ac6f5109bb7bf2ba281ccbae9fe7d7b2.ppt
- Количество слайдов: 59

New Technologies and Innovations in Laboratory Support of HIV/AIDS care and Treatment Dr. Thaweesak Tirawatnapong Chula Medical Research Center (Chula MRC)

Diagnostics Tests for HIV • 1 Tests to facilitate initial diagnosis • 2 Tests to stage the patients • 3 Tests to monitor the patients

Important of HIV Testing • Known HIV status for care and treatment • For prevention measure • Problem • Poor HIV testing coverage in resource- limited Setting

General Community Requirement • Need to increase the level of access to high quality diagnostic in resource-limited settings • To facilitate early detection and treatment of HIV/AIDS • Simplify and improve the efficiency of HIV testing • Tests design to be delivered at The point of care (POC) or Near POC
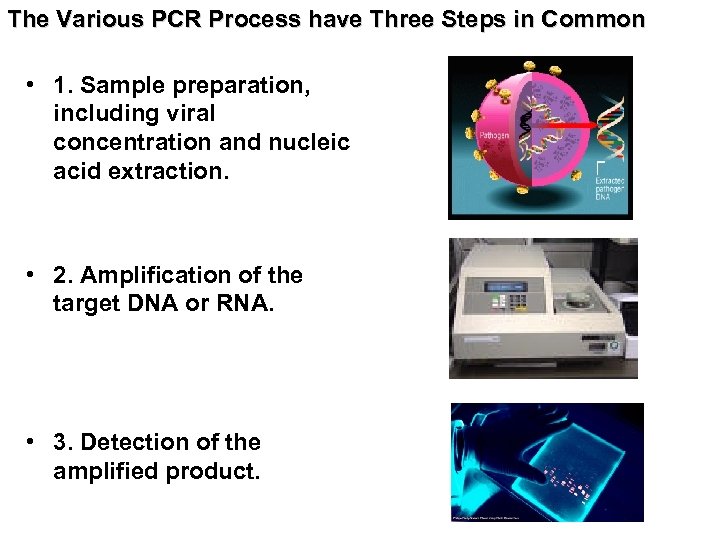
The Various PCR Process have Three Steps in Common • 1. Sample preparation, including viral concentration and nucleic acid extraction. • 2. Amplification of the target DNA or RNA. • 3. Detection of the amplified product.
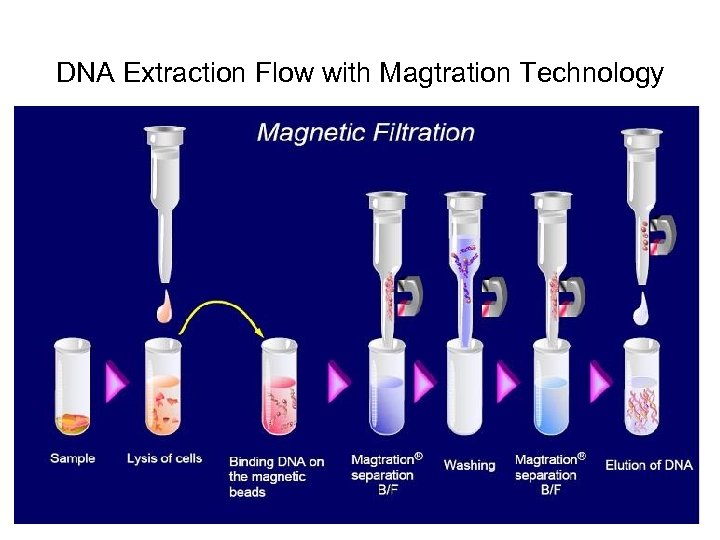
DNA Extraction Flow with Magtration Technology

