75dd59cf6e16e32ccabf2ae745d06562.ppt
- Количество слайдов: 19
 New stent technology Magnesium-alloy The Progress-AMS Study • • Raimund Erbel, M Haude, Th Konorza, D Boese Department of Cardiology West-German Heart Center Essen University Duisburg-Essen www. wdhz. de erbel@uk-essen. de Erbel et al. , Lancet 2007; 369(9576): 1869 -75
New stent technology Magnesium-alloy The Progress-AMS Study • • Raimund Erbel, M Haude, Th Konorza, D Boese Department of Cardiology West-German Heart Center Essen University Duisburg-Essen www. wdhz. de erbel@uk-essen. de Erbel et al. , Lancet 2007; 369(9576): 1869 -75
 Clinical Performance and Angiographic Results of the Coronary Stenting with Absorbable Metal Stents The PROGRESS-AMS Study • Purpose • Design • Hypotheses • To evaluate the clinical feasibility of an absorbable metal stent in the treatment of a single de novo lesion in a native coronary artery • Prospective, multi-center, consecutive, nonrandomized FIM (First In Man – coronary) study MACE rate after 4 months <30 % comparable to BMS Erbel et al. , Lancet 2007; 369(9576): 1869 -75
Clinical Performance and Angiographic Results of the Coronary Stenting with Absorbable Metal Stents The PROGRESS-AMS Study • Purpose • Design • Hypotheses • To evaluate the clinical feasibility of an absorbable metal stent in the treatment of a single de novo lesion in a native coronary artery • Prospective, multi-center, consecutive, nonrandomized FIM (First In Man – coronary) study MACE rate after 4 months <30 % comparable to BMS Erbel et al. , Lancet 2007; 369(9576): 1869 -75
