bf3ccea245c88d5ee7be9dab3cbdaa43.ppt
- Количество слайдов: 71
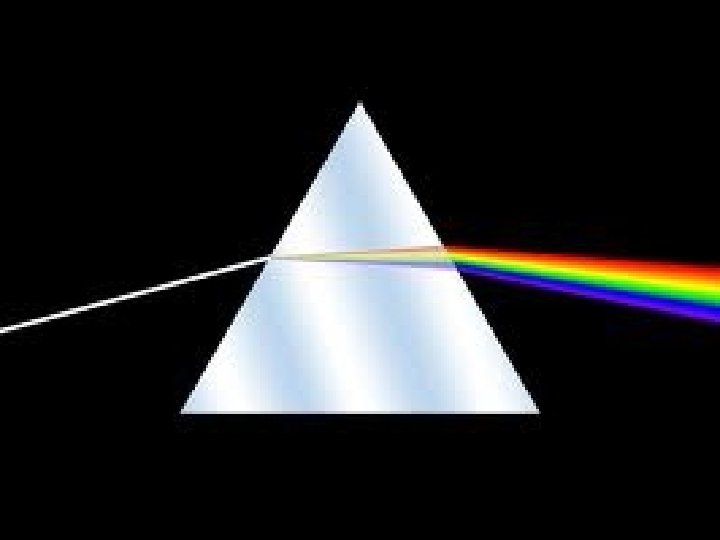
 New Unit! Energy in Earth Processes Question of the Day What is electromagnetic energy, and how does it affect me? What causes rainbows? 77
New Unit! Energy in Earth Processes Question of the Day What is electromagnetic energy, and how does it affect me? What causes rainbows? 77
 Energy • Ability to do work • Earth processes driven by 2 sources: 1) Major (external): the sun 2) Minor (internal): Earth’s interior
Energy • Ability to do work • Earth processes driven by 2 sources: 1) Major (external): the sun 2) Minor (internal): Earth’s interior
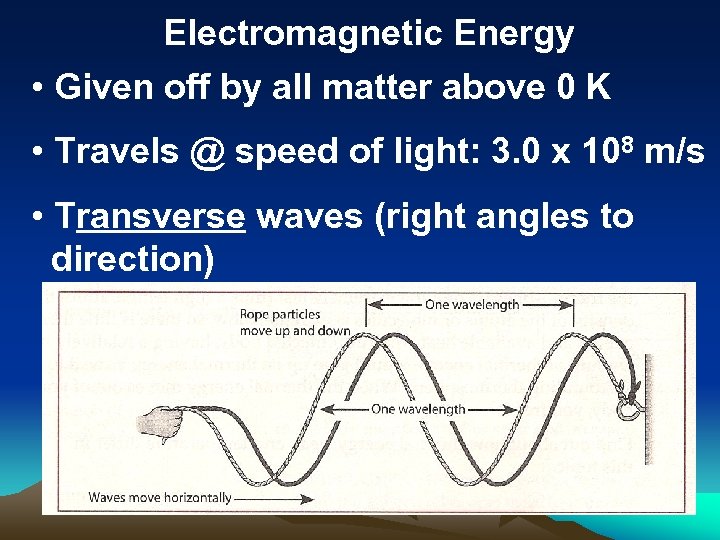 Electromagnetic Energy • Given off by all matter above 0 K • Travels @ speed of light: 3. 0 x 108 m/s • Transverse waves (right angles to direction)
Electromagnetic Energy • Given off by all matter above 0 K • Travels @ speed of light: 3. 0 x 108 m/s • Transverse waves (right angles to direction)
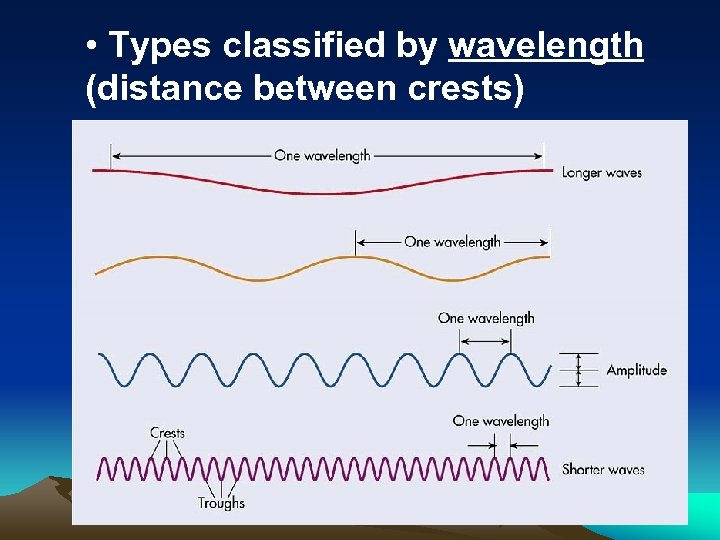 • Types classified by wavelength (distance between crests)
• Types classified by wavelength (distance between crests)
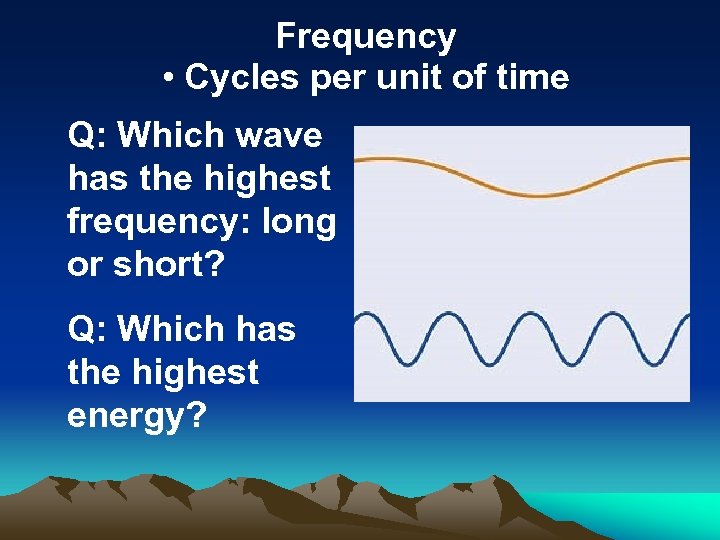 Frequency • Cycles per unit of time Q: Which wave has the highest frequency: long or short? Q: Which has the highest energy?
Frequency • Cycles per unit of time Q: Which wave has the highest frequency: long or short? Q: Which has the highest energy?