Automated Nucleic Acid Extraction

Extraction and PCR Set Up Cobas Ampriprep Qiagen Symphony

COBAS® S 201 System for Blood screening
Chiron Tigris for Blood screening

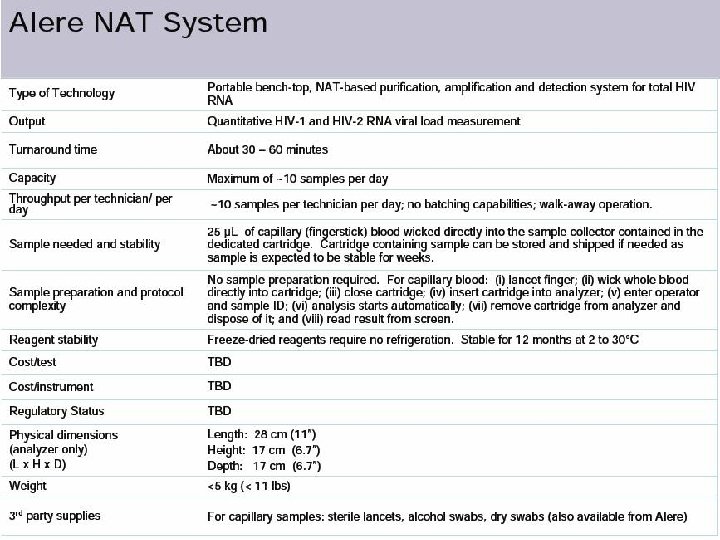
New Technologies for Viral Load Testing • Alere NAT system disposable cartridge that provides for sample collection, cell lysis, specific target capture, reverse transcription, polymerase chain reaction amplification and real time fluorescence detection based on reporter probe hybridization on an integrated micro probe array.
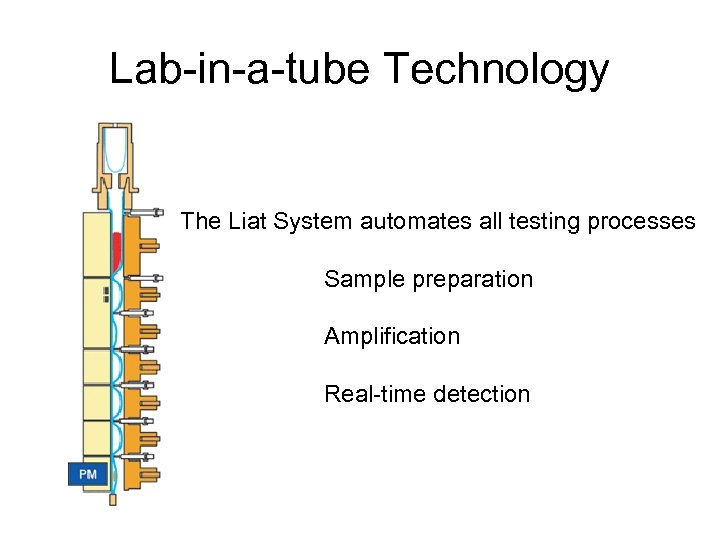
Lab-in-a-tube Technology The Liat System automates all testing processes Sample preparation Amplification Real-time detection

Portable Fully Automate real-time PCR
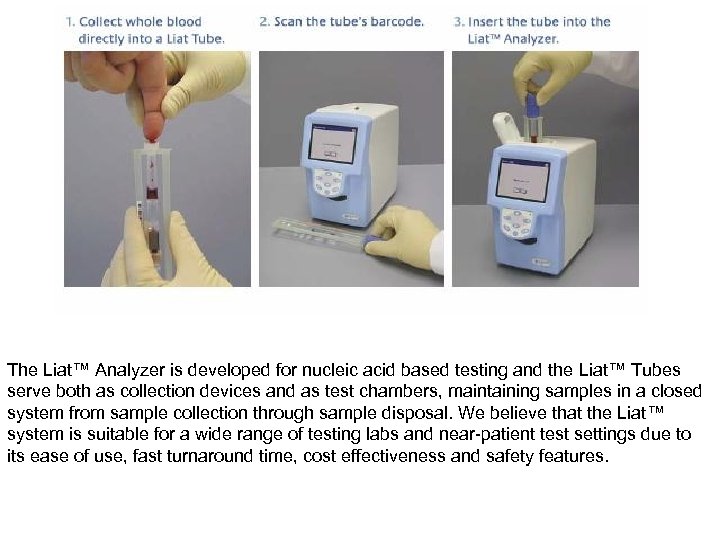
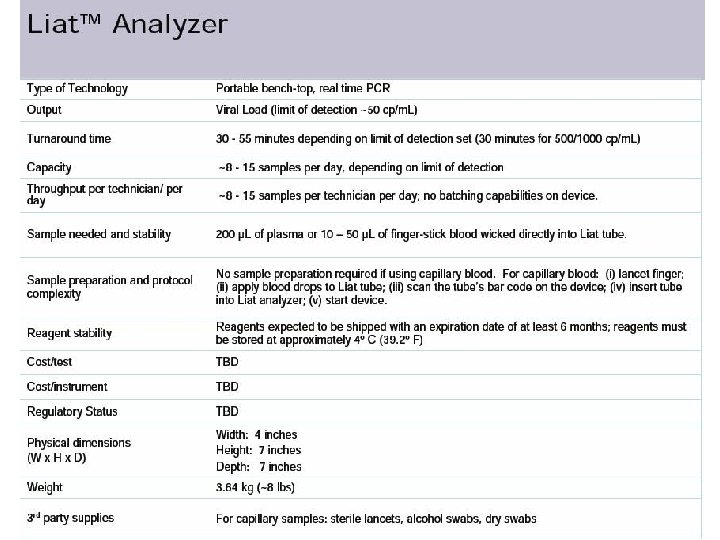
The Liat™ Analyzer is developed for nucleic acid based testing and the Liat™ Tubes serve both as collection devices and as test chambers, maintaining samples in a closed system from sample collection through sample disposal. We believe that the Liat™ system is suitable for a wide range of testing labs and near-patient test settings due to its ease of use, fast turnaround time, cost effectiveness and safety features.
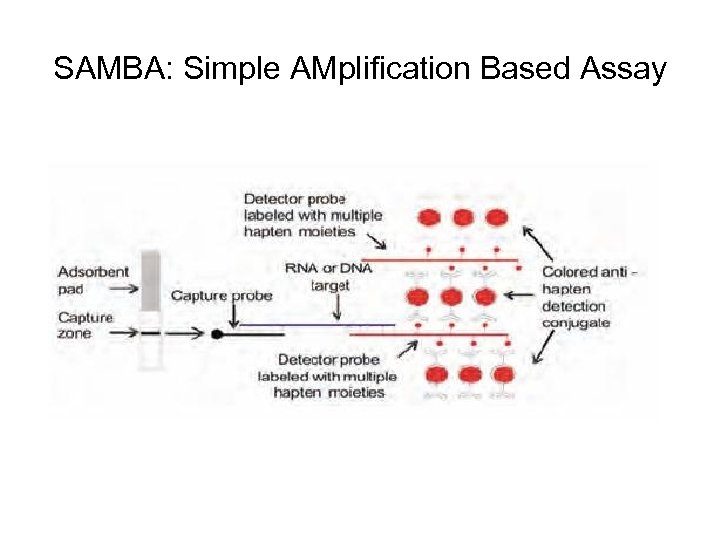
SAMBA: Simple AMplification Based Assay