 PROGRESS STUDY Principal Investigator Raimund Erbel, MD, Essen, Germany Co-Chairman Ron Waksman, MD, Washington, USA Steering Committee Raimund Erbel, MD, Essen, Germany Ron Waksman, MD, Washington, USA Bernd Heublein † , MD, Hannover, Germany CEC & DSMB IVUS Core laboratory Jan Bart Hak, Ph. D, Groningen, NL Martial Hamon, MD, Caen, France Rafael Beyar, MD, Haifa, Israel Neil J. Weissman, MD, Washington, USA QCA Core laboratory Cardialysis, Rotterdam, The Netherlands Data Coordinating Ron Waksman, MD, Washington, USA Study Coordination Stefan Wagner, Ph. D, Erlangen, Germany Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS STUDY Principal Investigator Raimund Erbel, MD, Essen, Germany Co-Chairman Ron Waksman, MD, Washington, USA Steering Committee Raimund Erbel, MD, Essen, Germany Ron Waksman, MD, Washington, USA Bernd Heublein † , MD, Hannover, Germany CEC & DSMB IVUS Core laboratory Jan Bart Hak, Ph. D, Groningen, NL Martial Hamon, MD, Caen, France Rafael Beyar, MD, Haifa, Israel Neil J. Weissman, MD, Washington, USA QCA Core laboratory Cardialysis, Rotterdam, The Netherlands Data Coordinating Ron Waksman, MD, Washington, USA Study Coordination Stefan Wagner, Ph. D, Erlangen, Germany Erbel et al. , Lancet 2007; 369(9576): 1869 -75
