6adea60d03981fee9ea61ea9bd23c30d.ppt
- Количество слайдов: 25

New Priorities for IAVI Dr. Seth Berkley President & CEO, International AIDS Vaccine Initiative 4 August 2008 XVII International AIDS Conference Mexico City, Mexico

An AIDS Vaccine is Possible Immune control is possible: Majority of HIV-infected individuals initially suppress viral load Populations resistant to HIV infection Long-term non-progressors: control infection for many years Highly exposed, uninfected: CSWs, MSMs Children of infected mothers Experimental candidates: Live attenuated SIV & challenge Broadly neutralizing antibodies in Macaques

AIDS VACCINE BLUEPRINT 2008 A Challenge to the Field A Roadmap for Progress

AIDS Vaccines: Global Update- August 2008 Summary & IAVI Assessment Successful development of an AIDS vaccine will likely require: Induction of broadly neutralizing antibodies Control of HIV as well as live attenuated SIV controls against homologous challenge e. g. as well as human “elite controllers” No candidate currently in the pipeline achieves these objectives New and improved functional T-cell assays beyond ELISPOT will help to prioritize candidates for development Success will likely require: Breakthrough in the solution to the HIV neutralizing antibody problem Development of more potent vector(s) e. g. replicating viral vectors Determination of the HIV antigenic inserts which must be included in a vaccine to confer protection against globally diverse subtypes of HIV
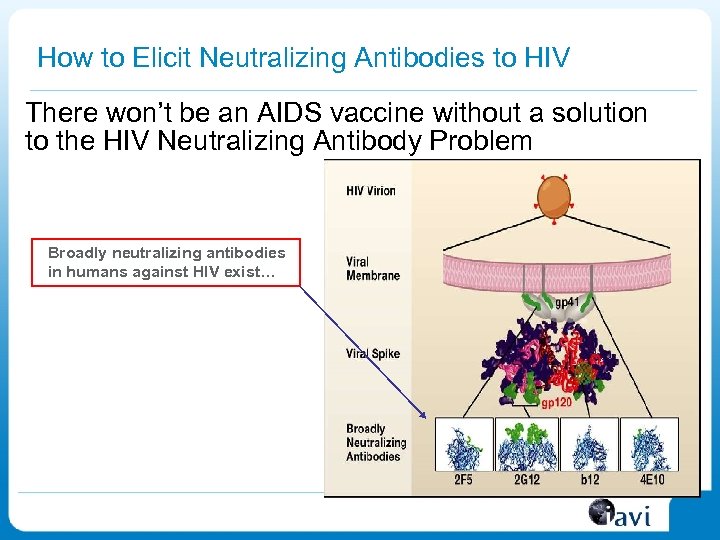
How to Elicit Neutralizing Antibodies to HIV There won’t be an AIDS vaccine without a solution to the HIV Neutralizing Antibody Problem Broadly neutralizing antibodies in humans against HIV exist…