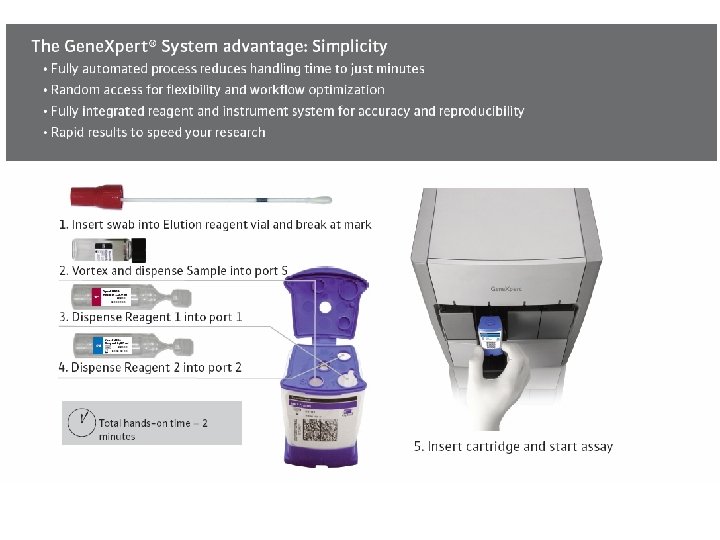
Cepheid Gene. Xpert System

Gene. Xpert Cartridge and Module
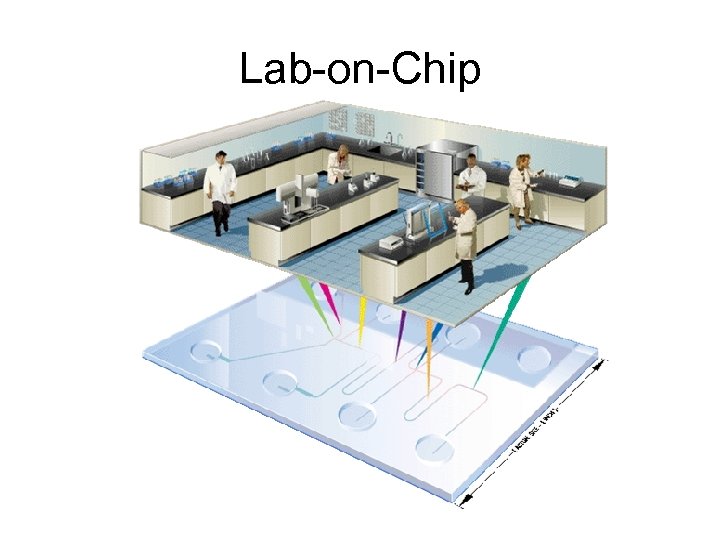
Lab-on-Chip