 PROGRESS STUDY Australia M Horrigan, Melbourne, AUS Belgium B de Bruyne & W Wijns, Aalst, BE Germany M Haude, S Sack, D Boese, R Erbel, DE Netherlands JJRM Bonnier & J Koolen, Eindhoven Switzerland F Eberli & T Lüscher, Zurich, CH P Erne, Luzern, CH UK C Di Mario & C Ilsley, London, UK USA R Waksman, Washington, USA Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS STUDY Australia M Horrigan, Melbourne, AUS Belgium B de Bruyne & W Wijns, Aalst, BE Germany M Haude, S Sack, D Boese, R Erbel, DE Netherlands JJRM Bonnier & J Koolen, Eindhoven Switzerland F Eberli & T Lüscher, Zurich, CH P Erne, Luzern, CH UK C Di Mario & C Ilsley, London, UK USA R Waksman, Washington, USA Erbel et al. , Lancet 2007; 369(9576): 1869 -75
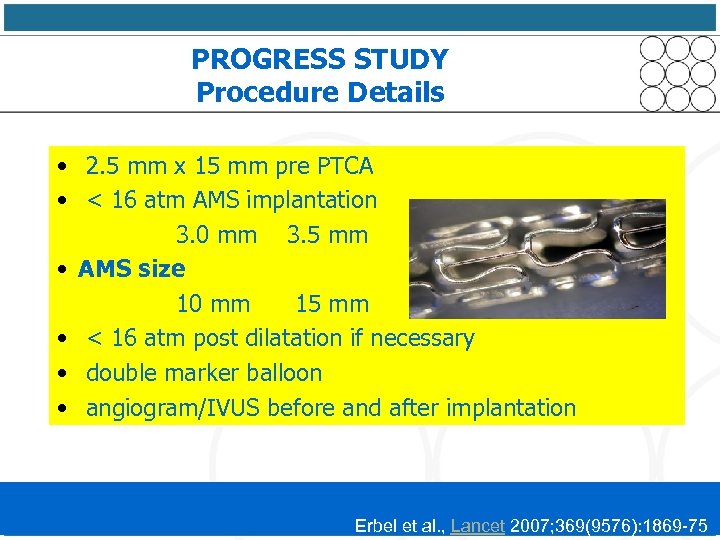 PROGRESS STUDY Procedure Details • 2. 5 mm x 15 mm pre PTCA • < 16 atm AMS implantation 3. 0 mm 3. 5 mm • AMS size 10 mm 15 mm • < 16 atm post dilatation if necessary • double marker balloon • angiogram/IVUS before and after implantation Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS STUDY Procedure Details • 2. 5 mm x 15 mm pre PTCA • < 16 atm AMS implantation 3. 0 mm 3. 5 mm • AMS size 10 mm 15 mm • < 16 atm post dilatation if necessary • double marker balloon • angiogram/IVUS before and after implantation Erbel et al. , Lancet 2007; 369(9576): 1869 -75
