c4edcc9dd61bcfd241ca241b856c9f8c.ppt
- Количество слайдов: 116
 New Concepts in the Evaluation and Treatment of Dyslipidemia Nathan D. Wong, Ph. D, FACC Professor and Director Heart Disease Prevention Program Division of Cardiology University of California, Irvine President, American Society for Preventive Cardiology
New Concepts in the Evaluation and Treatment of Dyslipidemia Nathan D. Wong, Ph. D, FACC Professor and Director Heart Disease Prevention Program Division of Cardiology University of California, Irvine President, American Society for Preventive Cardiology

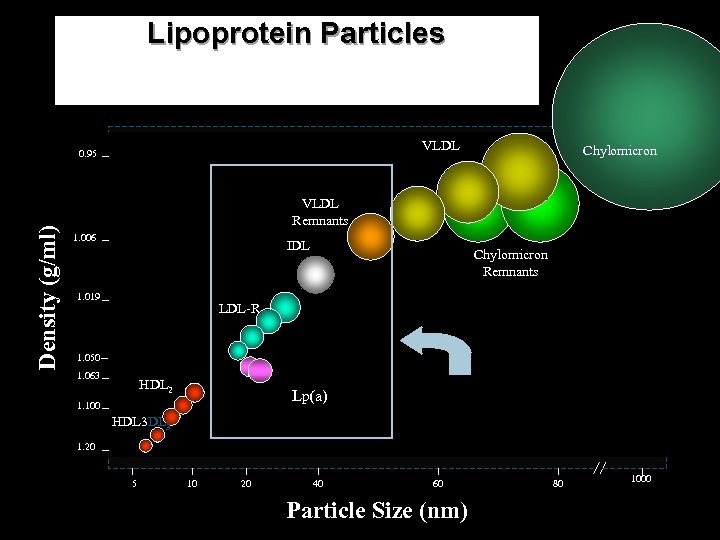 Lipoprotein Particles VLDL Density (g/ml) 0. 95 Chylomicron VLDL Remnants 1. 006 IDL 1. 019 Chylomicron Remnants LDL-R 1. 050 1. 063 HDL 2 Lp(a) 1. 100 HDL 3 Only these lipoprotein particles found in plaque at biopsy. 1. 20 5 10 20 40 60 Particle Size (nm) 80 1000
Lipoprotein Particles VLDL Density (g/ml) 0. 95 Chylomicron VLDL Remnants 1. 006 IDL 1. 019 Chylomicron Remnants LDL-R 1. 050 1. 063 HDL 2 Lp(a) 1. 100 HDL 3 Only these lipoprotein particles found in plaque at biopsy. 1. 20 5 10 20 40 60 Particle Size (nm) 80 1000
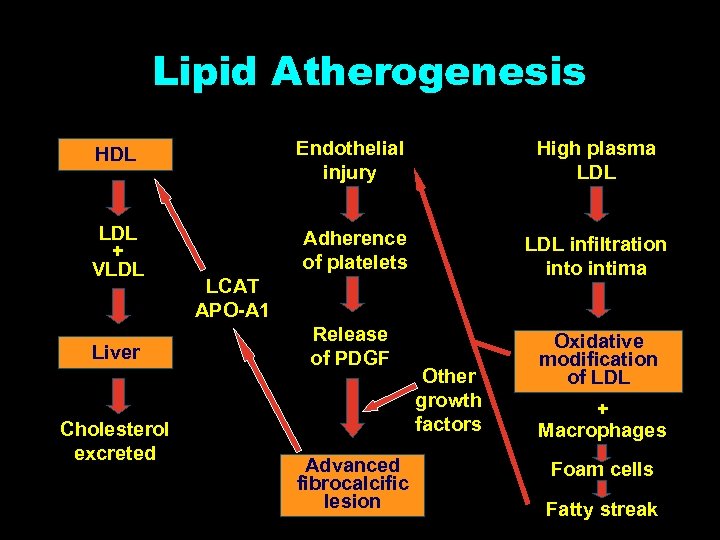 Lipid Atherogenesis HDL Endothelial injury High plasma LDL + VLDL Adherence of platelets LDL infiltration into intima Release of PDGF Oxidative modification of LDL Liver Cholesterol excreted LCAT APO-A 1 Advanced fibrocalcific lesion Other growth factors + Macrophages Foam cells Fatty streak
Lipid Atherogenesis HDL Endothelial injury High plasma LDL + VLDL Adherence of platelets LDL infiltration into intima Release of PDGF Oxidative modification of LDL Liver Cholesterol excreted LCAT APO-A 1 Advanced fibrocalcific lesion Other growth factors + Macrophages Foam cells Fatty streak
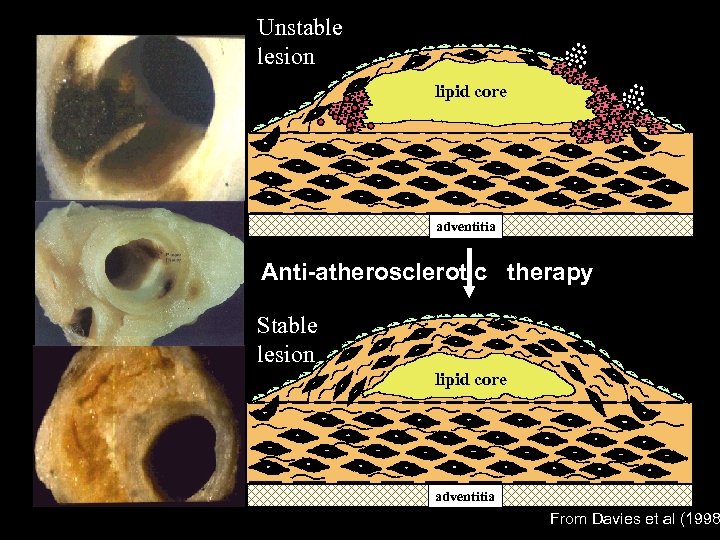 Unstable lesion lipid core adventitia Anti-atherosclerotic therapy Stable lesion lipid core adventitia From Davies et al (1998
Unstable lesion lipid core adventitia Anti-atherosclerotic therapy Stable lesion lipid core adventitia From Davies et al (1998
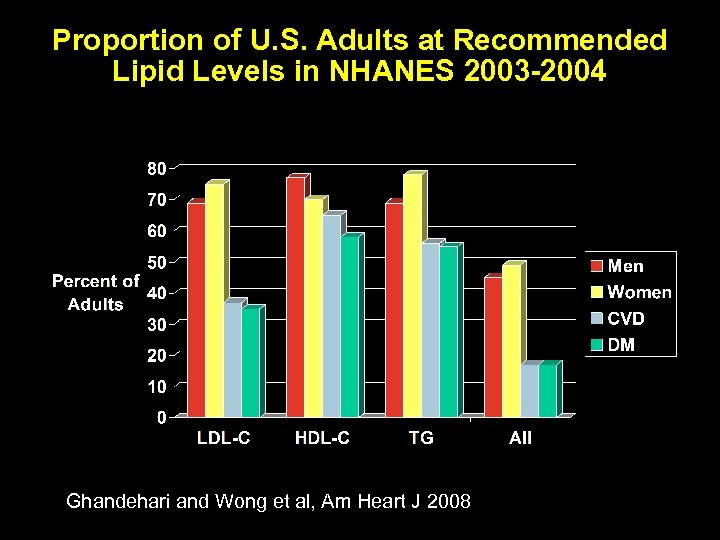 Proportion of U. S. Adults at Recommended Lipid Levels in NHANES 2003 -2004 Ghandehari and Wong et al, Am Heart J 2008
Proportion of U. S. Adults at Recommended Lipid Levels in NHANES 2003 -2004 Ghandehari and Wong et al, Am Heart J 2008
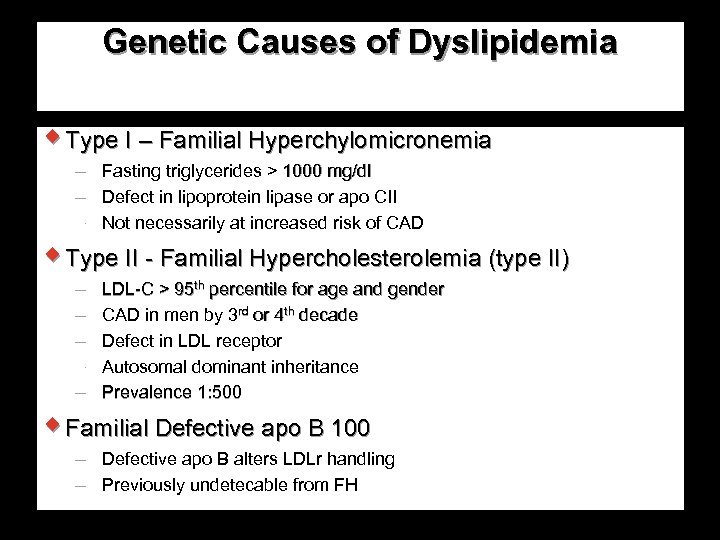 Genetic Causes of Dyslipidemia w Type I – Familial Hyperchylomicronemia Fasting triglycerides > 1000 mg/dl Defect in lipoprotein lipase or apo CII Not necessarily at increased risk of CAD w Type II - Familial Hypercholesterolemia (type II) LDL-C > 95 th percentile for age and gender CAD in men by 3 rd or 4 th decade Defect in LDL receptor Autosomal dominant inheritance Prevalence 1: 500 w Familial Defective apo B 100 Defective apo B alters LDLr handling Previously undetecable from FH
Genetic Causes of Dyslipidemia w Type I – Familial Hyperchylomicronemia Fasting triglycerides > 1000 mg/dl Defect in lipoprotein lipase or apo CII Not necessarily at increased risk of CAD w Type II - Familial Hypercholesterolemia (type II) LDL-C > 95 th percentile for age and gender CAD in men by 3 rd or 4 th decade Defect in LDL receptor Autosomal dominant inheritance Prevalence 1: 500 w Familial Defective apo B 100 Defective apo B alters LDLr handling Previously undetecable from FH
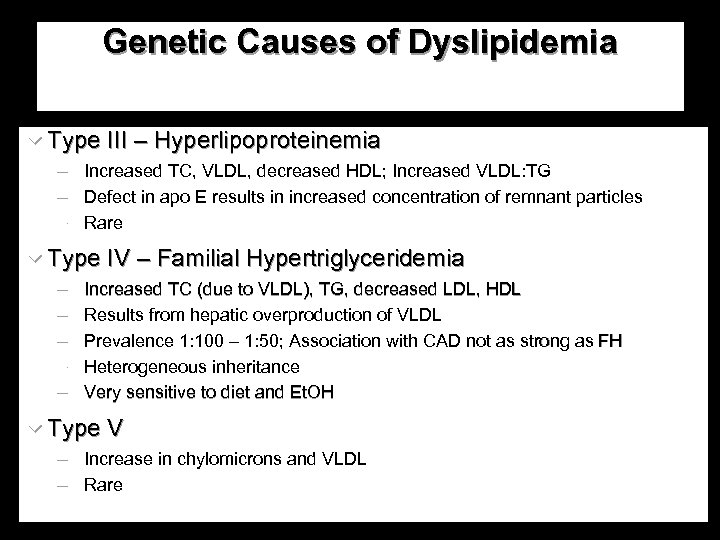 Genetic Causes of Dyslipidemia w Type III – Hyperlipoproteinemia Increased TC, VLDL, decreased HDL; Increased VLDL: TG Defect in apo E results in increased concentration of remnant particles Rare w Type IV – Familial Hypertriglyceridemia Increased TC (due to VLDL), TG, decreased LDL, HDL Results from hepatic overproduction of VLDL Prevalence 1: 100 – 1: 50; Association with CAD not as strong as FH Heterogeneous inheritance Very sensitive to diet and Et. OH w Type V Increase in chylomicrons and VLDL Rare
Genetic Causes of Dyslipidemia w Type III – Hyperlipoproteinemia Increased TC, VLDL, decreased HDL; Increased VLDL: TG Defect in apo E results in increased concentration of remnant particles Rare w Type IV – Familial Hypertriglyceridemia Increased TC (due to VLDL), TG, decreased LDL, HDL Results from hepatic overproduction of VLDL Prevalence 1: 100 – 1: 50; Association with CAD not as strong as FH Heterogeneous inheritance Very sensitive to diet and Et. OH w Type V Increase in chylomicrons and VLDL Rare
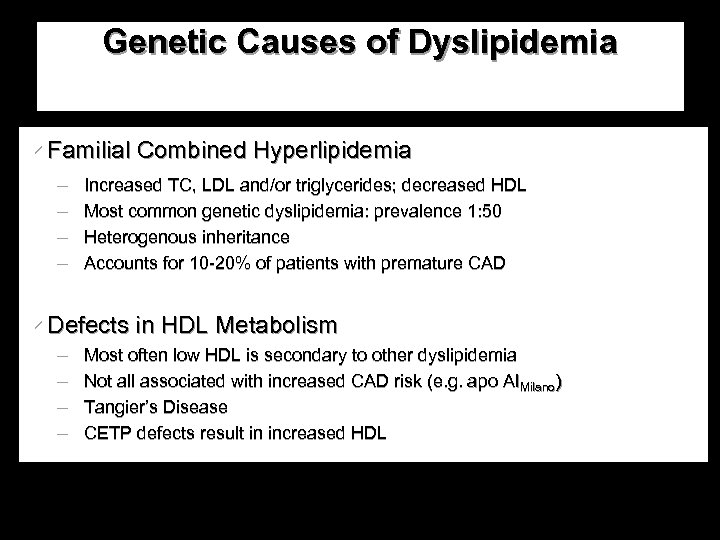 Genetic Causes of Dyslipidemia w Familial Combined Hyperlipidemia Increased TC, LDL and/or triglycerides; decreased HDL Most common genetic dyslipidemia: prevalence 1: 50 Heterogenous inheritance Accounts for 10 -20% of patients with premature CAD w Defects in HDL Metabolism Most often low HDL is secondary to other dyslipidemia Not all associated with increased CAD risk (e. g. apo AIMilano) Tangier’s Disease CETP defects result in increased HDL
Genetic Causes of Dyslipidemia w Familial Combined Hyperlipidemia Increased TC, LDL and/or triglycerides; decreased HDL Most common genetic dyslipidemia: prevalence 1: 50 Heterogenous inheritance Accounts for 10 -20% of patients with premature CAD w Defects in HDL Metabolism Most often low HDL is secondary to other dyslipidemia Not all associated with increased CAD risk (e. g. apo AIMilano) Tangier’s Disease CETP defects result in increased HDL
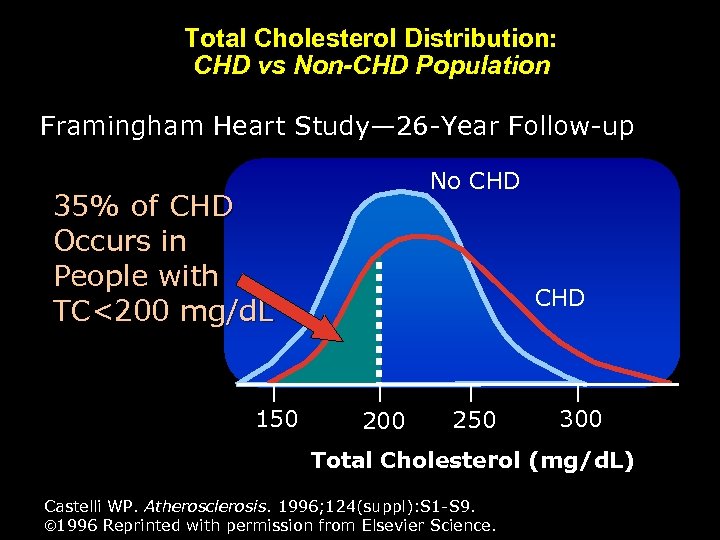 Total Cholesterol Distribution: CHD vs Non-CHD Population Framingham Heart Study— 26 -Year Follow-up No CHD 35% of CHD Occurs in People with TC<200 mg/d. L 150 CHD 200 250 300 Total Cholesterol (mg/d. L) Castelli WP. Atherosclerosis. 1996; 124(suppl): S 1 -S 9. 1996 Reprinted with permission from Elsevier Science.
Total Cholesterol Distribution: CHD vs Non-CHD Population Framingham Heart Study— 26 -Year Follow-up No CHD 35% of CHD Occurs in People with TC<200 mg/d. L 150 CHD 200 250 300 Total Cholesterol (mg/d. L) Castelli WP. Atherosclerosis. 1996; 124(suppl): S 1 -S 9. 1996 Reprinted with permission from Elsevier Science.
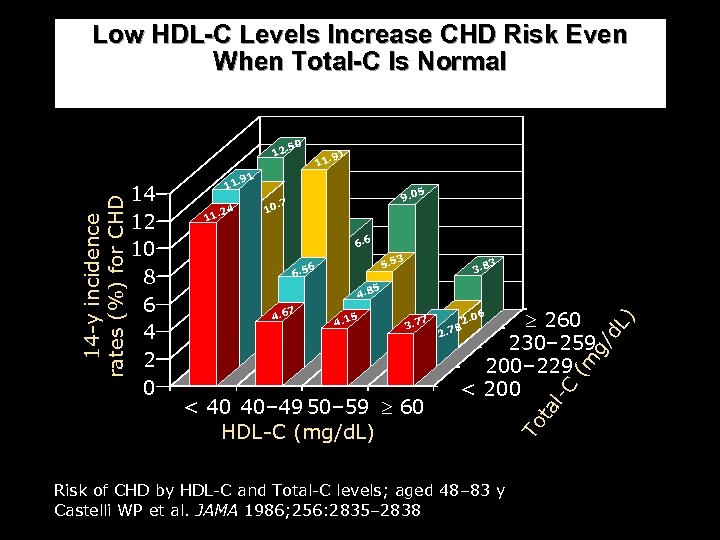 Low HDL-C Levels Increase CHD Risk Even When Total-C Is Normal 11 . 91 11. 24 11 9. 0 0. 7 1 5 6. 6 5. 5 6 6. 5 5 3. 7 7 2 260 230– 259 200– 229 < 200 . 78 2. 0 6 ( Risk of CHD by HDL-C and Total-C levels; aged 48– 83 y Castelli WP et al. JAMA 1986; 256: 2835– 2838 l-C < 40 40– 49 50– 59 60 HDL-C (mg/d. L) ) 4. 1 5 d. L 7 3 m 4. 6 3. 8 g/ 4. 8 3 ta 14 12 10 8 6 4 2 0 . 50 To 14 -y incidence rates (%) for CHD 12
Low HDL-C Levels Increase CHD Risk Even When Total-C Is Normal 11 . 91 11. 24 11 9. 0 0. 7 1 5 6. 6 5. 5 6 6. 5 5 3. 7 7 2 260 230– 259 200– 229 < 200 . 78 2. 0 6 ( Risk of CHD by HDL-C and Total-C levels; aged 48– 83 y Castelli WP et al. JAMA 1986; 256: 2835– 2838 l-C < 40 40– 49 50– 59 60 HDL-C (mg/d. L) ) 4. 1 5 d. L 7 3 m 4. 6 3. 8 g/ 4. 8 3 ta 14 12 10 8 6 4 2 0 . 50 To 14 -y incidence rates (%) for CHD 12
 Why is HDL the New Frontier? Multiple Beneficial Properties of HDL 1. Reverse cholesterol transport 2. Anti-oxidant 3. Inhibit vascular inflammation: Adhesion molecules and monocyte infiltration 4. Anti-thrombotic / profibrinolytic: Increase fibrinolysis; decrease platelet aggregation 5. Endothelial stabilization Apo A-I, major protein of HDL is the major player in all these properties. It is the vehicle for cholesterol removal. Thus, current concepts dictate that HDL therapy should be Apo A-I based rather than HDL Kashyap et al 2008 Cholesterol based.
Why is HDL the New Frontier? Multiple Beneficial Properties of HDL 1. Reverse cholesterol transport 2. Anti-oxidant 3. Inhibit vascular inflammation: Adhesion molecules and monocyte infiltration 4. Anti-thrombotic / profibrinolytic: Increase fibrinolysis; decrease platelet aggregation 5. Endothelial stabilization Apo A-I, major protein of HDL is the major player in all these properties. It is the vehicle for cholesterol removal. Thus, current concepts dictate that HDL therapy should be Apo A-I based rather than HDL Kashyap et al 2008 Cholesterol based.
