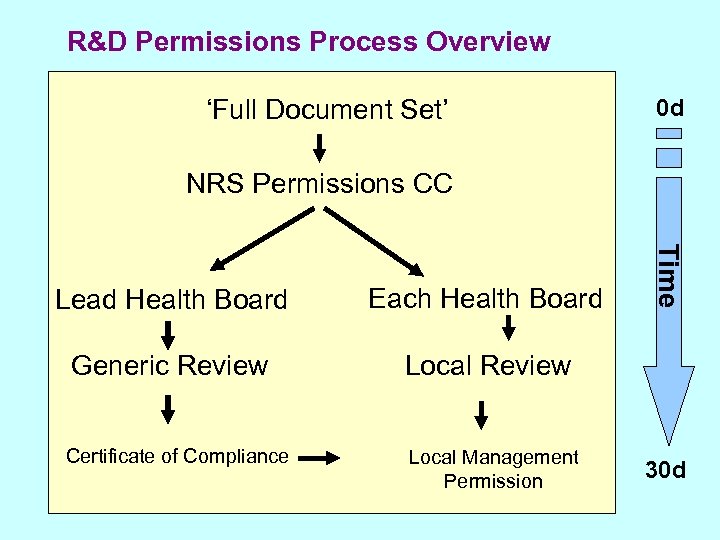
R&D Permissions Process Overview ‘Full Document Set’ 0 d NRS Permissions CC Each Health Board Generic Review Local Review Certificate of Compliance Local Management Permission Time Lead Health Board 30 d

NRS R&D Permission Process(es) R&D permissions process in Scotland is simple, but can vary depending on: • single- or multicentre? • UK-wide study? • lead R&D office?
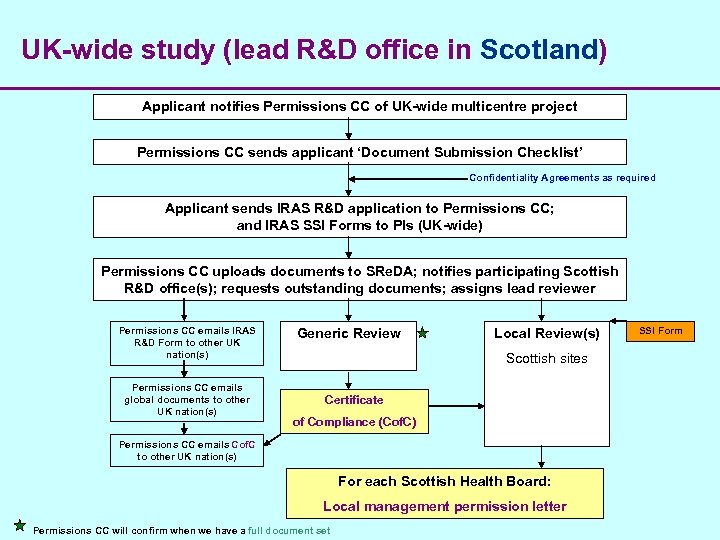
UK-wide study (lead R&D office in Scotland) Applicant notifies Permissions CC of UK-wide multicentre project Permissions CC sends applicant ‘Document Submission Checklist’ Confidentiality Agreements as required Applicant sends IRAS R&D application to Permissions CC; and IRAS SSI Forms to PIs (UK-wide) Permissions CC uploads documents to SRe. DA; notifies participating Scottish R&D office(s); requests outstanding documents; assigns lead reviewer Permissions CC emails IRAS R&D Form to other UK nation(s) Permissions CC emails global documents to other UK nation(s) Generic Review Local Review(s) Scottish sites Certificate of Compliance (Cof. C) Permissions CC emails Cof. C to other UK nation(s) For each Scottish Health Board: Local management permission letter Permissions CC will confirm when we have a full document set SSI Form
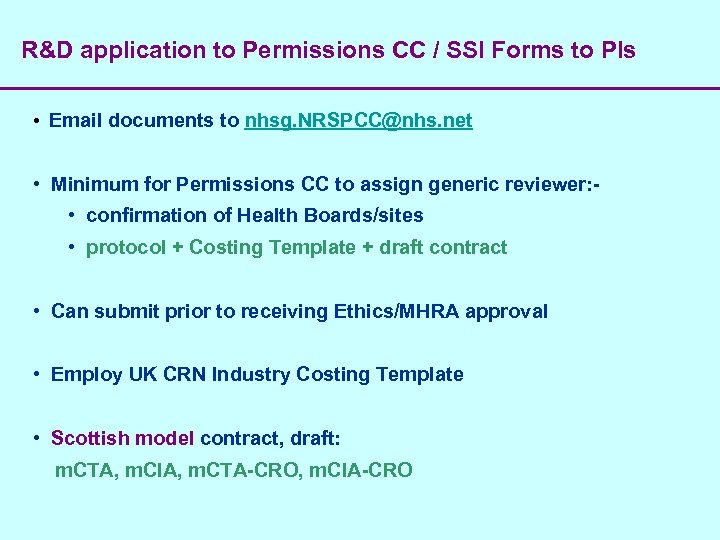
R&D application to Permissions CC / SSI Forms to PIs • Email documents to nhsg. NRSPCC@nhs. net • Minimum for Permissions CC to assign generic reviewer: • confirmation of Health Boards/sites • protocol + Costing Template + draft contract • Can submit prior to receiving Ethics/MHRA approval • Employ UK CRN Industry Costing Template • Scottish model contract, draft: m. CTA, m. CIA, m. CTA-CRO, m. CIA-CRO