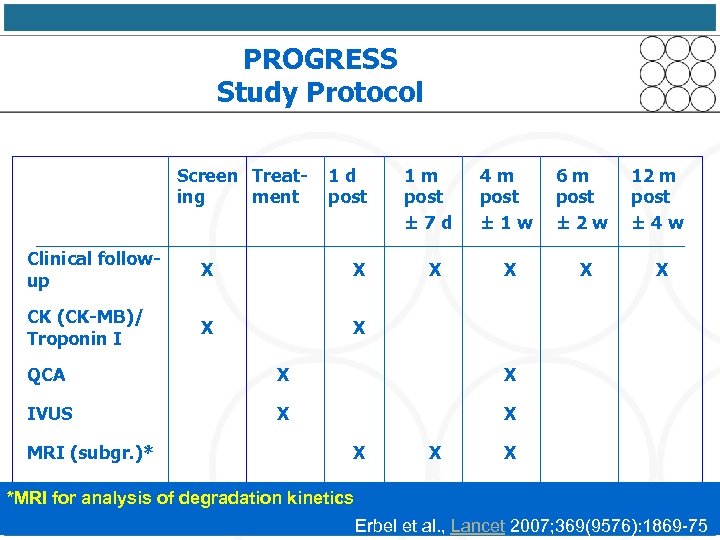 PROGRESS Study Protocol Screen Treating ment 1 d post Clinical followup X X CK (CK-MB)/ Troponin I X 1 m post ± 7 d 4 m post ± 1 w 6 m post ± 2 w 12 m post ± 4 w X X X QCA X X IVUS X X MRI (subgr. )* X X X *MRI for analysis of degradation kinetics Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS Study Protocol Screen Treating ment 1 d post Clinical followup X X CK (CK-MB)/ Troponin I X 1 m post ± 7 d 4 m post ± 1 w 6 m post ± 2 w 12 m post ± 4 w X X X QCA X X IVUS X X MRI (subgr. )* X X X *MRI for analysis of degradation kinetics Erbel et al. , Lancet 2007; 369(9576): 1869 -75
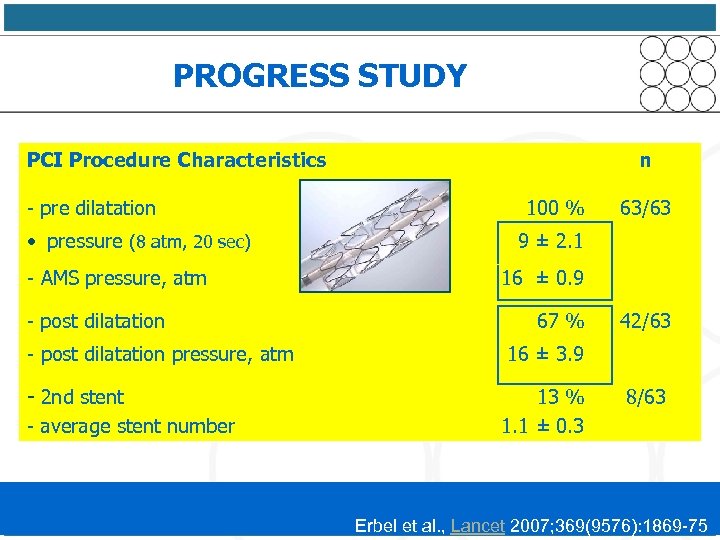 PROGRESS STUDY PCI Procedure Characteristics n - pre dilatation 100 % • pressure (8 atm, 20 sec) 63/63 9 ± 2. 1 - AMS pressure, atm 16 ± 0. 9 - post dilatation 67 % 42/63 - post dilatation pressure, atm 16 ± 3. 9 - 2 nd stent - average stent number 13 % 8/63 1. 1 ± 0. 3 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS STUDY PCI Procedure Characteristics n - pre dilatation 100 % • pressure (8 atm, 20 sec) 63/63 9 ± 2. 1 - AMS pressure, atm 16 ± 0. 9 - post dilatation 67 % 42/63 - post dilatation pressure, atm 16 ± 3. 9 - 2 nd stent - average stent number 13 % 8/63 1. 1 ± 0. 3 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