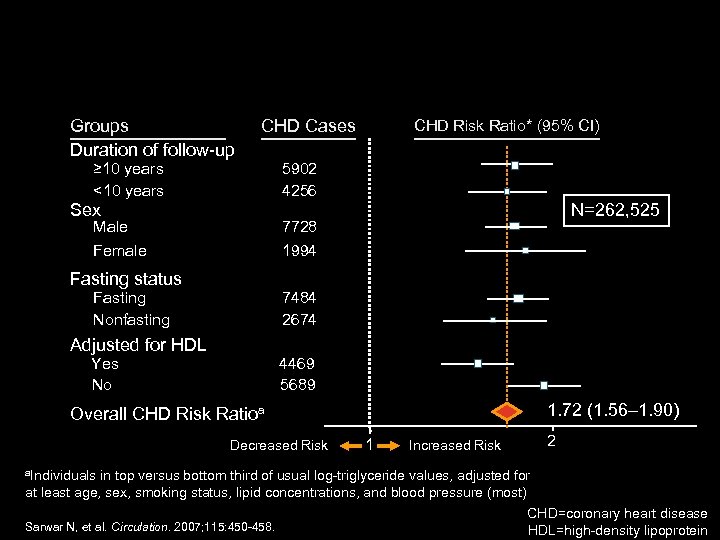 Groups Duration of follow-up CHD Cases ≥ 10 years <10 years CHD Risk Ratio* (95% CI) 5902 4256 Sex Male Female N=262, 525 7728 1994 Fasting status Fasting Nonfasting 7484 2674 Adjusted for HDL Yes No 4469 5689 1. 72 (1. 56– 1. 90) Overall CHD Risk Ratioa Decreased Risk a. Individuals 1 Increased Risk 2 in top versus bottom third of usual log-triglyceride values, adjusted for at least age, sex, smoking status, lipid concentrations, and blood pressure (most) CHD=coronary heart disease Sarwar N, et al. Circulation. 2007; 115: 450 -458. HDL=high-density lipoprotein
Groups Duration of follow-up CHD Cases ≥ 10 years <10 years CHD Risk Ratio* (95% CI) 5902 4256 Sex Male Female N=262, 525 7728 1994 Fasting status Fasting Nonfasting 7484 2674 Adjusted for HDL Yes No 4469 5689 1. 72 (1. 56– 1. 90) Overall CHD Risk Ratioa Decreased Risk a. Individuals 1 Increased Risk 2 in top versus bottom third of usual log-triglyceride values, adjusted for at least age, sex, smoking status, lipid concentrations, and blood pressure (most) CHD=coronary heart disease Sarwar N, et al. Circulation. 2007; 115: 450 -458. HDL=high-density lipoprotein
 w Triglyceride-rich lipoproteins carry cholesterol and promote atherosclerosis* w Very–low-density lipoprotein (VLDL) is precursor to low-density lipoprotein (LDL) w Hypertriglyceridemia (HTG) drives Cholesterol esters enrichment of VLDL (more atherogenic) ↓ LDL size (small, dense LDL are more atherogenic)* ↓ LDL-C (small, dense LDL carry less cholesterol)* ↓ High-density lipoprotein (HDL) size (small, dense HDL are unstable) Insulin resistance Proinflammatory state Prothrombotic state Prooxidative state Endothelial dysfunction w HTG is linked to other proatherogenic states* *Reasons why non–HDL-C is stronger than LDL-C as predictor of cardiovascular disease
w Triglyceride-rich lipoproteins carry cholesterol and promote atherosclerosis* w Very–low-density lipoprotein (VLDL) is precursor to low-density lipoprotein (LDL) w Hypertriglyceridemia (HTG) drives Cholesterol esters enrichment of VLDL (more atherogenic) ↓ LDL size (small, dense LDL are more atherogenic)* ↓ LDL-C (small, dense LDL carry less cholesterol)* ↓ High-density lipoprotein (HDL) size (small, dense HDL are unstable) Insulin resistance Proinflammatory state Prothrombotic state Prooxidative state Endothelial dysfunction w HTG is linked to other proatherogenic states* *Reasons why non–HDL-C is stronger than LDL-C as predictor of cardiovascular disease
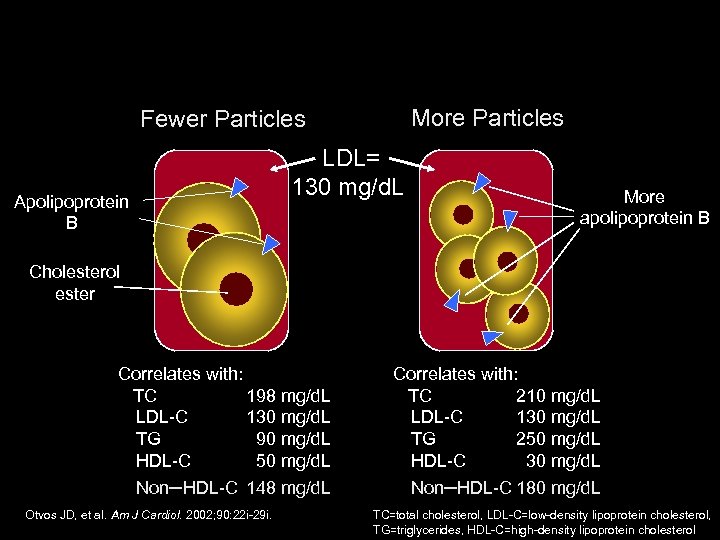 More Particles Fewer Particles LDL= 130 mg/d. L Apolipoprotein B More apolipoprotein B Cholesterol ester Correlates with: TC 198 mg/d. L LDL-C 130 mg/d. L TG 90 mg/d. L HDL-C 50 mg/d. L Correlates with: TC 210 mg/d. L LDL-C 130 mg/d. L TG 250 mg/d. L HDL-C 30 mg/d. L Non–HDL-C 148 mg/d. L Non–HDL-C 180 mg/d. L Otvos JD, et al. Am J Cardiol. 2002; 90: 22 i-29 i. TC=total cholesterol, LDL-C=low-density lipoprotein cholesterol, TG=triglycerides, HDL-C=high-density lipoprotein cholesterol
More Particles Fewer Particles LDL= 130 mg/d. L Apolipoprotein B More apolipoprotein B Cholesterol ester Correlates with: TC 198 mg/d. L LDL-C 130 mg/d. L TG 90 mg/d. L HDL-C 50 mg/d. L Correlates with: TC 210 mg/d. L LDL-C 130 mg/d. L TG 250 mg/d. L HDL-C 30 mg/d. L Non–HDL-C 148 mg/d. L Non–HDL-C 180 mg/d. L Otvos JD, et al. Am J Cardiol. 2002; 90: 22 i-29 i. TC=total cholesterol, LDL-C=low-density lipoprotein cholesterol, TG=triglycerides, HDL-C=high-density lipoprotein cholesterol
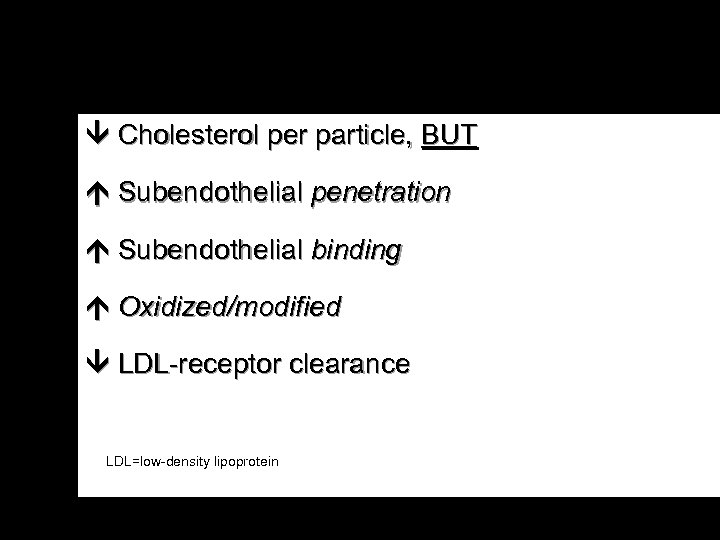 Cholesterol per particle, BUT Subendothelial penetration Subendothelial binding Oxidized/modified LDL-receptor clearance LDL=low-density lipoprotein
Cholesterol per particle, BUT Subendothelial penetration Subendothelial binding Oxidized/modified LDL-receptor clearance LDL=low-density lipoprotein
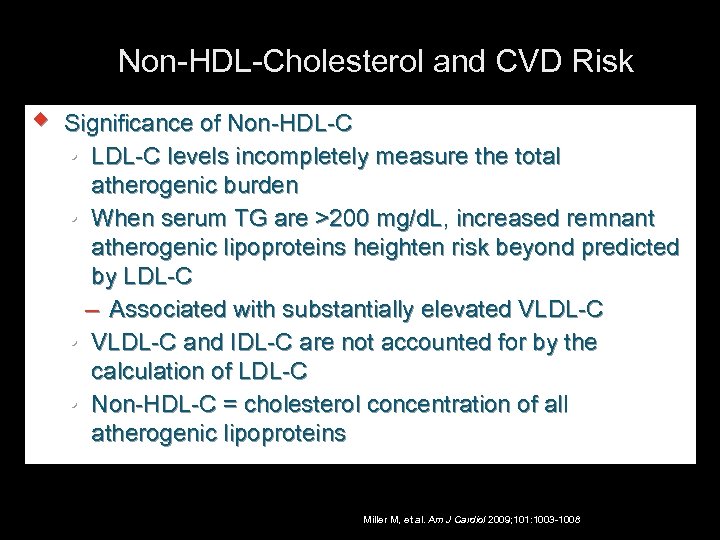 Non-HDL-Cholesterol and CVD Risk w Significance of Non-HDL-C LDL-C levels incompletely measure the total atherogenic burden When serum TG are >200 mg/d. L, increased remnant atherogenic lipoproteins heighten risk beyond predicted by LDL-C – Associated with substantially elevated VLDL-C and IDL-C are not accounted for by the calculation of LDL-C Non-HDL-C = cholesterol concentration of all atherogenic lipoproteins Miller M, et al. Am J Cardiol 2009; 101: 1003 -1008
Non-HDL-Cholesterol and CVD Risk w Significance of Non-HDL-C LDL-C levels incompletely measure the total atherogenic burden When serum TG are >200 mg/d. L, increased remnant atherogenic lipoproteins heighten risk beyond predicted by LDL-C – Associated with substantially elevated VLDL-C and IDL-C are not accounted for by the calculation of LDL-C Non-HDL-C = cholesterol concentration of all atherogenic lipoproteins Miller M, et al. Am J Cardiol 2009; 101: 1003 -1008
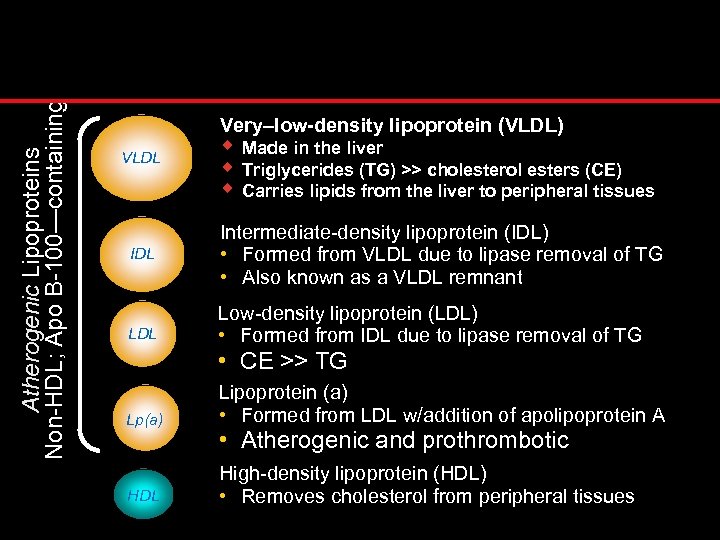 Atherogenic Lipoproteins Non-HDL; Apo B-100—containing Very–low-density lipoprotein (VLDL) VLDL IDL LDL w Made in the liver w Triglycerides (TG) >> cholesterol esters (CE) w Carries lipids from the liver to peripheral tissues Intermediate-density lipoprotein (IDL) • Formed from VLDL due to lipase removal of TG • Also known as a VLDL remnant Low-density lipoprotein (LDL) • Formed from IDL due to lipase removal of TG • CE >> TG Lp(a) Lipoprotein (a) • Formed from LDL w/addition of apolipoprotein A HDL High-density lipoprotein (HDL) • Removes cholesterol from peripheral tissues • Atherogenic and prothrombotic
Atherogenic Lipoproteins Non-HDL; Apo B-100—containing Very–low-density lipoprotein (VLDL) VLDL IDL LDL w Made in the liver w Triglycerides (TG) >> cholesterol esters (CE) w Carries lipids from the liver to peripheral tissues Intermediate-density lipoprotein (IDL) • Formed from VLDL due to lipase removal of TG • Also known as a VLDL remnant Low-density lipoprotein (LDL) • Formed from IDL due to lipase removal of TG • CE >> TG Lp(a) Lipoprotein (a) • Formed from LDL w/addition of apolipoprotein A HDL High-density lipoprotein (HDL) • Removes cholesterol from peripheral tissues • Atherogenic and prothrombotic
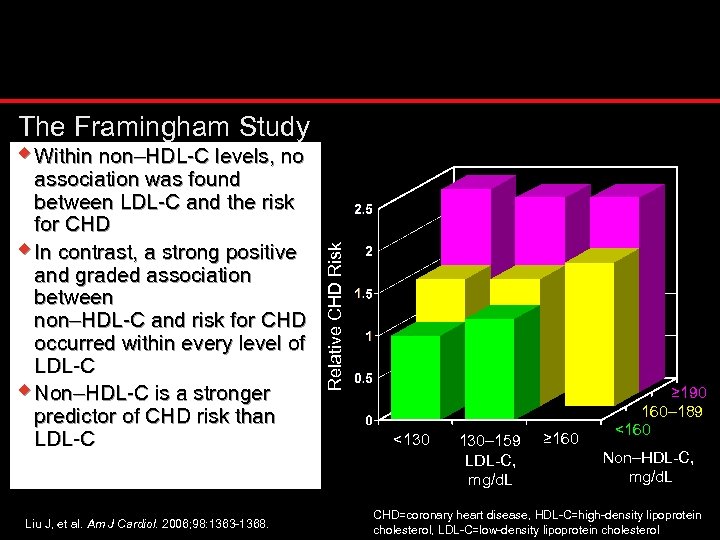 association was found between LDL-C and the risk for CHD w In contrast, a strong positive and graded association between non–HDL-C and risk for CHD occurred within every level of LDL-C w Non–HDL-C is a stronger predictor of CHD risk than LDL-C Liu J, et al. Am J Cardiol. 2006; 98: 1363 -1368. Relative CHD Risk The Framingham Study w Within non–HDL-C levels, no <130 130– 159 LDL-C, mg/d. L ≥ 160 ≥ 190 160– 189 <160 Non–HDL-C, mg/d. L CHD=coronary heart disease, HDL-C=high-density lipoprotein cholesterol, LDL-C=low-density lipoprotein cholesterol
association was found between LDL-C and the risk for CHD w In contrast, a strong positive and graded association between non–HDL-C and risk for CHD occurred within every level of LDL-C w Non–HDL-C is a stronger predictor of CHD risk than LDL-C Liu J, et al. Am J Cardiol. 2006; 98: 1363 -1368. Relative CHD Risk The Framingham Study w Within non–HDL-C levels, no <130 130– 159 LDL-C, mg/d. L ≥ 160 ≥ 190 160– 189 <160 Non–HDL-C, mg/d. L CHD=coronary heart disease, HDL-C=high-density lipoprotein cholesterol, LDL-C=low-density lipoprotein cholesterol
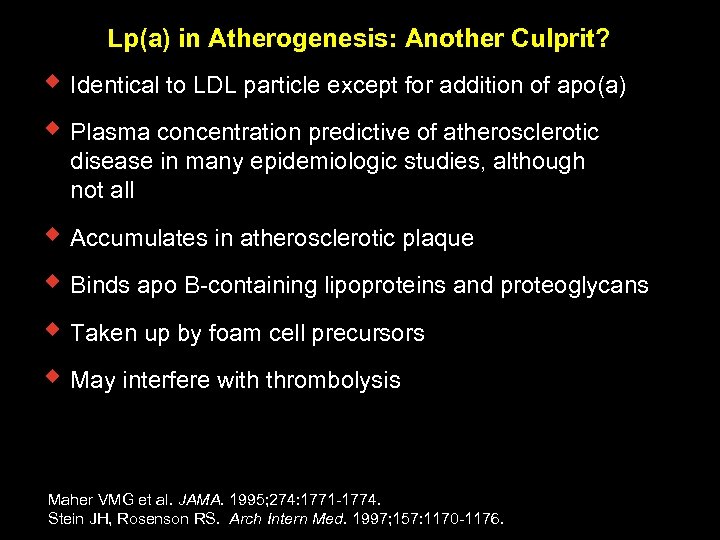 Lp(a) in Atherogenesis: Another Culprit? w Identical to LDL particle except for addition of apo(a) w Plasma concentration predictive of atherosclerotic disease in many epidemiologic studies, although not all w Accumulates in atherosclerotic plaque w Binds apo B-containing lipoproteins and proteoglycans w Taken up by foam cell precursors w May interfere with thrombolysis Maher VMG et al. JAMA. 1995; 274: 1771 -1774. Stein JH, Rosenson RS. Arch Intern Med. 1997; 157: 1170 -1176.
Lp(a) in Atherogenesis: Another Culprit? w Identical to LDL particle except for addition of apo(a) w Plasma concentration predictive of atherosclerotic disease in many epidemiologic studies, although not all w Accumulates in atherosclerotic plaque w Binds apo B-containing lipoproteins and proteoglycans w Taken up by foam cell precursors w May interfere with thrombolysis Maher VMG et al. JAMA. 1995; 274: 1771 -1774. Stein JH, Rosenson RS. Arch Intern Med. 1997; 157: 1170 -1176.
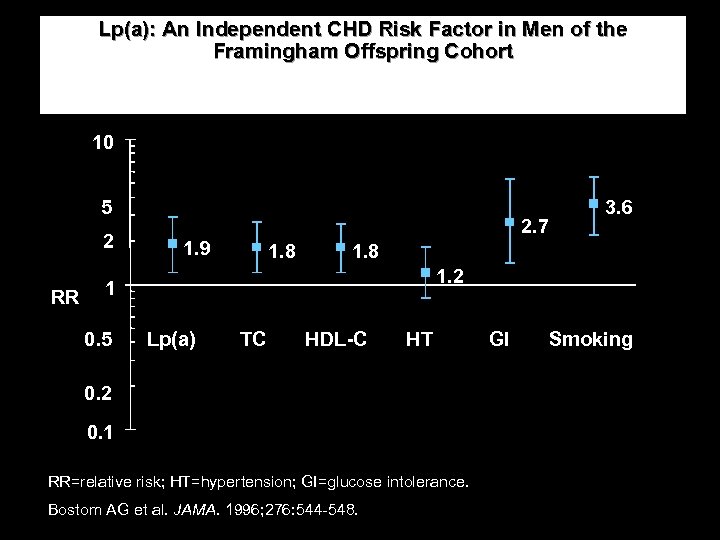 Lp(a): An Independent CHD Risk Factor in Men of the Framingham Offspring Cohort 10 5 2 RR 1. 9 2. 7 1. 8 1. 2 1 0. 5 3. 6 Lp(a) TC HDL-C HT GI Smoking 0. 2 0. 1 RR=relative risk; HT=hypertension; GI=glucose intolerance. Bostom AG et al. JAMA. 1996; 276: 544 -548.
Lp(a): An Independent CHD Risk Factor in Men of the Framingham Offspring Cohort 10 5 2 RR 1. 9 2. 7 1. 8 1. 2 1 0. 5 3. 6 Lp(a) TC HDL-C HT GI Smoking 0. 2 0. 1 RR=relative risk; HT=hypertension; GI=glucose intolerance. Bostom AG et al. JAMA. 1996; 276: 544 -548.
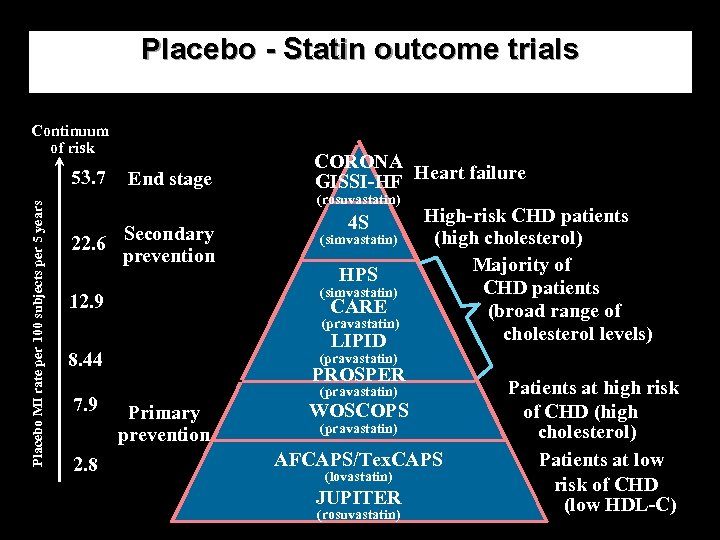 Placebo - Statin outcome trials Continuum of risk Placebo MI rate per 100 subjects per 5 years 53. 7 End stage CORONA GISSI-HF Heart failure (rosuvastatin) 22. 6 Secondary prevention 4 S (simvastatin) HPS (simvastatin) 12. 9 CARE (pravastatin) LIPID 8. 44 7. 9 2. 8 High-risk CHD patients (high cholesterol) Majority of CHD patients (broad range of cholesterol levels) (pravastatin) PROSPER (pravastatin) Primary prevention WOSCOPS (pravastatin) AFCAPS/Tex. CAPS (lovastatin) JUPITER (rosuvastatin) Patients at high risk of CHD (high cholesterol) Patients at low risk of CHD (low HDL-C)
Placebo - Statin outcome trials Continuum of risk Placebo MI rate per 100 subjects per 5 years 53. 7 End stage CORONA GISSI-HF Heart failure (rosuvastatin) 22. 6 Secondary prevention 4 S (simvastatin) HPS (simvastatin) 12. 9 CARE (pravastatin) LIPID 8. 44 7. 9 2. 8 High-risk CHD patients (high cholesterol) Majority of CHD patients (broad range of cholesterol levels) (pravastatin) PROSPER (pravastatin) Primary prevention WOSCOPS (pravastatin) AFCAPS/Tex. CAPS (lovastatin) JUPITER (rosuvastatin) Patients at high risk of CHD (high cholesterol) Patients at low risk of CHD (low HDL-C)
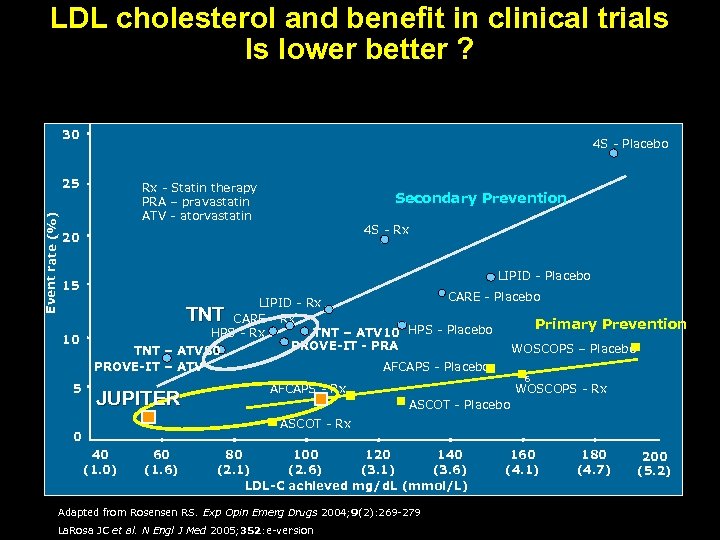 LDL cholesterol and benefit in clinical trials Is lower better ? 30 4 S - Placebo Event rate (%) 25 Rx - Statin therapy PRA – pravastatin ATV - atorvastatin Secondary Prevention 4 S - Rx 20 15 LIPID - Placebo CARE - Placebo LIPID - Rx CARE - Rx Primary Prevention HPS - Rx TNT – ATV 10 HPS - Placebo PROVE-IT - PRA WOSCOPS – Placebo TNT – ATV 80 PROVE-IT – ATV AFCAPS - Placebo TNT 10 5 JUPITER 6 AFCAPS - Rx WOSCOPS - Rx ASCOT - Placebo ASCOT - Rx 0 40 (1. 0) 60 (1. 6) 80 100 120 140 (2. 1) (2. 6) (3. 1) (3. 6) LDL-C achieved mg/d. L (mmol/L) Adapted from Rosensen RS. Exp Opin Emerg Drugs 2004; 9(2): 269 -279 La. Rosa JC et al. N Engl J Med 2005; 352: e-version 160 (4. 1) 180 (4. 7) 200 (5. 2)
