bc1325bc0a74fb0e11a9f771acc9380e.ppt
- Количество слайдов: 25

Newborn Screening- who is doing what and why and where is it going? ? ? Sara Copeland, MD Department of Health and Human Services Health Resources and Services Administration Medical Officer, Genetics Services Branch
Public Health and Genetics HRSA CDC NIH

Timeline of Genetics in HRSA • 1912 - Established Nation Children’s Bureau • 1935 -Establishment of Title V of the Social Security Act • 1950 s, Congress earmarked funds for children with developmental delays (mental retardation) and congenital heart disease • 1972 -the Sickle Cell Anemia Control Act • Prompted the MCH program to improve access to genetic services • 1976 - The National Sickle Cell Anemia, Cooley's Anemia, Tay-Sachs and Genetic Disease Act of • expansion of MCHB's genetics activities beyond sickle cell disease programs. • 1999 - NBS Taskforce • 2000 - MCH Heritable Disorders Program authorized • Implemented in 2004 • 2008 - Newborn Screening Saves Lives Act

Genetics Services Branch-Legislation: • Heritable Disorders Program- Public Health Services Act- 2000 and 2008 • Congenital Conditions- Prenatally and Postnatally Diagnosed Conditions Awareness Act- 2008 • Special Projects of Regional and National Significance (SPRANS)- Title V- Social Security Act – Genetic Services – Sickle Cell Disease and Newborn Screening- earmark – Hemophilia disease • Sickle Cell Disease and Treatment Program- Sickle Cell Act

Legislation • Some specify HRSA, some do not • NBSSLA is for everyone to benefit from and contribute to- has been specified who administers the funds • Authorization to act does not mean we have the funding to proceed (appropriations)

Legislation • Title XXVI of the Children’s Health Care Act of 2000 (Title XI of PHS act) - Establishes a program to improve the ability of States to provide newborn and child screening for heritable disorders. - Three Sections establishing grant programs and the Advisory Committee on Heritable Disorders in Newborns and Children

Provisions of Public Law 110 -204 Newborn Screening Saves Lives Act of 2008 • This statute amends the Public Health Service Act to facilitate the creation of Federal guidelines on newborn screening – To assist State newborn screening programs in meeting federal guidelines – To establish grant programs to provide for education and outreach on newborn screening and follow-up care once newborn screening has been conducted – To reauthorize programs under Part A of Title XI of the Act
Provisions of Public Law 110 -204 Newborn Screening Saves Lives Act of 2008 • The Act reauthorizes and expands the role of the Adv. Committee on Heritable Disorders in Newborns and Children (ACHDNC) • Establishes an Interagency Coordinating Committee on Newborn and Child Screening • Creates an internet-based information clearinghouse to provide information about newborn and child screening for heritable disorders

Provisions of Public Law 110 -204 Newborn Screening Saves Lives Act of 2008 • Bill requires the Secretary of HHS – To ensure the quality of laboratories involved in NBS activities – To develop a national contingency plan for newborn screening • Bill gives NIH the authority to carry out research in newborn screening, including identifying new screening technologies and researching diseases management strategies for the conditions that can be detected through screening (NIH program to be known as the Hunter Kelly Newborn Screening Research Program)

Section 1111 (SACHDNC) • Make systematic evidence-based and peer-reviewed recommendations that include the heritable disorders that have the potential to significantly impact public health for which all newborns should be screened, including secondary conditions that may be identified as a result of the laboratory methods used for screening • Develop a model decision-matrix for newborn screening expansion, including an evaluation of the potential public health impact of such expansion and periodically update the recommended uniform screening panel, as appropriate, based on such decision-matrix