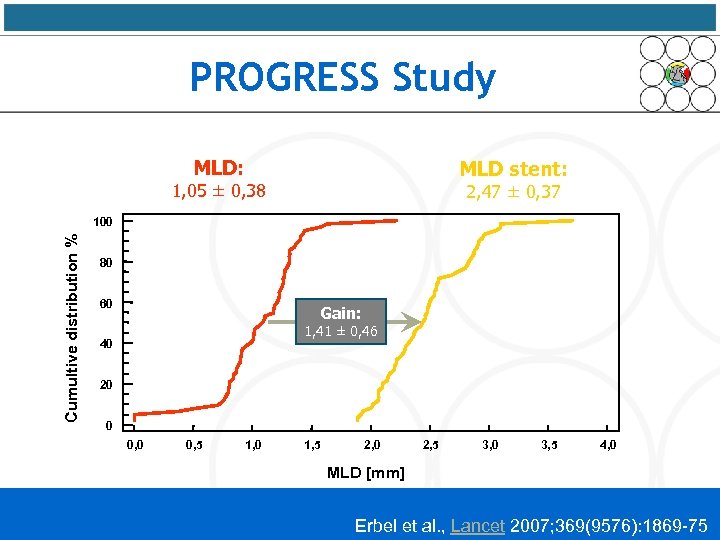 PROGRESS Study MLD: MLD stent: 1, 05 ± 0, 38 2, 47 ± 0, 37 Cumultive distribution % 100 80 60 Gain: 1, 41 ± 0, 46 40 20 0 0, 5 1, 0 1, 5 2, 0 2, 5 3, 0 3, 5 4, 0 MLD [mm] Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS Study MLD: MLD stent: 1, 05 ± 0, 38 2, 47 ± 0, 37 Cumultive distribution % 100 80 60 Gain: 1, 41 ± 0, 46 40 20 0 0, 5 1, 0 1, 5 2, 0 2, 5 3, 0 3, 5 4, 0 MLD [mm] Erbel et al. , Lancet 2007; 369(9576): 1869 -75
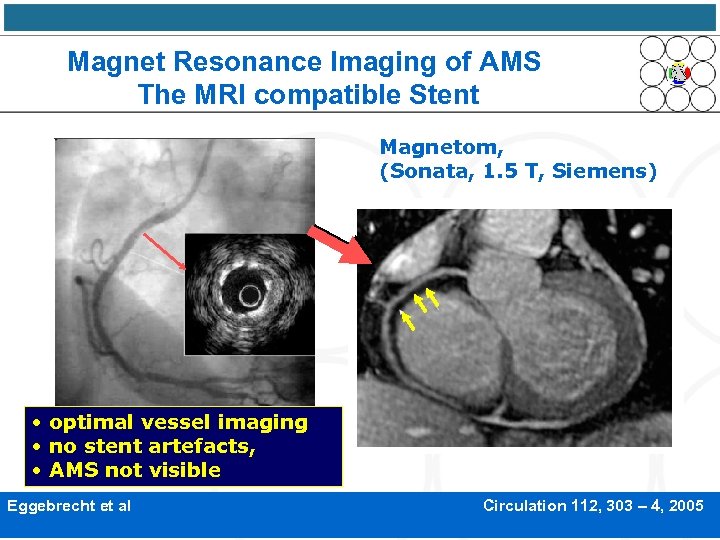 Magnet Resonance Imaging of AMS The MRI compatible Stent Magnetom, (Sonata, 1. 5 T, Siemens) • optimal vessel imaging • no stent artefacts, • AMS not visible Eggebrecht et al Circulation 112, 303 – 4, 2005
Magnet Resonance Imaging of AMS The MRI compatible Stent Magnetom, (Sonata, 1. 5 T, Siemens) • optimal vessel imaging • no stent artefacts, • AMS not visible Eggebrecht et al Circulation 112, 303 – 4, 2005
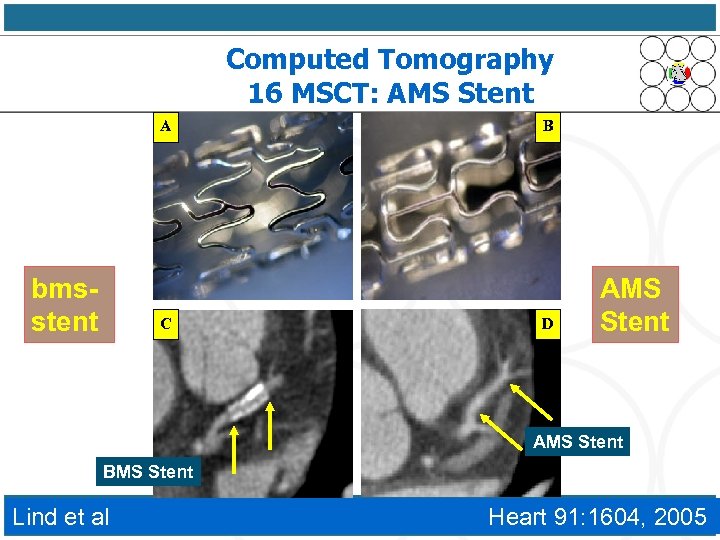 Computed Tomography 16 MSCT: AMS Stent A bmsstent C B D AMS Stent BMS Stent Lind et al Heart 91: 1604, 2005
Computed Tomography 16 MSCT: AMS Stent A bmsstent C B D AMS Stent BMS Stent Lind et al Heart 91: 1604, 2005
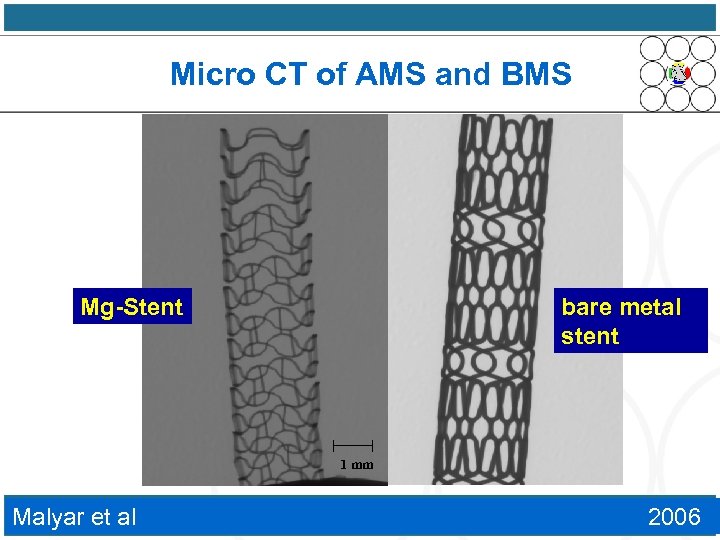 Micro CT of AMS and BMS Mg-Stent bare metal stent 1 mm Malyar et al 2006
Micro CT of AMS and BMS Mg-Stent bare metal stent 1 mm Malyar et al 2006