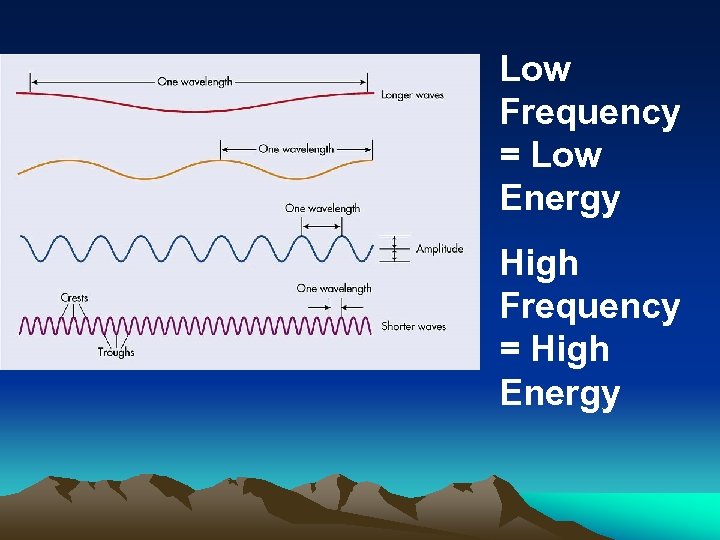 Low Frequency = Low Energy High Frequency = High Energy
Low Frequency = Low Energy High Frequency = High Energy
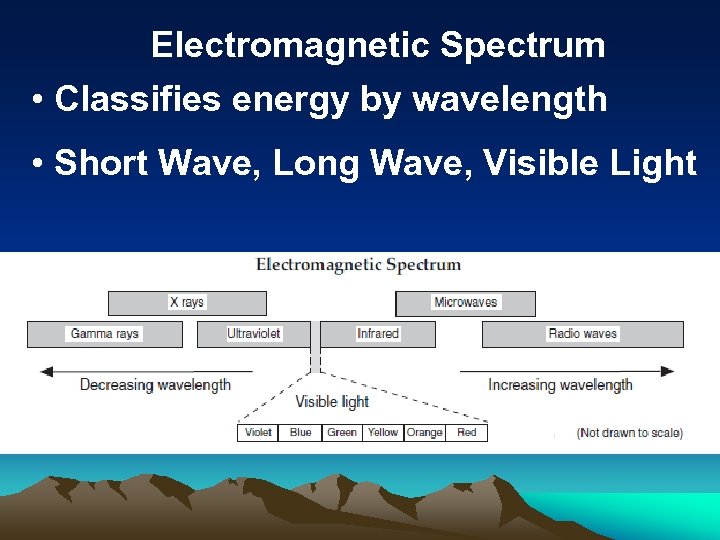 Electromagnetic Spectrum • Classifies energy by wavelength • Short Wave, Long Wave, Visible Light
Electromagnetic Spectrum • Classifies energy by wavelength • Short Wave, Long Wave, Visible Light
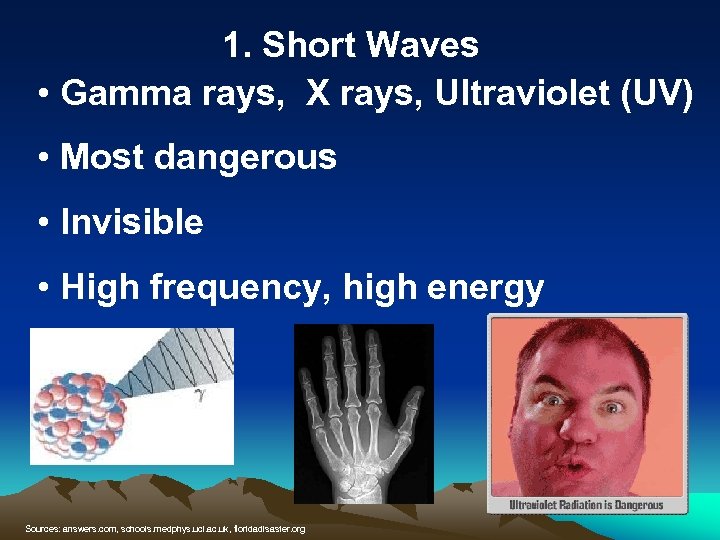 1. Short Waves • Gamma rays, X rays, Ultraviolet (UV) • Most dangerous • Invisible • High frequency, high energy Sources: answers. com, schools. medphys. ucl. ac. uk, floridadisaster. org
1. Short Waves • Gamma rays, X rays, Ultraviolet (UV) • Most dangerous • Invisible • High frequency, high energy Sources: answers. com, schools. medphys. ucl. ac. uk, floridadisaster. org
 2. Long Waves • Infrared, Microwaves, Radio Waves • Least dangerous • Invisible • Low frequency, low energy Sources: target. com, sci-toys. com, abledata. com
2. Long Waves • Infrared, Microwaves, Radio Waves • Least dangerous • Invisible • Low frequency, low energy Sources: target. com, sci-toys. com, abledata. com
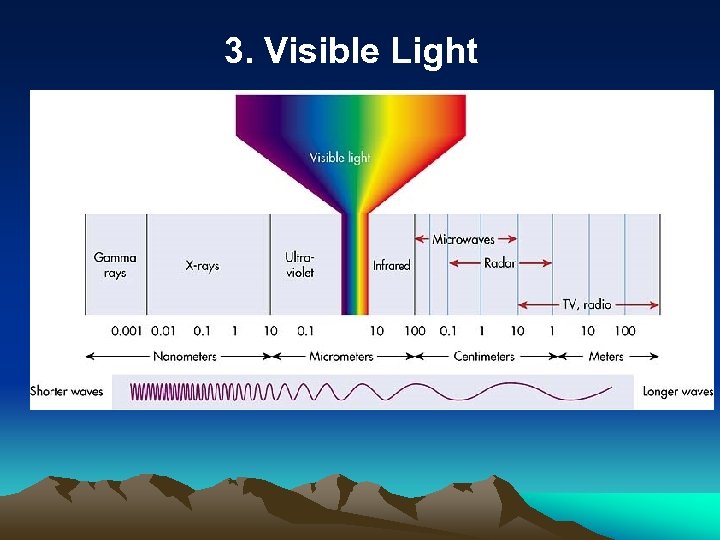 3. Visible Light
3. Visible Light
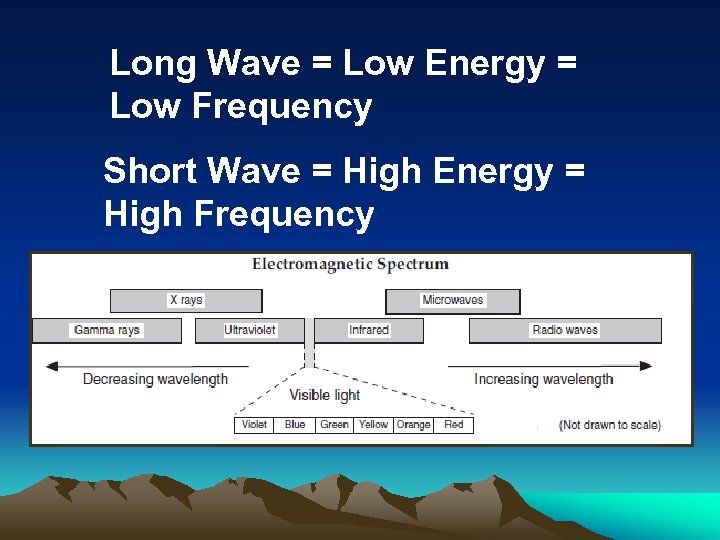 Long Wave = Low Energy = Low Frequency Short Wave = High Energy = High Frequency
Long Wave = Low Energy = Low Frequency Short Wave = High Energy = High Frequency
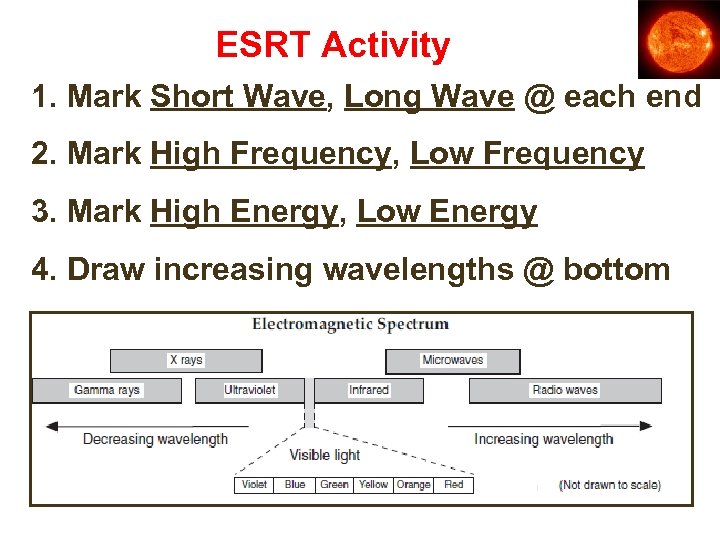 ESRT Activity 1. Mark Short Wave, Long Wave @ each end 2. Mark High Frequency, Low Frequency 3. Mark High Energy, Low Energy 4. Draw increasing wavelengths @ bottom
ESRT Activity 1. Mark Short Wave, Long Wave @ each end 2. Mark High Frequency, Low Frequency 3. Mark High Energy, Low Energy 4. Draw increasing wavelengths @ bottom
 1. Which has a shorter wavelength than red light: Radio waves, microwaves, infrared, UV? 2. Which color of the visible spectrum has the shortest wavelength? 3. Which has the lowest frequency: radio waves, infrared rays, red light, gamma rays? 4. Compared to the speed of gamma rays, infrared is: faster, slower, the same 5. Electromagnetic spectrum classifies by: temperature, speed, source, wavelength?
1. Which has a shorter wavelength than red light: Radio waves, microwaves, infrared, UV? 2. Which color of the visible spectrum has the shortest wavelength? 3. Which has the lowest frequency: radio waves, infrared rays, red light, gamma rays? 4. Compared to the speed of gamma rays, infrared is: faster, slower, the same 5. Electromagnetic spectrum classifies by: temperature, speed, source, wavelength?