Handy Lab
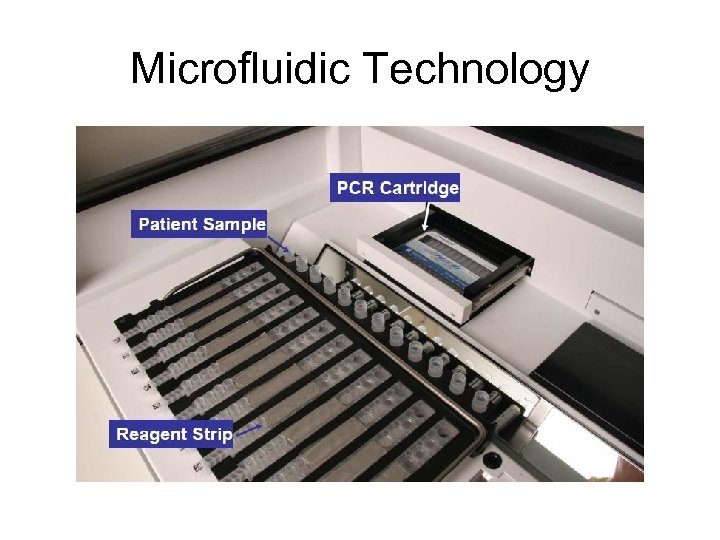
Microfluidic Technology

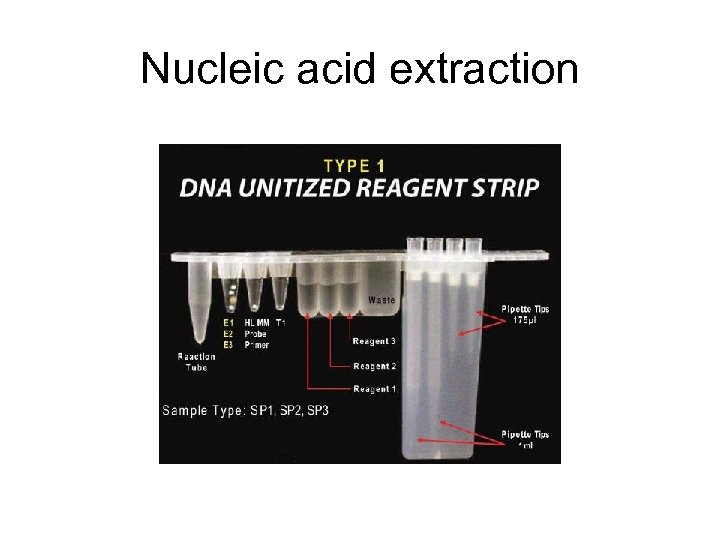
Nucleic acid extraction

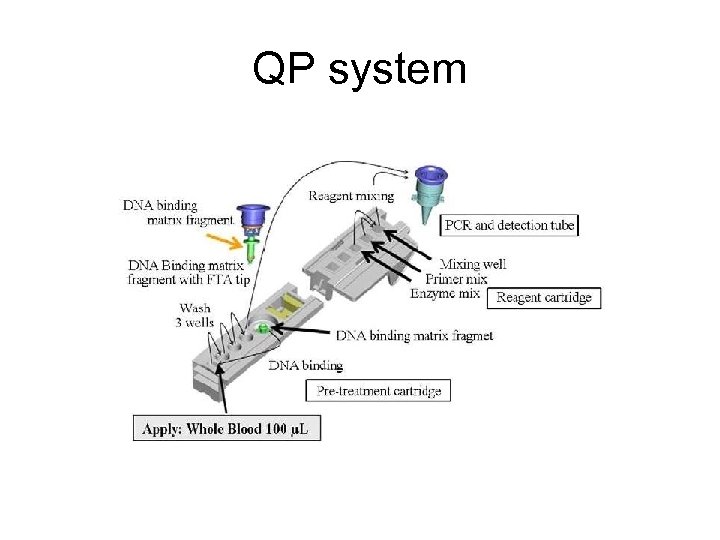
QP system



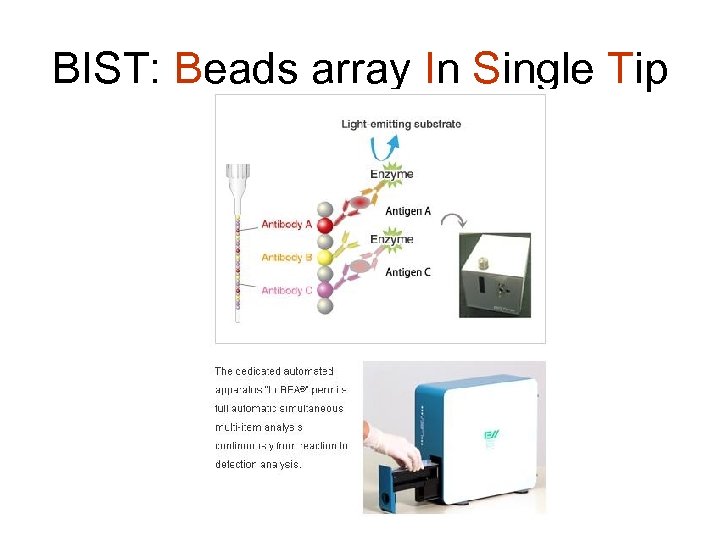
BIST: Beads array In Single Tip

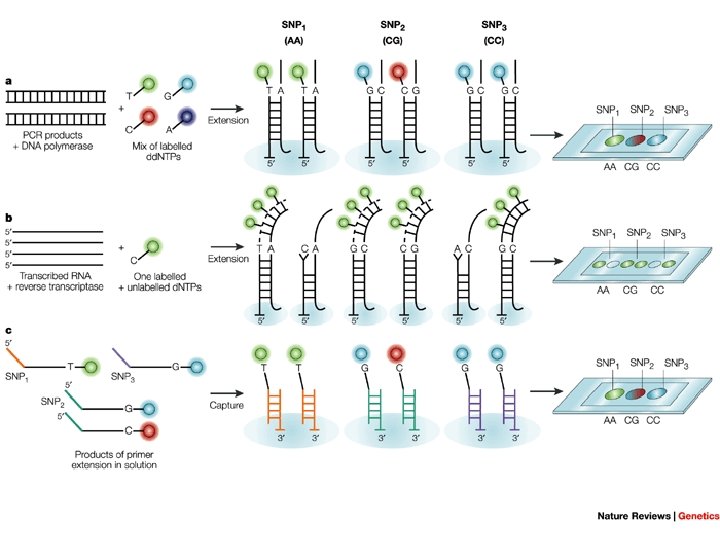
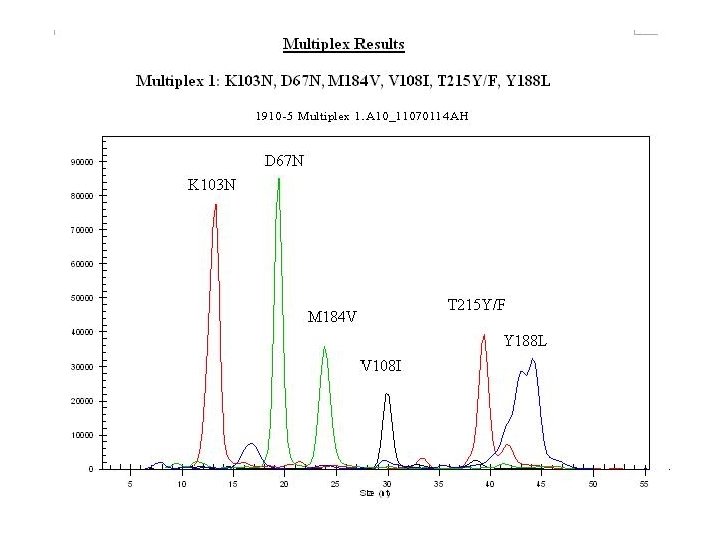
New HIV Drug resistant testing • Minisequencing or Single base extension
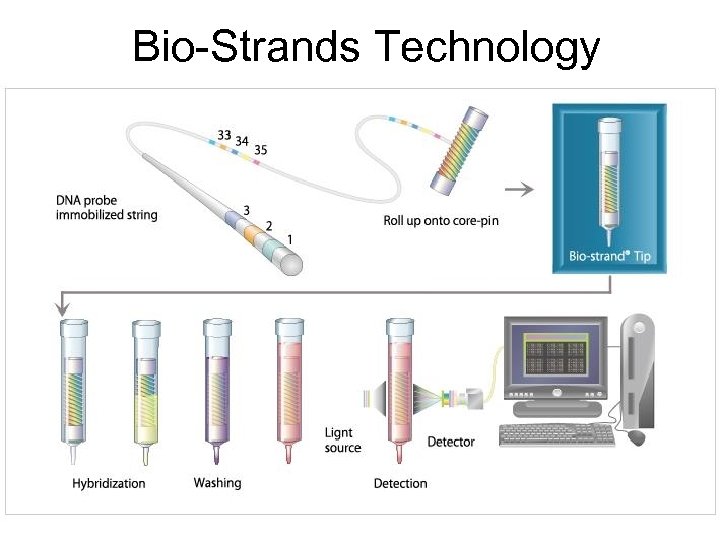