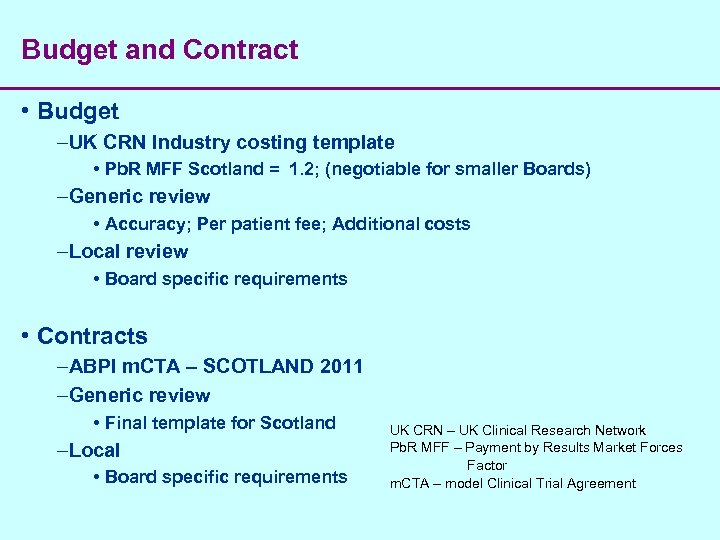
Budget and Contract • Budget –UK CRN Industry costing template • Pb. R MFF Scotland = 1. 2; (negotiable for smaller Boards) –Generic review • Accuracy; Per patient fee; Additional costs –Local review • Board specific requirements • Contracts –ABPI m. CTA – SCOTLAND 2011 –Generic review • Final template for Scotland –Local • Board specific requirements UK CRN – UK Clinical Research Network Pb. R MFF – Payment by Results Market Forces Factor m. CTA – model Clinical Trial Agreement
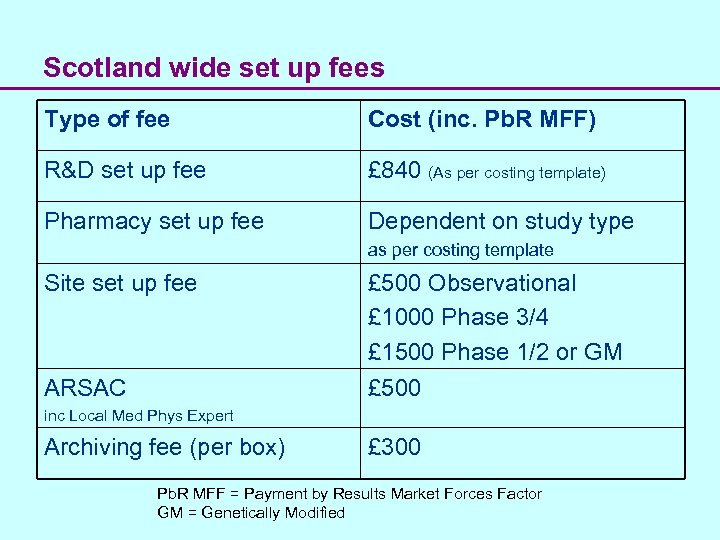
Scotland wide set up fees Type of fee Cost (inc. Pb. R MFF) R&D set up fee £ 840 (As per costing template) Pharmacy set up fee Dependent on study type as per costing template Site set up fee ARSAC £ 500 Observational £ 1000 Phase 3/4 £ 1500 Phase 1/2 or GM £ 500 inc Local Med Phys Expert Archiving fee (per box) £ 300 Pb. R MFF = Payment by Results Market Forces Factor GM = Genetically Modified
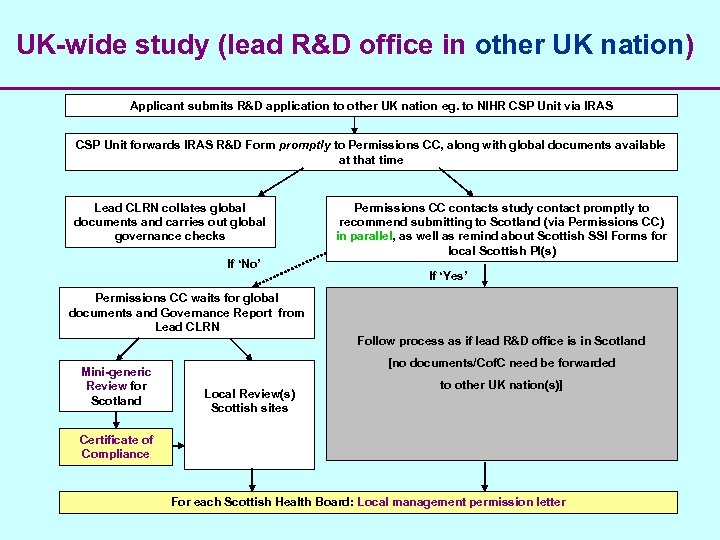
UK-wide study (lead R&D office in other UK nation) Applicant submits R&D application to other UK nation eg. to NIHR CSP Unit via IRAS CSP Unit forwards IRAS R&D Form promptly to Permissions CC, along with global documents available at that time Lead CLRN collates global documents and carries out global governance checks If ‘No’ Permissions CC contacts study contact promptly to recommend submitting to Scotland (via Permissions CC) in parallel, as well as remind about Scottish SSI Forms for local Scottish PI(s) If ‘Yes’ Permissions