Research Consortia focus on Key Challenges e. g. Neutralizing Antibody Consortium Broadly neutralizing High thru-put immunogen design antibodies Determining structure Assays to rapidly screen immunogens of novel antigens Characterize Sera and Identify BN-Mabs Protocol G Structural Biology High thru-put Robot Immunogen Design Proteins Peptides Sugars “needle in haystack” Immunogen Screening Major Block Slow Immunogen Screen Clinical Dev.
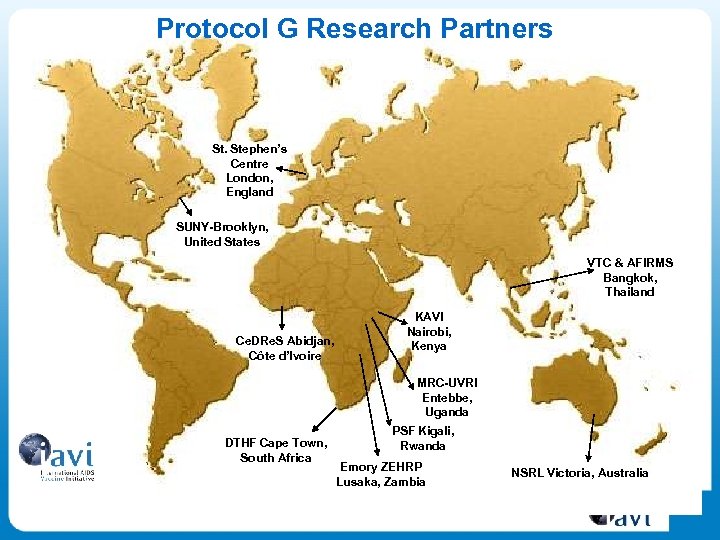
Protocol G Research Partners St. Stephen’s Centre London, England SUNY-Brooklyn, United States VTC & AFIRMS Bangkok, Thailand Ce. DRe. S Abidjan, Côte d’Ivoire KAVI Nairobi, Kenya MRC-UVRI Entebbe, Uganda DTHF Cape Town, South Africa PSF Kigali, Rwanda Emory ZEHRP Lusaka, Zambia NSRL Victoria, Australia
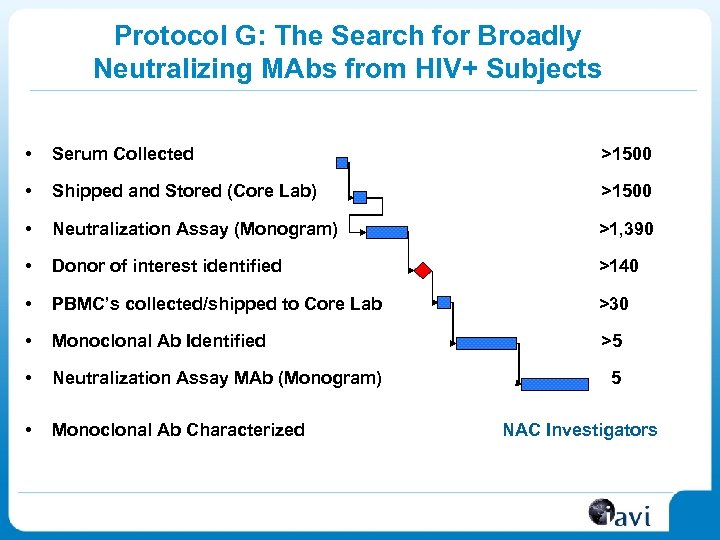
Protocol G: The Search for Broadly Neutralizing MAbs from HIV+ Subjects • Serum Collected >1500 • Shipped and Stored (Core Lab) >1500 • Neutralization Assay (Monogram) >1, 390 • Donor of interest identified >140 • PBMC’s collected/shipped to Core Lab >30 • Monoclonal Ab Identified >5 • Neutralization Assay MAb (Monogram) • Monoclonal Ab Characterized 5 NAC Investigators

IAVI Neutralizing Antibody and Discovery Laboratory Network Oxford Karolinska Institutet U. Marseilles Instituto di Recerca in Biomedicina U. Wisconsin U. Washington Academia Sinica Scripps International Centre for Genetic Engineering and Biotechnology Indian Institute of Science Cornell University Dana Farber Harvard IAVI U. Pennsylvania NIH Vaccine Research Center