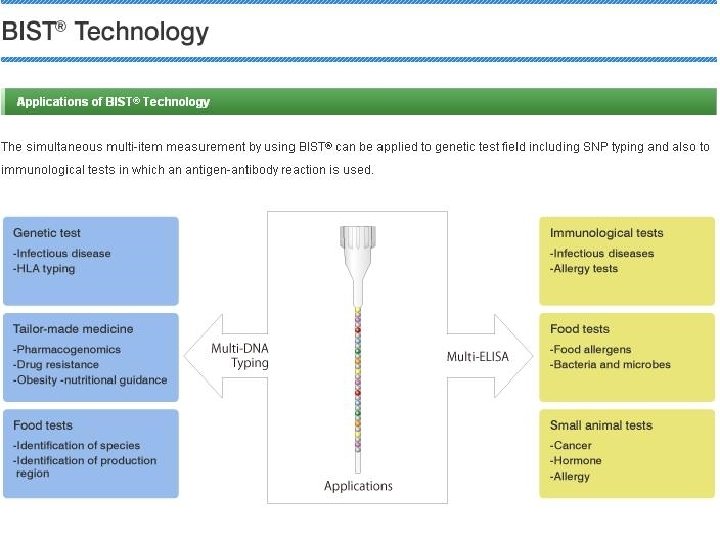
Bio-Strands Technology
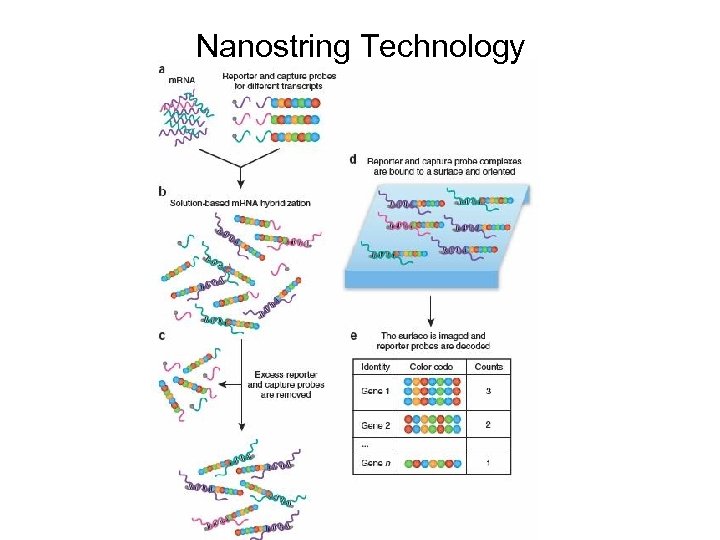
Nanostring Technology
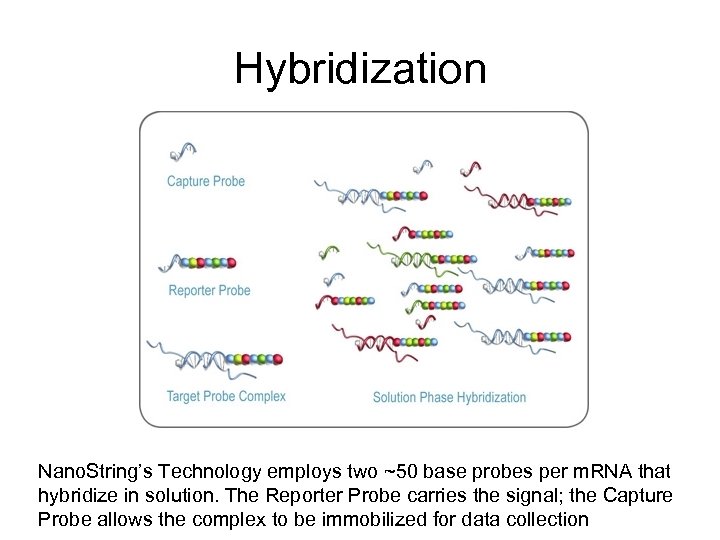
Hybridization Nano. String’s Technology employs two ~50 base probes per m. RNA that hybridize in solution. The Reporter Probe carries the signal; the Capture Probe allows the complex to be immobilized for data collection
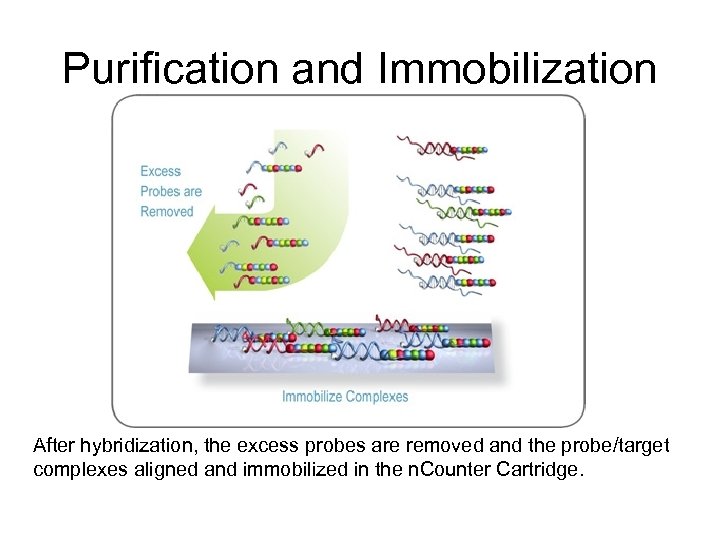