CC waits for global documents and Governance Report from Lead CLRN Follow process as if lead R&D office is in Scotland Mini-generic Review for Scotland [no documents/Cof. C need be forwarded Local Review(s) Scottish sites to other UK nation(s)] Certificate of Compliance For each Scottish Health Board: Local management permission letter

How you can help speed up the process ? • • Apply for R&D permission prior to receiving REC/MHRA approval Documents you send to a REC, send also to Permissions CC (incl. interim) Use the NRS Document Submission Checklist Send correct versions of necessary documents to NRS Permissions CC electronically • Employ Scottish model contracts “as published” • Obtain PIs’ support prior to sending out Site-Specific Information (SSI) Forms and let them know that the SSI Form is on it’s way • Submit amendments sent to a REC to NRS Permissions CC also, and at the same time Commercial customers should also: • Employ the UK CRN Costing Template • Get in touch with NRS Permissions CC early to discuss CDAs • Get in touch early on to initiate contract / budget discussions with the Commercial Manager of the lead R&D office

Processes for: Amendments, New Sites Amendments • Permissions CC coordinates amendments for NRS projects • Permissions CC should receive documentation, to upload and notify participating Health Boards / other UK nation New sites • Adding new Scottish site to Scottish multicentre study, post R&D permission • Adding new Scottish site to single-Scottish-site study, post R&D permission • Adding 1 st Scottish site to UK study
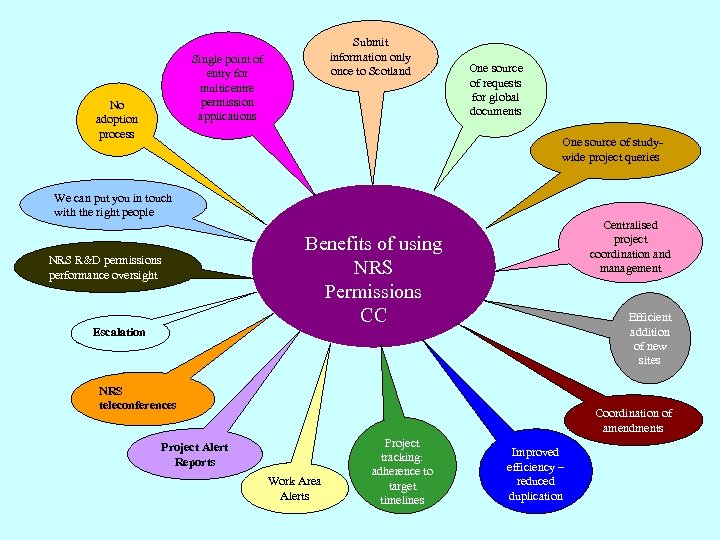