LDL cholesterol and benefit in clinical trials Is lower better ? 30 4 S - Placebo Event rate (%) 25 Rx - Statin therapy PRA – pravastatin ATV - atorvastatin Secondary Prevention 4 S - Rx 20 15 LIPID - Placebo CARE - Placebo LIPID - Rx CARE - Rx Primary Prevention HPS - Rx TNT – ATV 10 HPS - Placebo PROVE-IT - PRA WOSCOPS – Placebo TNT – ATV 80 PROVE-IT – ATV AFCAPS - Placebo TNT 10 5 JUPITER 6 AFCAPS - Rx WOSCOPS - Rx ASCOT - Placebo ASCOT - Rx 0 40 (1. 0) 60 (1. 6) 80 100 120 140 (2. 1) (2. 6) (3. 1) (3. 6) LDL-C achieved mg/d. L (mmol/L) Adapted from Rosensen RS. Exp Opin Emerg Drugs 2004; 9(2): 269 -279 La. Rosa JC et al. N Engl J Med 2005; 352: e-version 160 (4. 1) 180 (4. 7) 200 (5. 2)
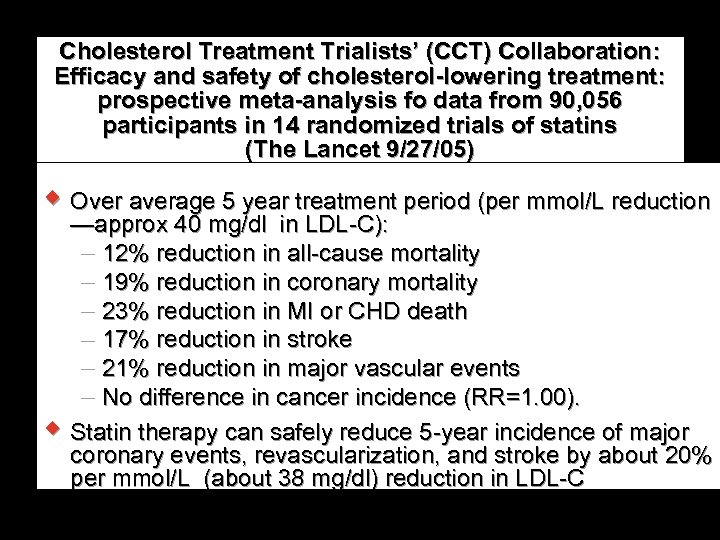 Cholesterol Treatment Trialists’ (CCT) Collaboration: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis fo data from 90, 056 participants in 14 randomized trials of statins (The Lancet 9/27/05) w Over average 5 year treatment period (per mmol/L reduction —approx 40 mg/dl in LDL-C): 12% reduction in all-cause mortality 19% reduction in coronary mortality 23% reduction in MI or CHD death 17% reduction in stroke 21% reduction in major vascular events No difference in cancer incidence (RR=1. 00). w Statin therapy can safely reduce 5 -year incidence of major coronary events, revascularization, and stroke by about 20% per mmol/L (about 38 mg/dl) reduction in LDL-C
Cholesterol Treatment Trialists’ (CCT) Collaboration: Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis fo data from 90, 056 participants in 14 randomized trials of statins (The Lancet 9/27/05) w Over average 5 year treatment period (per mmol/L reduction —approx 40 mg/dl in LDL-C): 12% reduction in all-cause mortality 19% reduction in coronary mortality 23% reduction in MI or CHD death 17% reduction in stroke 21% reduction in major vascular events No difference in cancer incidence (RR=1. 00). w Statin therapy can safely reduce 5 -year incidence of major coronary events, revascularization, and stroke by about 20% per mmol/L (about 38 mg/dl) reduction in LDL-C
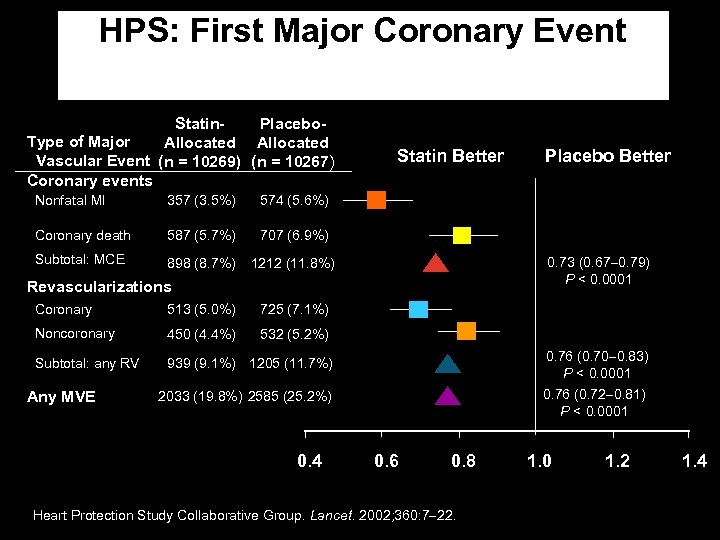 HPS: First Major Coronary Event Statin. Placebo. Type of Major Allocated Vascular Event (n = 10269) (n = 10267) Coronary events Nonfatal MI 357 (3. 5%) 587 (5. 7%) 707 (6. 9%) Subtotal: MCE 898 (8. 7%) 1212 (11. 8%) Placebo Better 574 (5. 6%) Coronary death Statin Better 0. 73 (0. 67 0. 79) P < 0. 0001 Revascularizations Coronary 513 (5. 0%) 725 (7. 1%) Noncoronary 450 (4. 4%) 532 (5. 2%) Subtotal: any RV 939 (9. 1%) 1205 (11. 7%) Any MVE 0. 76 (0. 70 0. 83) P < 0. 0001 0. 76 (0. 72 0. 81) P < 0. 0001 2033 (19. 8%) 2585 (25. 2%) 0. 4 0. 6 0. 8 Heart Protection Study Collaborative Group. Lancet. 2002; 360: 7 22. 1. 0 1. 2 1. 4
HPS: First Major Coronary Event Statin. Placebo. Type of Major Allocated Vascular Event (n = 10269) (n = 10267) Coronary events Nonfatal MI 357 (3. 5%) 587 (5. 7%) 707 (6. 9%) Subtotal: MCE 898 (8. 7%) 1212 (11. 8%) Placebo Better 574 (5. 6%) Coronary death Statin Better 0. 73 (0. 67 0. 79) P < 0. 0001 Revascularizations Coronary 513 (5. 0%) 725 (7. 1%) Noncoronary 450 (4. 4%) 532 (5. 2%) Subtotal: any RV 939 (9. 1%) 1205 (11. 7%) Any MVE 0. 76 (0. 70 0. 83) P < 0. 0001 0. 76 (0. 72 0. 81) P < 0. 0001 2033 (19. 8%) 2585 (25. 2%) 0. 4 0. 6 0. 8 Heart Protection Study Collaborative Group. Lancet. 2002; 360: 7 22. 1. 0 1. 2 1. 4
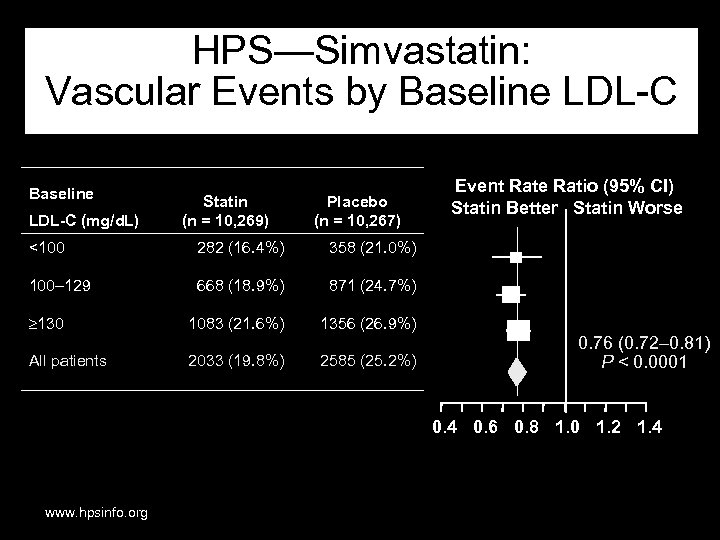 HPS—Simvastatin: Vascular Events by Baseline LDL-C (mg/d. L) Statin (n = 10, 269) Placebo (n = 10, 267) <100 282 (16. 4%) 358 (21. 0%) 100– 129 668 (18. 9%) 871 (24. 7%) 130 1083 (21. 6%) 1356 (26. 9%) All patients 2033 (19. 8%) 2585 (25. 2%) Event Rate Ratio (95% CI) Statin Better Statin Worse 0. 76 (0. 72– 0. 81) P < 0. 0001 0. 4 0. 6 0. 8 1. 0 1. 2 1. 4 www. hpsinfo. org
HPS—Simvastatin: Vascular Events by Baseline LDL-C (mg/d. L) Statin (n = 10, 269) Placebo (n = 10, 267) <100 282 (16. 4%) 358 (21. 0%) 100– 129 668 (18. 9%) 871 (24. 7%) 130 1083 (21. 6%) 1356 (26. 9%) All patients 2033 (19. 8%) 2585 (25. 2%) Event Rate Ratio (95% CI) Statin Better Statin Worse 0. 76 (0. 72– 0. 81) P < 0. 0001 0. 4 0. 6 0. 8 1. 0 1. 2 1. 4 www. hpsinfo. org
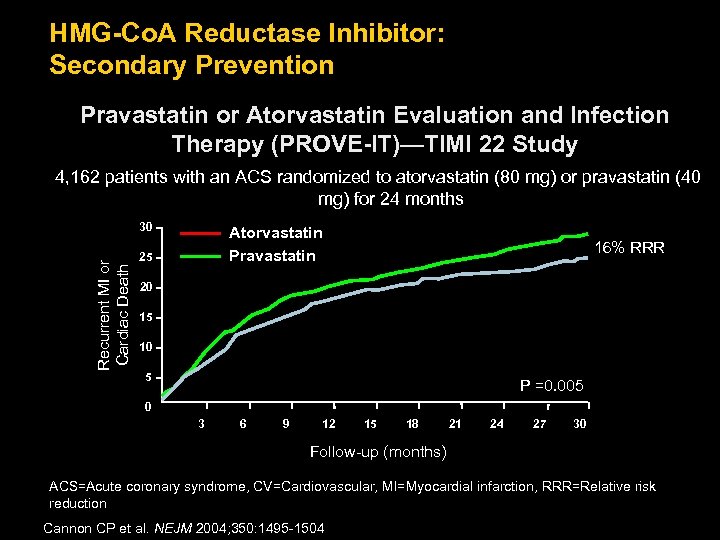 HMG-Co. A Reductase Inhibitor: Secondary Prevention Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT)—TIMI 22 Study 4, 162 patients with an ACS randomized to atorvastatin (80 mg) or pravastatin (40 mg) for 24 months Recurrent MI or Cardiac Death 30 Atorvastatin Pravastatin 25 16% RRR 20 15 10 5 P =0. 005 0 3 6 9 12 15 18 21 24 27 30 Follow-up (months) ACS=Acute coronary syndrome, CV=Cardiovascular, MI=Myocardial infarction, RRR=Relative risk reduction Cannon CP et al. NEJM 2004; 350: 1495 -1504
HMG-Co. A Reductase Inhibitor: Secondary Prevention Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT)—TIMI 22 Study 4, 162 patients with an ACS randomized to atorvastatin (80 mg) or pravastatin (40 mg) for 24 months Recurrent MI or Cardiac Death 30 Atorvastatin Pravastatin 25 16% RRR 20 15 10 5 P =0. 005 0 3 6 9 12 15 18 21 24 27 30 Follow-up (months) ACS=Acute coronary syndrome, CV=Cardiovascular, MI=Myocardial infarction, RRR=Relative risk reduction Cannon CP et al. NEJM 2004; 350: 1495 -1504
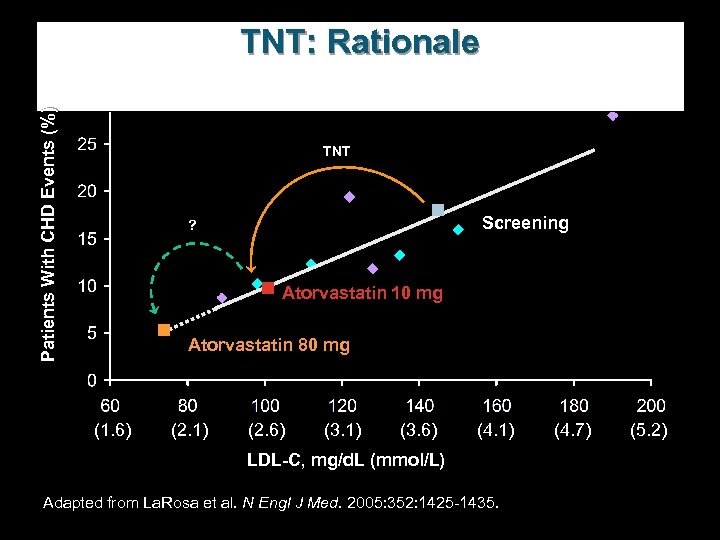 Patients With CHD Events (%) TNT: Rationale TNT Screening ? Atorvastatin 10 mg Atorvastatin 80 mg (1. 6) (2. 1) (2. 6) (3. 1) (3. 6) (4. 1) LDL-C, mg/d. L (mmol/L) Adapted from La. Rosa et al. N Engl J Med. 2005: 352: 1425 -1435. (4. 7) (5. 2)
Patients With CHD Events (%) TNT: Rationale TNT Screening ? Atorvastatin 10 mg Atorvastatin 80 mg (1. 6) (2. 1) (2. 6) (3. 1) (3. 6) (4. 1) LDL-C, mg/d. L (mmol/L) Adapted from La. Rosa et al. N Engl J Med. 2005: 352: 1425 -1435. (4. 7) (5. 2)
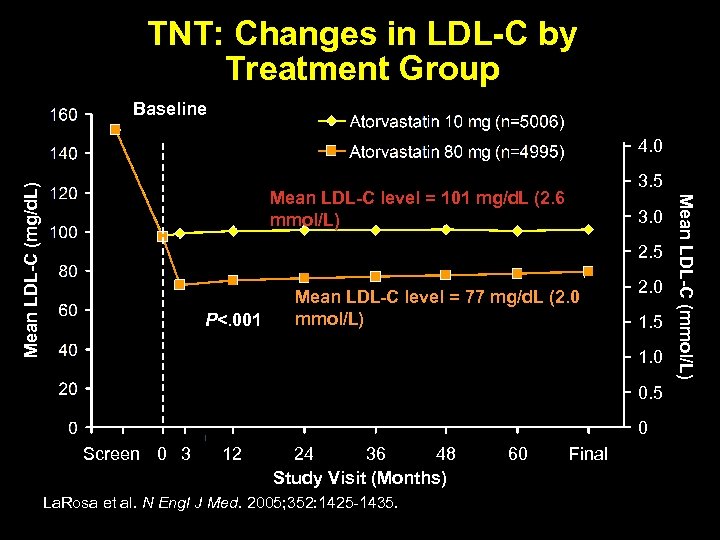 TNT: Changes in LDL-C by Treatment Group Baseline Mean LDL-C level = 101 mg/d. L (2. 6 mmol/L) 3. 5 3. 0 2. 5 P<. 001 Mean LDL-C level = 77 mg/d. L (2. 0 mmol/L) 2. 0 1. 5 1. 0 0. 5 0 Screen 0 3 12 24 36 48 Study Visit (Months) La. Rosa et al. N Engl J Med. 2005; 352: 1425 -1435. 60 Final Mean LDL-C (mmol/L) Mean LDL-C (mg/d. L) 4. 0
TNT: Changes in LDL-C by Treatment Group Baseline Mean LDL-C level = 101 mg/d. L (2. 6 mmol/L) 3. 5 3. 0 2. 5 P<. 001 Mean LDL-C level = 77 mg/d. L (2. 0 mmol/L) 2. 0 1. 5 1. 0 0. 5 0 Screen 0 3 12 24 36 48 Study Visit (Months) La. Rosa et al. N Engl J Med. 2005; 352: 1425 -1435. 60 Final Mean LDL-C (mmol/L) Mean LDL-C (mg/d. L) 4. 0
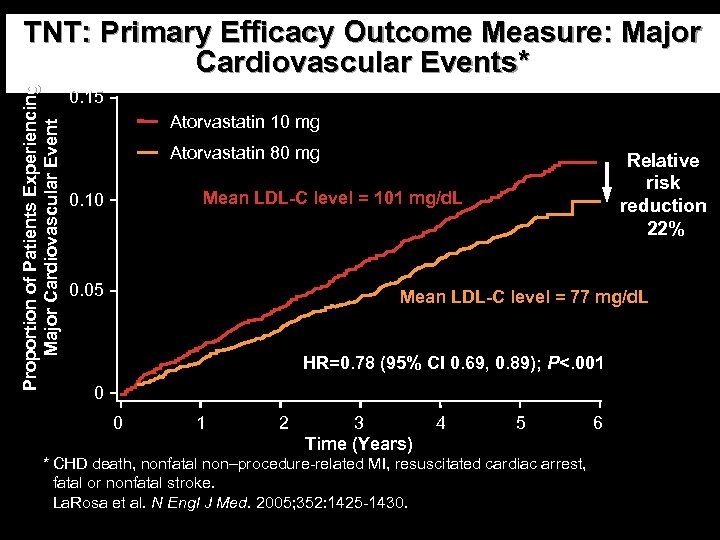 Proportion of Patients Experiencing Major Cardiovascular Event TNT: Primary Efficacy Outcome Measure: Major Cardiovascular Events* 0. 15 Atorvastatin 10 mg Atorvastatin 80 mg Relative risk reduction 22% Mean LDL-C level = 101 mg/d. L 0. 10 0. 05 Mean LDL-C level = 77 mg/d. L HR=0. 78 (95% CI 0. 69, 0. 89); P<. 001 0 0 1 2 3 Time (Years) 4 5 * CHD death, nonfatal non–procedure-related MI, resuscitated cardiac arrest, fatal or nonfatal stroke. La. Rosa et al. N Engl J Med. 2005; 352: 1425 -1430. 6
Proportion of Patients Experiencing Major Cardiovascular Event TNT: Primary Efficacy Outcome Measure: Major Cardiovascular Events* 0. 15 Atorvastatin 10 mg Atorvastatin 80 mg Relative risk reduction 22% Mean LDL-C level = 101 mg/d. L 0. 10 0. 05 Mean LDL-C level = 77 mg/d. L HR=0. 78 (95% CI 0. 69, 0. 89); P<. 001 0 0 1 2 3 Time (Years) 4 5 * CHD death, nonfatal non–procedure-related MI, resuscitated cardiac arrest, fatal or nonfatal stroke. La. Rosa et al. N Engl J Med. 2005; 352: 1425 -1430. 6
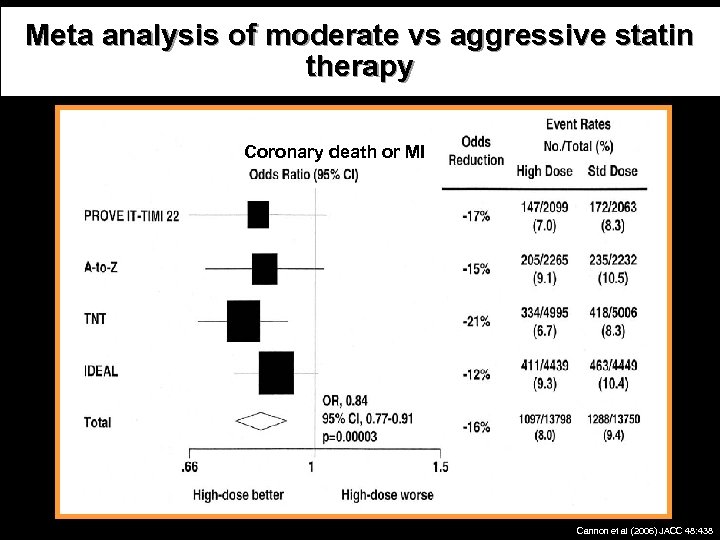 Meta analysis of moderate vs aggressive statin therapy Coronary death or MI ACS Stable CHD Cannon et al (2006) JACC 48: 438
Meta analysis of moderate vs aggressive statin therapy Coronary death or MI ACS Stable CHD Cannon et al (2006) JACC 48: 438
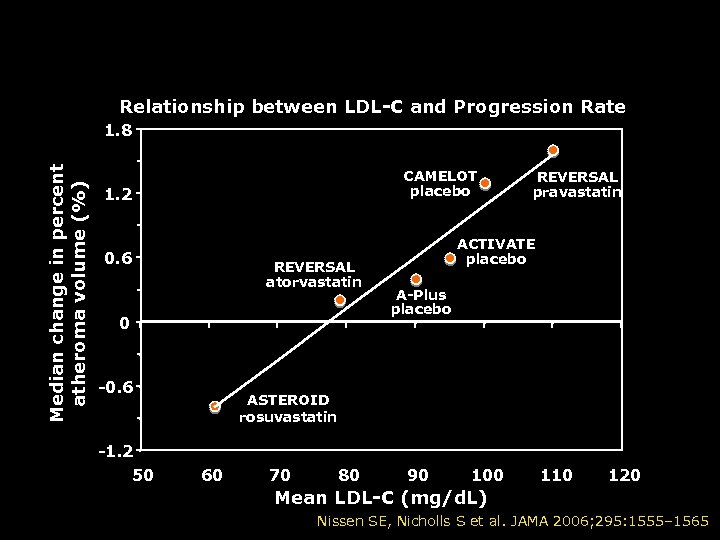 Recent Coronary IVUS Progression Trials Relationship between LDL-C and Progression Rate Median change in percent atheroma volume (%) 1. 8 CAMELOT placebo 1. 2 0. 6 REVERSAL atorvastatin 0 -0. 6 -1. 2 50 REVERSAL pravastatin ACTIVATE placebo A-Plus placebo ASTEROID rosuvastatin 60 70 80 90 100 110 120 Mean LDL-C (mg/d. L) Nissen SE, Nicholls S et al. JAMA 2006; 295: 1555– 1565
Recent Coronary IVUS Progression Trials Relationship between LDL-C and Progression Rate Median change in percent atheroma volume (%) 1. 8 CAMELOT placebo 1. 2 0. 6 REVERSAL atorvastatin 0 -0. 6 -1. 2 50 REVERSAL pravastatin ACTIVATE placebo A-Plus placebo ASTEROID rosuvastatin 60 70 80 90 100 110 120 Mean LDL-C (mg/d. L) Nissen SE, Nicholls S et al. JAMA 2006; 295: 1555– 1565
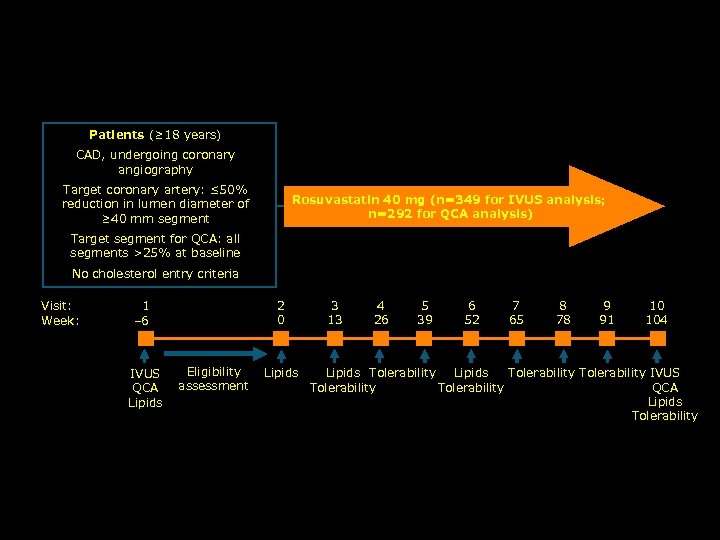 ASTEROID: Study Design Patients (≥ 18 years) CAD, undergoing coronary angiography Target coronary artery: ≤ 50% reduction in lumen diameter of ≥ 40 mm segment Rosuvastatin 40 mg (n=349 for IVUS analysis; n=292 for QCA analysis) Target segment for QCA: all segments >25% at baseline No cholesterol entry criteria Visit: Week: 2 0 1 – 6 IVUS QCA Lipids Eligibility assessment Lipids 3 13 4 26 5 39 6 52 7 65 8 78 9 91 10 104 Lipids Tolerability IVUS QCA Tolerability Lipids Tolerability
ASTEROID: Study Design Patients (≥ 18 years) CAD, undergoing coronary angiography Target coronary artery: ≤ 50% reduction in lumen diameter of ≥ 40 mm segment Rosuvastatin 40 mg (n=349 for IVUS analysis; n=292 for QCA analysis) Target segment for QCA: all segments >25% at baseline No cholesterol entry criteria Visit: Week: 2 0 1 – 6 IVUS QCA Lipids Eligibility assessment Lipids 3 13 4 26 5 39 6 52 7 65 8 78 9 91 10 104 Lipids Tolerability IVUS QCA Tolerability Lipids Tolerability
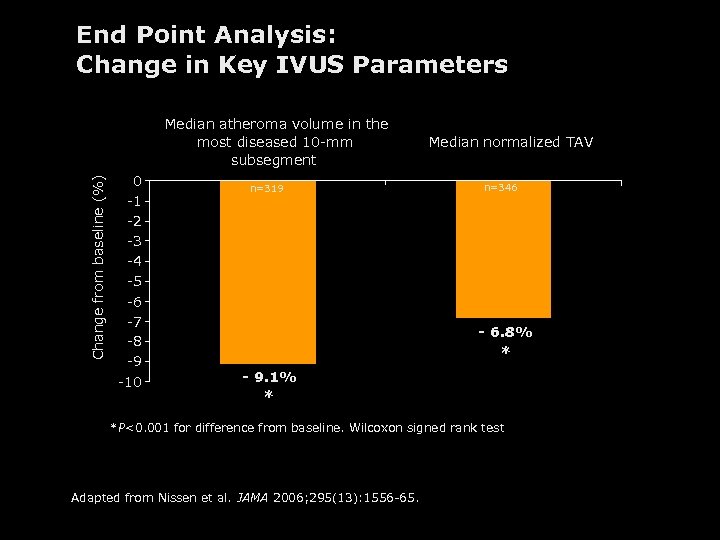 End Point Analysis: Change in Key IVUS Parameters Change from baseline (%) Median atheroma volume in the most diseased 10 -mm subsegment 0 -1 -2 -3 -4 -5 -6 -7 -8 -9 -10 n=319 Median normalized TAV n=346 - 6. 8% * - 9. 1% * *P<0. 001 for difference from baseline. Wilcoxon signed rank test Adapted from Nissen et al. JAMA 2006; 295(13): 1556 -65.
End Point Analysis: Change in Key IVUS Parameters Change from baseline (%) Median atheroma volume in the most diseased 10 -mm subsegment 0 -1 -2 -3 -4 -5 -6 -7 -8 -9 -10 n=319 Median normalized TAV n=346 - 6. 8% * - 9. 1% * *P<0. 001 for difference from baseline. Wilcoxon signed rank test Adapted from Nissen et al. JAMA 2006; 295(13): 1556 -65.
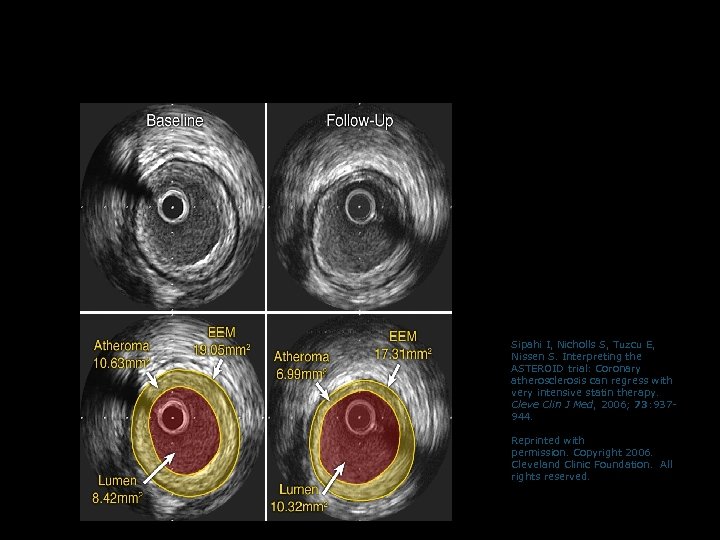 Example of Regression of Atherosclerosis with Rosuvastatin in ASTEROID (measured by IVUS) Sipahi I, Nicholls S, Tuzcu E, Nissen S. Interpreting the ASTEROID trial: Coronary atherosclerosis can regress with very intensive statin therapy. Cleve Clin J Med, 2006; 73: 937944. Reprinted with permission. Copyright 2006. Cleveland Clinic Foundation. All rights reserved.
Example of Regression of Atherosclerosis with Rosuvastatin in ASTEROID (measured by IVUS) Sipahi I, Nicholls S, Tuzcu E, Nissen S. Interpreting the ASTEROID trial: Coronary atherosclerosis can regress with very intensive statin therapy. Cleve Clin J Med, 2006; 73: 937944. Reprinted with permission. Copyright 2006. Cleveland Clinic Foundation. All rights reserved.