NAC Membership • • Scientific Director Dennis Burton-TSRI Executive Director Wayne Koff- IAVI • Structural Virology, Crystallography, Protein Chemistry, Immunology, Immunogen Modeling Dennis Burton - TSRI Ian Wilson - TSRI Peter Kwong - VRC (NIAID) Richard Wyatt - VRC (NIAID) Gary Nabel - VRC (NIAID) Joseph Sodroski – Harvard Medical School John Moore – Cornell University Robert Doms – U. Pennsylvania David Baker – U. Washington Bill Schief – U. Washington • • • • Mechanisms of Neutralization and Resistance Pascal Poignard – University of Marseilles Quentin Sattentau – University of Oxford • • B Cell Immunobiology/Monoclonal Antibody Identification Antonio Lanzavecchia - IRB, Bellinzona • • • Non-Human Primate Models Ronald Desrosiers – Harvard Medical School David Watkins – University of Wisconsin • • • Carbohydrate Antigen Design / Glycobiology Raymond Dwek – University of Oxford Ben Davis – University of Oxford Chi-Huey Wong – TSRI Protein Antigen Design / Medicinal Chemistry Phil Dawson - TSRI Stephen Kaminsky – IAVI Tim Zamb – IAVI Raghavan Varadarajan- Indian Inst. Sciences VS Chauhan, ICGEB, India
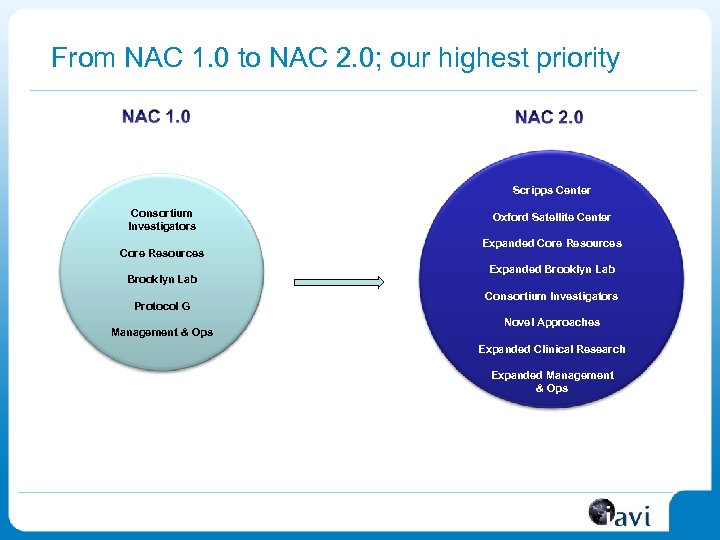
From NAC 1. 0 to NAC 2. 0; our highest priority Scripps Center Consortium Investigators Core Resources Brooklyn Lab Protocol G Management & Ops Oxford Satellite Center Expanded Core Resources Expanded Brooklyn Lab Consortium Investigators Novel Approaches Expanded Clinical Research Expanded Management & Ops
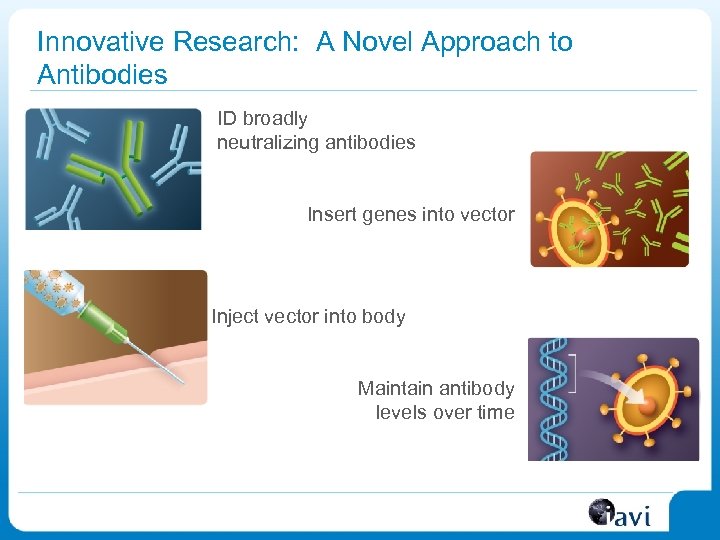
Innovative Research: A Novel Approach to Antibodies ID broadly neutralizing antibodies Insert genes into vector Inject vector into body Maintain antibody levels over time
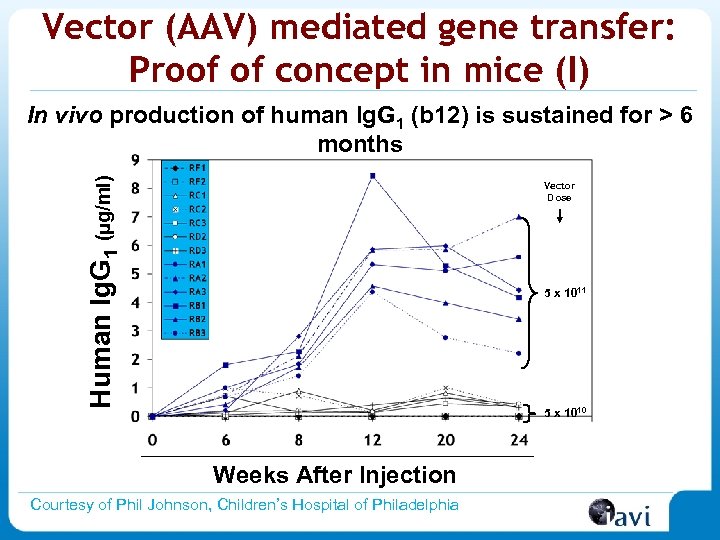
Vector (AAV) mediated gene transfer: Proof of concept in mice (I) Human Ig. G 1 (µg/ml) In vivo production of human Ig. G 1 (b 12) is sustained for > 6 months Vector Dose 5 x 1011 5 x 1010 Weeks After Injection Courtesy of Phil Johnson, Children’s Hospital of Philadelphia