Submit information only once to Scotland Single point of entry for multicentre permission applications No adoption process One source of requests for global documents One source of studywide project queries We can put you in touch with the right people Centralised project coordination and management Benefits of using NRS Permissions CC NRS R&D permissions performance oversight Escalation Efficient addition of new sites NRS teleconferences Coordination of amendments Project Alert Reports Work Area Alerts Project tracking: adherence to target timelines Improved efficiency – reduced duplication

Active project management • Circulation of ‘Project Alert Report’ every 2 weeks • NRS teleconference every 2 weeks, to discuss projects with key R&D office staff from each Node - chaired by Permissions CC, representation from CSO • Permissions CC team chases updates / actions / resolution • Escalation procedure • SRe. DA ‘Work Area’ alerts – at 20 and 30 calendar days
How is Scotland performing? NRS R&D permission times measured from: - receipt of NRS full document set, to issue of local Management permission at each participating Health Board R&D office [as ‘Net NHS time’].
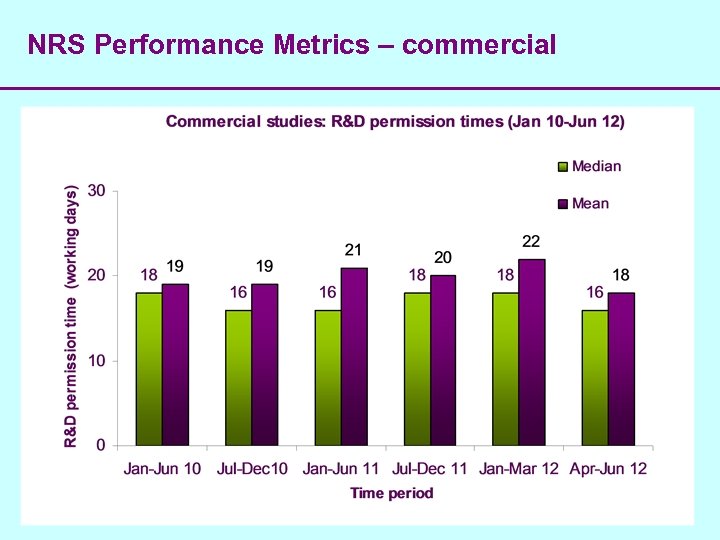
NRS Performance Metrics – commercial
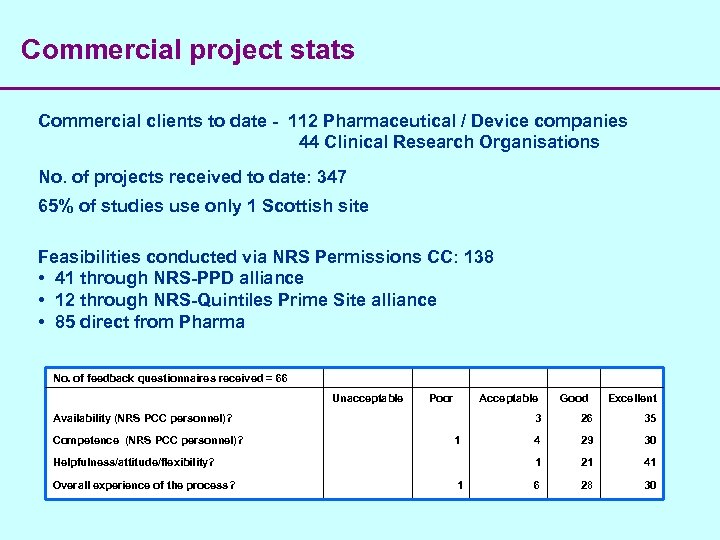
Commercial project stats Commercial clients to date - 112 Pharmaceutical / Device companies 44 Clinical Research Organisations No. of projects received to date: 347 65% of studies use only 1 Scottish site Feasibilities conducted via NRS Permissions CC: 138 • 41 through NRS-PPD alliance • 12 through NRS-Quintiles Prime Site alliance • 85 direct from Pharma No. of feedback questionnaires received = 66 Unacceptable Availability (NRS PCC personnel)? Competence (NRS PCC personnel)? 