Funded Programs- Heritable Disorders Program • • Regional Collaboratives Effective Follow-up Newborn Screening Clearinghouse SACHDNC
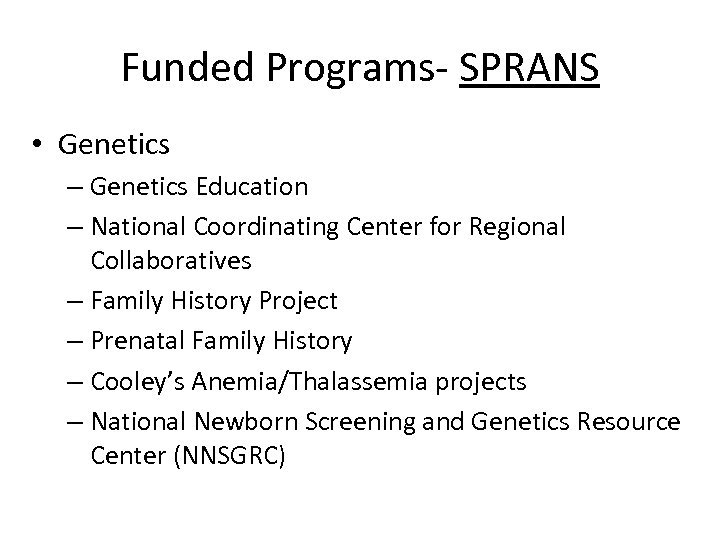
Funded Programs- SPRANS • Genetics – Genetics Education – National Coordinating Center for Regional Collaboratives – Family History Project – Prenatal Family History – Cooley’s Anemia/Thalassemia projects – National Newborn Screening and Genetics Resource Center (NNSGRC)

Funded Programs- SPRANS • Hemophilia – Treatment and Comprehensive Care Centers • Sickle Cell and NBS – Follow-up and community involvement projects

Funded Programs- SCDTP • Sickle cell disease and treatment programs – 9 networks – Comprehensive care for people living with SCD, evidence based treatment in a multi-disciplinary setting- including CBO – 1 coordinating center