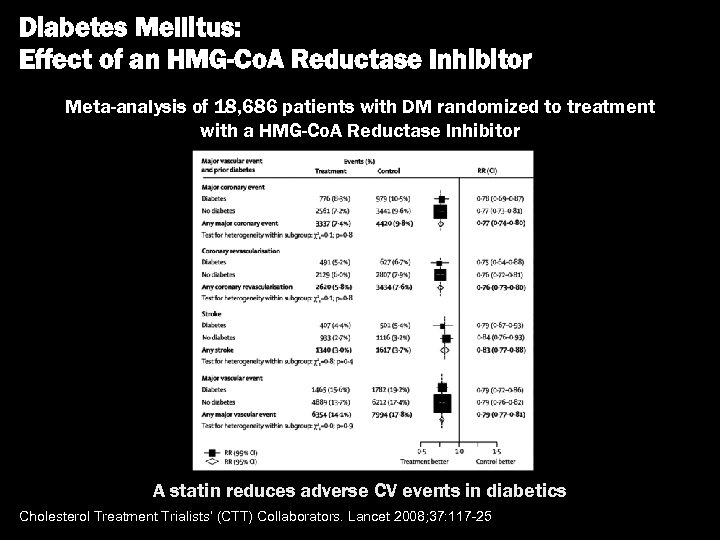 Diabetes Mellitus: Effect of an HMG-Co. A Reductase Inhibitor Meta-analysis of 18, 686 patients with DM randomized to treatment with a HMG-Co. A Reductase Inhibitor A statin reduces adverse CV events in diabetics Cholesterol Treatment Trialists’ (CTT) Collaborators. Lancet 2008; 37: 117 -25
Diabetes Mellitus: Effect of an HMG-Co. A Reductase Inhibitor Meta-analysis of 18, 686 patients with DM randomized to treatment with a HMG-Co. A Reductase Inhibitor A statin reduces adverse CV events in diabetics Cholesterol Treatment Trialists’ (CTT) Collaborators. Lancet 2008; 37: 117 -25
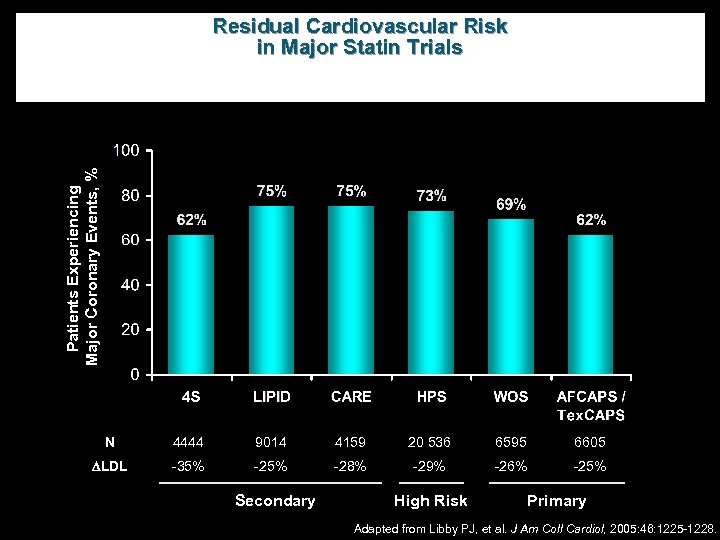 Patients Experiencing Major Coronary Events, % Residual Cardiovascular Risk in Major Statin Trials N 4444 9014 4159 20 536 6595 6605 LDL -35% -28% -29% -26% -25% Secondary High Risk Primary Adapted from Libby PJ, et al. J Am Coll Cardiol, 2005: 46: 1225 -1228.
Patients Experiencing Major Coronary Events, % Residual Cardiovascular Risk in Major Statin Trials N 4444 9014 4159 20 536 6595 6605 LDL -35% -28% -29% -26% -25% Secondary High Risk Primary Adapted from Libby PJ, et al. J Am Coll Cardiol, 2005: 46: 1225 -1228.
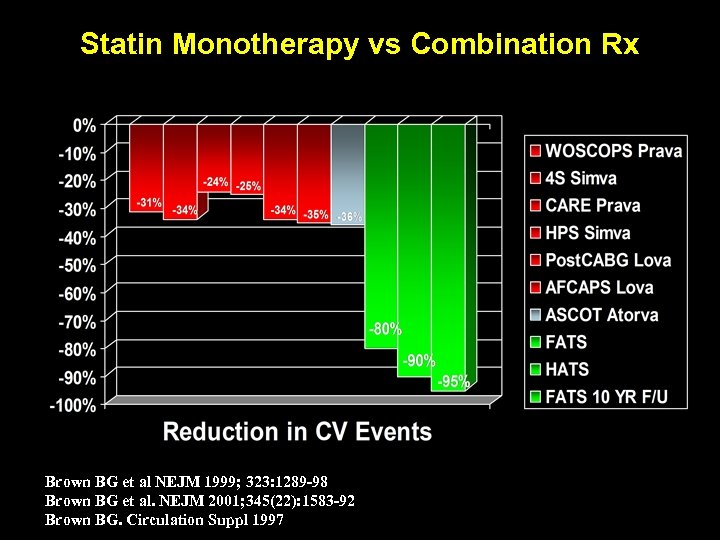 Statin Monotherapy vs Combination Rx Brown BG et al NEJM 1999; 323: 1289 -98 Brown BG et al. NEJM 2001; 345(22): 1583 -92 Brown BG. Circulation Suppl 1997
Statin Monotherapy vs Combination Rx Brown BG et al NEJM 1999; 323: 1289 -98 Brown BG et al. NEJM 2001; 345(22): 1583 -92 Brown BG. Circulation Suppl 1997
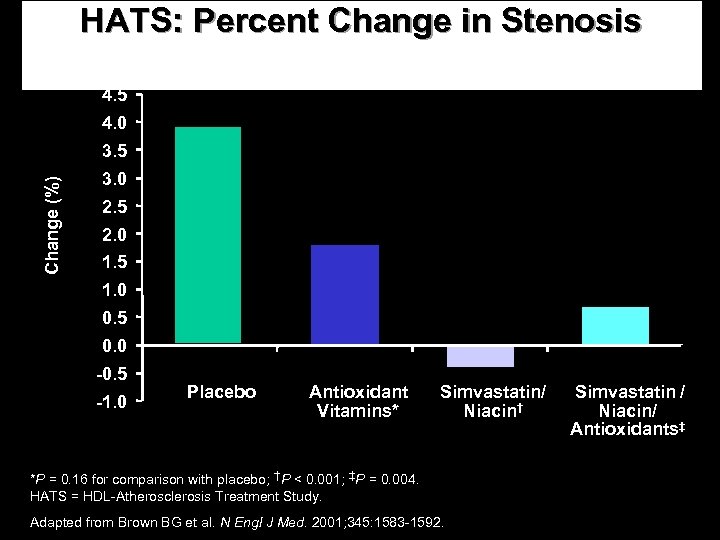 HATS: Percent Change in Stenosis 4. 5 4. 0 Change (%) 3. 5 3. 0 2. 5 2. 0 1. 5 1. 0 0. 5 0. 0 -0. 5 -1. 0 Placebo Antioxidant Vitamins* Simvastatin/ Niacin† *P = 0. 16 for comparison with placebo; †P < 0. 001; ‡P = 0. 004. HATS = HDL-Atherosclerosis Treatment Study. Adapted from Brown BG et al. N Engl J Med. 2001; 345: 1583 -1592. Simvastatin / Niacin/ Antioxidants‡
HATS: Percent Change in Stenosis 4. 5 4. 0 Change (%) 3. 5 3. 0 2. 5 2. 0 1. 5 1. 0 0. 5 0. 0 -0. 5 -1. 0 Placebo Antioxidant Vitamins* Simvastatin/ Niacin† *P = 0. 16 for comparison with placebo; †P < 0. 001; ‡P = 0. 004. HATS = HDL-Atherosclerosis Treatment Study. Adapted from Brown BG et al. N Engl J Med. 2001; 345: 1583 -1592. Simvastatin / Niacin/ Antioxidants‡
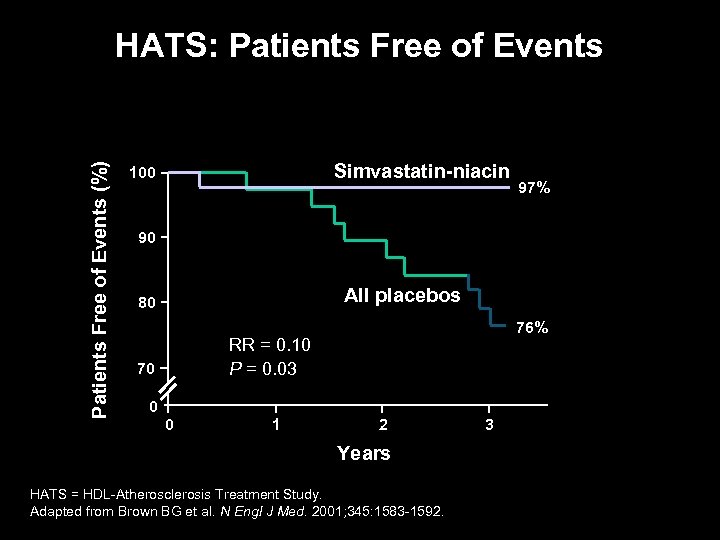 Patients Free of Events (%) HATS: Patients Free of Events Simvastatin-niacin 100 97% 90 All placebos 80 76% RR = 0. 10 P = 0. 03 70 0 0 1 2 Years HATS = HDL-Atherosclerosis Treatment Study. Adapted from Brown BG et al. N Engl J Med. 2001; 345: 1583 -1592. 3
Patients Free of Events (%) HATS: Patients Free of Events Simvastatin-niacin 100 97% 90 All placebos 80 76% RR = 0. 10 P = 0. 03 70 0 0 1 2 Years HATS = HDL-Atherosclerosis Treatment Study. Adapted from Brown BG et al. N Engl J Med. 2001; 345: 1583 -1592. 3
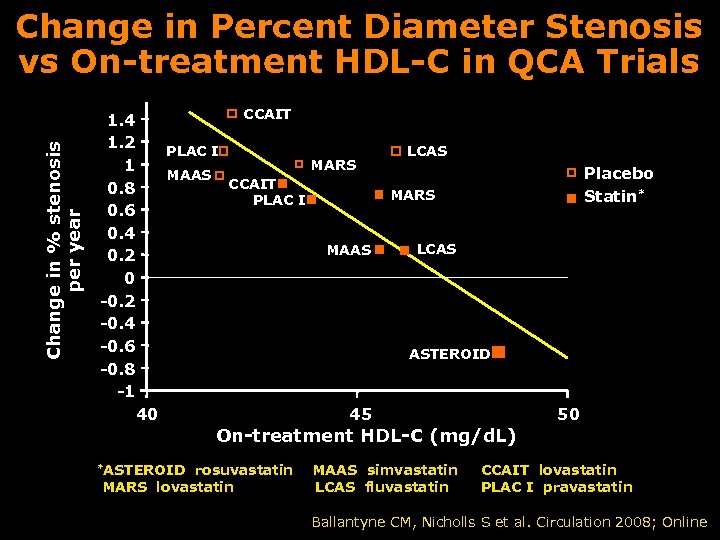 Change in % stenosis per year Change in Percent Diameter Stenosis vs On-treatment HDL-C in QCA Trials CCAIT 1. 4 1. 2 PLAC I MARS 1 MAAS CCAIT 0. 8 PLAC I 0. 6 0. 4 MAAS 0. 2 0 -0. 2 -0. 4 -0. 6 -0. 8 -1 40 45 LCAS Placebo Statin* MARS LCAS ASTEROID 50 On-treatment HDL-C (mg/d. L) *ASTEROID rosuvastatin MARS lovastatin MAAS simvastatin LCAS fluvastatin CCAIT lovastatin PLAC I pravastatin Ballantyne CM, Nicholls S et al. Circulation 2008; Online
Change in % stenosis per year Change in Percent Diameter Stenosis vs On-treatment HDL-C in QCA Trials CCAIT 1. 4 1. 2 PLAC I MARS 1 MAAS CCAIT 0. 8 PLAC I 0. 6 0. 4 MAAS 0. 2 0 -0. 2 -0. 4 -0. 6 -0. 8 -1 40 45 LCAS Placebo Statin* MARS LCAS ASTEROID 50 On-treatment HDL-C (mg/d. L) *ASTEROID rosuvastatin MARS lovastatin MAAS simvastatin LCAS fluvastatin CCAIT lovastatin PLAC I pravastatin Ballantyne CM, Nicholls S et al. Circulation 2008; Online
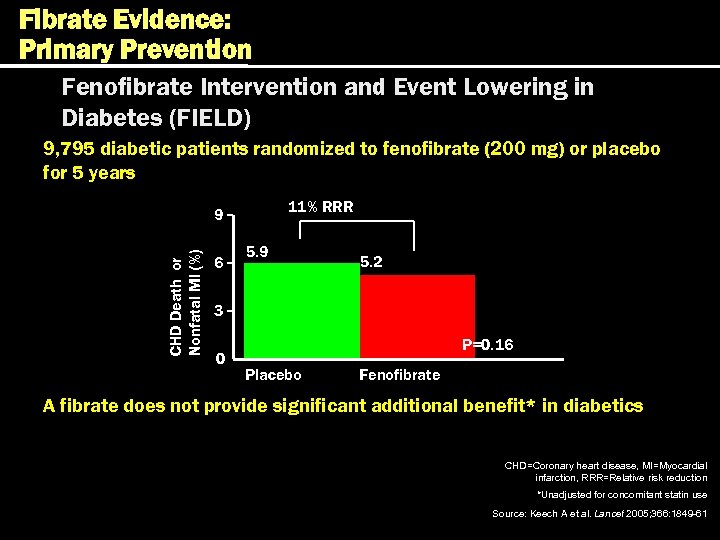 Fibrate Evidence: Primary Prevention Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) 9, 795 diabetic patients randomized to fenofibrate (200 mg) or placebo for 5 years 11% RRR CHD Death or Nonfatal MI (%) 9 6 5. 9 5. 2 3 0 P=0. 16 Placebo Fenofibrate A fibrate does not provide significant additional benefit* in diabetics CHD=Coronary heart disease, MI=Myocardial infarction, RRR=Relative risk reduction *Unadjusted for concomitant statin use Source: Keech A et al. Lancet 2005; 366: 1849 -61
Fibrate Evidence: Primary Prevention Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) 9, 795 diabetic patients randomized to fenofibrate (200 mg) or placebo for 5 years 11% RRR CHD Death or Nonfatal MI (%) 9 6 5. 9 5. 2 3 0 P=0. 16 Placebo Fenofibrate A fibrate does not provide significant additional benefit* in diabetics CHD=Coronary heart disease, MI=Myocardial infarction, RRR=Relative risk reduction *Unadjusted for concomitant statin use Source: Keech A et al. Lancet 2005; 366: 1849 -61
 ACCORD Lipid Study Results (NEJM 2010; 362: 1563 -74) w 5518 patients with type 2 DM treated with open label simvastatin randomly assigned to fenofibrate or placebo and followed for 4. 7 years. w Annual rate of primary outcome of nonfatal MI, stroke or CVD death 2. 2% in fenofibrate group vs. 1. 6% in placebo group (HR=0. 91, p=0. 33). w Pre-specified subgroup analyses showed possible benefit in men vs. women and those with high triglycerides and low HDL-C. w Results support statin therapy alone to reduce CVD risk in high risk type 2 DM patients.
ACCORD Lipid Study Results (NEJM 2010; 362: 1563 -74) w 5518 patients with type 2 DM treated with open label simvastatin randomly assigned to fenofibrate or placebo and followed for 4. 7 years. w Annual rate of primary outcome of nonfatal MI, stroke or CVD death 2. 2% in fenofibrate group vs. 1. 6% in placebo group (HR=0. 91, p=0. 33). w Pre-specified subgroup analyses showed possible benefit in men vs. women and those with high triglycerides and low HDL-C. w Results support statin therapy alone to reduce CVD risk in high risk type 2 DM patients.
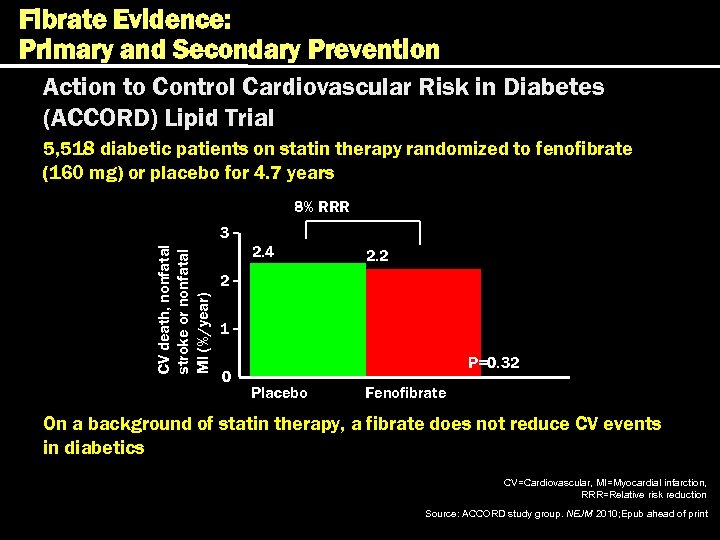 Fibrate Evidence: Primary and Secondary Prevention Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid Trial 5, 518 diabetic patients on statin therapy randomized to fenofibrate (160 mg) or placebo for 4. 7 years 8% RRR CV death, nonfatal stroke or nonfatal MI (%/year) 3 2. 4 2. 2 2 1 0 P=0. 32 Placebo Fenofibrate On a background of statin therapy, a fibrate does not reduce CV events in diabetics CV=Cardiovascular, MI=Myocardial infarction, RRR=Relative risk reduction Source: ACCORD study group. NEJM 2010; Epub ahead of print
Fibrate Evidence: Primary and Secondary Prevention Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid Trial 5, 518 diabetic patients on statin therapy randomized to fenofibrate (160 mg) or placebo for 4. 7 years 8% RRR CV death, nonfatal stroke or nonfatal MI (%/year) 3 2. 4 2. 2 2 1 0 P=0. 32 Placebo Fenofibrate On a background of statin therapy, a fibrate does not reduce CV events in diabetics CV=Cardiovascular, MI=Myocardial infarction, RRR=Relative risk reduction Source: ACCORD study group. NEJM 2010; Epub ahead of print
 AIM-HIGH NEJM 11/15/2011
AIM-HIGH NEJM 11/15/2011
 Objective To determine whether the residual risk associated with low levels of HDL-C in patients with established CHD whose LDL-C therapy was optimized with statins ± ezetimibe would be mitigated with extended-release niacin vs. placebo during long-term follow-up
Objective To determine whether the residual risk associated with low levels of HDL-C in patients with established CHD whose LDL-C therapy was optimized with statins ± ezetimibe would be mitigated with extended-release niacin vs. placebo during long-term follow-up
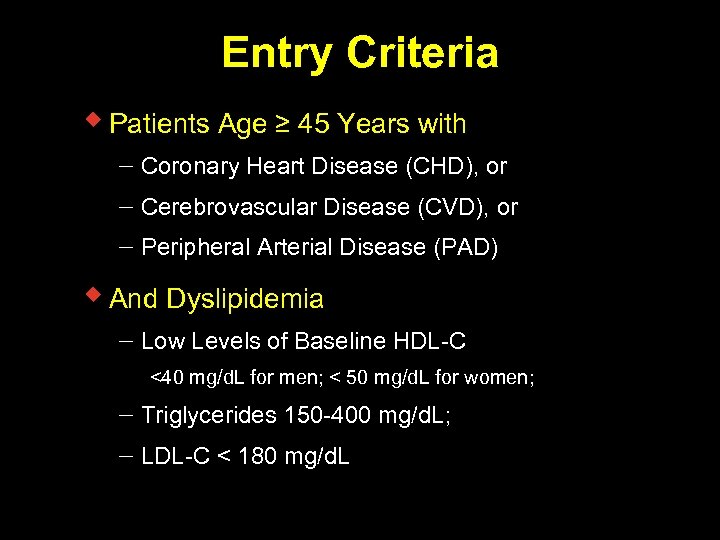 Entry Criteria w Patients Age ≥ 45 Years with Coronary Heart Disease (CHD), or Cerebrovascular Disease (CVD), or Peripheral Arterial Disease (PAD) w And Dyslipidemia Low Levels of Baseline HDL-C <40 mg/d. L for men; < 50 mg/d. L for women; Triglycerides 150 -400 mg/d. L; LDL-C < 180 mg/d. L
Entry Criteria w Patients Age ≥ 45 Years with Coronary Heart Disease (CHD), or Cerebrovascular Disease (CVD), or Peripheral Arterial Disease (PAD) w And Dyslipidemia Low Levels of Baseline HDL-C <40 mg/d. L for men; < 50 mg/d. L for women; Triglycerides 150 -400 mg/d. L; LDL-C < 180 mg/d. L
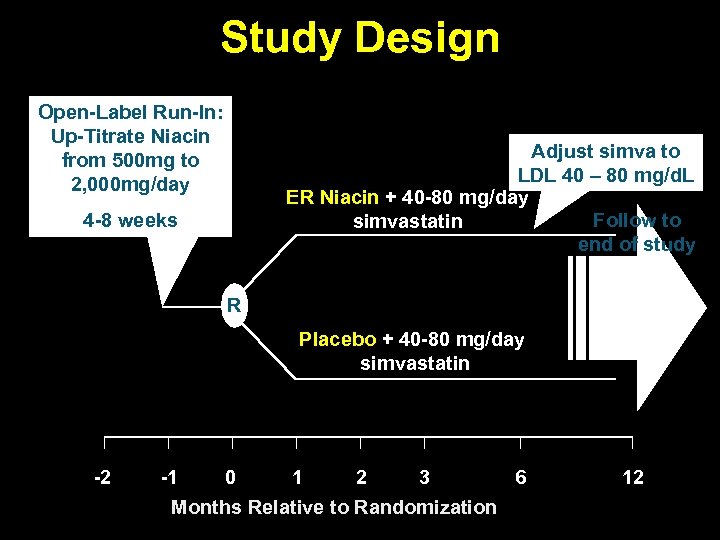 Study Design Open-Label Run-In: Up-Titrate Niacin from 500 mg to 2, 000 mg/day 4 -8 weeks Adjust simva to LDL 40 – 80 mg/d. L ER Niacin + 40 -80 mg/day Follow to simvastatin end of study R Placebo + 40 -80 mg/day simvastatin -2 -1 0 1 2 3 6 Months Relative to Randomization 12
Study Design Open-Label Run-In: Up-Titrate Niacin from 500 mg to 2, 000 mg/day 4 -8 weeks Adjust simva to LDL 40 – 80 mg/d. L ER Niacin + 40 -80 mg/day Follow to simvastatin end of study R Placebo + 40 -80 mg/day simvastatin -2 -1 0 1 2 3 6 Months Relative to Randomization 12
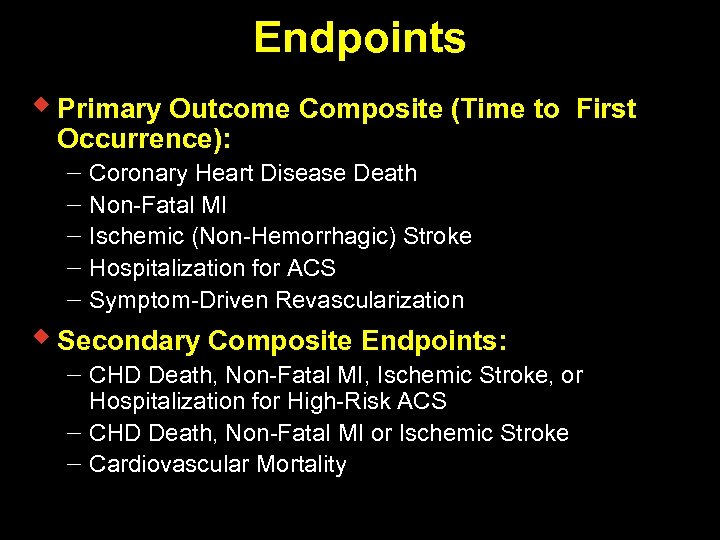 Endpoints w Primary Outcome Composite (Time to First Occurrence): Coronary Heart Disease Death Non-Fatal MI Ischemic (Non-Hemorrhagic) Stroke Hospitalization for ACS Symptom-Driven Revascularization w Secondary Composite Endpoints: CHD Death, Non-Fatal MI, Ischemic Stroke, or Hospitalization for High-Risk ACS CHD Death, Non-Fatal MI or Ischemic Stroke Cardiovascular Mortality
Endpoints w Primary Outcome Composite (Time to First Occurrence): Coronary Heart Disease Death Non-Fatal MI Ischemic (Non-Hemorrhagic) Stroke Hospitalization for ACS Symptom-Driven Revascularization w Secondary Composite Endpoints: CHD Death, Non-Fatal MI, Ischemic Stroke, or Hospitalization for High-Risk ACS CHD Death, Non-Fatal MI or Ischemic Stroke Cardiovascular Mortality
