8b3e08da45c744c5c59525d5d1635032.ppt
- Количество слайдов: 17


New Unit—Atoms, their structure and the Periodic Table Announcements: Note: It is your responsibility to check to see that the grade you have matches the grades I post. Also, those who missed the test must take it today!! Do you think I can create water that is invisible? Copy this assignment!! Read page 73 -78 Do 1 -6 page 78

Goals for today l To understand the concept of using models to represent something so small it can’t be seen. l To explore the early models of the atom. l Understand how new discoveries helped to change and refine the model.

Chapter 3: Atoms and their structure. Section 1 - Atoms

Early models of the atom - 400 BC – Democritus of Abdera - Suggested that all matter was made of indivisible units called “atoma” Atoma in greek means “indivisible”

Things began to change As science progressed, we began to experiment “better”. We had small amounts of electric current and knowledge was spreading. Two scientists, Priestly and Lavoisier, did experiments to help another scientist—John Dalton to “put it together”. They provided the insights for Dalton to “play with”. Dalton thought this was entertaining. (scientists can sure have fun in weird ways can’t they? ) Let’s look into Daltons Playhouse
Priestly demonstrates through experimentation what we cannot see with our eyes! The Law of Conservation of Mass Lavoisier demonstrates through experimentation, what we cannot see with our eyes. the Law of Definite Proportions!! Any sample of a compound is in the same proportion by mass. They also have the same formula no matter where or how you got the sample! Ex. C 6 H 12 O 6 Similar is the Law of Multiple Proportions Ex NO vs NO 2 When a formula two different substances are made of the same elements, they change by whole number multiples of the elements.

But scientists did not know these things……. they were struggling to figure them out!! Enter John Dalton to take the research and put together the early story.

Early models of the atom - 1766 -1844 - John Dalton - Dalton’s atomic theory: 1) All elements are composed of tiny indivisible particles called atoms 2) Atoms of the same element are identical. The atoms of any one element are different from those of any other elements.
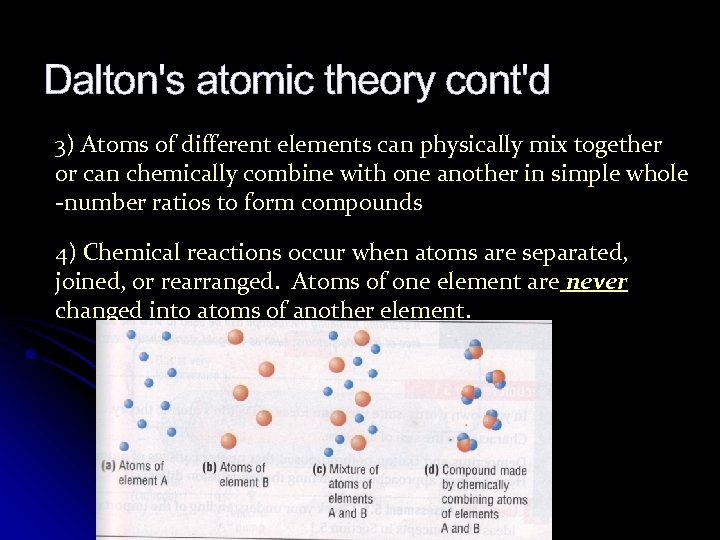
Dalton's atomic theory cont'd 3) Atoms of different elements can physically mix together or can chemically combine with one another in simple whole -number ratios to form compounds 4) Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element are never changed into atoms of another element.

How Small is an atom? - Atom – smallest particle of an element that retains the properties of that element - A pure copper coin contains 2. 4 x 1022 atoms - There are 6 x 109 people on earth

What can you see atoms with?
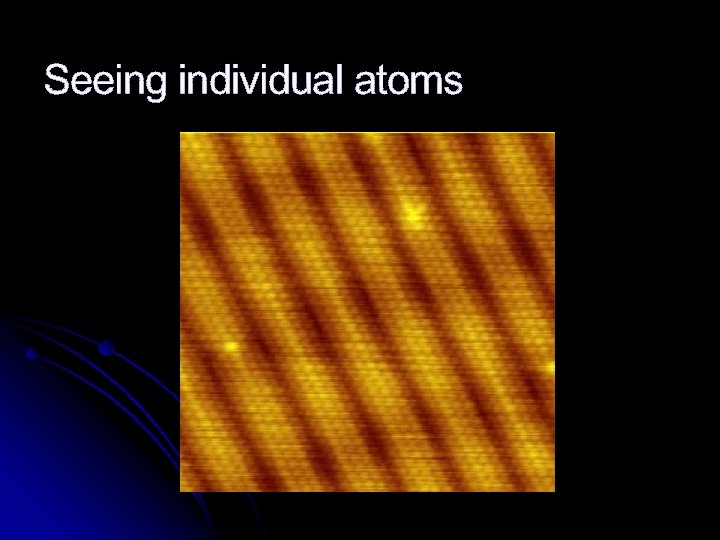
Seeing individual atoms

End

And now…. Without further ado……. An amazing fireman trick…. The “Invisible Water”!!!!!! Yes ladies and gentleman we have a The Great entertain us !!! famous magician to Leggioni today!! Then who? Who ? Is it…. . Penn and Teller? Who else? its…. . David Copperfield? No!! Doug Henning? No!!

Homework review--quick

e Pleas his t study How to create equalities for conversions Divide!! How do centigrams and milligrams relate? Centi means “a hundredth” and milli means a “thousandth” Drop the “th” and there a hundred centigrams in a gram and a thousand milligrams in a gram. How do those relate? this ually rk Act wo ould w too Convert 357 milligrams to centigrams. 100 cg = 1000 mg now reduce (divide by 100) 1 cg = 10 mg Make the equality 1 cg = 10 mg Make the conversions 1 cg = 10 mg 1 cg Solve: 357 mg X 1 cg = 35. 7 cg 1 10 mg
8b3e08da45c744c5c59525d5d1635032.ppt