Funded Programs- Congenital Conditions • Evidence based information and services to parents receiving a positive diagnosis for a prenatally or postnatally diagnosed condition (i. e. dwarfism, spina bifida, down syndrome, trisomy)
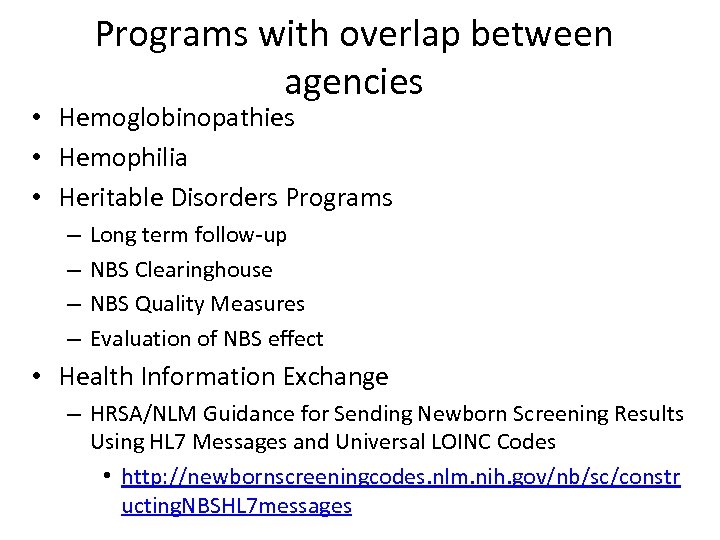
Programs with overlap between agencies • Hemoglobinopathies • Hemophilia • Heritable Disorders Programs – – Long term follow-up NBS Clearinghouse NBS Quality Measures Evaluation of NBS effect • Health Information Exchange – HRSA/NLM Guidance for Sending Newborn Screening Results Using HL 7 Messages and Universal LOINC Codes • http: //newbornscreeningcodes. nlm. nih. gov/nb/sc/constr ucting. NBSHL 7 messages
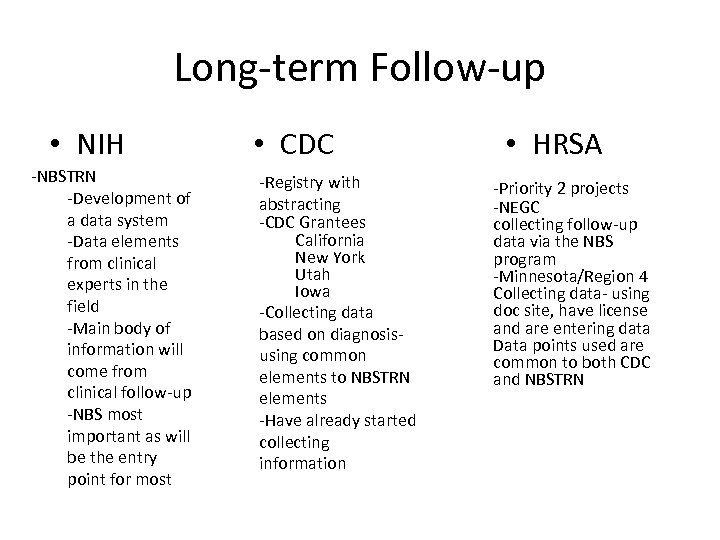
Long-term Follow-up • NIH -NBSTRN -Development of a data system -Data elements from clinical experts in the field -Main body of information will come from clinical follow-up -NBS most important as will be the entry point for most • CDC -Registry with abstracting -CDC Grantees California New York Utah Iowa -Collecting data based on diagnosisusing common elements to NBSTRN elements -Have already started collecting information • HRSA -Priority 2 projects -NEGC collecting follow-up data via the NBS program -Minnesota/Region 4 Collecting data- using doc site, have license and are entering data Data points used are common to both CDC and NBSTRN
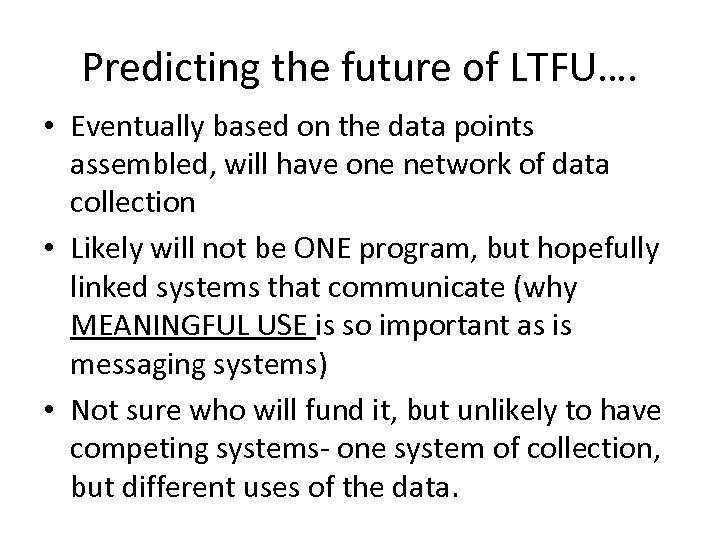
Predicting the future of LTFU…. • Eventually based on the data points assembled, will have one network of data collection • Likely will not be ONE program, but hopefully linked systems that communicate (why MEANINGFUL USE is so important as is messaging systems) • Not sure who will fund it, but unlikely to have competing systems- one system of collection, but different uses of the data.
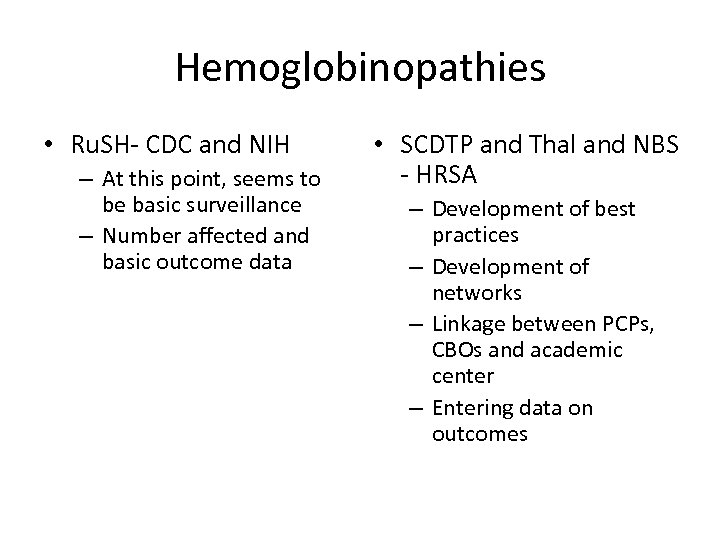
Hemoglobinopathies • Ru. SH- CDC and NIH – At this point, seems to be basic surveillance – Number affected and basic outcome data • SCDTP and Thal and NBS - HRSA – Development of best practices – Development of networks – Linkage between PCPs, CBOs and academic center – Entering data on outcomes
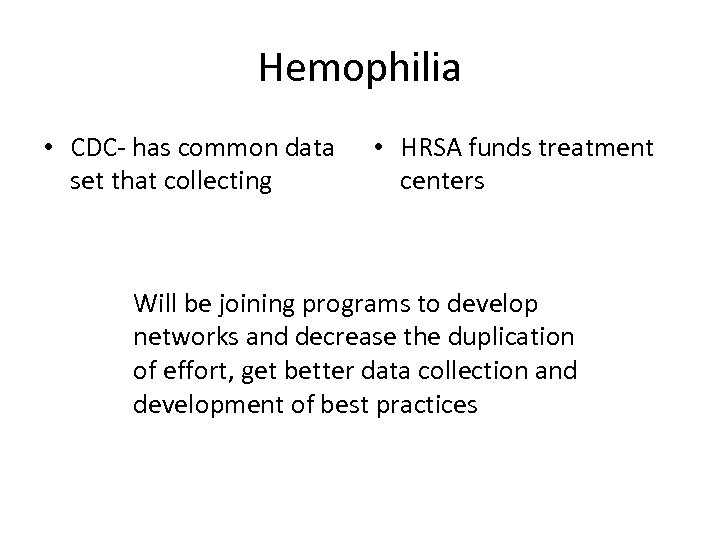
Hemophilia • CDC- has common data set that collecting • HRSA funds treatment centers Will be joining programs to develop networks and decrease the duplication of effort, get better data collection and development of best practices