1 Helpfulness/attitude/flexibility? Overall experience of the process? 1 Poor Acceptable Good Excellent 3 26 35 4 29 1 21 6 28 30 41 30
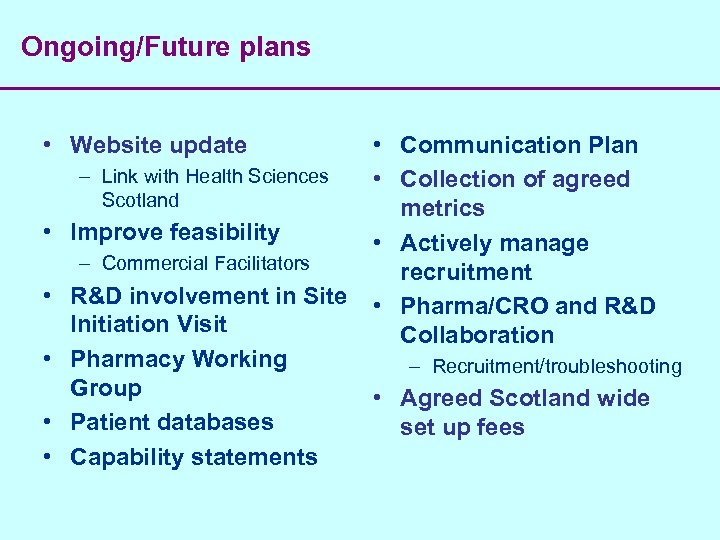
Ongoing/Future plans • Website update • • • Communication Plan – Link with Health Sciences • Collection of agreed Scotland metrics Improve feasibility • Actively manage – Commercial Facilitators recruitment R&D involvement in Site • Pharma/CRO and R&D Initiation Visit Collaboration Pharmacy Working – Recruitment/troubleshooting Group • Agreed Scotland wide Patient databases set up fees Capability statements

Supporting clinical studies • Commercial Facilitators – 2 in each node (primary and secondary care) – Assist with feasibility, start up and recruitment • Nurses – Dedicated research nurses – Help with resourcing nurses – Expert support for investigators • Imaging infrastructure – Dedicated scanners and reporting

NRS Permissions CC Contact details Dr Alison Walker National Coordinator NHS Research Scotland Permissions Coordinating Centre (NRS Permissions CC) alisonwalker 1@nhs. net Tel: 01224 554051 NRS Permissions CC Office nhsg. NRSPCC@nhs. net Tel: 01224 552690 Website: http: //www. NRSPCC. org

Health Science Scotland is uniquely placed to set up collaborations between companies and researchers. Graeme Boyle: Senior Program Manager g. boyle@healthsciencescotland. com www. healthsciencescotland. com - Researchers - Research areas and assets - Our population - Case studies
8ef6a1e02927e7e03f66e16c3877c3de.ppt