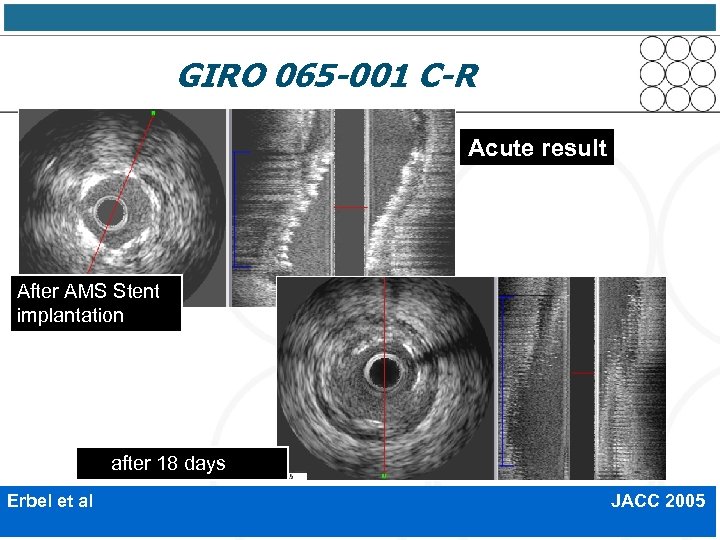 GIRO 065 -001 C-R Acute result After AMS Stent implantation after 18 days Erbel et al JACC 2005
GIRO 065 -001 C-R Acute result After AMS Stent implantation after 18 days Erbel et al JACC 2005
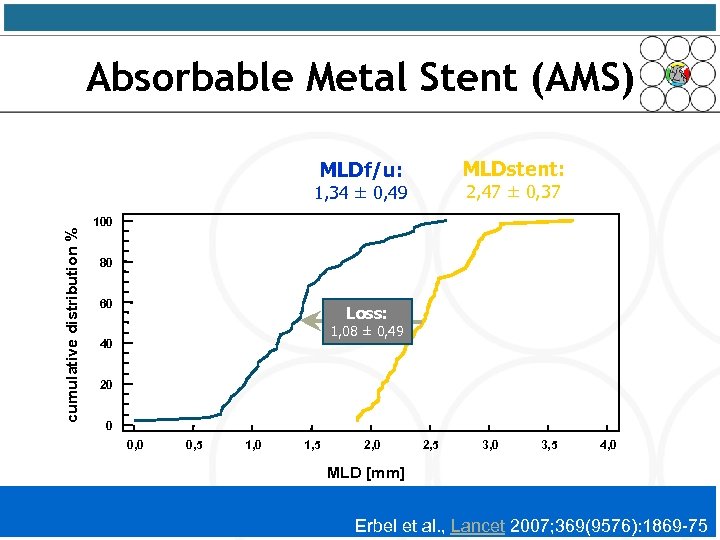 Absorbable Metal Stent (AMS) MLDstent: MLDf/u: 2, 47 ± 0, 37 cumulative distribution % 1, 34 ± 0, 49 100 80 60 Loss: 1, 08 ± 0, 49 40 20 0 0, 5 1, 0 1, 5 2, 0 2, 5 3, 0 3, 5 4, 0 MLD [mm] Erbel et al. , Lancet 2007; 369(9576): 1869 -75
Absorbable Metal Stent (AMS) MLDstent: MLDf/u: 2, 47 ± 0, 37 cumulative distribution % 1, 34 ± 0, 49 100 80 60 Loss: 1, 08 ± 0, 49 40 20 0 0, 5 1, 0 1, 5 2, 0 2, 5 3, 0 3, 5 4, 0 MLD [mm] Erbel et al. , Lancet 2007; 369(9576): 1869 -75
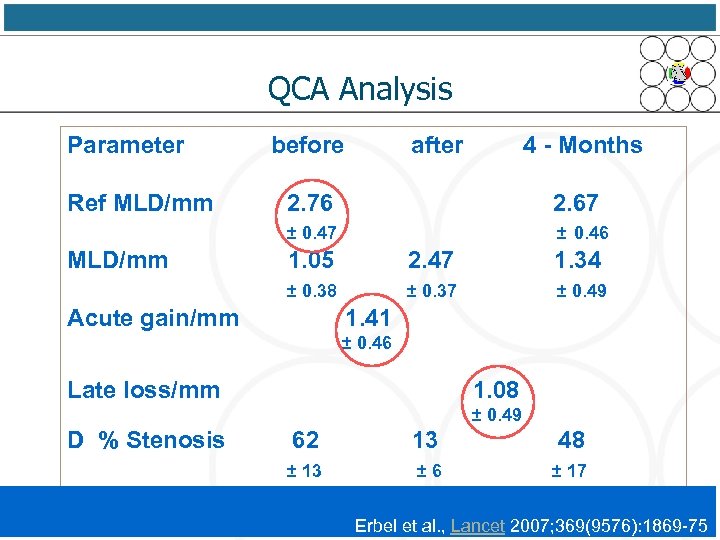 QCA Analysis Parameter Ref MLD/mm before after 4 - Months 2. 67 ± 0. 47 MLD/mm 2. 76 ± 0. 46 1. 05 2. 47 1. 34 ± 0. 38 ± 0. 37 ± 0. 49 Acute gain/mm 1. 41 ± 0. 46 Late loss/mm 1. 08 ± 0. 49 D % Stenosis 62 13 48 ± 13 ± 6 ± 17 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
QCA Analysis Parameter Ref MLD/mm before after 4 - Months 2. 67 ± 0. 47 MLD/mm 2. 76 ± 0. 46 1. 05 2. 47 1. 34 ± 0. 38 ± 0. 37 ± 0. 49 Acute gain/mm 1. 41 ± 0. 46 Late loss/mm 1. 08 ± 0. 49 D % Stenosis 62 13 48 ± 13 ± 6 ± 17 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