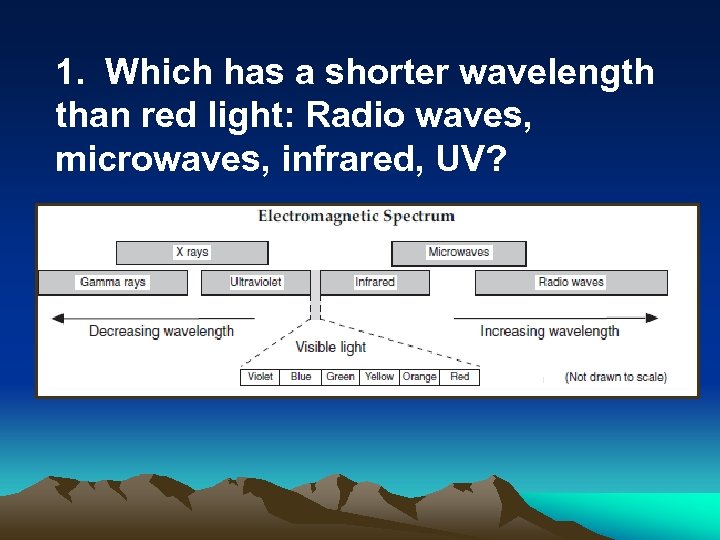 1. Which has a shorter wavelength than red light: Radio waves, microwaves, infrared, UV?
1. Which has a shorter wavelength than red light: Radio waves, microwaves, infrared, UV?
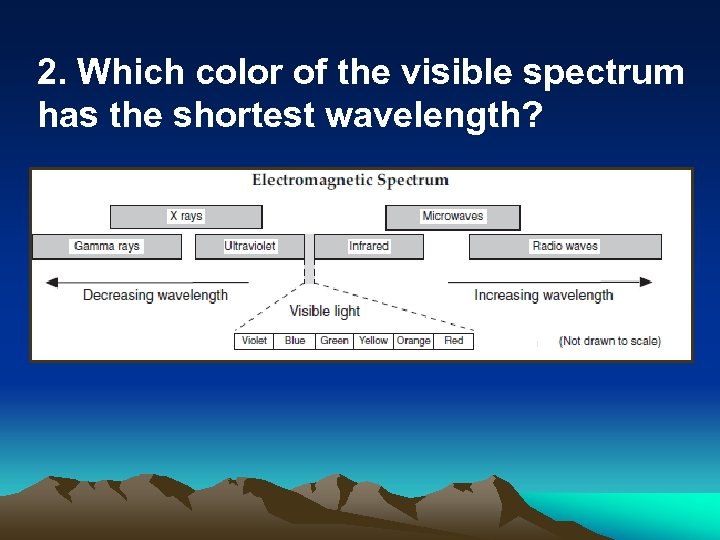 2. Which color of the visible spectrum has the shortest wavelength?
2. Which color of the visible spectrum has the shortest wavelength?
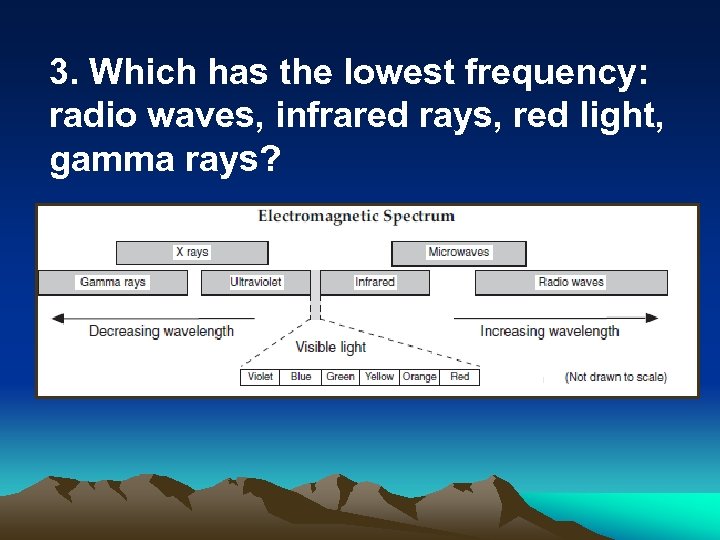 3. Which has the lowest frequency: radio waves, infrared rays, red light, gamma rays?
3. Which has the lowest frequency: radio waves, infrared rays, red light, gamma rays?
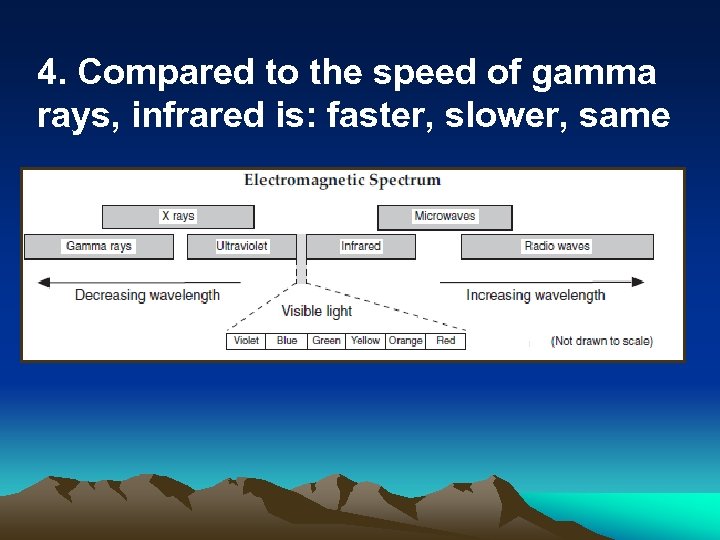 4. Compared to the speed of gamma rays, infrared is: faster, slower, same
4. Compared to the speed of gamma rays, infrared is: faster, slower, same
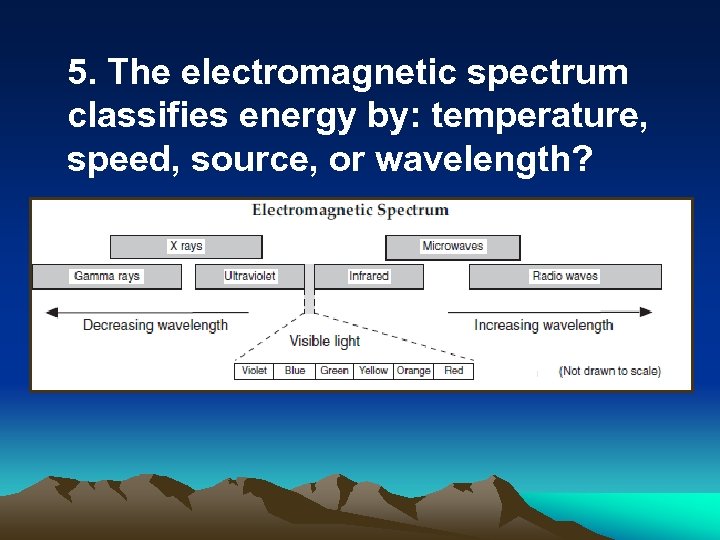 5. The electromagnetic spectrum classifies energy by: temperature, speed, source, or wavelength?