Programs with overlap within HRSA • • Effective Follow-up NNSGRC Newborn screening clearinghouse Early and continuous screening through the medical home

Effective Follow-up -Focuses on HIE -Means to develop information exchange -Proof of concept, that data transfer can work from the public health departments to labs or PCPs etc. -Not a long-term followup system -Likely will not be ongoing, more of developmental project Early and continuous screening -Not necessarily based on NBS screening -Means to improve overall screening for developmental problems in medical home setting NNSGRC -Will be changing since introduction of Clearinghouse -Focus will be development of a state program resource center -Will be working to develop new systems for quality assessment of programs, expand the program visits, revise the PEAS and work with CDC for a full NBS system quality enhancement program -Currently houses the NNSIS- will be moved to own program in 2012 Newborn screening Clearinghous -Existence is legislated -Focus will be development of a provider and consumer resources -For next couple of years will be working with HRSA to gather information about what new NNSIS will look like- but will not HOUSE the NNSIS -No newborn screening patient information will be linked to this site

NNSIS- National Newborn Screening Information System • Is currently housed in the NNSGRC • Is voluntary and will remain so- reporting about state incidence etc. • We need to update and upgrade this system – Upgraded system will be developed- implemented in FY 2012 – Will be separate from other systems, not housed in any other program • Will have several tiers of access – State access – Federal CQE access (voluntary) – Public face- only aggregate data to meet the legislative needswant to be able to provide some quality measurements

Upcoming Programs • ICC- Interagency Collaborative Committee on NBS – Legislated in NBSSLA – Will include all major HHS entities • Federal agencies will make up the membership – Will provide guidance for Secretary of HHS – Will work with SACHDNC – Will be shared delegation by CDC and HRSA • NBS CQE Program – – – Voluntary program to enhance quality of NBS SYSTEMS Collaboration with CDC NSQAP and PT programs Will include pre and post analytical systems Outgrowth of NNSGRC program evaluation Needs to be shaped by state needs Hope to be able to get certification that impacts CHIPRA and Medicaid reimbursement

Contact Information Sara Copeland, MD Genetics Services Branch, Medical Officer 301 -443 -8860 scopeland@hrsa. gov
bc1325bc0a74fb0e11a9f771acc9380e.ppt