Vector Mediated Ab Gene Transfer Protection Against SIV Plasma Viral Load (log 10) Naive 4 L 6 5 L 7 N 4 Courtesy of Phil Johnson, Wks post Children’s Hospital of Philadelphia SIVmac 316 i. v. Challenge
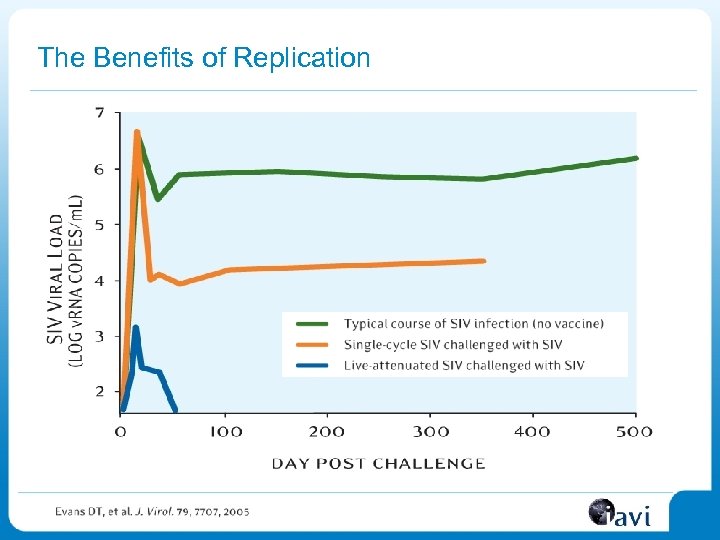
The Benefits of Replication
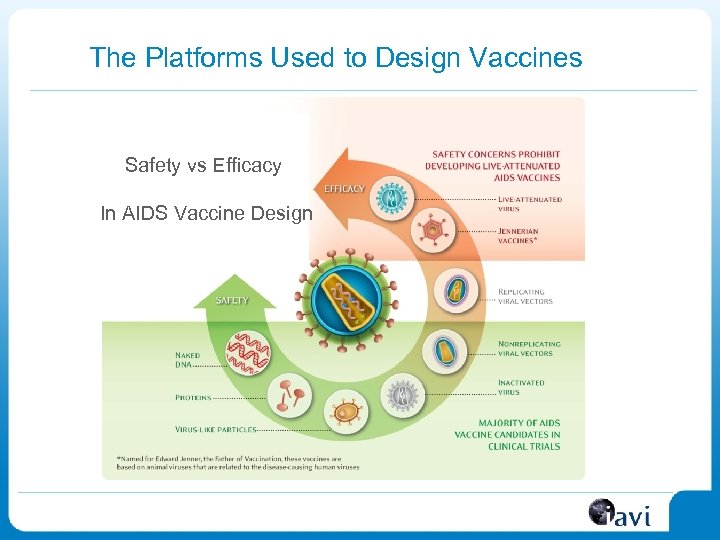
The Platforms Used to Design Vaccines Safety vs Efficacy In AIDS Vaccine Design

Replicating Vector Portfolio VSV CDV NDV VEEV HSV CMV Reo Se. V VEEV CDV VSV Reo In vitro expression, Stability screen NDV Small animal models IAVI / Gates CMV IAVI / Academic Partner Non-IAVI MV Measles virus GSK / Crucell HSV IAVI / Biotech Partnership IAVI / Biotech Exploratory VSV Ad Adenovirus 5 / 7 NCI Vaccinia virus (Tiantan) National Center for AIDS Beijing NHP model Attenuated VSV Wyeth / Profectus VV Se. V Construct vectors Primary candidate emerges Clinical Candidates

IAVI AIDS Vaccine Development Laboratory Ø 2006 -2008: SUNY-Downstate Ø 4 Q 2008: Brooklyn Army Terminal (30, 000 sq feet)