Purification and Immobilization After hybridization, the excess probes are removed and the probe/target complexes aligned and immobilized in the n. Counter Cartridge.
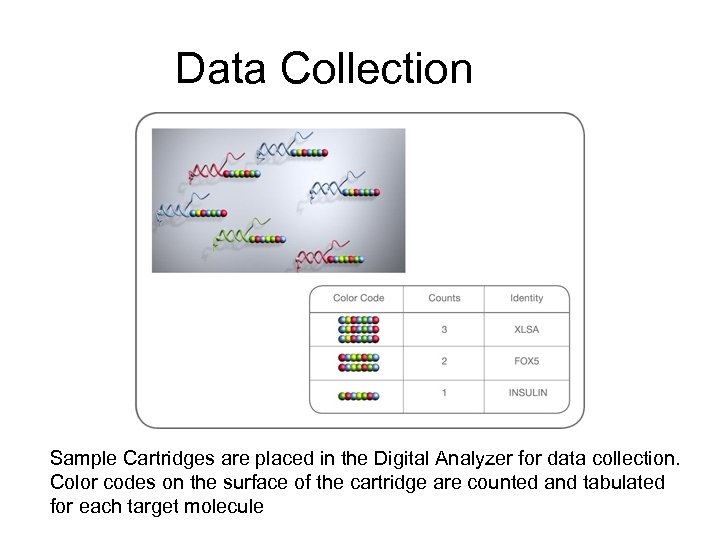
Data Collection Sample Cartridges are placed in the Digital Analyzer for data collection. Color codes on the surface of the cartridge are counted and tabulated for each target molecule
n. Counter Analysis System • • High level of precision High level of sensitivity (<1 copy per cell) No enzyme processing or Amplification Quantification