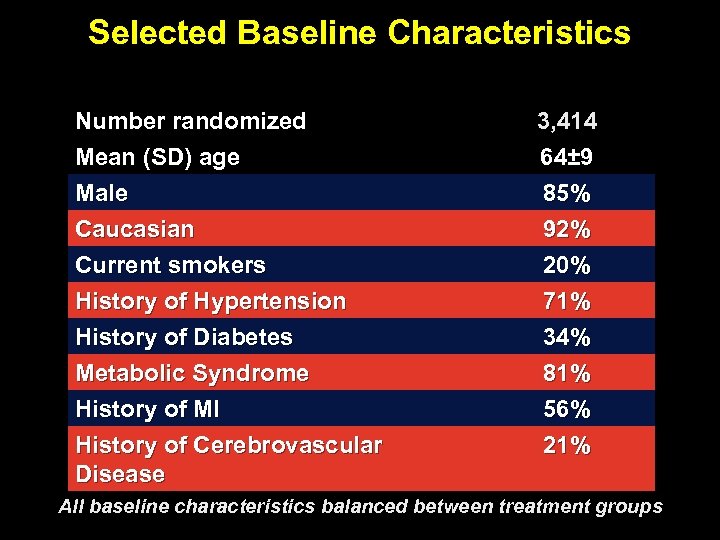 Selected Baseline Characteristics Number randomized Mean (SD) age Male Caucasian 3, 414 64± 9 85% 92% Current smokers History of Hypertension History of Diabetes Metabolic Syndrome History of MI History of Cerebrovascular Disease 20% 71% 34% 81% 56% 21% All baseline characteristics balanced between treatment groups
Selected Baseline Characteristics Number randomized Mean (SD) age Male Caucasian 3, 414 64± 9 85% 92% Current smokers History of Hypertension History of Diabetes Metabolic Syndrome History of MI History of Cerebrovascular Disease 20% 71% 34% 81% 56% 21% All baseline characteristics balanced between treatment groups
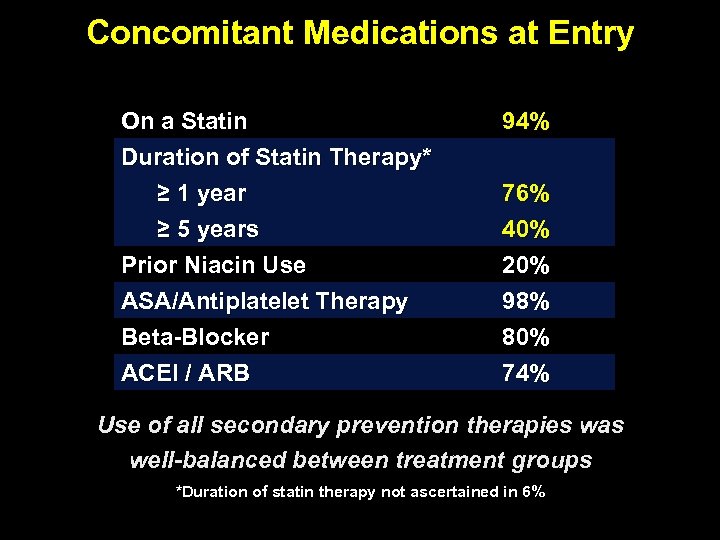 Concomitant Medications at Entry On a Statin Duration of Statin Therapy* ≥ 1 year ≥ 5 years 94% Prior Niacin Use ASA/Antiplatelet Therapy Βeta-Blocker ACEI / ARB 20% 98% 80% 74% 76% 40% Use of all secondary prevention therapies was well-balanced between treatment groups *Duration of statin therapy not ascertained in 6%
Concomitant Medications at Entry On a Statin Duration of Statin Therapy* ≥ 1 year ≥ 5 years 94% Prior Niacin Use ASA/Antiplatelet Therapy Βeta-Blocker ACEI / ARB 20% 98% 80% 74% 76% 40% Use of all secondary prevention therapies was well-balanced between treatment groups *Duration of statin therapy not ascertained in 6%
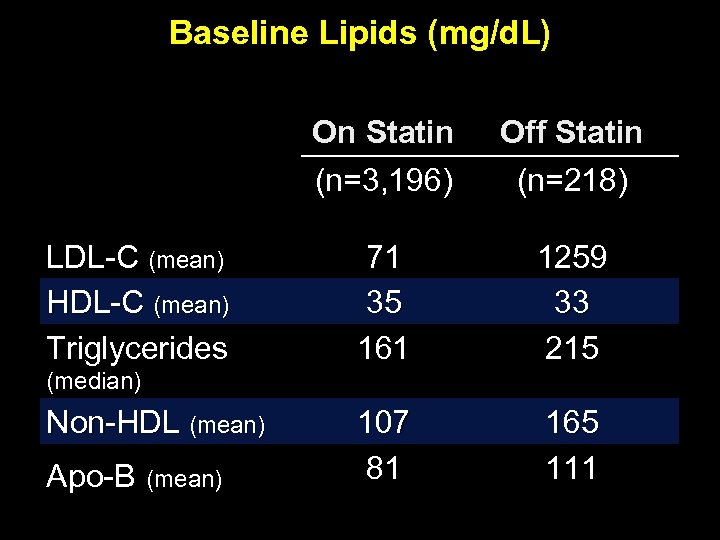 Baseline Lipids (mg/d. L) On Statin (n=3, 196) LDL-C (mean) HDL-C (mean) Triglycerides Off Statin (n=218) 71 35 161 1259 33 215 107 81 165 111 (median) Non-HDL (mean) Apo-B (mean)
Baseline Lipids (mg/d. L) On Statin (n=3, 196) LDL-C (mean) HDL-C (mean) Triglycerides Off Statin (n=218) 71 35 161 1259 33 215 107 81 165 111 (median) Non-HDL (mean) Apo-B (mean)
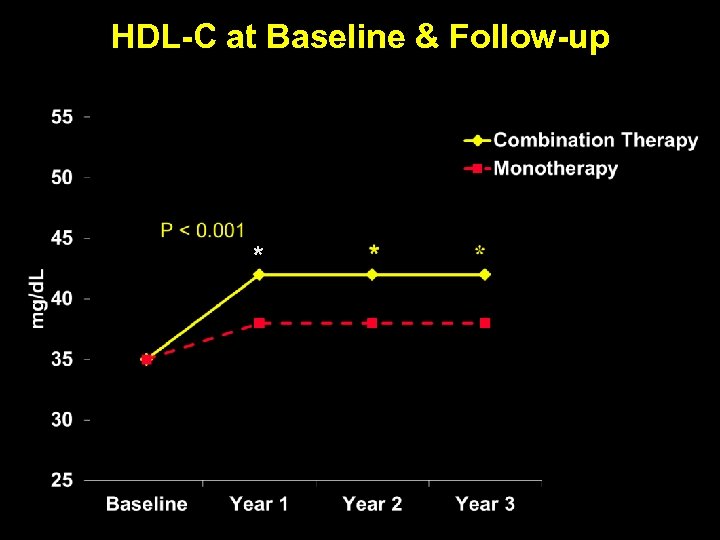 HDL-C at Baseline & Follow-up *
HDL-C at Baseline & Follow-up *
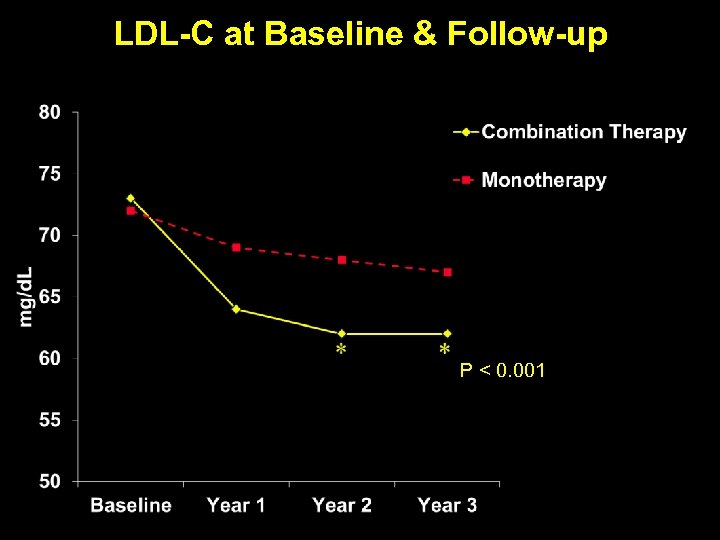 LDL-C at Baseline & Follow-up * P < 0. 001
LDL-C at Baseline & Follow-up * P < 0. 001
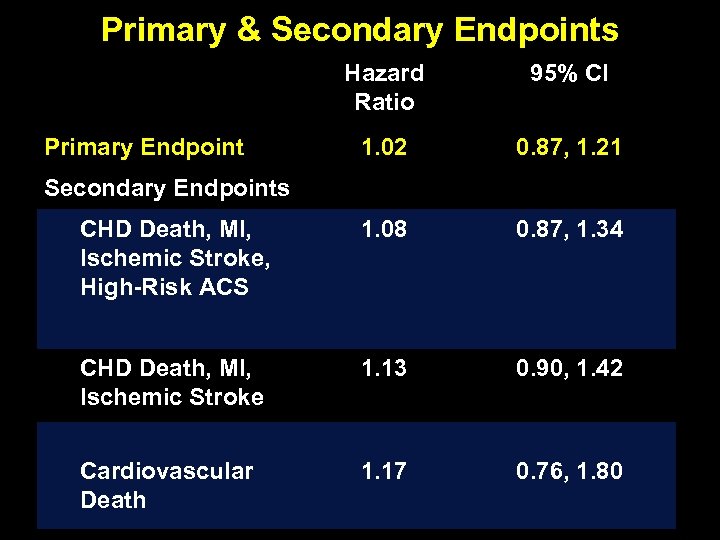 Primary & Secondary Endpoints Hazard Ratio 95% CI 1. 02 0. 87, 1. 21 CHD Death, MI, Ischemic Stroke, High-Risk ACS 1. 08 0. 87, 1. 34 CHD Death, MI, Ischemic Stroke 1. 13 0. 90, 1. 42 Cardiovascular Death 1. 17 0. 76, 1. 80 Primary Endpoint Secondary Endpoints
Primary & Secondary Endpoints Hazard Ratio 95% CI 1. 02 0. 87, 1. 21 CHD Death, MI, Ischemic Stroke, High-Risk ACS 1. 08 0. 87, 1. 34 CHD Death, MI, Ischemic Stroke 1. 13 0. 90, 1. 42 Cardiovascular Death 1. 17 0. 76, 1. 80 Primary Endpoint Secondary Endpoints
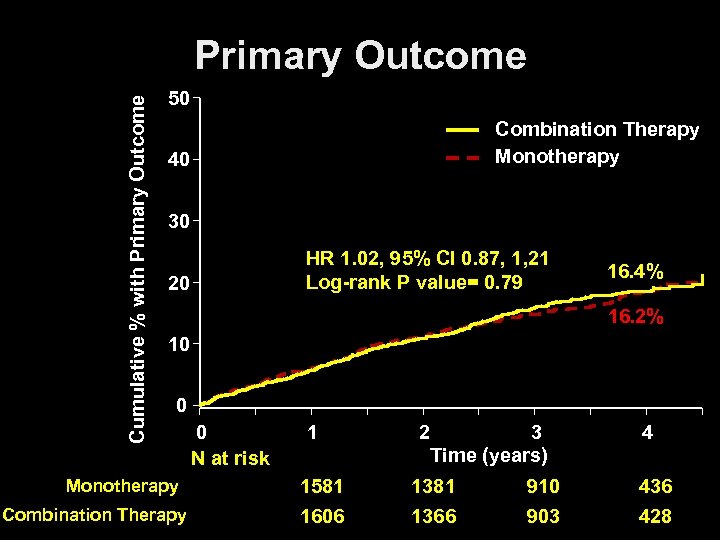 Cumulative % with Primary Outcome 50 Combination Therapy Monotherapy 40 30 20 HR 1. 02, 95% CI 0. 87, 1, 21 Log-rank P value= 0. 79 16. 4% 16. 2% 10 0 0 N at risk Monotherapy 1696 Combination Therapy 1718 1 1581 1606 2 3 Time (years) 1381 1366 910 903 4 436 428
Cumulative % with Primary Outcome 50 Combination Therapy Monotherapy 40 30 20 HR 1. 02, 95% CI 0. 87, 1, 21 Log-rank P value= 0. 79 16. 4% 16. 2% 10 0 0 N at risk Monotherapy 1696 Combination Therapy 1718 1 1581 1606 2 3 Time (years) 1381 1366 910 903 4 436 428
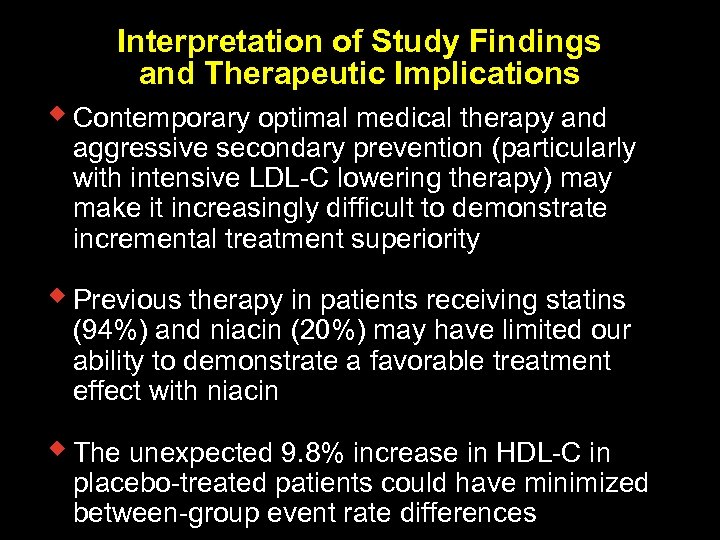 Interpretation of Study Findings and Therapeutic Implications w Contemporary optimal medical therapy and aggressive secondary prevention (particularly with intensive LDL-C lowering therapy) may make it increasingly difficult to demonstrate incremental treatment superiority w Previous therapy in patients receiving statins (94%) and niacin (20%) may have limited our ability to demonstrate a favorable treatment effect with niacin w The unexpected 9. 8% increase in HDL-C in placebo-treated patients could have minimized between-group event rate differences
Interpretation of Study Findings and Therapeutic Implications w Contemporary optimal medical therapy and aggressive secondary prevention (particularly with intensive LDL-C lowering therapy) may make it increasingly difficult to demonstrate incremental treatment superiority w Previous therapy in patients receiving statins (94%) and niacin (20%) may have limited our ability to demonstrate a favorable treatment effect with niacin w The unexpected 9. 8% increase in HDL-C in placebo-treated patients could have minimized between-group event rate differences
 Interpretation of Study Findings and Therapeutic Implications v? Intensive use of statin therapy for ≥ 1 year in ~ 75% of patients may have caused “delipidation” of lipid-rich necrotic cores, converting high-risk vulnerable plaques → stable, quiescent plaques v. Residual risk in AIM-HIGH patients during follow-up was appreciable (5. 4% event rate/year), but was not mitigated by niacin v. Whether niacin benefit might have been discerned during a longer follow-up remains uncertain
Interpretation of Study Findings and Therapeutic Implications v? Intensive use of statin therapy for ≥ 1 year in ~ 75% of patients may have caused “delipidation” of lipid-rich necrotic cores, converting high-risk vulnerable plaques → stable, quiescent plaques v. Residual risk in AIM-HIGH patients during follow-up was appreciable (5. 4% event rate/year), but was not mitigated by niacin v. Whether niacin benefit might have been discerned during a longer follow-up remains uncertain
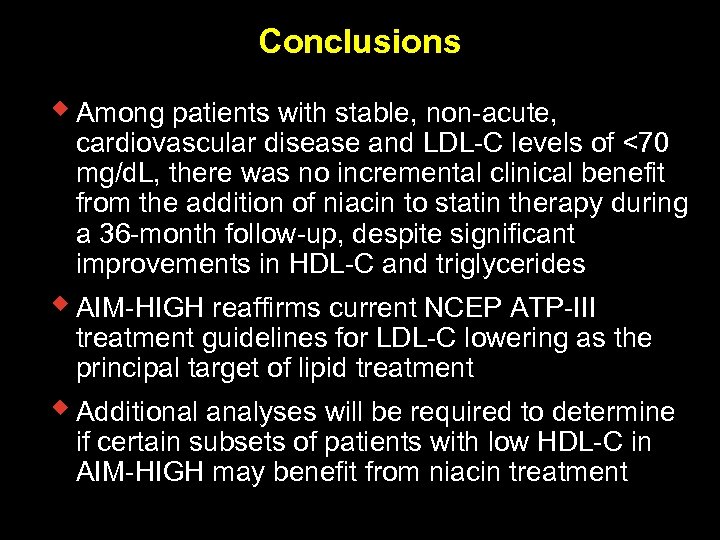 Conclusions w Among patients with stable, non-acute, cardiovascular disease and LDL-C levels of <70 mg/d. L, there was no incremental clinical benefit from the addition of niacin to statin therapy during a 36 -month follow-up, despite significant improvements in HDL-C and triglycerides w AIM-HIGH reaffirms current NCEP ATP-III treatment guidelines for LDL-C lowering as the principal target of lipid treatment w Additional analyses will be required to determine if certain subsets of patients with low HDL-C in AIM-HIGH may benefit from niacin treatment
Conclusions w Among patients with stable, non-acute, cardiovascular disease and LDL-C levels of <70 mg/d. L, there was no incremental clinical benefit from the addition of niacin to statin therapy during a 36 -month follow-up, despite significant improvements in HDL-C and triglycerides w AIM-HIGH reaffirms current NCEP ATP-III treatment guidelines for LDL-C lowering as the principal target of lipid treatment w Additional analyses will be required to determine if certain subsets of patients with low HDL-C in AIM-HIGH may benefit from niacin treatment
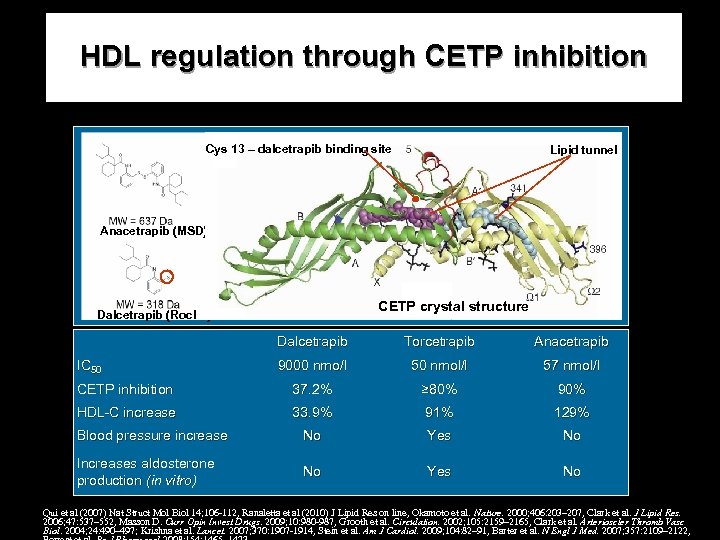 HDL regulation through CETP inhibition Cys 13 – dalcetrapib binding site Lipid tunnel Anacetrapib (MSD) CETP crystal structure Dalcetrapib (Roche) Dalcetrapib Torcetrapib Anacetrapib 9000 nmo/l 50 nmol/l 57 nmol/l CETP inhibition 37. 2% ≥ 80% 90% HDL-C increase 33. 9% 91% 129% Blood pressure increase No Yes No Increases aldosterone production (in vitro) No Yes No IC 50 Qui et al (2007) Nat Struct Mol Biol 14; 106 -112, Ranaletta et al (2010) J Lipid Res on line, Okamoto et al. Nature. 2000; 406: 203– 207, Clark et al. J Lipid Res. 2006; 47: 537– 552, Masson D. Curr Opin Invest Drugs. 2009; 10: 980 -987, Grooth et al. Circulation. 2002; 105: 2159– 2165, Clark et al. Arterioscler Thromb Vasc Biol. 2004; 24: 490– 497; Krishna et al. Lancet. 2007; 370: 1907 -1914, Stein et al. Am J Cardiol. 2009; 104: 82– 91, Barter et al. N Engl J Med. 2007; 357: 2109– 2122,
HDL regulation through CETP inhibition Cys 13 – dalcetrapib binding site Lipid tunnel Anacetrapib (MSD) CETP crystal structure Dalcetrapib (Roche) Dalcetrapib Torcetrapib Anacetrapib 9000 nmo/l 50 nmol/l 57 nmol/l CETP inhibition 37. 2% ≥ 80% 90% HDL-C increase 33. 9% 91% 129% Blood pressure increase No Yes No Increases aldosterone production (in vitro) No Yes No IC 50 Qui et al (2007) Nat Struct Mol Biol 14; 106 -112, Ranaletta et al (2010) J Lipid Res on line, Okamoto et al. Nature. 2000; 406: 203– 207, Clark et al. J Lipid Res. 2006; 47: 537– 552, Masson D. Curr Opin Invest Drugs. 2009; 10: 980 -987, Grooth et al. Circulation. 2002; 105: 2159– 2165, Clark et al. Arterioscler Thromb Vasc Biol. 2004; 24: 490– 497; Krishna et al. Lancet. 2007; 370: 1907 -1914, Stein et al. Am J Cardiol. 2009; 104: 82– 91, Barter et al. N Engl J Med. 2007; 357: 2109– 2122,
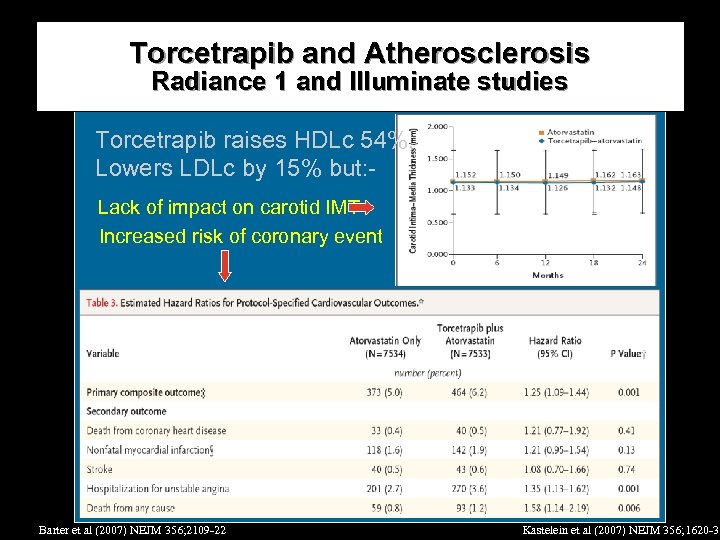 Torcetrapib and Atherosclerosis Radiance 1 and Illuminate studies Torcetrapib raises HDLc 54%, Lowers LDLc by 15% but: Lack of impact on carotid IMT Increased risk of coronary event Barter et al (2007) NEJM 356; 2109 -22 Kastelein et al (2007) NEJM 356; 1620 -30
Torcetrapib and Atherosclerosis Radiance 1 and Illuminate studies Torcetrapib raises HDLc 54%, Lowers LDLc by 15% but: Lack of impact on carotid IMT Increased risk of coronary event Barter et al (2007) NEJM 356; 2109 -22 Kastelein et al (2007) NEJM 356; 1620 -30
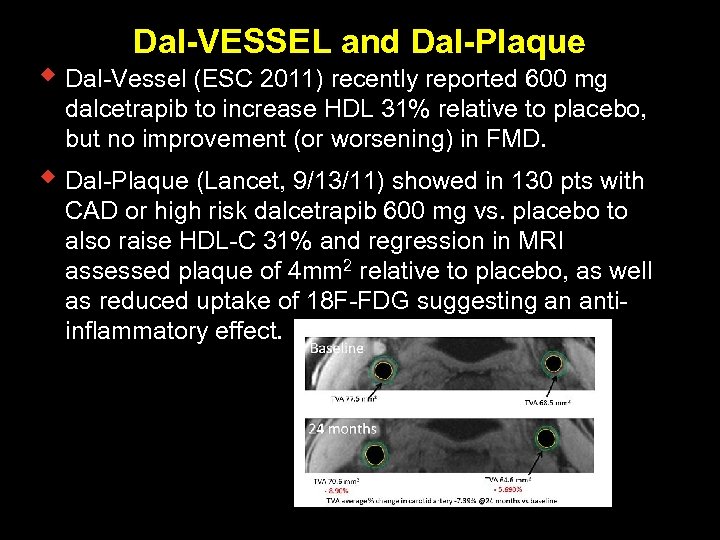 Dal-VESSEL and Dal-Plaque w Dal-Vessel (ESC 2011) recently reported 600 mg dalcetrapib to increase HDL 31% relative to placebo, but no improvement (or worsening) in FMD. w Dal-Plaque (Lancet, 9/13/11) showed in 130 pts with CAD or high risk dalcetrapib 600 mg vs. placebo to also raise HDL-C 31% and regression in MRI assessed plaque of 4 mm 2 relative to placebo, as well as reduced uptake of 18 F-FDG suggesting an antiinflammatory effect.
Dal-VESSEL and Dal-Plaque w Dal-Vessel (ESC 2011) recently reported 600 mg dalcetrapib to increase HDL 31% relative to placebo, but no improvement (or worsening) in FMD. w Dal-Plaque (Lancet, 9/13/11) showed in 130 pts with CAD or high risk dalcetrapib 600 mg vs. placebo to also raise HDL-C 31% and regression in MRI assessed plaque of 4 mm 2 relative to placebo, as well as reduced uptake of 18 F-FDG suggesting an antiinflammatory effect.