5. The electromagnetic spectrum classifies energy by: temperature, speed, source, or wavelength?

 Question of the Day How does energy interact with surfaces? In the summer, which is hotter when you touch it: a black car or white car? Why? 78
Question of the Day How does energy interact with surfaces? In the summer, which is hotter when you touch it: a black car or white car? Why? 78
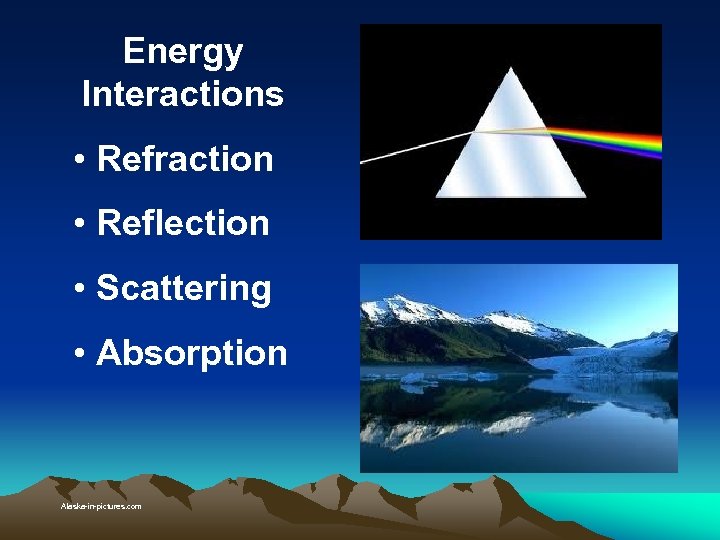 Energy Interactions • Refraction • Reflection • Scattering • Absorption Alaska-in-pictures. com
Energy Interactions • Refraction • Reflection • Scattering • Absorption Alaska-in-pictures. com
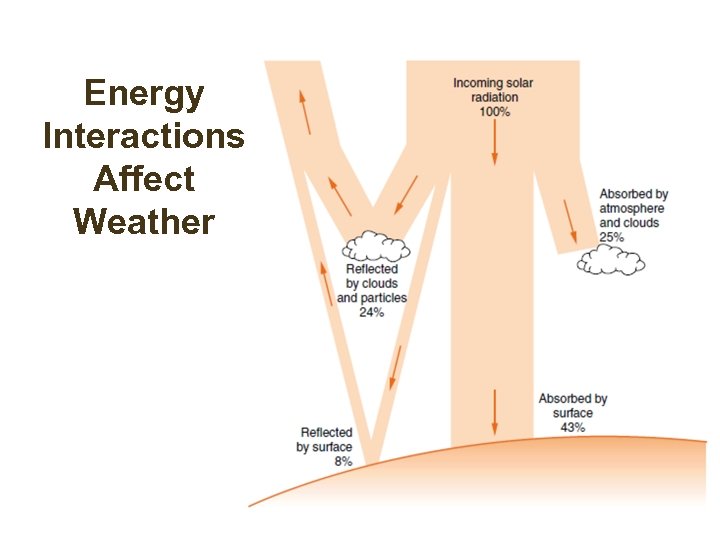 Energy Interactions Affect Weather
Energy Interactions Affect Weather
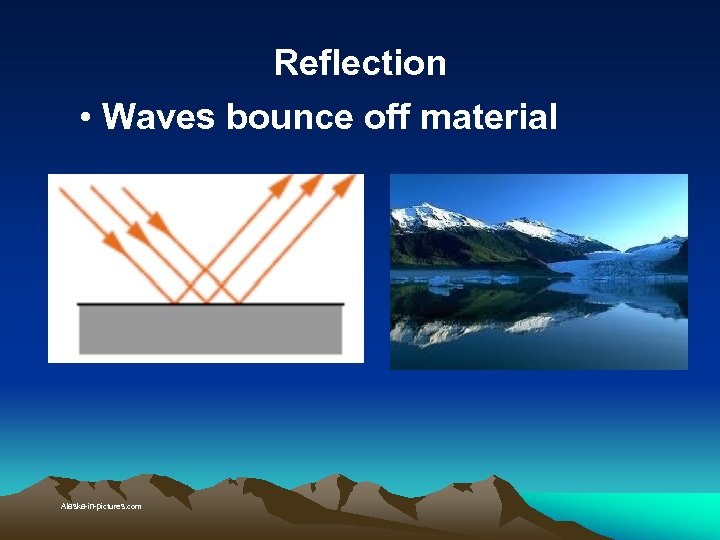 Reflection • Waves bounce off material Alaska-in-pictures. com
Reflection • Waves bounce off material Alaska-in-pictures. com
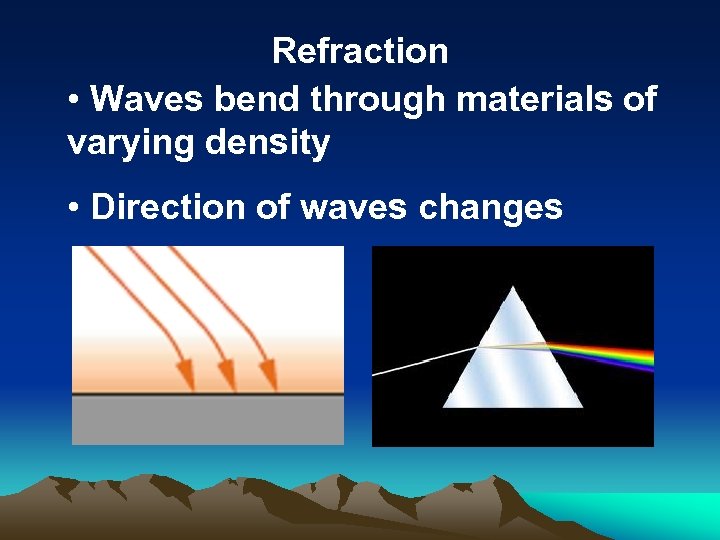 Refraction • Waves bend through materials of varying density • Direction of waves changes
Refraction • Waves bend through materials of varying density • Direction of waves changes
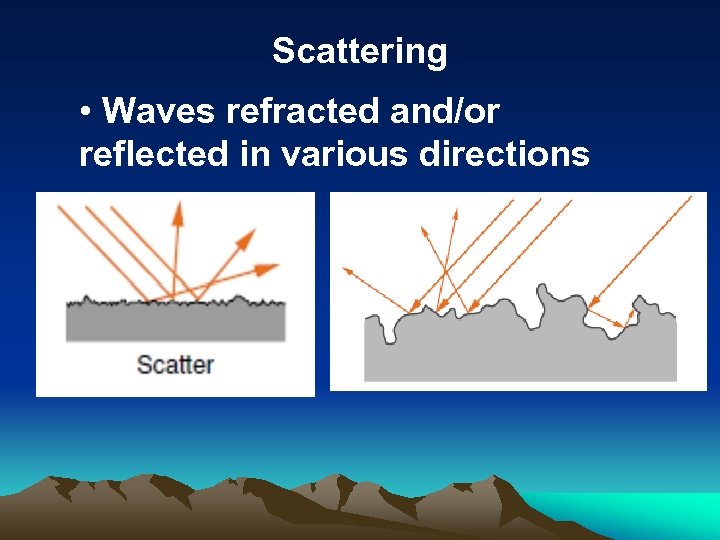 Scattering • Waves refracted and/or reflected in various directions
Scattering • Waves refracted and/or reflected in various directions
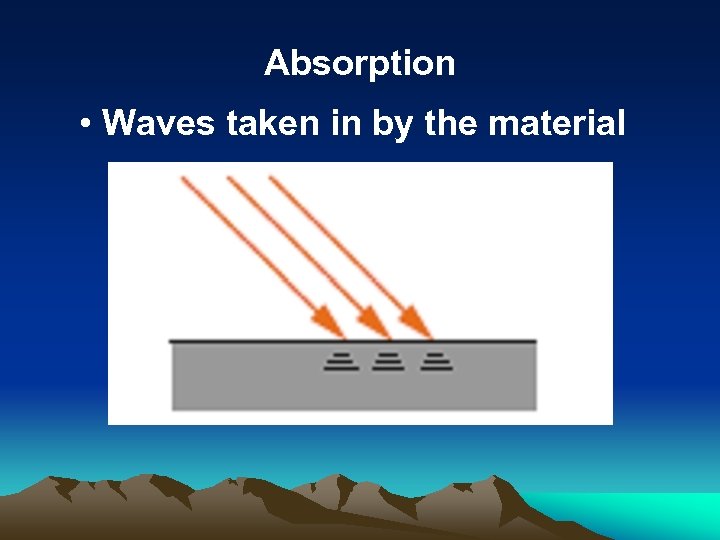 Absorption • Waves taken in by the material
Absorption • Waves taken in by the material
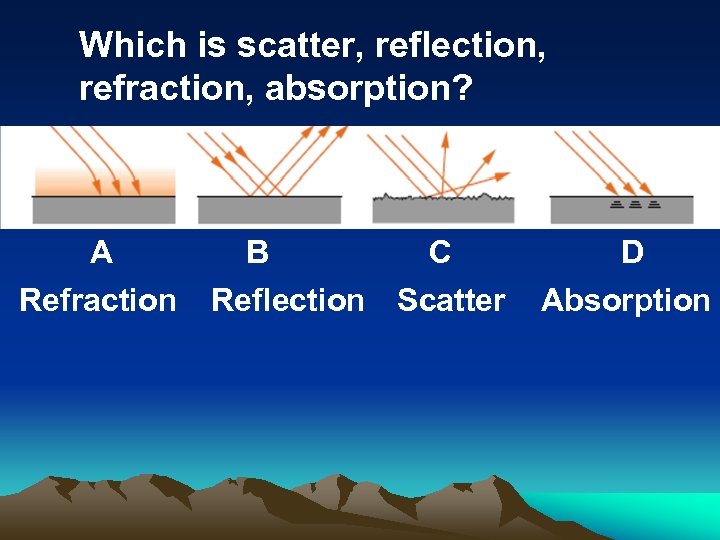 Which is scatter, reflection, refraction, absorption? A Refraction B C Reflection Scatter D Absorption
Which is scatter, reflection, refraction, absorption? A Refraction B C Reflection Scatter D Absorption
 Characteristics That Affect Absorption Color • Dark colors absorb better than light • Dark colors heat up and cool off faster • Fast absorption = Fast radiation (give off energy)
Characteristics That Affect Absorption Color • Dark colors absorb better than light • Dark colors heat up and cool off faster • Fast absorption = Fast radiation (give off energy)
 Texture • Rough vs. smooth • Rough absorbs more, reflects less Would a mirror absorb more or less than a rock?