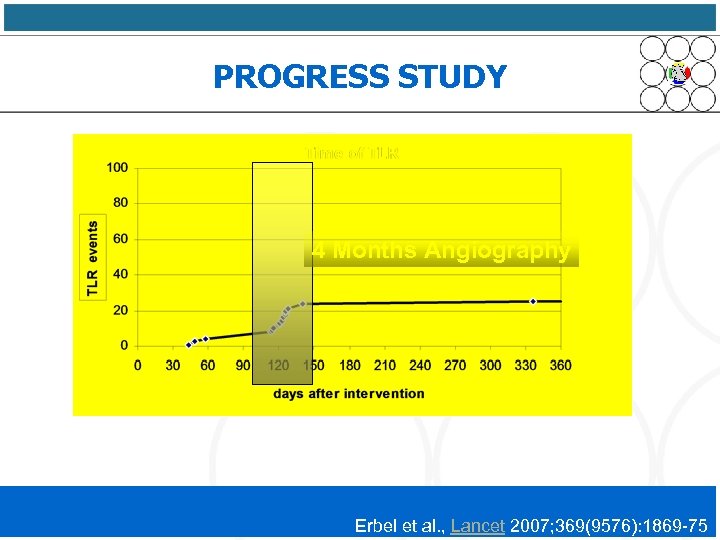 PROGRESS STUDY 4 Months Angiography Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS STUDY 4 Months Angiography Erbel et al. , Lancet 2007; 369(9576): 1869 -75
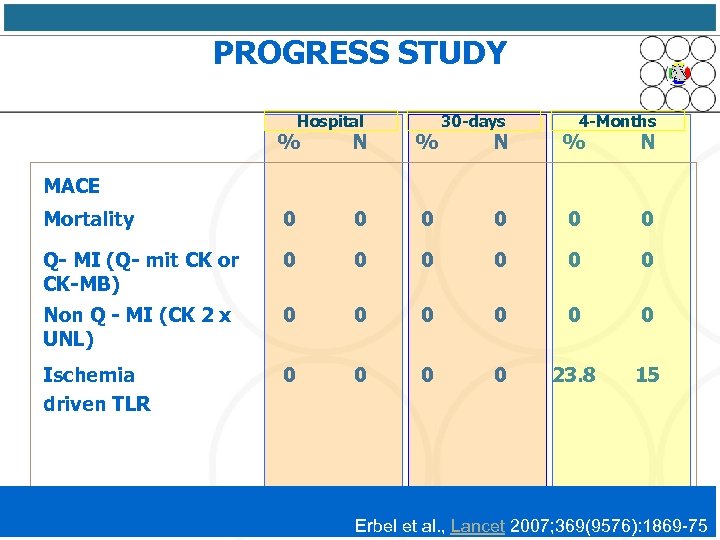 PROGRESS STUDY Hospital % N % Mortality 0 0 Q- MI (Q- mit CK or CK-MB) 0 Non Q - MI (CK 2 x UNL) Ischemia driven TLR 30 -days 4 -Months N % N 0 0 0 0 0 23. 8 15 MACE Erbel et al. , Lancet 2007; 369(9576): 1869 -75
PROGRESS STUDY Hospital % N % Mortality 0 0 Q- MI (Q- mit CK or CK-MB) 0 Non Q - MI (CK 2 x UNL) Ischemia driven TLR 30 -days 4 -Months N % N 0 0 0 0 0 23. 8 15 MACE Erbel et al. , Lancet 2007; 369(9576): 1869 -75
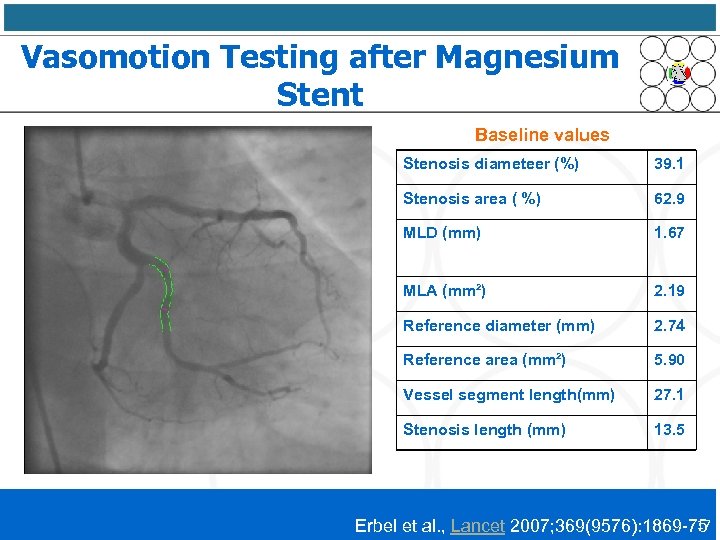 Vasomotion Testing after Magnesium Stent Baseline values Stenosis diameteer (%) 39. 1 Stenosis area ( %) 62. 9 MLD (mm) 1. 67 MLA (mm²) 2. 19 Reference diameter (mm) 2. 74 Reference area (mm²) 5. 90 Vessel segment length(mm) 27. 1 Stenosis length (mm) 13. 5 17 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
Vasomotion Testing after Magnesium Stent Baseline values Stenosis diameteer (%) 39. 1 Stenosis area ( %) 62. 9 MLD (mm) 1. 67 MLA (mm²) 2. 19 Reference diameter (mm) 2. 74 Reference area (mm²) 5. 90 Vessel segment length(mm) 27. 1 Stenosis length (mm) 13. 5 17 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