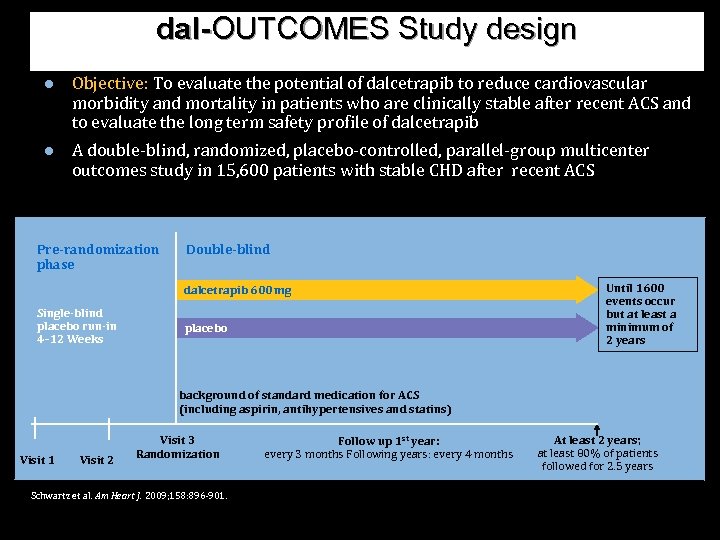 dal-OUTCOMES Study design ● Objective: To evaluate the potential of dalcetrapib to reduce cardiovascular morbidity and mortality in patients who are clinically stable after recent ACS and to evaluate the long term safety profile of dalcetrapib ● A double-blind, randomized, placebo-controlled, parallel-group multicenter outcomes study in 15, 600 patients with stable CHD after recent ACS Pre-randomization phase Double-blind dalcetrapib 600 mg Single-blind placebo run-in 4– 12 Weeks placebo Until 1600 events occur but at least a minimum of 2 years background of standard medication for ACS (including aspirin, antihypertensives and statins) Visit 1 Visit 2 Visit 3 Randomization Schwartz et al. Am Heart J. 2009; 158: 896 -901. Follow up 1 st year: every 3 months Following years: every 4 months At least 2 years; at least 80% of patients followed for 2. 5 years
dal-OUTCOMES Study design ● Objective: To evaluate the potential of dalcetrapib to reduce cardiovascular morbidity and mortality in patients who are clinically stable after recent ACS and to evaluate the long term safety profile of dalcetrapib ● A double-blind, randomized, placebo-controlled, parallel-group multicenter outcomes study in 15, 600 patients with stable CHD after recent ACS Pre-randomization phase Double-blind dalcetrapib 600 mg Single-blind placebo run-in 4– 12 Weeks placebo Until 1600 events occur but at least a minimum of 2 years background of standard medication for ACS (including aspirin, antihypertensives and statins) Visit 1 Visit 2 Visit 3 Randomization Schwartz et al. Am Heart J. 2009; 158: 896 -901. Follow up 1 st year: every 3 months Following years: every 4 months At least 2 years; at least 80% of patients followed for 2. 5 years
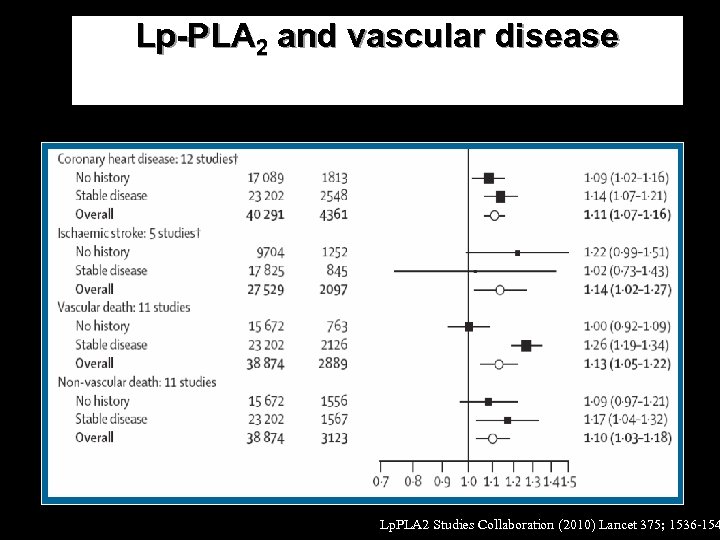 Lp-PLA 2 and vascular disease Lp. PLA 2 Studies Collaboration (2010) Lancet 375; 1536 -154
Lp-PLA 2 and vascular disease Lp. PLA 2 Studies Collaboration (2010) Lancet 375; 1536 -154
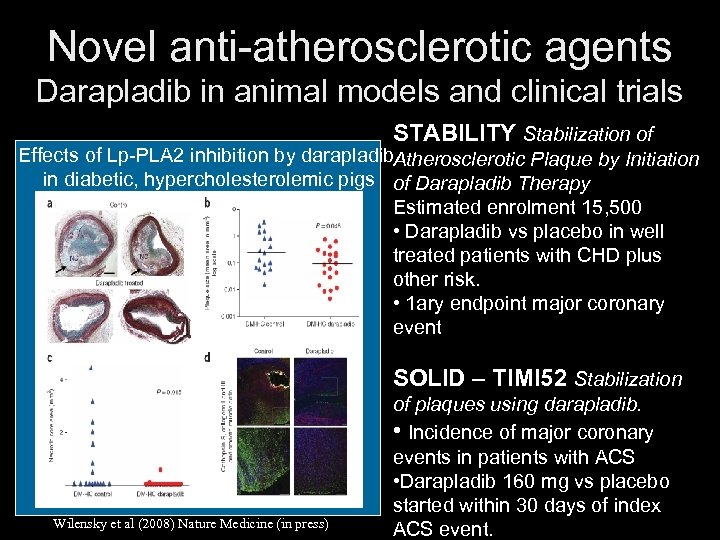 Novel anti-atherosclerotic agents Darapladib in animal models and clinical trials STABILITY Stabilization of Effects of Lp-PLA 2 inhibition by darapladib. Atherosclerotic Plaque by Initiation in diabetic, hypercholesterolemic pigs of Darapladib Therapy Estimated enrolment 15, 500 • Darapladib vs placebo in well treated patients with CHD plus other risk. • 1 ary endpoint major coronary event SOLID – TIMI 52 Stabilization Wilensky et al (2008) Nature Medicine (in press) of plaques using darapladib. • Incidence of major coronary events in patients with ACS • Darapladib 160 mg vs placebo started within 30 days of index ACS event.
Novel anti-atherosclerotic agents Darapladib in animal models and clinical trials STABILITY Stabilization of Effects of Lp-PLA 2 inhibition by darapladib. Atherosclerotic Plaque by Initiation in diabetic, hypercholesterolemic pigs of Darapladib Therapy Estimated enrolment 15, 500 • Darapladib vs placebo in well treated patients with CHD plus other risk. • 1 ary endpoint major coronary event SOLID – TIMI 52 Stabilization Wilensky et al (2008) Nature Medicine (in press) of plaques using darapladib. • Incidence of major coronary events in patients with ACS • Darapladib 160 mg vs placebo started within 30 days of index ACS event.
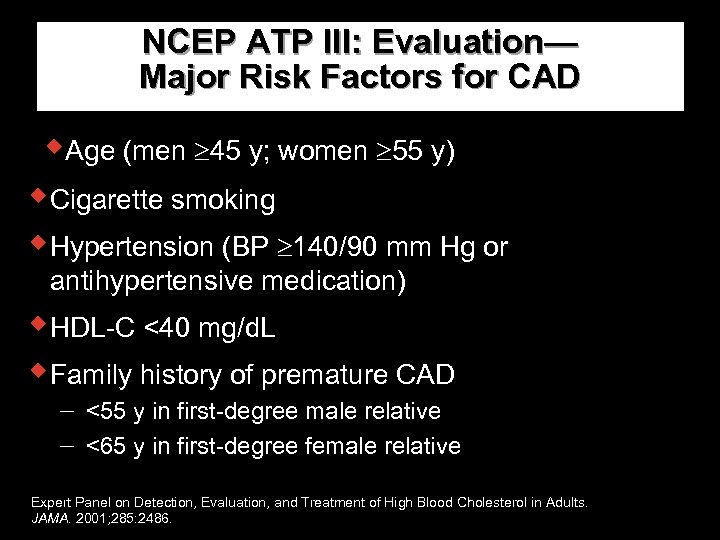 NCEP ATP III: Evaluation— Major Risk Factors for CAD w. Age (men 45 y; women 55 y) w. Cigarette smoking w. Hypertension (BP 140/90 mm Hg or antihypertensive medication) w. HDL-C <40 mg/d. L w. Family history of premature CAD <55 y in first-degree male relative <65 y in first-degree female relative Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
NCEP ATP III: Evaluation— Major Risk Factors for CAD w. Age (men 45 y; women 55 y) w. Cigarette smoking w. Hypertension (BP 140/90 mm Hg or antihypertensive medication) w. HDL-C <40 mg/d. L w. Family history of premature CAD <55 y in first-degree male relative <65 y in first-degree female relative Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
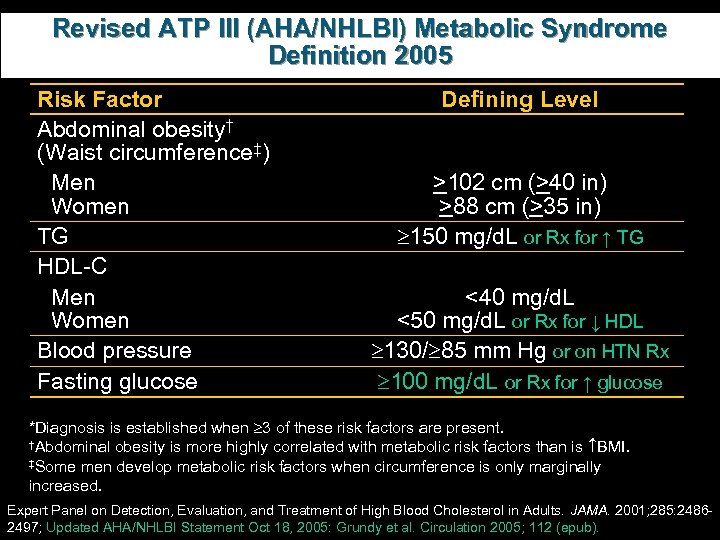 Revised ATP III (AHA/NHLBI) Metabolic Syndrome Definition 2005 Risk Factor Abdominal obesity† (Waist circumference‡) Men Women TG HDL-C Men Women Blood pressure Fasting glucose Defining Level >102 cm (>40 in) >88 cm (>35 in) 150 mg/d. L or Rx for ↑ TG <40 mg/d. L <50 mg/d. L or Rx for ↓ HDL 130/ 85 mm Hg or on HTN Rx 100 mg/d. L or Rx for ↑ glucose *Diagnosis is established when 3 of these risk factors are present. †Abdominal obesity is more highly correlated with metabolic risk factors than is BMI. ‡Some men develop metabolic risk factors when circumference is only marginally increased. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 24862497; Updated AHA/NHLBI Statement Oct 18, 2005: Grundy et al. Circulation 2005; 112 (epub).
Revised ATP III (AHA/NHLBI) Metabolic Syndrome Definition 2005 Risk Factor Abdominal obesity† (Waist circumference‡) Men Women TG HDL-C Men Women Blood pressure Fasting glucose Defining Level >102 cm (>40 in) >88 cm (>35 in) 150 mg/d. L or Rx for ↑ TG <40 mg/d. L <50 mg/d. L or Rx for ↓ HDL 130/ 85 mm Hg or on HTN Rx 100 mg/d. L or Rx for ↑ glucose *Diagnosis is established when 3 of these risk factors are present. †Abdominal obesity is more highly correlated with metabolic risk factors than is BMI. ‡Some men develop metabolic risk factors when circumference is only marginally increased. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 24862497; Updated AHA/NHLBI Statement Oct 18, 2005: Grundy et al. Circulation 2005; 112 (epub).
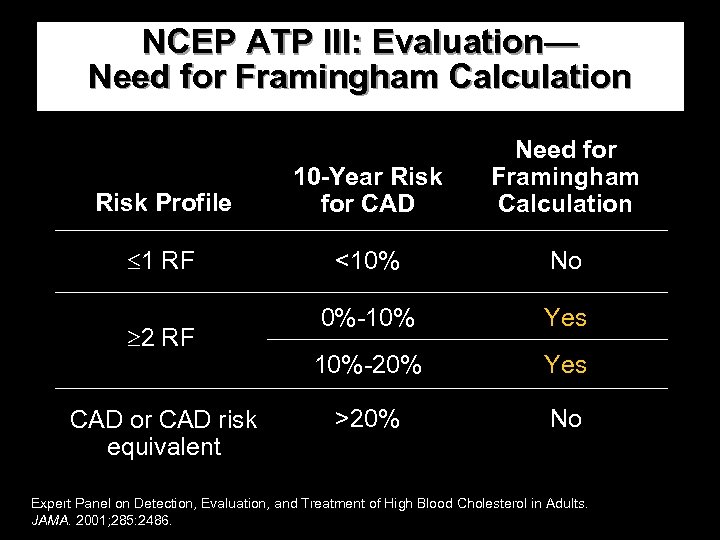 NCEP ATP III: Evaluation— Need for Framingham Calculation Risk Profile 10 -Year Risk for CAD Need for Framingham Calculation 1 RF <10% No 0%-10% Yes 10%-20% Yes >20% No 2 RF CAD or CAD risk equivalent Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
NCEP ATP III: Evaluation— Need for Framingham Calculation Risk Profile 10 -Year Risk for CAD Need for Framingham Calculation 1 RF <10% No 0%-10% Yes 10%-20% Yes >20% No 2 RF CAD or CAD risk equivalent Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
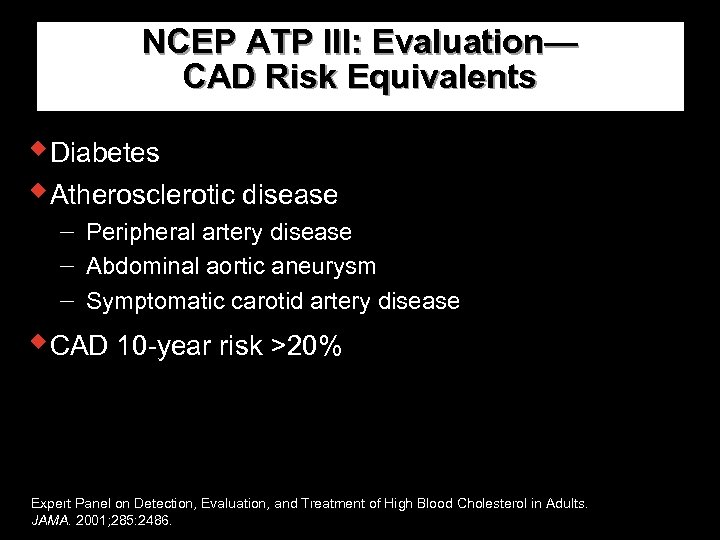 NCEP ATP III: Evaluation— CAD Risk Equivalents w. Diabetes w. Atherosclerotic disease Peripheral artery disease Abdominal aortic aneurysm Symptomatic carotid artery disease w. CAD 10 -year risk >20% Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
NCEP ATP III: Evaluation— CAD Risk Equivalents w. Diabetes w. Atherosclerotic disease Peripheral artery disease Abdominal aortic aneurysm Symptomatic carotid artery disease w. CAD 10 -year risk >20% Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
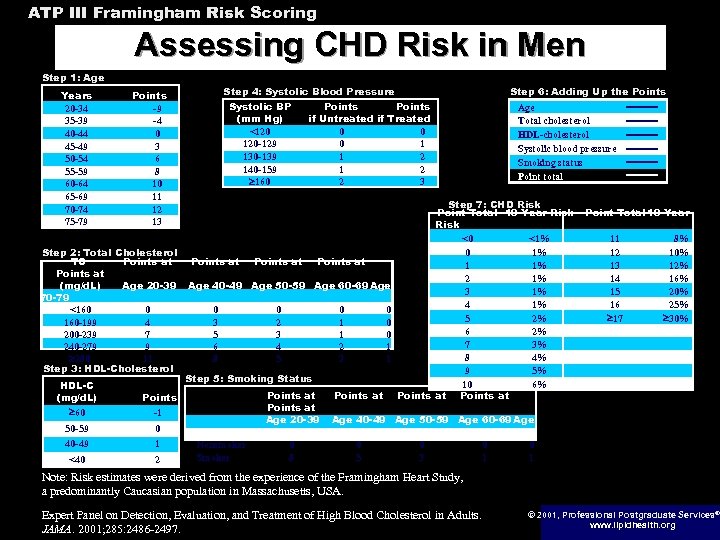 ATP III Framingham Risk Scoring Assessing CHD Risk in Men Step 1: Age Years 20 -34 35 -39 40 -44 45 -49 50 -54 55 -59 60 -64 65 -69 70 -74 75 -79 Step 4: Systolic Blood Pressure Points -9 -4 0 3 6 8 10 11 12 13 Step 2: Total Cholesterol TC Points at (mg/d. L) Age 20 -39 70 -79 <160 0 160 -199 4 200 -239 7 240 -279 9 280 11 Step 3: HDL-Cholesterol HDL-C (mg/d. L) 60 0 40 -49 1 <40 2 Points at Points if Untreated if Treated 0 0 0 1 1 2 2 3 Points at Age 40 -49 Age 50 -59 Age 60 -69 Age 0 3 5 6 8 0 2 3 4 5 0 1 1 2 3 0 0 0 1 1 Step 5: Smoking Status Points -1 50 -59 Systolic BP (mm Hg) <120 120 -129 130 -139 140 -159 160 70 -79 Nonsmoker Smoker Points at Age 20 -39 0 8 Step 6: Adding Up the Points at Age Total cholesterol HDL-cholesterol Systolic blood pressure Smoking status Point total Step 7: CHD Risk Point Total 10 -Year Risk <0 <1% 0 1% 1 1% 2 1% 3 1% 4 1% 5 2% 6 2% 7 3% 8 4% 9 5% 10 6% Points at Point Total 10 -Year 11 12 13 14 15 16 17 8% 10% 12% 16% 20% 25% 30% Age 40 -49 Age 50 -59 Age 60 -69 Age 0 5 0 3 0 1 Note: Risk estimates were derived from the experience of the Framingham Heart Study, a predominantly Caucasian population in Massachusetts, USA. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486 -2497. © 2001, Professional Postgraduate Services® www. lipidhealth. org
ATP III Framingham Risk Scoring Assessing CHD Risk in Men Step 1: Age Years 20 -34 35 -39 40 -44 45 -49 50 -54 55 -59 60 -64 65 -69 70 -74 75 -79 Step 4: Systolic Blood Pressure Points -9 -4 0 3 6 8 10 11 12 13 Step 2: Total Cholesterol TC Points at (mg/d. L) Age 20 -39 70 -79 <160 0 160 -199 4 200 -239 7 240 -279 9 280 11 Step 3: HDL-Cholesterol HDL-C (mg/d. L) 60 0 40 -49 1 <40 2 Points at Points if Untreated if Treated 0 0 0 1 1 2 2 3 Points at Age 40 -49 Age 50 -59 Age 60 -69 Age 0 3 5 6 8 0 2 3 4 5 0 1 1 2 3 0 0 0 1 1 Step 5: Smoking Status Points -1 50 -59 Systolic BP (mm Hg) <120 120 -129 130 -139 140 -159 160 70 -79 Nonsmoker Smoker Points at Age 20 -39 0 8 Step 6: Adding Up the Points at Age Total cholesterol HDL-cholesterol Systolic blood pressure Smoking status Point total Step 7: CHD Risk Point Total 10 -Year Risk <0 <1% 0 1% 1 1% 2 1% 3 1% 4 1% 5 2% 6 2% 7 3% 8 4% 9 5% 10 6% Points at Point Total 10 -Year 11 12 13 14 15 16 17 8% 10% 12% 16% 20% 25% 30% Age 40 -49 Age 50 -59 Age 60 -69 Age 0 5 0 3 0 1 Note: Risk estimates were derived from the experience of the Framingham Heart Study, a predominantly Caucasian population in Massachusetts, USA. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486 -2497. © 2001, Professional Postgraduate Services® www. lipidhealth. org
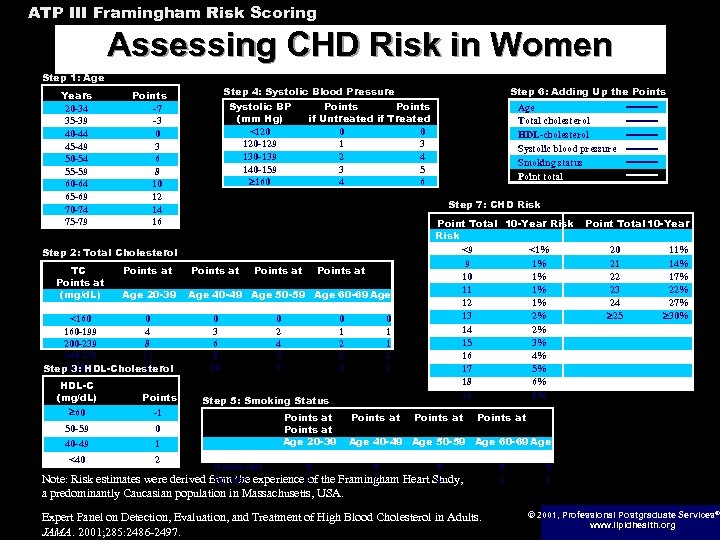 ATP III Framingham Risk Scoring Assessing CHD Risk in Women Step 1: Age Years 20 -34 35 -39 40 -44 45 -49 50 -54 55 -59 60 -64 65 -69 70 -74 75 -79 Step 4: Systolic Blood Pressure Points -7 -3 0 3 6 8 10 12 14 16 Systolic BP (mm Hg) <120 120 -129 130 -139 140 -159 160 Points if Untreated if Treated 0 0 1 3 2 4 3 5 4 6 HDL-C (mg/d. L) 60 Points -1 50 -59 0 40 -49 1 <40 2 Age Total cholesterol HDL-cholesterol Systolic blood pressure Smoking status Point total Step 7: CHD Risk Step 2: Total Cholesterol TC Points at (mg/d. L) Age 20 -39 70 -79 <160 0 160 -199 4 200 -239 8 240 -279 11 13 Step 280 3: HDL-Cholesterol Step 6: Adding Up the Points at Age 40 -49 Age 50 -59 Age 60 -69 Age 0 3 6 8 10 0 2 4 5 7 0 1 2 3 4 0 1 1 2 2 Step 5: Smoking Status Points at Age 20 -39 70 -79 Nonsmoker 0 Smoker from the experience 9 of Points at Point Total 10 -Year Risk <9 <1% 9 1% 10 1% 11 1% 12 1% 13 2% 14 2% 15 3% 16 4% 17 5% 18 6% 19 8% Points at Point Total 10 -Year 20 21 22 23 24 25 11% 14% 17% 22% 27% 30% Points at Age 40 -49 Age 50 -59 Age 60 -69 Age 0 0 7 4 Note: Risk estimates were derived the Framingham Heart Study, a predominantly Caucasian population in Massachusetts, USA. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486 -2497. 0 2 0 1 © 2001, Professional Postgraduate Services® www. lipidhealth. org
ATP III Framingham Risk Scoring Assessing CHD Risk in Women Step 1: Age Years 20 -34 35 -39 40 -44 45 -49 50 -54 55 -59 60 -64 65 -69 70 -74 75 -79 Step 4: Systolic Blood Pressure Points -7 -3 0 3 6 8 10 12 14 16 Systolic BP (mm Hg) <120 120 -129 130 -139 140 -159 160 Points if Untreated if Treated 0 0 1 3 2 4 3 5 4 6 HDL-C (mg/d. L) 60 Points -1 50 -59 0 40 -49 1 <40 2 Age Total cholesterol HDL-cholesterol Systolic blood pressure Smoking status Point total Step 7: CHD Risk Step 2: Total Cholesterol TC Points at (mg/d. L) Age 20 -39 70 -79 <160 0 160 -199 4 200 -239 8 240 -279 11 13 Step 280 3: HDL-Cholesterol Step 6: Adding Up the Points at Age 40 -49 Age 50 -59 Age 60 -69 Age 0 3 6 8 10 0 2 4 5 7 0 1 2 3 4 0 1 1 2 2 Step 5: Smoking Status Points at Age 20 -39 70 -79 Nonsmoker 0 Smoker from the experience 9 of Points at Point Total 10 -Year Risk <9 <1% 9 1% 10 1% 11 1% 12 1% 13 2% 14 2% 15 3% 16 4% 17 5% 18 6% 19 8% Points at Point Total 10 -Year 20 21 22 23 24 25 11% 14% 17% 22% 27% 30% Points at Age 40 -49 Age 50 -59 Age 60 -69 Age 0 0 7 4 Note: Risk estimates were derived the Framingham Heart Study, a predominantly Caucasian population in Massachusetts, USA. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486 -2497. 0 2 0 1 © 2001, Professional Postgraduate Services® www. lipidhealth. org
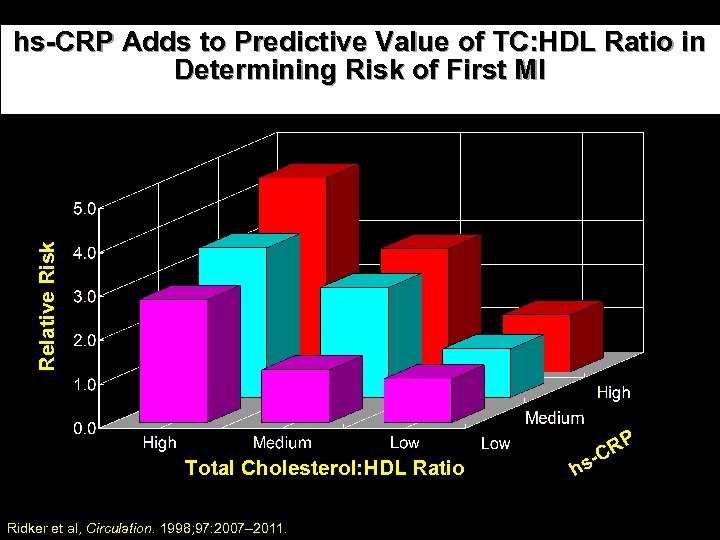 Relative Risk hs-CRP Adds to Predictive Value of TC: HDL Ratio in Determining Risk of First MI Total Cholesterol: HDL Ratio Ridker et al, Circulation. 1998; 97: 2007– 2011. RP s-C h
Relative Risk hs-CRP Adds to Predictive Value of TC: HDL Ratio in Determining Risk of First MI Total Cholesterol: HDL Ratio Ridker et al, Circulation. 1998; 97: 2007– 2011. RP s-C h
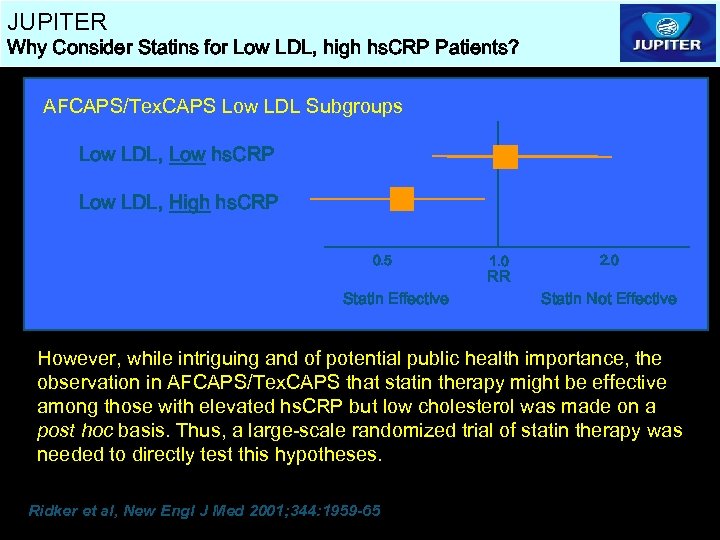 JUPITER Why Consider Statins for Low LDL, high hs. CRP Patients? AFCAPS/Tex. CAPS Low LDL Subgroups Low LDL, Low hs. CRP [A] Low LDL, High hs. CRP [B] 0. 5 Statin Effective 1. 0 RR 2. 0 Statin Not Effective However, while intriguing and of potential public health importance, the observation in AFCAPS/Tex. CAPS that statin therapy might be effective among those with elevated hs. CRP but low cholesterol was made on a post hoc basis. Thus, a large-scale randomized trial of statin therapy was needed to directly test this hypotheses. Ridker et al, New Engl J Med 2001; 344: 1959 -65
JUPITER Why Consider Statins for Low LDL, high hs. CRP Patients? AFCAPS/Tex. CAPS Low LDL Subgroups Low LDL, Low hs. CRP [A] Low LDL, High hs. CRP [B] 0. 5 Statin Effective 1. 0 RR 2. 0 Statin Not Effective However, while intriguing and of potential public health importance, the observation in AFCAPS/Tex. CAPS that statin therapy might be effective among those with elevated hs. CRP but low cholesterol was made on a post hoc basis. Thus, a large-scale randomized trial of statin therapy was needed to directly test this hypotheses. Ridker et al, New Engl J Med 2001; 344: 1959 -65
 JUPITER Trial Design JUPITER Multi-National Randomized Double Blind Placebo Controlled Trial of Rosuvastatin in the Prevention of Cardiovascular Events Among Individuals With Low LDL and Elevated hs. CRP Rosuvastatin 20 mg (N=8901) No Prior CVD or DM Men >50, Women >60 LDL <130 mg/d. L hs. CRP >2 mg/L 4 -week run-in Placebo (N=8901) MI Stroke Unstable Angina CVD Death CABG/PTCA Argentina, Belgium, Brazil, Bulgaria, Canada, Chile, Colombia, Costa Rica, Denmark, El Salvador, Estonia, Germany, Israel, Mexico, Netherlands, Norway, Panama, Poland, Romania, Russia, South Africa, Switzerland, United Kingdom, Uruguay, United States, Venezuela Ridker et al, Circulation 2003; 108: 2292 -2297.