Texture • Rough vs. smooth • Rough absorbs more, reflects less Would a mirror absorb more or less than a rock?
 1. Which is the best absorber of energy: red, white, black, or pink? 2. Painting a house a lighter color will reduce the solar energy: absorbed, scattered, reflected, or refracted? 3. Compared with a dull, rough rock, a shiny smooth rock will cause sunlight to be: reflected, refracted, scattered, or absorbed?
1. Which is the best absorber of energy: red, white, black, or pink? 2. Painting a house a lighter color will reduce the solar energy: absorbed, scattered, reflected, or refracted? 3. Compared with a dull, rough rock, a shiny smooth rock will cause sunlight to be: reflected, refracted, scattered, or absorbed?
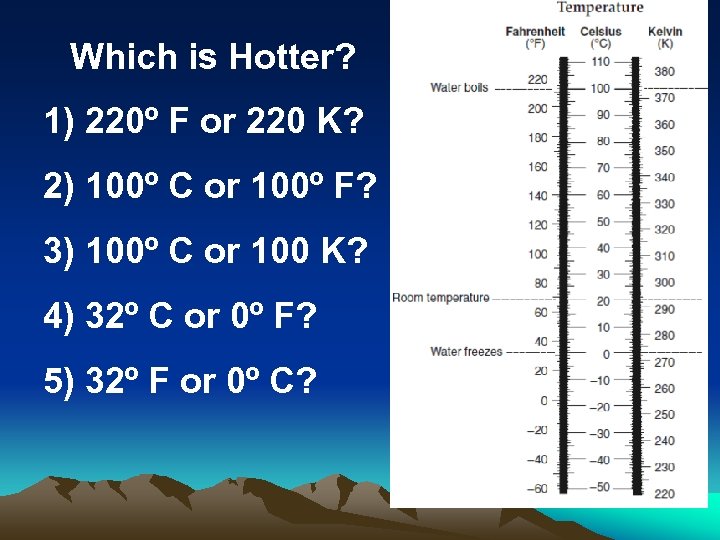 Which is Hotter? 1) 220º F or 220 K? 2) 100º C or 100º F? 3) 100º C or 100 K? 4) 32º C or 0º F? 5) 32º F or 0º C?
Which is Hotter? 1) 220º F or 220 K? 2) 100º C or 100º F? 3) 100º C or 100 K? 4) 32º C or 0º F? 5) 32º F or 0º C?

 Question of the Day How does energy move? If you’re warm and hold hands with someone cold, what happens? 79
Question of the Day How does energy move? If you’re warm and hold hands with someone cold, what happens? 79
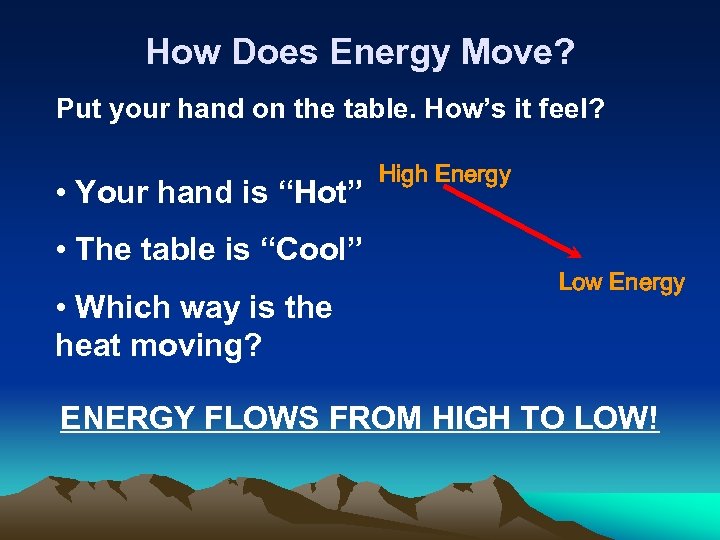 How Does Energy Move? Put your hand on the table. How’s it feel? • Your hand is “Hot” • The table is “Cool” • Which way is the heat moving? High Energy Low Energy ENERGY FLOWS FROM HIGH TO LOW!
How Does Energy Move? Put your hand on the table. How’s it feel? • Your hand is “Hot” • The table is “Cool” • Which way is the heat moving? High Energy Low Energy ENERGY FLOWS FROM HIGH TO LOW!
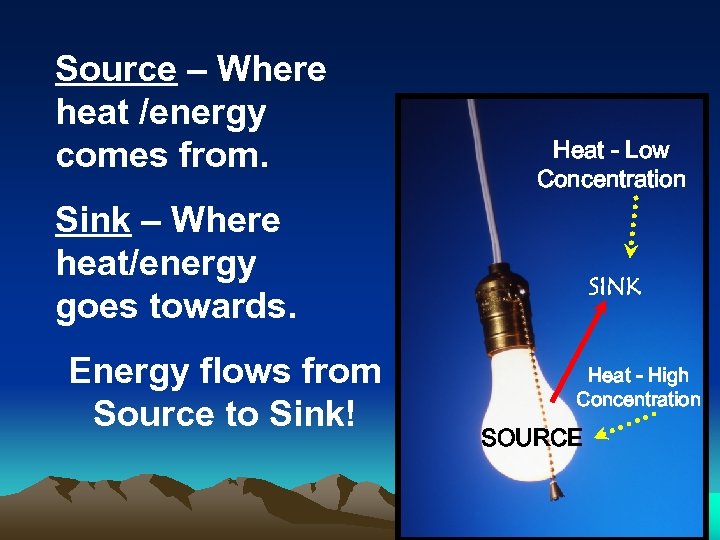 Source – Where heat /energy comes from. Heat - Low Concentration Sink – Where heat/energy goes towards. Energy flows from Source to Sink! SINK Heat - High Concentration SOURCE
Source – Where heat /energy comes from. Heat - Low Concentration Sink – Where heat/energy goes towards. Energy flows from Source to Sink! SINK Heat - High Concentration SOURCE
 Dynamic Equilibrium (Radiative Balance) • Heat moves from source to sink until energies are equal • Temperature remains constant
Dynamic Equilibrium (Radiative Balance) • Heat moves from source to sink until energies are equal • Temperature remains constant