Multiplexed Diagnostic • LLNL Awarded $2. 5 M to Move Pathogen Detection Tests to Nano. String Platform • June 07, 2011 • The National Institutes of Health has awarded $2. 5 million to researchers at Lawrence Livermore National Laboratory to support the development of pathogen detection assays on Nano. String Technologies' n. Counter system. • The project aims to develop high-content, multiplex assays capable of simultaneously detecting 35 National Institute of Allergy and Infectious Diseases category A, B, and C viral agents, plus quantifying cytokine and chemokine responses in patients,
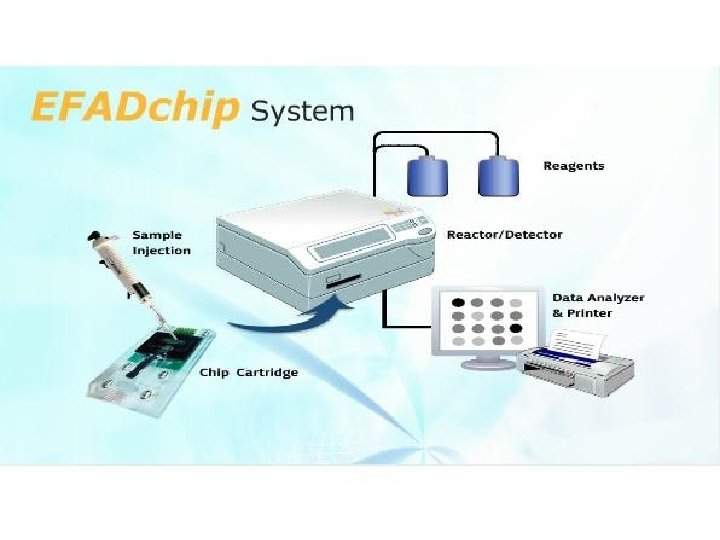
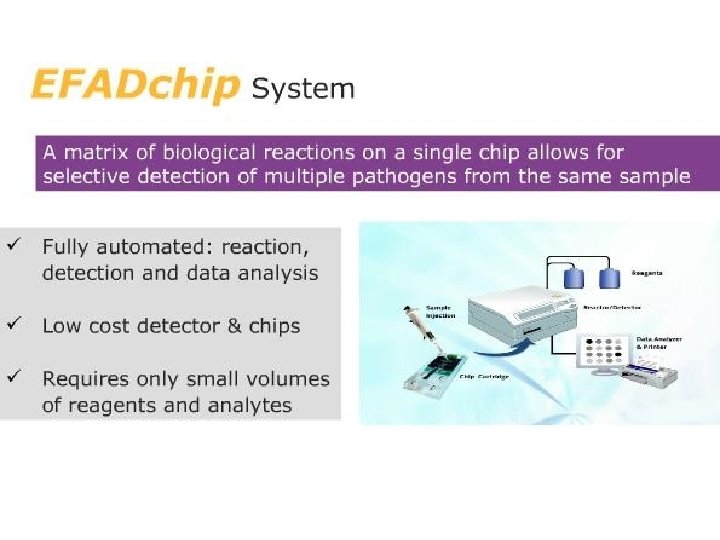
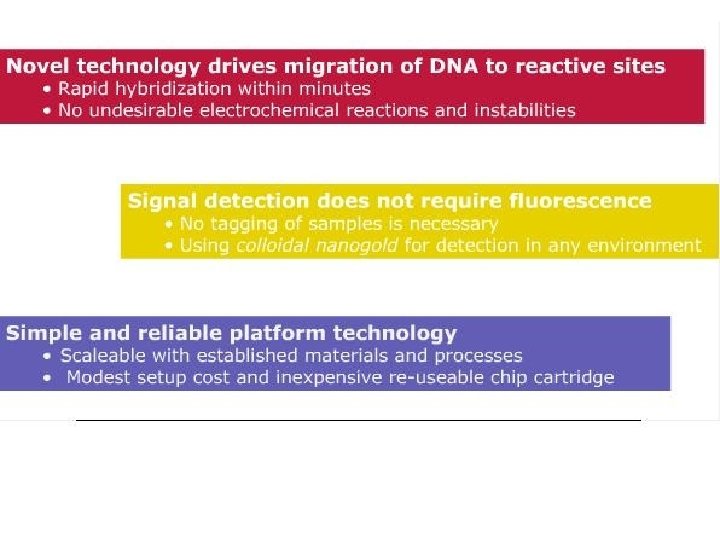
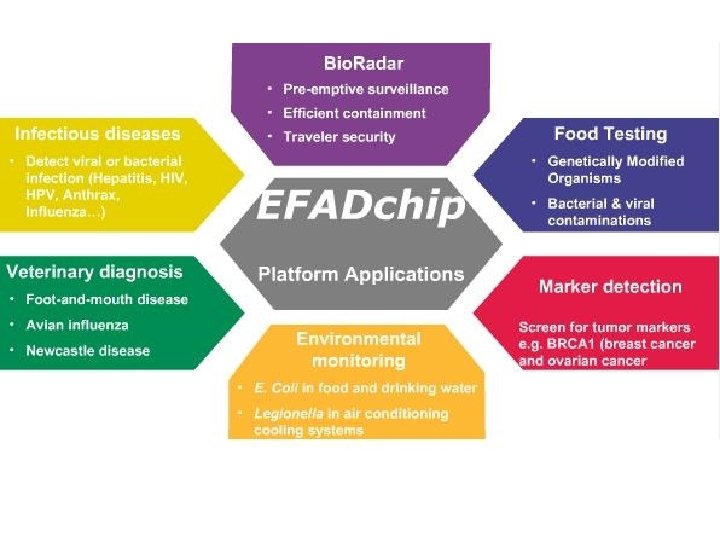