JUPITER Trial Design JUPITER Multi-National Randomized Double Blind Placebo Controlled Trial of Rosuvastatin in the Prevention of Cardiovascular Events Among Individuals With Low LDL and Elevated hs. CRP Rosuvastatin 20 mg (N=8901) No Prior CVD or DM Men >50, Women >60 LDL <130 mg/d. L hs. CRP >2 mg/L 4 -week run-in Placebo (N=8901) MI Stroke Unstable Angina CVD Death CABG/PTCA Argentina, Belgium, Brazil, Bulgaria, Canada, Chile, Colombia, Costa Rica, Denmark, El Salvador, Estonia, Germany, Israel, Mexico, Netherlands, Norway, Panama, Poland, Romania, Russia, South Africa, Switzerland, United Kingdom, Uruguay, United States, Venezuela Ridker et al, Circulation 2003; 108: 2292 -2297.
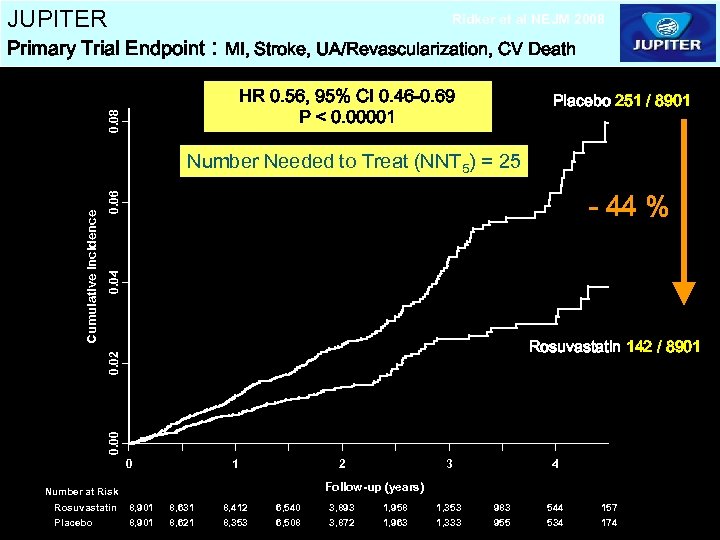 JUPITER Ridker et al NEJM 2008 Primary Trial Endpoint : MI, Stroke, UA/Revascularization, CV Death 0. 08 HR 0. 56, 95% CI 0. 46 -0. 69 P < 0. 00001 Placebo 251 / 8901 0. 04 0. 06 - 44 % Rosuvastatin 142 / 8901 0. 00 0. 02 Cumulative Incidence Number Needed to Treat (NNT 5) = 25 0 1 2 4 Follow-up (years) Number at Risk Rosuvastatin Placebo 3 8, 901 8, 631 8, 621 8, 412 8, 353 6, 540 6, 508 3, 893 3, 872 1, 958 1, 963 1, 353 1, 333 983 955 544 534 157 174
JUPITER Ridker et al NEJM 2008 Primary Trial Endpoint : MI, Stroke, UA/Revascularization, CV Death 0. 08 HR 0. 56, 95% CI 0. 46 -0. 69 P < 0. 00001 Placebo 251 / 8901 0. 04 0. 06 - 44 % Rosuvastatin 142 / 8901 0. 00 0. 02 Cumulative Incidence Number Needed to Treat (NNT 5) = 25 0 1 2 4 Follow-up (years) Number at Risk Rosuvastatin Placebo 3 8, 901 8, 631 8, 621 8, 412 8, 353 6, 540 6, 508 3, 893 3, 872 1, 958 1, 963 1, 353 1, 333 983 955 544 534 157 174
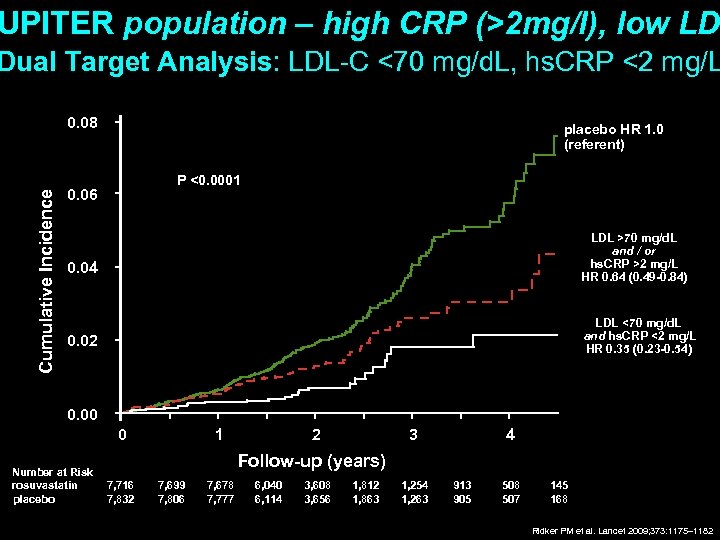 UPITER population – high CRP (>2 mg/l), low LD Dual Target Analysis: LDL-C <70 mg/d. L, hs. CRP <2 mg/L Cumulative Incidence 0. 08 placebo HR 1. 0 (referent) P <0. 0001 0. 06 LDL >70 mg/d. L and / or hs. CRP >2 mg/L HR 0. 64 (0. 49 -0. 84) 0. 04 LDL <70 mg/d. L and hs. CRP <2 mg/L HR 0. 35 (0. 23 -0. 54) 0. 02 0. 00 0 Number at Risk rosuvastatin placebo 1 2 3 4 Follow-up (years) 7, 716 7, 832 7, 699 7, 806 7, 678 7, 777 6, 040 6, 114 3, 608 3, 656 1, 812 1, 863 1, 254 1, 263 913 905 508 507 145 168 Ridker PM et al. Lancet 2009; 373: 1175– 1182
UPITER population – high CRP (>2 mg/l), low LD Dual Target Analysis: LDL-C <70 mg/d. L, hs. CRP <2 mg/L Cumulative Incidence 0. 08 placebo HR 1. 0 (referent) P <0. 0001 0. 06 LDL >70 mg/d. L and / or hs. CRP >2 mg/L HR 0. 64 (0. 49 -0. 84) 0. 04 LDL <70 mg/d. L and hs. CRP <2 mg/L HR 0. 35 (0. 23 -0. 54) 0. 02 0. 00 0 Number at Risk rosuvastatin placebo 1 2 3 4 Follow-up (years) 7, 716 7, 832 7, 699 7, 806 7, 678 7, 777 6, 040 6, 114 3, 608 3, 656 1, 812 1, 863 1, 254 1, 263 913 905 508 507 145 168 Ridker PM et al. Lancet 2009; 373: 1175– 1182
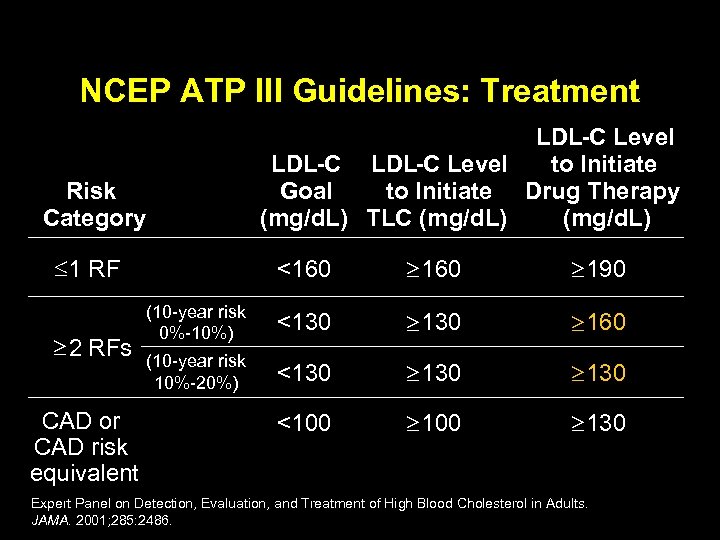 NCEP ATP III Guidelines: Treatment Risk Category 1 RF 2 RFs CAD or CAD risk equivalent LDL-C Level to Initiate Goal to Initiate Drug Therapy (mg/d. L) TLC (mg/d. L) <160 190 (10 -year risk 0%-10%) <130 160 (10 -year risk 10%-20%) <130 <100 130 Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
NCEP ATP III Guidelines: Treatment Risk Category 1 RF 2 RFs CAD or CAD risk equivalent LDL-C Level to Initiate Goal to Initiate Drug Therapy (mg/d. L) TLC (mg/d. L) <160 190 (10 -year risk 0%-10%) <130 160 (10 -year risk 10%-20%) <130 <100 130 Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
 Statins in ACS - Guidelines • Who - Initiate therapy regardless of baseline LDL. • When – Pre-discharge; but no difference in benefit when initiated immediately or days post event (ESC <4 days). • What – Evidence base is for high dose statin (but not 80 mg simvastatin). • Goal - <70 mg/dl (2. 0 mmol/l) LDL cholesterol. ACC/ AHA 2007 in JACC (2008) 51; 210 -47 2007 in Eur Heart J (2007) 28; 1598 -1660 ESC
Statins in ACS - Guidelines • Who - Initiate therapy regardless of baseline LDL. • When – Pre-discharge; but no difference in benefit when initiated immediately or days post event (ESC <4 days). • What – Evidence base is for high dose statin (but not 80 mg simvastatin). • Goal - <70 mg/dl (2. 0 mmol/l) LDL cholesterol. ACC/ AHA 2007 in JACC (2008) 51; 210 -47 2007 in Eur Heart J (2007) 28; 1598 -1660 ESC
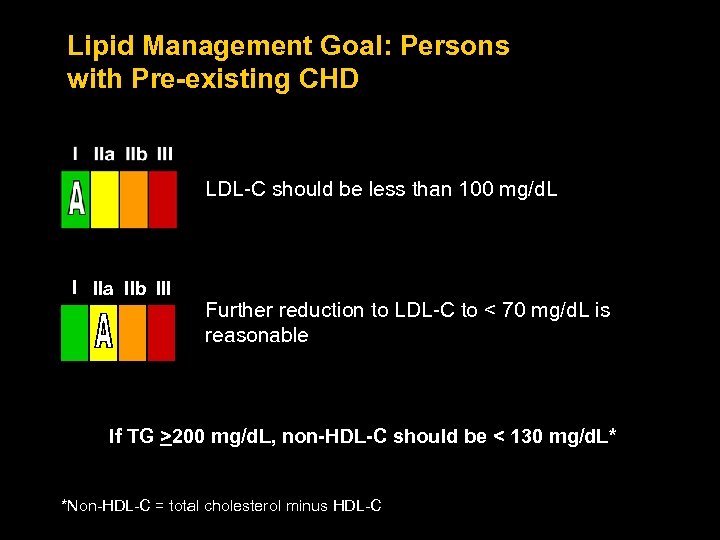 Lipid Management Goal: Persons with Pre-existing CHD LDL-C should be less than 100 mg/d. L I IIa IIb III Further reduction to LDL-C to < 70 mg/d. L is reasonable If TG >200 mg/d. L, non-HDL-C should be < 130 mg/d. L* *Non-HDL-C = total cholesterol minus HDL-C
Lipid Management Goal: Persons with Pre-existing CHD LDL-C should be less than 100 mg/d. L I IIa IIb III Further reduction to LDL-C to < 70 mg/d. L is reasonable If TG >200 mg/d. L, non-HDL-C should be < 130 mg/d. L* *Non-HDL-C = total cholesterol minus HDL-C
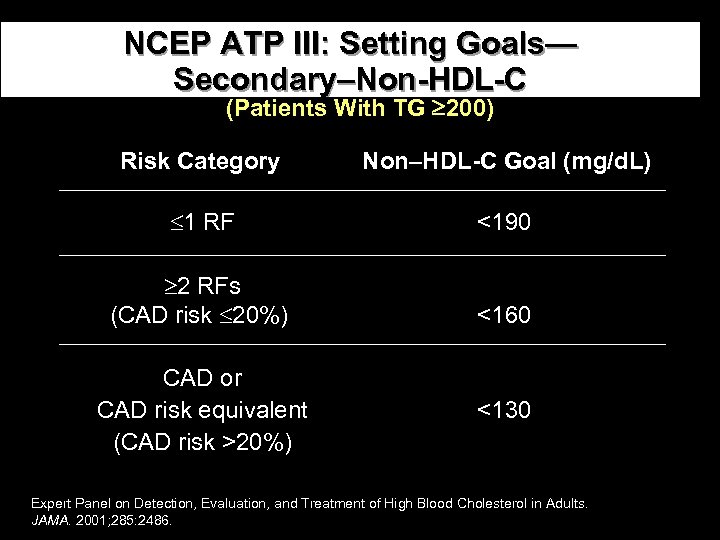 NCEP ATP III: Setting Goals— Secondary–Non-HDL-C (Patients With TG 200) Risk Category Non–HDL-C Goal (mg/d. L) 1 RF <190 2 RFs (CAD risk 20%) <160 CAD or CAD risk equivalent (CAD risk >20%) <130 Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
NCEP ATP III: Setting Goals— Secondary–Non-HDL-C (Patients With TG 200) Risk Category Non–HDL-C Goal (mg/d. L) 1 RF <190 2 RFs (CAD risk 20%) <160 CAD or CAD risk equivalent (CAD risk >20%) <130 Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
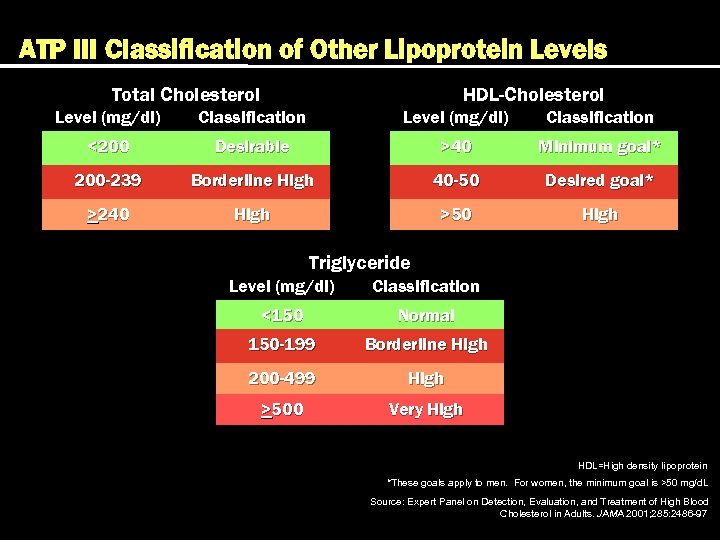 ATP III Classification of Other Lipoprotein Levels Total Cholesterol HDL-Cholesterol Level (mg/dl) Classification <200 Desirable >40 Minimum goal* 200 -239 Borderline High 40 -50 Desired goal* >240 High >50 High Triglyceride Level (mg/dl) Classification <150 Normal 150 -199 Borderline High 200 -499 High >500 Very High HDL=High density lipoprotein *These goals apply to men. For women, the minimum goal is >50 mg/d. L Source: Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA 2001; 285: 2486 -97
ATP III Classification of Other Lipoprotein Levels Total Cholesterol HDL-Cholesterol Level (mg/dl) Classification <200 Desirable >40 Minimum goal* 200 -239 Borderline High 40 -50 Desired goal* >240 High >50 High Triglyceride Level (mg/dl) Classification <150 Normal 150 -199 Borderline High 200 -499 High >500 Very High HDL=High density lipoprotein *These goals apply to men. For women, the minimum goal is >50 mg/d. L Source: Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA 2001; 285: 2486 -97
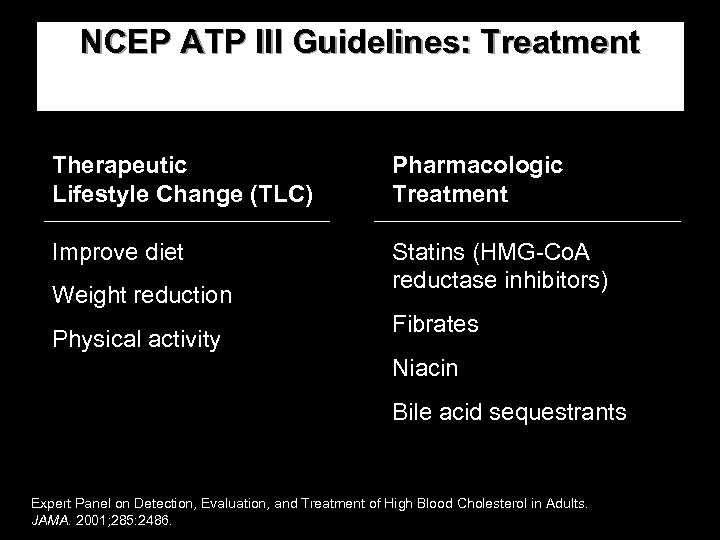 NCEP ATP III Guidelines: Treatment Therapeutic Lifestyle Change (TLC) Pharmacologic Treatment Improve diet Statins (HMG-Co. A reductase inhibitors) Weight reduction Physical activity Fibrates Niacin Bile acid sequestrants Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
NCEP ATP III Guidelines: Treatment Therapeutic Lifestyle Change (TLC) Pharmacologic Treatment Improve diet Statins (HMG-Co. A reductase inhibitors) Weight reduction Physical activity Fibrates Niacin Bile acid sequestrants Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.


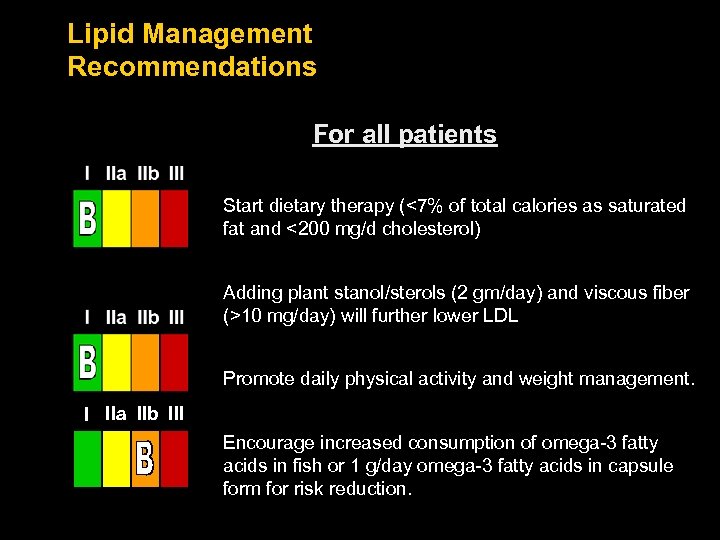 Lipid Management Recommendations For all patients Start dietary therapy (<7% of total calories as saturated fat and <200 mg/d cholesterol) Adding plant stanol/sterols (2 gm/day) and viscous fiber (>10 mg/day) will further lower LDL Promote daily physical activity and weight management. I IIa IIb III Encourage increased consumption of omega-3 fatty acids in fish or 1 g/day omega-3 fatty acids in capsule form for risk reduction.
Lipid Management Recommendations For all patients Start dietary therapy (<7% of total calories as saturated fat and <200 mg/d cholesterol) Adding plant stanol/sterols (2 gm/day) and viscous fiber (>10 mg/day) will further lower LDL Promote daily physical activity and weight management. I IIa IIb III Encourage increased consumption of omega-3 fatty acids in fish or 1 g/day omega-3 fatty acids in capsule form for risk reduction.