 Question of the Day What are the 3 methods that energy moves by? 1. Does increasing temperature decrease or increase density? 2. Does decreasing temperature decrease or increase density? 80
Question of the Day What are the 3 methods that energy moves by? 1. Does increasing temperature decrease or increase density? 2. Does decreasing temperature decrease or increase density? 80

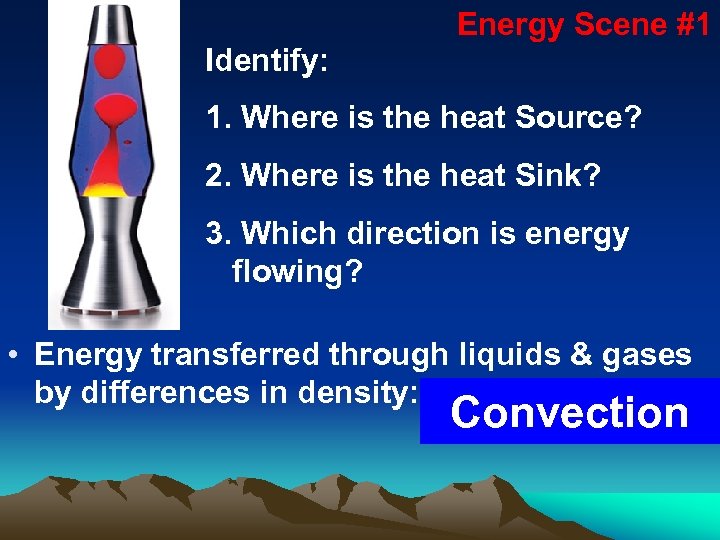 Energy Scene #1 Identify: 1. Where is the heat Source? 2. Where is the heat Sink? 3. Which direction is energy flowing? • Energy transferred through liquids & gases by differences in density: Convection
Energy Scene #1 Identify: 1. Where is the heat Source? 2. Where is the heat Sink? 3. Which direction is energy flowing? • Energy transferred through liquids & gases by differences in density: Convection
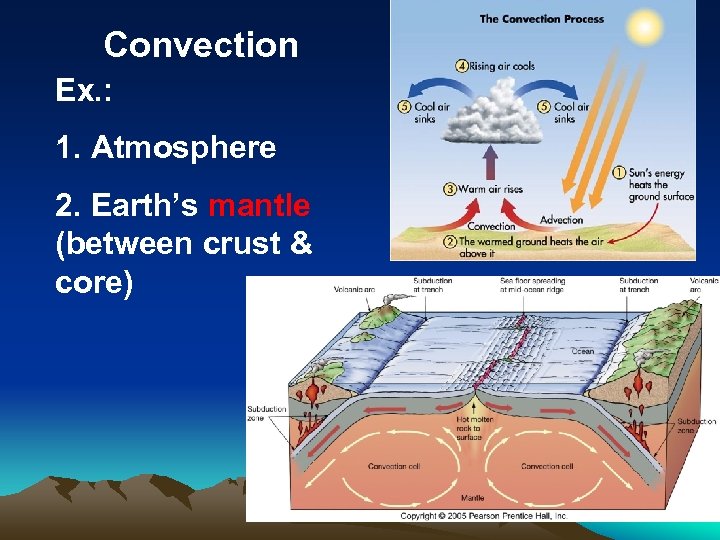 Convection Ex. : 1. Atmosphere 2. Earth’s mantle (between crust & core)
Convection Ex. : 1. Atmosphere 2. Earth’s mantle (between crust & core)
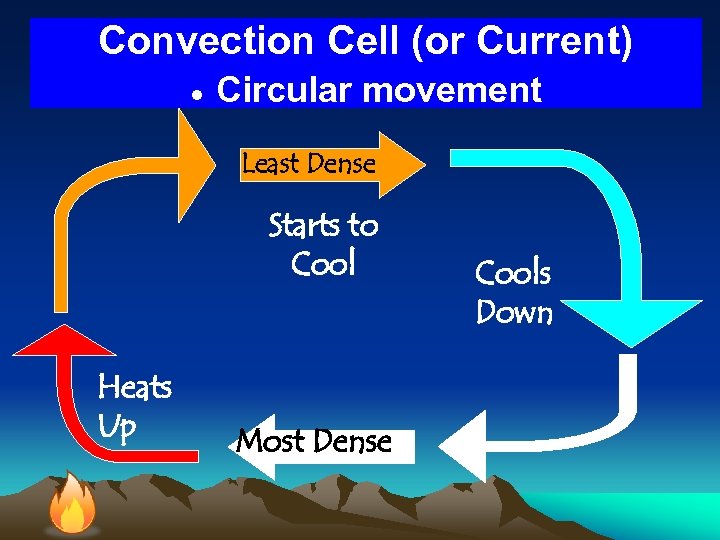 Convection Cell (or Current) ● Circular movement Least Dense Starts to Cool Heats Up Most Dense Cools Down
Convection Cell (or Current) ● Circular movement Least Dense Starts to Cool Heats Up Most Dense Cools Down
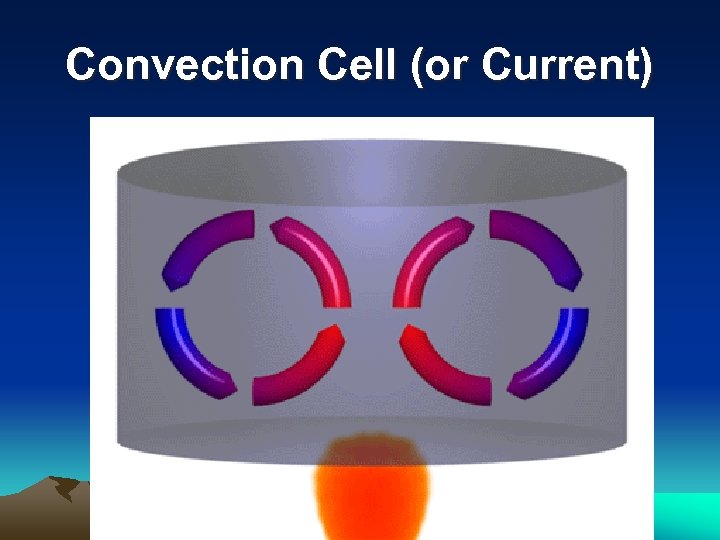 Convection Cell (or Current)
Convection Cell (or Current)
 Warm Up Cool Down
Warm Up Cool Down
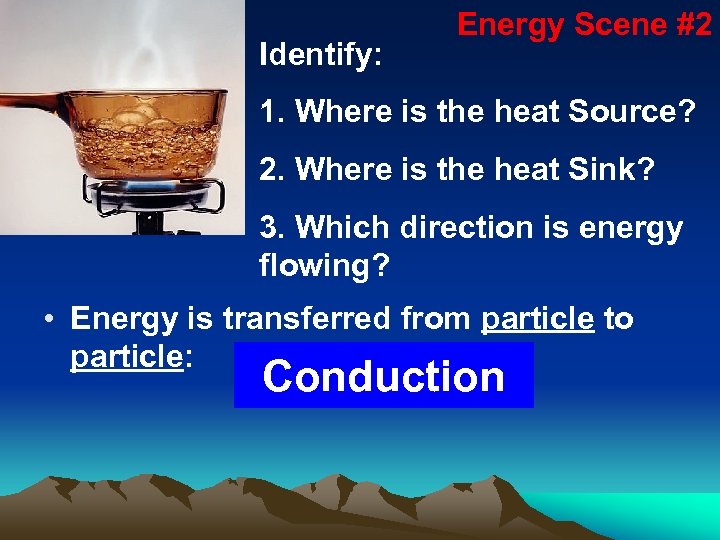 Identify: Energy Scene #2 1. Where is the heat Source? 2. Where is the heat Sink? 3. Which direction is energy flowing? • Energy is transferred from particle to particle: Conduction
Identify: Energy Scene #2 1. Where is the heat Source? 2. Where is the heat Sink? 3. Which direction is energy flowing? • Energy is transferred from particle to particle: Conduction
 Conduction • Most effective in solids, especially metals, since particles are close together Ex. : 1. Getting burnt by touching stove. 2. Melting ice in hand. 3. Fire poker getting hot.
Conduction • Most effective in solids, especially metals, since particles are close together Ex. : 1. Getting burnt by touching stove. 2. Melting ice in hand. 3. Fire poker getting hot.