• Science 24 June 2011: Vol. 332 no. 6037 p. 1497 • BIOTECHNOLOGY Lab-on-a-Chip Maker Looks to Put Hong Kong on Biotech Map

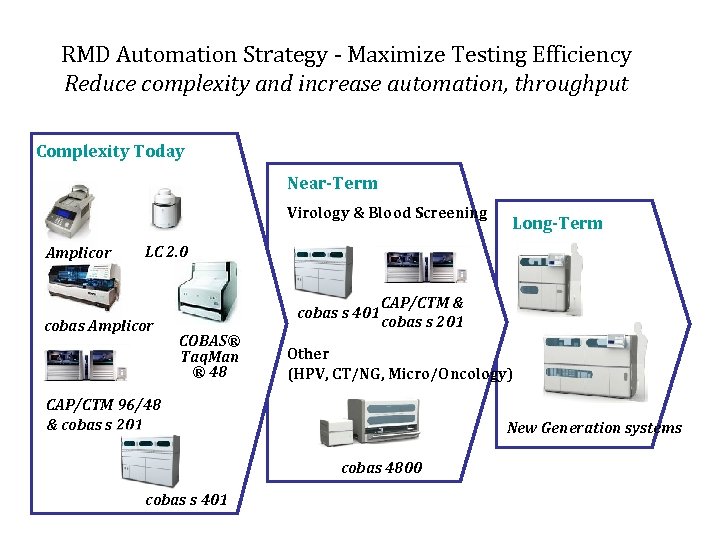
RMD Automation Strategy - Maximize Testing Efficiency Reduce complexity and increase automation, throughput Complexity Today Near-Term Virology & Blood Screening Amplicor Long-Term LC 2. 0 cobas Amplicor cobas s 401 COBAS® Taq. Man ® 48 CAP/CTM & cobas s 201 Other (HPV, CT/NG, Micro/Oncology) CAP/CTM 96/48 & cobas s 201 New Generation systems cobas 4800 cobas s 401

ac6f5109bb7bf2ba281ccbae9fe7d7b2.ppt