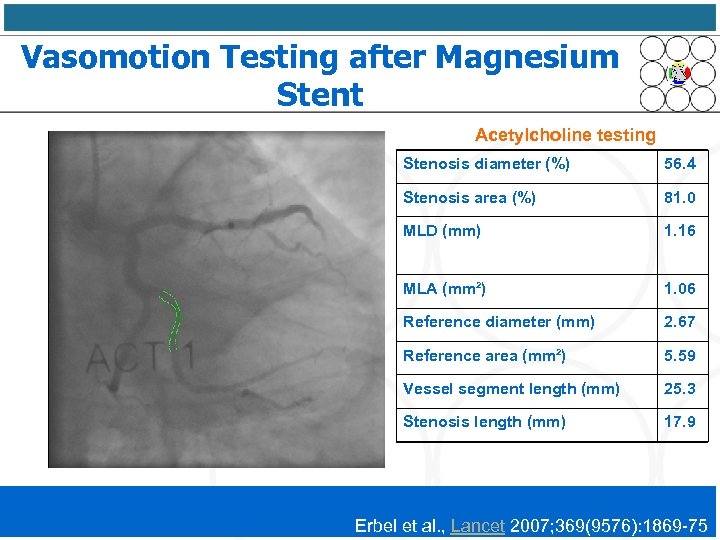 Vasomotion Testing after Magnesium Stent Acetylcholine testing Stenosis diameter (%) 56. 4 Stenosis area (%) 81. 0 MLD (mm) 1. 16 MLA (mm²) 1. 06 Reference diameter (mm) 2. 67 Reference area (mm²) 5. 59 Vessel segment length (mm) 25. 3 Stenosis length (mm) 17. 9 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
Vasomotion Testing after Magnesium Stent Acetylcholine testing Stenosis diameter (%) 56. 4 Stenosis area (%) 81. 0 MLD (mm) 1. 16 MLA (mm²) 1. 06 Reference diameter (mm) 2. 67 Reference area (mm²) 5. 59 Vessel segment length (mm) 25. 3 Stenosis length (mm) 17. 9 Erbel et al. , Lancet 2007; 369(9576): 1869 -75
 Magnesium Stent Conclusion • AMS realized with low recoil • High technical sucess • AMS permits MRT and CT based imaging • No acute or subacute stent thrombosis • i. TLR rate comparable to BMS • IVUS detected degradation within 4 M • Vasomotion reactivation Drug elution and AMS delayed degradation – Dream concept* * Please listen to R Waksman in the next session, room 6
Magnesium Stent Conclusion • AMS realized with low recoil • High technical sucess • AMS permits MRT and CT based imaging • No acute or subacute stent thrombosis • i. TLR rate comparable to BMS • IVUS detected degradation within 4 M • Vasomotion reactivation Drug elution and AMS delayed degradation – Dream concept* * Please listen to R Waksman in the next session, room 6