 Energy Scene #3 Identify: 1. Where’s the heat Source? 2. Where’s the heat Sink? 3. Which direction is energy flowing? • Energy transferred through space or gases: Radiation
Energy Scene #3 Identify: 1. Where’s the heat Source? 2. Where’s the heat Sink? 3. Which direction is energy flowing? • Energy transferred through space or gases: Radiation
 Radiation • Higher an object’s temperature, more energy it radiates Ex. : 1. The Sun 2. Light bulb 3. Radiator • Good radiators = good absorbers
Radiation • Higher an object’s temperature, more energy it radiates Ex. : 1. The Sun 2. Light bulb 3. Radiator • Good radiators = good absorbers
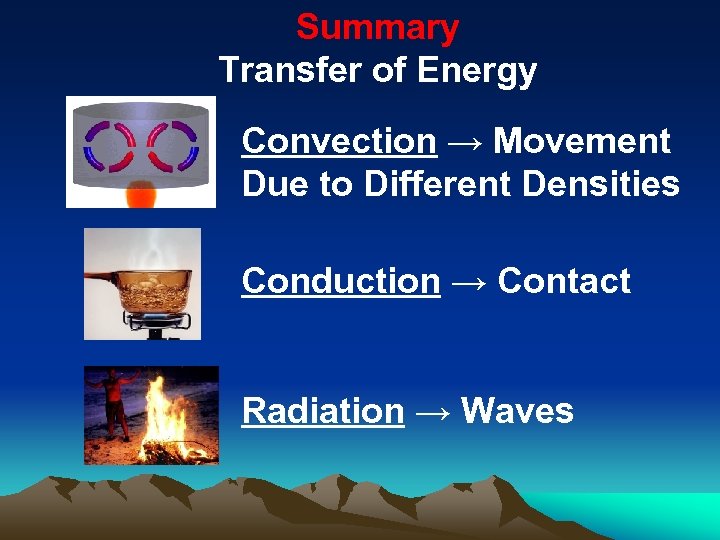 Summary Transfer of Energy Convection → Movement Due to Different Densities Conduction → Contact Radiation → Waves
Summary Transfer of Energy Convection → Movement Due to Different Densities Conduction → Contact Radiation → Waves
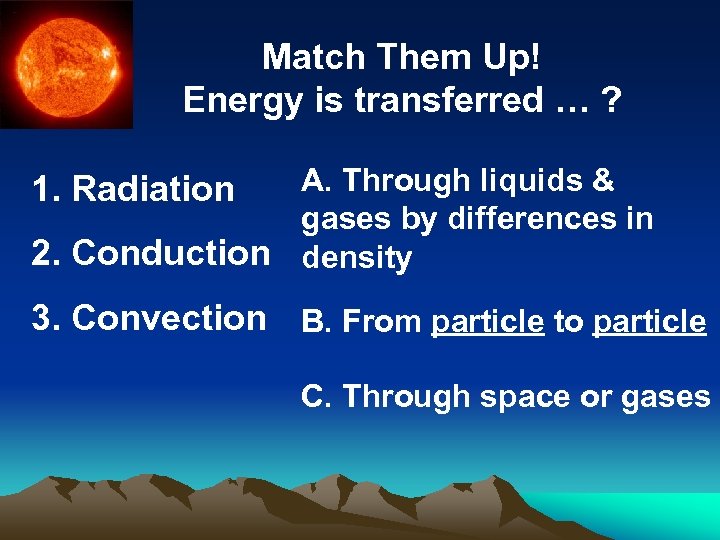 Match Them Up! Energy is transferred … ? A. Through liquids & gases by differences in 2. Conduction density 1. Radiation 3. Convection B. From particle to particle C. Through space or gases
Match Them Up! Energy is transferred … ? A. Through liquids & gases by differences in 2. Conduction density 1. Radiation 3. Convection B. From particle to particle C. Through space or gases

 Question of the Day How is energy transformed? Which is… 1) Radiation 2) Conduction 3) Convection 82
Question of the Day How is energy transformed? Which is… 1) Radiation 2) Conduction 3) Convection 82
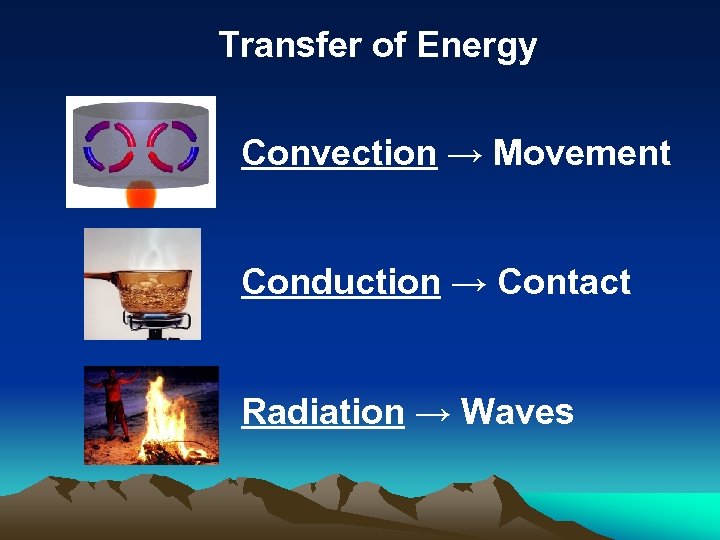 Transfer of Energy Convection → Movement Conduction → Contact Radiation → Waves
Transfer of Energy Convection → Movement Conduction → Contact Radiation → Waves
 Kinetic vs. Potential Energy Kinetic Energy: object in motion • Faster something moves, greater its kinetic energy • More mass = greater kinetic energy
Kinetic vs. Potential Energy Kinetic Energy: object in motion • Faster something moves, greater its kinetic energy • More mass = greater kinetic energy
 Potential Energy: “stored energy” • Related to position or phase • Ex. : higher object is above Earth, greater its potential to fall = greater potential energy. • More mass = more potential energy
Potential Energy: “stored energy” • Related to position or phase • Ex. : higher object is above Earth, greater its potential to fall = greater potential energy. • More mass = more potential energy
 Transformation • Potential energy can be transformed into kinetic • Ex. : Waterfall Water at top - high potential Water falling - some potential energy → some kinetic energy, resulting in an increase in speed.
Transformation • Potential energy can be transformed into kinetic • Ex. : Waterfall Water at top - high potential Water falling - some potential energy → some kinetic energy, resulting in an increase in speed.
 Heat Production Friction • Glaciers – Kinetic energy transformed into heat energy at interface (boundary) between glacier and valley walls.
Heat Production Friction • Glaciers – Kinetic energy transformed into heat energy at interface (boundary) between glacier and valley walls.
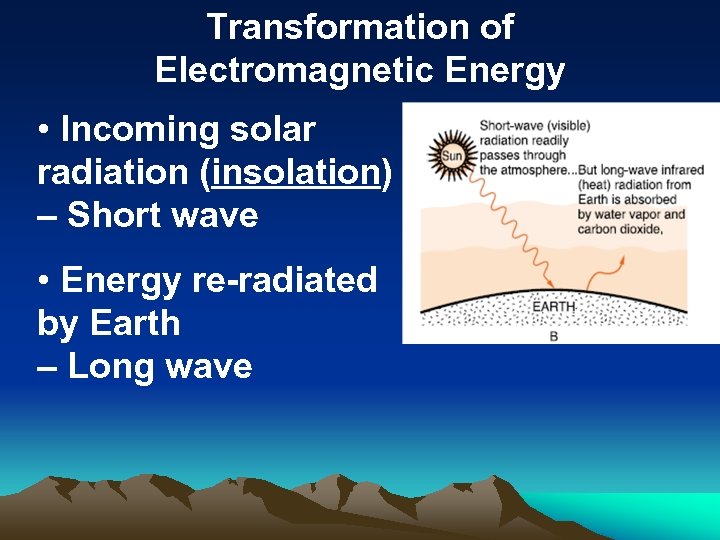 Transformation of Electromagnetic Energy • Incoming solar radiation (insolation) – Short wave • Energy re-radiated by Earth – Long wave
Transformation of Electromagnetic Energy • Incoming solar radiation (insolation) – Short wave • Energy re-radiated by Earth – Long wave
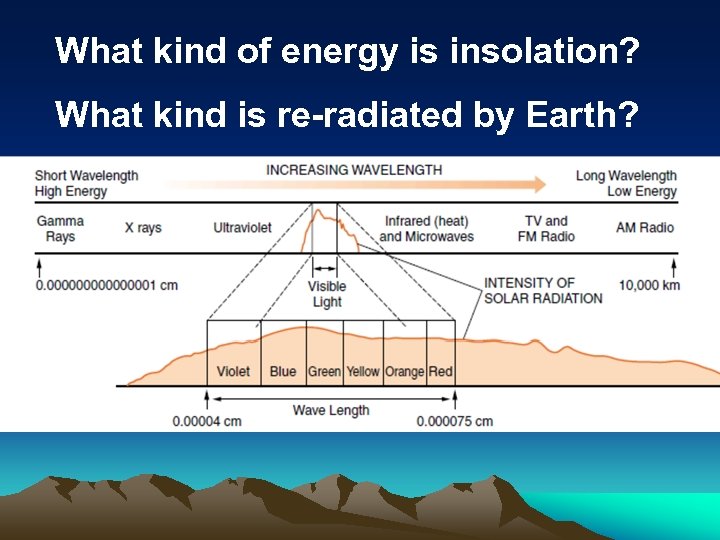 What kind of energy is insolation? What kind is re-radiated by Earth?
What kind of energy is insolation? What kind is re-radiated by Earth?
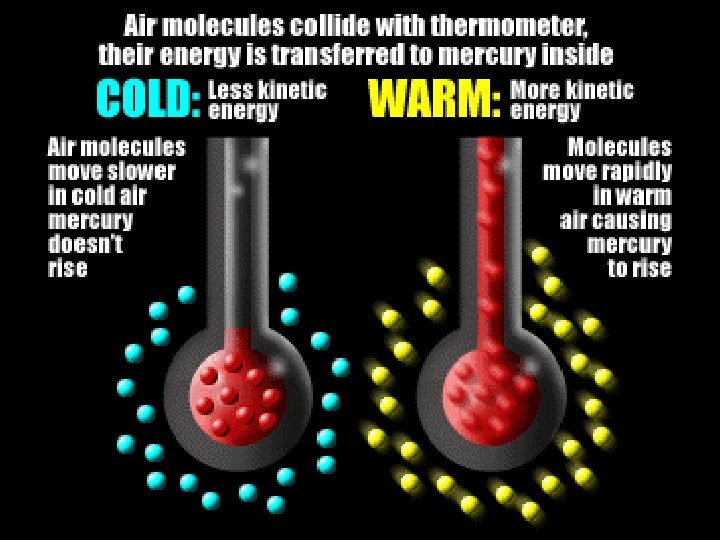
 Temperature: measure of average kinetic energy of particles in matter. • Greater kinetic energy, higher temperature.