IAVI AIDS Vaccine Development Laboratory (Brooklyn) • A unique industrial-style, not for profit, applied Research laboratory fully dedicated to AIDS Vaccine Discovery and Development Replicating Viral Vectors: The next generation of candidate AIDS vaccines Preclinical Immunology: Systematic comparison and prioritization of vaccine candidates Integrates IAVI’s Neutralizing Antibody and Control of HIV programs Process Development to accelerate Concept to Clinic
Mechanisms of Innovation are now more important than ever Pushing the envelop with a new model…. Partnership with the BMGF Move beyond mainstream HIV vaccine research; Venture capital approach Venture Advisory Committee Brook Byers (Kleiner Perkins Caufield & Byers), Paul Klingenstein (Aberdare), Mike Powell (Sofinnova), Bryan Roberts (Venrock), Greg Weinhoff (CHL Medical) ‘Seed capital’ grants for feasibility studies Non dilutive, minimal diligence, speed Welcome creativity, ‘unproven’ ideas Draw from other disciplines Cross fertilization of ideas Adopt advances, not trying to recreate the wheel
Innovation Fund Completed Grants Current pipeline reflects Fund focus areas Ø Novel assay / high throughput screening technologies Ø Vax. Design (US) Ø In vitro mimic of human immune system for rapid vaccine evaluation Ø Spaltudaq (US) Ø Human B cell screening technology for identification of new bn. MAbs Ø Novel Antigen Discovery/Delivery Ø Lipoxen (UK) Ø Liposome delivery technology for antigen presentation Ø Strand (India) Ø In silico protein structure modeling to design immunogens mimicking the 4 E 10 epitope
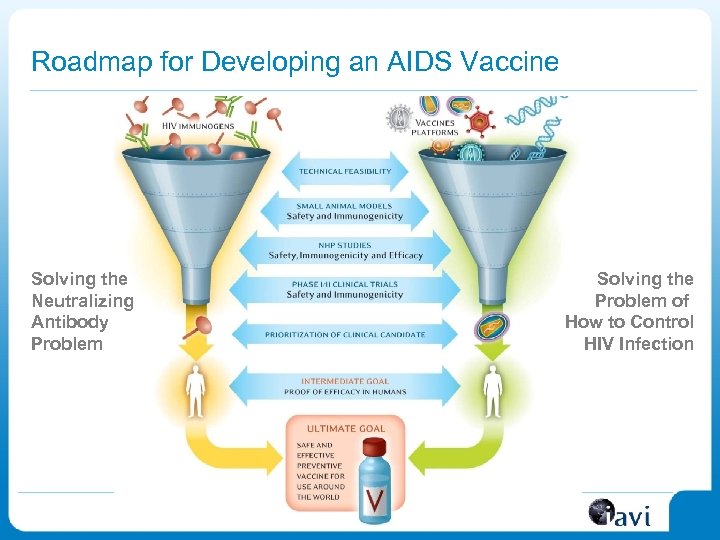
Roadmap for Developing an AIDS Vaccine Solving the Neutralizing Antibody Problem Solving the Problem of How to Control HIV Infection
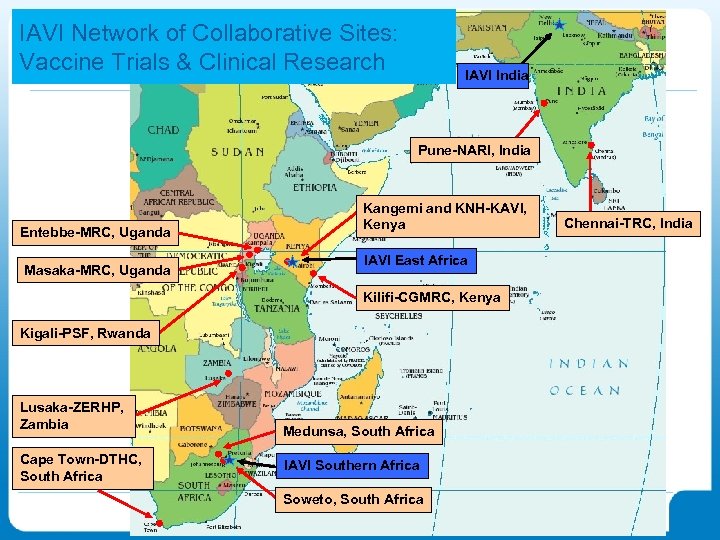
IAVI Network of Collaborative Sites: Vaccine Trials & Clinical Research IAVI India Pune-NARI, India Entebbe-MRC, Uganda Masaka-MRC, Uganda Kangemi and KNH-KAVI, Kenya IAVI East Africa Kilifi-CGMRC, Kenya Kigali-PSF, Rwanda Lusaka-ZERHP, Zambia Cape Town-DTHC, South Africa Medunsa, South Africa IAVI Southern Africa Soweto, South Africa Chennai-TRC, India

IAVI Gratefully Acknowledges the support of our Donors

IMAGINE a World Without AIDS
6adea60d03981fee9ea61ea9bd23c30d.ppt