Temperature: measure of average kinetic energy of particles in matter. • Greater kinetic energy, higher temperature.
 Heat Energy Heat energy (thermal energy): energy of motion of particles of matter. • Heat is transferred b/c of difference in temperature • Measured in joules: metric unit of energy
Heat Energy Heat energy (thermal energy): energy of motion of particles of matter. • Heat is transferred b/c of difference in temperature • Measured in joules: metric unit of energy
 Conservation of Energy • Energy can neither be created nor destroyed. • Energy can only be transformed from one state to another. • Ex. : potential → kinetic.
Conservation of Energy • Energy can neither be created nor destroyed. • Energy can only be transformed from one state to another. • Ex. : potential → kinetic.
 1. An interface is: a) change in state of environment; b) region below Earth’s surface; c) region with no changes; d) boundary across which energy may be exchanged. 2. Friction at an interface always produces: a) transformation of energy; b) form of pollution; c) chemical change; d) phase change. 3. Electromagnetic energy that reaches Earth from sun is called: a) insolation; b) conduction; c) specific heat; d) terrestrial radiation.
1. An interface is: a) change in state of environment; b) region below Earth’s surface; c) region with no changes; d) boundary across which energy may be exchanged. 2. Friction at an interface always produces: a) transformation of energy; b) form of pollution; c) chemical change; d) phase change. 3. Electromagnetic energy that reaches Earth from sun is called: a) insolation; b) conduction; c) specific heat; d) terrestrial radiation.

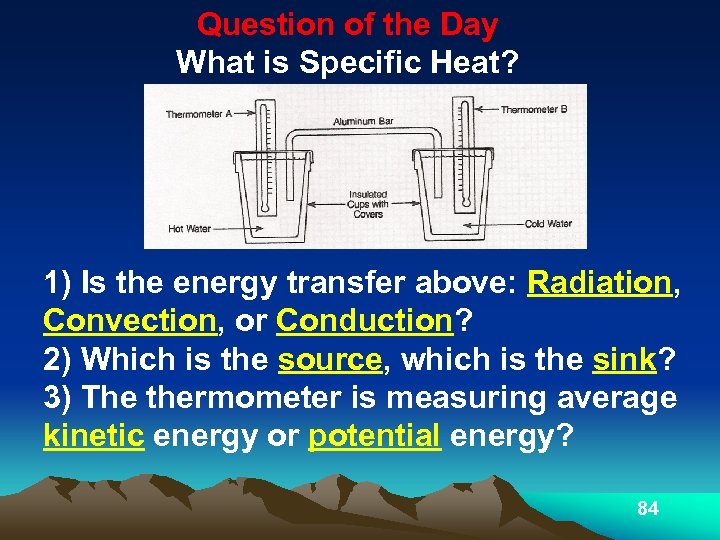 Question of the Day What is Specific Heat? 1) Is the energy transfer above: Radiation, Convection, or Conduction? 2) Which is the source, which is the sink? 3) The thermometer is measuring average kinetic energy or potential energy? 84
Question of the Day What is Specific Heat? 1) Is the energy transfer above: Radiation, Convection, or Conduction? 2) Which is the source, which is the sink? 3) The thermometer is measuring average kinetic energy or potential energy? 84
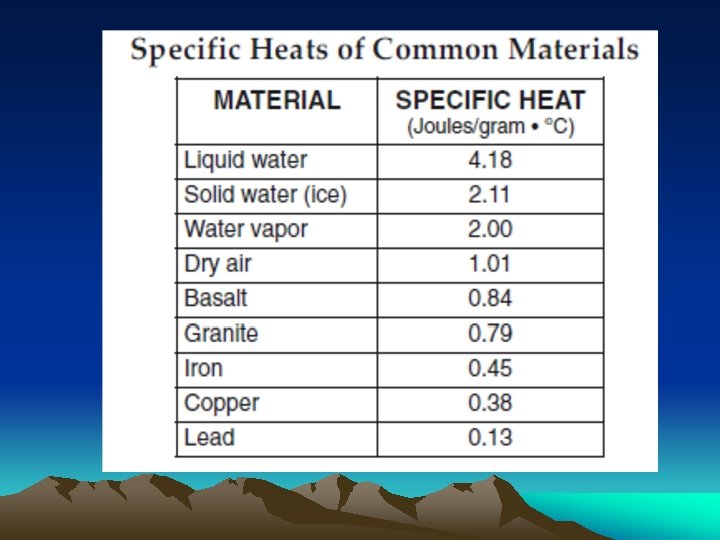
 What is Specific Heat? • Quantity of heat needed to raise temp. of 1 gm. of any substance 1º C. • Resistance to heating up or cooling off. High Specific Heat Low Specific Heat Need More Energy Need Less Energy
What is Specific Heat? • Quantity of heat needed to raise temp. of 1 gm. of any substance 1º C. • Resistance to heating up or cooling off. High Specific Heat Low Specific Heat Need More Energy Need Less Energy
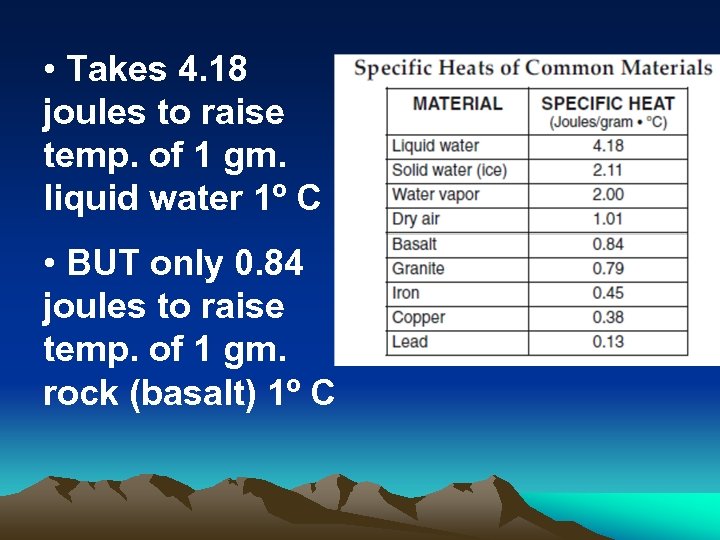 • Takes 4. 18 joules to raise temp. of 1 gm. liquid water 1º C • BUT only 0. 84 joules to raise temp. of 1 gm. rock (basalt) 1º C
• Takes 4. 18 joules to raise temp. of 1 gm. liquid water 1º C • BUT only 0. 84 joules to raise temp. of 1 gm. rock (basalt) 1º C
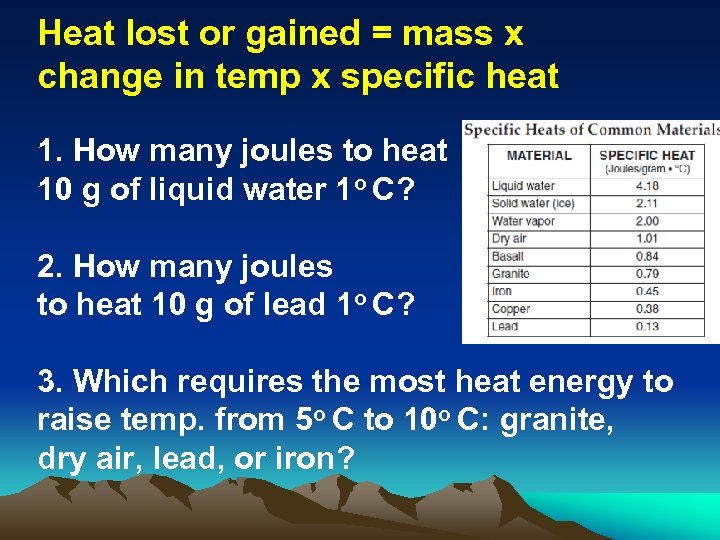 Heat lost or gained = mass x change in temp x specific heat 1. How many joules to heat 10 g of liquid water 1 o C? 2. How many joules to heat 10 g of lead 1 o C? 3. Which requires the most heat energy to raise temp. from 5 o C to 10 o C: granite, dry air, lead, or iron?
Heat lost or gained = mass x change in temp x specific heat 1. How many joules to heat 10 g of liquid water 1 o C? 2. How many joules to heat 10 g of lead 1 o C? 3. Which requires the most heat energy to raise temp. from 5 o C to 10 o C: granite, dry air, lead, or iron?