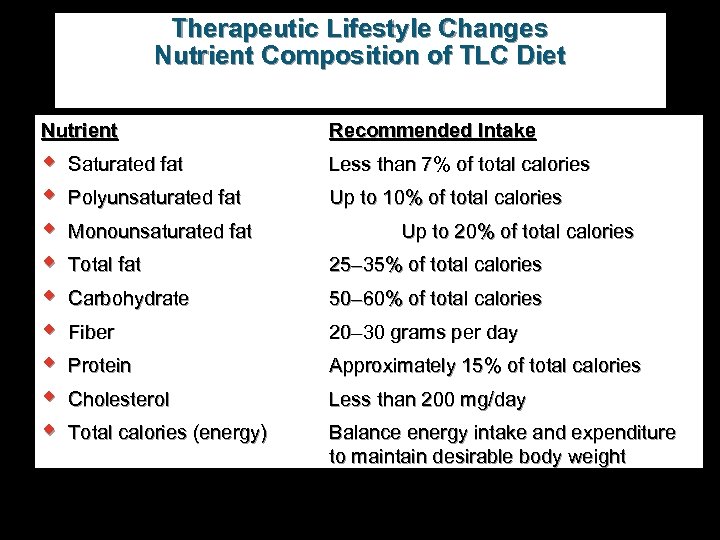 Therapeutic Lifestyle Changes Nutrient Composition of TLC Diet Nutrient Recommended Intake w w w w w Saturated fat Less than 7% of total calories Polyunsaturated fat Up to 10% of total calories Monounsaturated fat Up to 20% of total calories Total fat 25– 35% of total calories Carbohydrate 50– 60% of total calories Fiber 20– 30 grams per day Protein Approximately 15% of total calories Cholesterol Less than 200 mg/day Total calories (energy) Balance energy intake and expenditure to maintain desirable body weight
Therapeutic Lifestyle Changes Nutrient Composition of TLC Diet Nutrient Recommended Intake w w w w w Saturated fat Less than 7% of total calories Polyunsaturated fat Up to 10% of total calories Monounsaturated fat Up to 20% of total calories Total fat 25– 35% of total calories Carbohydrate 50– 60% of total calories Fiber 20– 30 grams per day Protein Approximately 15% of total calories Cholesterol Less than 200 mg/day Total calories (energy) Balance energy intake and expenditure to maintain desirable body weight
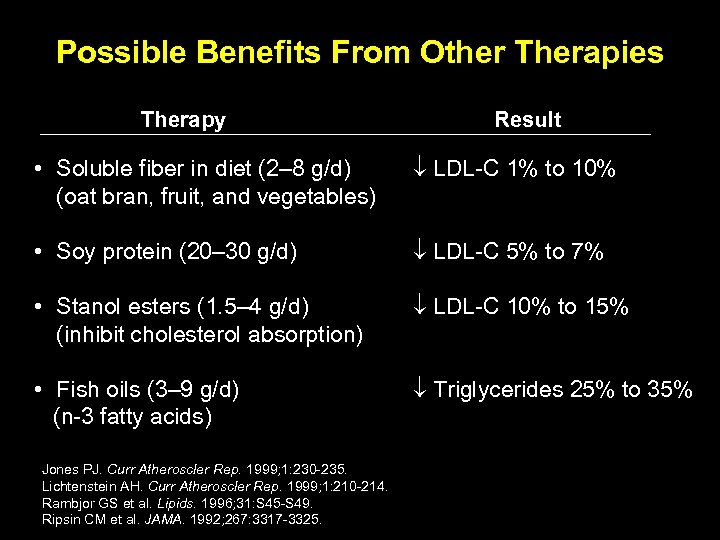 Possible Benefits From Other Therapies Therapy Result • Soluble fiber in diet (2– 8 g/d) (oat bran, fruit, and vegetables) LDL-C 1% to 10% • Soy protein (20– 30 g/d) LDL-C 5% to 7% • Stanol esters (1. 5– 4 g/d) (inhibit cholesterol absorption) LDL-C 10% to 15% • Fish oils (3– 9 g/d) (n-3 fatty acids) Triglycerides 25% to 35% Jones PJ. Curr Atheroscler Rep. 1999; 1: 230 -235. Lichtenstein AH. Curr Atheroscler Rep. 1999; 1: 210 -214. Rambjor GS et al. Lipids. 1996; 31: S 45 -S 49. Ripsin CM et al. JAMA. 1992; 267: 3317 -3325.
Possible Benefits From Other Therapies Therapy Result • Soluble fiber in diet (2– 8 g/d) (oat bran, fruit, and vegetables) LDL-C 1% to 10% • Soy protein (20– 30 g/d) LDL-C 5% to 7% • Stanol esters (1. 5– 4 g/d) (inhibit cholesterol absorption) LDL-C 10% to 15% • Fish oils (3– 9 g/d) (n-3 fatty acids) Triglycerides 25% to 35% Jones PJ. Curr Atheroscler Rep. 1999; 1: 230 -235. Lichtenstein AH. Curr Atheroscler Rep. 1999; 1: 210 -214. Rambjor GS et al. Lipids. 1996; 31: S 45 -S 49. Ripsin CM et al. JAMA. 1992; 267: 3317 -3325.
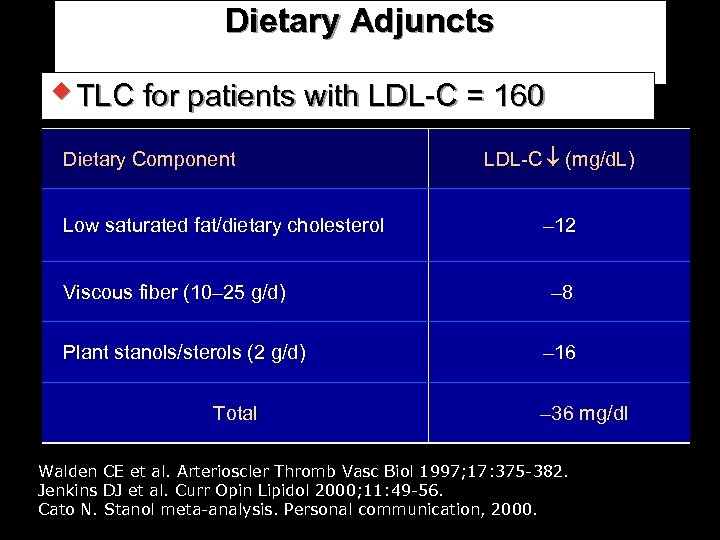 Dietary Adjuncts w TLC for patients with LDL-C = 160 Dietary Component Low saturated fat/dietary cholesterol Viscous fiber (10– 25 g/d) Plant stanols/sterols (2 g/d) Total LDL-C (mg/d. L) – 12 – 8 – 16 – 36 mg/dl Walden CE et al. Arterioscler Thromb Vasc Biol 1997; 17: 375 -382. Jenkins DJ et al. Curr Opin Lipidol 2000; 11: 49 -56. Cato N. Stanol meta-analysis. Personal communication, 2000.
Dietary Adjuncts w TLC for patients with LDL-C = 160 Dietary Component Low saturated fat/dietary cholesterol Viscous fiber (10– 25 g/d) Plant stanols/sterols (2 g/d) Total LDL-C (mg/d. L) – 12 – 8 – 16 – 36 mg/dl Walden CE et al. Arterioscler Thromb Vasc Biol 1997; 17: 375 -382. Jenkins DJ et al. Curr Opin Lipidol 2000; 11: 49 -56. Cato N. Stanol meta-analysis. Personal communication, 2000.
 THE REAL NIGHTMARE Moderate physical activity at least 30 -60 minutes 5 days a week or longer will help to raise HDL-C, lower total and LDL-C, lower TG, lower glucose, insulin, and blood pressure levels.
THE REAL NIGHTMARE Moderate physical activity at least 30 -60 minutes 5 days a week or longer will help to raise HDL-C, lower total and LDL-C, lower TG, lower glucose, insulin, and blood pressure levels.
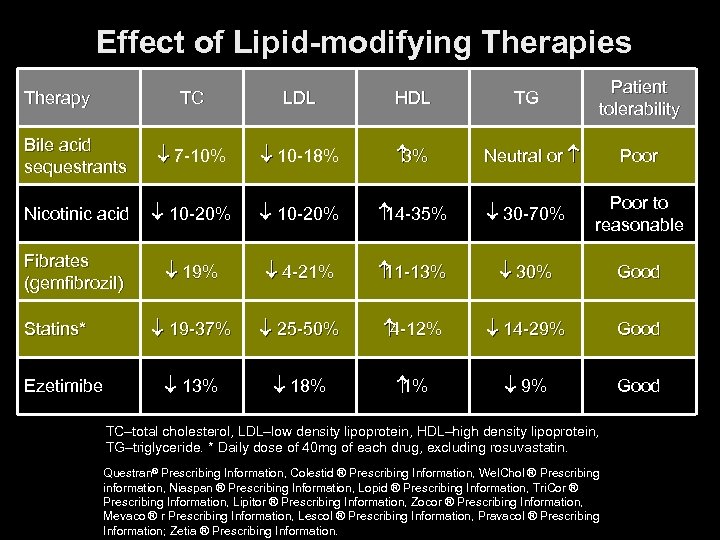 Effect of Lipid-modifying Therapies Therapy LDL HDL Bile acid sequestrants 7 -10% 10 -18% 3% Nicotinic acid 10 -20% 14 -35% 30 -70% Poor to reasonable Fibrates (gemfibrozil) 19% 4 -21% 11 -13% 30% Good 19 -37% 25 -50% 4 -12% 14 -29% Good 13% 18% 1% 9% Good Statins* Ezetimibe TG Patient tolerability TC Neutral or Poor TC–total cholesterol, LDL–low density lipoprotein, HDL–high density lipoprotein, TG–triglyceride. * Daily dose of 40 mg of each drug, excluding rosuvastatin. Questran® Prescribing Information, Colestid ® Prescribing Information, Wel. Chol ® Prescribing information, Niaspan ® Prescribing Information, Lopid ® Prescribing Information, Tri. Cor ® Prescribing Information, Lipitor ® Prescribing Information, Zocor ® Prescribing Information, Mevaco ® r Prescribing Information, Lescol ® Prescribing Information, Pravacol ® Prescribing Information; Zetia ® Prescribing Information.
Effect of Lipid-modifying Therapies Therapy LDL HDL Bile acid sequestrants 7 -10% 10 -18% 3% Nicotinic acid 10 -20% 14 -35% 30 -70% Poor to reasonable Fibrates (gemfibrozil) 19% 4 -21% 11 -13% 30% Good 19 -37% 25 -50% 4 -12% 14 -29% Good 13% 18% 1% 9% Good Statins* Ezetimibe TG Patient tolerability TC Neutral or Poor TC–total cholesterol, LDL–low density lipoprotein, HDL–high density lipoprotein, TG–triglyceride. * Daily dose of 40 mg of each drug, excluding rosuvastatin. Questran® Prescribing Information, Colestid ® Prescribing Information, Wel. Chol ® Prescribing information, Niaspan ® Prescribing Information, Lopid ® Prescribing Information, Tri. Cor ® Prescribing Information, Lipitor ® Prescribing Information, Zocor ® Prescribing Information, Mevaco ® r Prescribing Information, Lescol ® Prescribing Information, Pravacol ® Prescribing Information; Zetia ® Prescribing Information.
 When LDL-lowering drug therapy is employed in high-risk or moderately high risk patients, intensity of therapy should be sufficient to achieve a 30– 40% reduction in LDL-C levels.
When LDL-lowering drug therapy is employed in high-risk or moderately high risk patients, intensity of therapy should be sufficient to achieve a 30– 40% reduction in LDL-C levels.
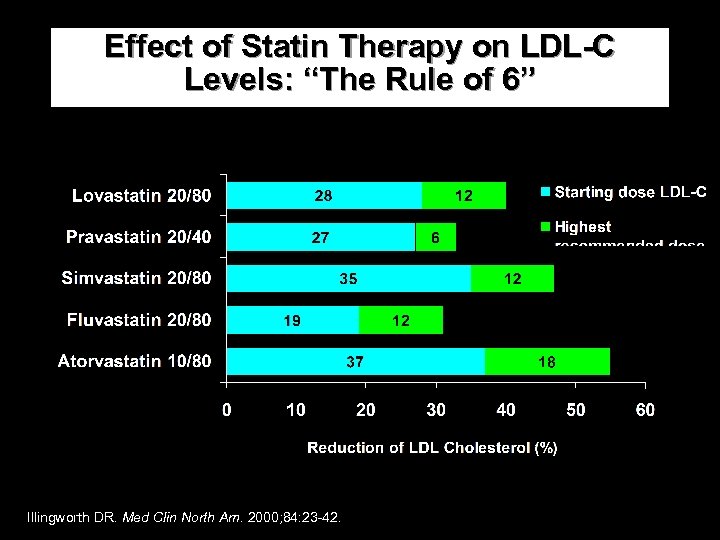 Effect of Statin Therapy on LDL-C Levels: “The Rule of 6” Illingworth DR. Med Clin North Am. 2000; 84: 23 -42.
Effect of Statin Therapy on LDL-C Levels: “The Rule of 6” Illingworth DR. Med Clin North Am. 2000; 84: 23 -42.
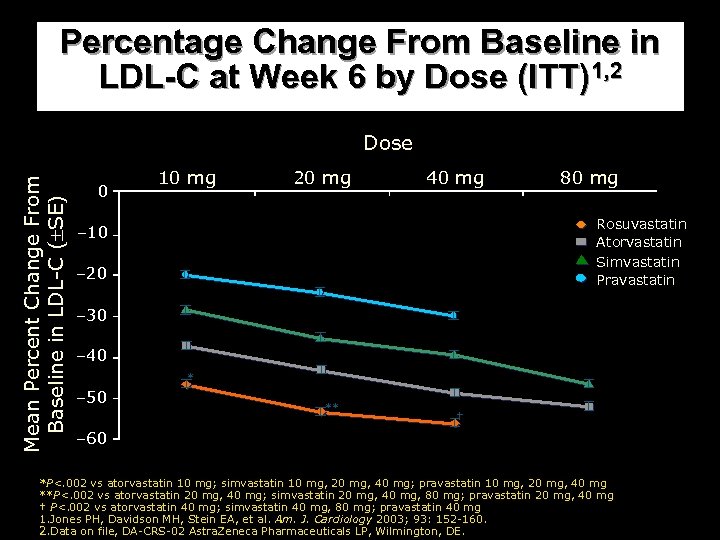 Percentage Change From Baseline in LDL-C at Week 6 by Dose (ITT)1, 2 Mean Percent Change From Baseline in LDL-C ( SE) Dose 0 10 mg 20 mg 40 mg 80 mg Rosuvastatin Atorvastatin Simvastatin Pravastatin – 10 – 20 – 30 – 40 * – 50 ** † – 60 *P<. 002 vs atorvastatin 10 mg; simvastatin 10 mg, 20 mg, 40 mg; pravastatin 10 mg, 20 mg, 40 mg **P<. 002 vs atorvastatin 20 mg, 40 mg; simvastatin 20 mg, 40 mg, 80 mg; pravastatin 20 mg, 40 mg † P<. 002 vs atorvastatin 40 mg; simvastatin 40 mg, 80 mg; pravastatin 40 mg 1. Jones PH, Davidson MH, Stein EA, et al. Am. J. Cardiology 2003; 93: 152 -160. 2. Data on file, DA-CRS-02 Astra. Zeneca Pharmaceuticals LP, Wilmington, DE.
Percentage Change From Baseline in LDL-C at Week 6 by Dose (ITT)1, 2 Mean Percent Change From Baseline in LDL-C ( SE) Dose 0 10 mg 20 mg 40 mg 80 mg Rosuvastatin Atorvastatin Simvastatin Pravastatin – 10 – 20 – 30 – 40 * – 50 ** † – 60 *P<. 002 vs atorvastatin 10 mg; simvastatin 10 mg, 20 mg, 40 mg; pravastatin 10 mg, 20 mg, 40 mg **P<. 002 vs atorvastatin 20 mg, 40 mg; simvastatin 20 mg, 40 mg, 80 mg; pravastatin 20 mg, 40 mg † P<. 002 vs atorvastatin 40 mg; simvastatin 40 mg, 80 mg; pravastatin 40 mg 1. Jones PH, Davidson MH, Stein EA, et al. Am. J. Cardiology 2003; 93: 152 -160. 2. Data on file, DA-CRS-02 Astra. Zeneca Pharmaceuticals LP, Wilmington, DE.
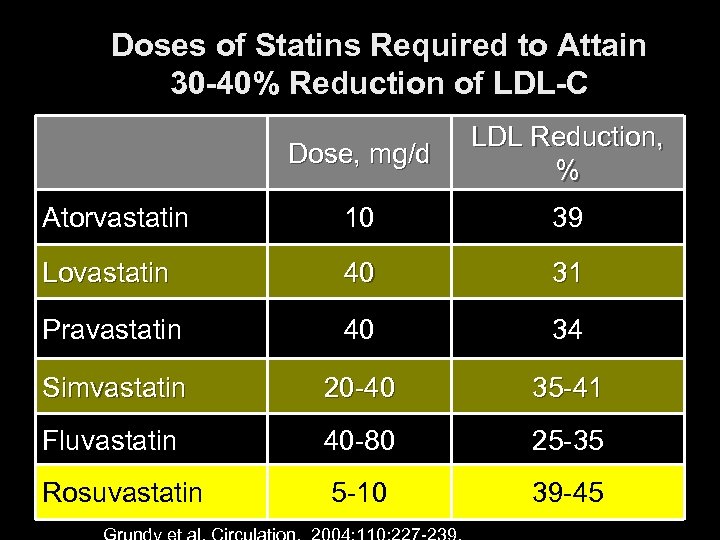 Doses of Statins Required to Attain 30 -40% Reduction of LDL-C Dose, mg/d LDL Reduction, % Atorvastatin 10 39 Lovastatin 40 31 Pravastatin 40 34 Simvastatin 20 -40 35 -41 Fluvastatin 40 -80 25 -35 Rosuvastatin 5 -10 39 -45
Doses of Statins Required to Attain 30 -40% Reduction of LDL-C Dose, mg/d LDL Reduction, % Atorvastatin 10 39 Lovastatin 40 31 Pravastatin 40 34 Simvastatin 20 -40 35 -41 Fluvastatin 40 -80 25 -35 Rosuvastatin 5 -10 39 -45
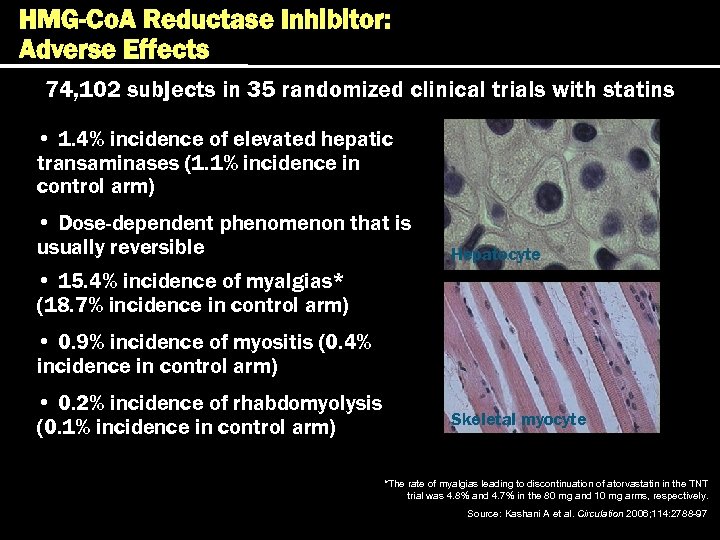 HMG-Co. A Reductase Inhibitor: Adverse Effects 74, 102 subjects in 35 randomized clinical trials with statins • 1. 4% incidence of elevated hepatic transaminases (1. 1% incidence in control arm) • Dose-dependent phenomenon that is usually reversible Hepatocyte • 15. 4% incidence of myalgias* (18. 7% incidence in control arm) • 0. 9% incidence of myositis (0. 4% incidence in control arm) • 0. 2% incidence of rhabdomyolysis (0. 1% incidence in control arm) Skeletal myocyte *The rate of myalgias leading to discontinuation of atorvastatin in the TNT trial was 4. 8% and 4. 7% in the 80 mg and 10 mg arms, respectively. Source: Kashani A et al. Circulation 2006; 114: 2788 -97
HMG-Co. A Reductase Inhibitor: Adverse Effects 74, 102 subjects in 35 randomized clinical trials with statins • 1. 4% incidence of elevated hepatic transaminases (1. 1% incidence in control arm) • Dose-dependent phenomenon that is usually reversible Hepatocyte • 15. 4% incidence of myalgias* (18. 7% incidence in control arm) • 0. 9% incidence of myositis (0. 4% incidence in control arm) • 0. 2% incidence of rhabdomyolysis (0. 1% incidence in control arm) Skeletal myocyte *The rate of myalgias leading to discontinuation of atorvastatin in the TNT trial was 4. 8% and 4. 7% in the 80 mg and 10 mg arms, respectively. Source: Kashani A et al. Circulation 2006; 114: 2788 -97
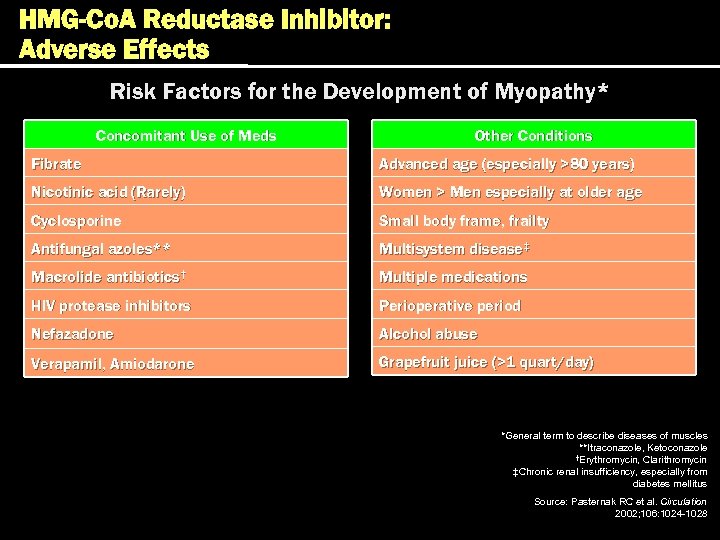 HMG-Co. A Reductase Inhibitor: Adverse Effects Risk Factors for the Development of Myopathy* Concomitant Use of Meds Other Conditions Fibrate Advanced age (especially >80 years) Nicotinic acid (Rarely) Women > Men especially at older age Cyclosporine Small body frame, frailty Antifungal azoles** Multisystem disease‡ Macrolide antibiotics† Multiple medications HIV protease inhibitors Perioperative period Nefazadone Alcohol abuse Verapamil, Amiodarone Grapefruit juice (>1 quart/day) *General term to describe diseases of muscles **Itraconazole, Ketoconazole †Erythromycin, Clarithromycin ‡Chronic renal insufficiency, especially from diabetes mellitus Source: Pasternak RC et al. Circulation 2002; 106: 1024 -1028
HMG-Co. A Reductase Inhibitor: Adverse Effects Risk Factors for the Development of Myopathy* Concomitant Use of Meds Other Conditions Fibrate Advanced age (especially >80 years) Nicotinic acid (Rarely) Women > Men especially at older age Cyclosporine Small body frame, frailty Antifungal azoles** Multisystem disease‡ Macrolide antibiotics† Multiple medications HIV protease inhibitors Perioperative period Nefazadone Alcohol abuse Verapamil, Amiodarone Grapefruit juice (>1 quart/day) *General term to describe diseases of muscles **Itraconazole, Ketoconazole †Erythromycin, Clarithromycin ‡Chronic renal insufficiency, especially from diabetes mellitus Source: Pasternak RC et al. Circulation 2002; 106: 1024 -1028
 Why combination therapy? w Few patients achieve LDL-C goal on monotherapy w Uptitration of dosage is rare w LDL-C goals are getting more aggressive w High-dose statins increase risk of side effects w Can address mixed dyslipidemia (e. g. , few pts achieve adequate control of HDL-C and triglycerides on monotherapy)
Why combination therapy? w Few patients achieve LDL-C goal on monotherapy w Uptitration of dosage is rare w LDL-C goals are getting more aggressive w High-dose statins increase risk of side effects w Can address mixed dyslipidemia (e. g. , few pts achieve adequate control of HDL-C and triglycerides on monotherapy)
 Options for Patients who Fail to Reach LDL-C Goal on Statin Monotherapy Addition of: • Niacin • Bile acid sequestrant • Cholesterol absorption inhibitor
Options for Patients who Fail to Reach LDL-C Goal on Statin Monotherapy Addition of: • Niacin • Bile acid sequestrant • Cholesterol absorption inhibitor
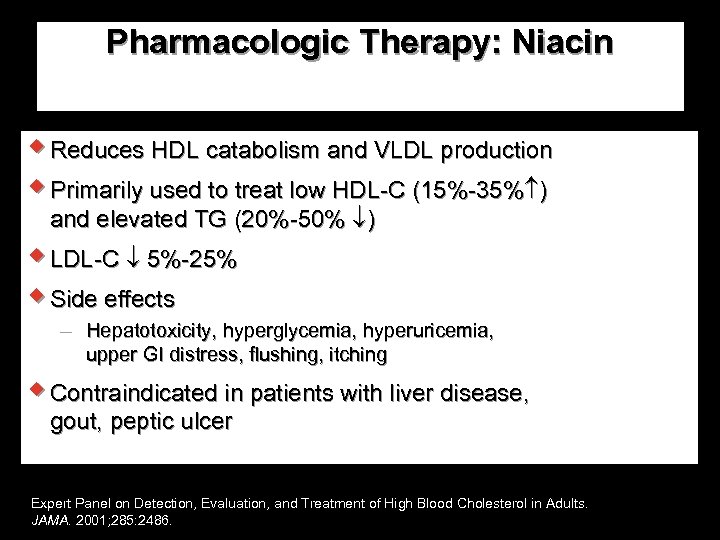 Pharmacologic Therapy: Niacin w Reduces HDL catabolism and VLDL production w Primarily used to treat low HDL-C (15%-35% ) and elevated TG (20%-50% ) w LDL-C 5%-25% w Side effects Hepatotoxicity, hyperglycemia, hyperuricemia, upper GI distress, flushing, itching w Contraindicated in patients with liver disease, gout, peptic ulcer Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
Pharmacologic Therapy: Niacin w Reduces HDL catabolism and VLDL production w Primarily used to treat low HDL-C (15%-35% ) and elevated TG (20%-50% ) w LDL-C 5%-25% w Side effects Hepatotoxicity, hyperglycemia, hyperuricemia, upper GI distress, flushing, itching w Contraindicated in patients with liver disease, gout, peptic ulcer Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
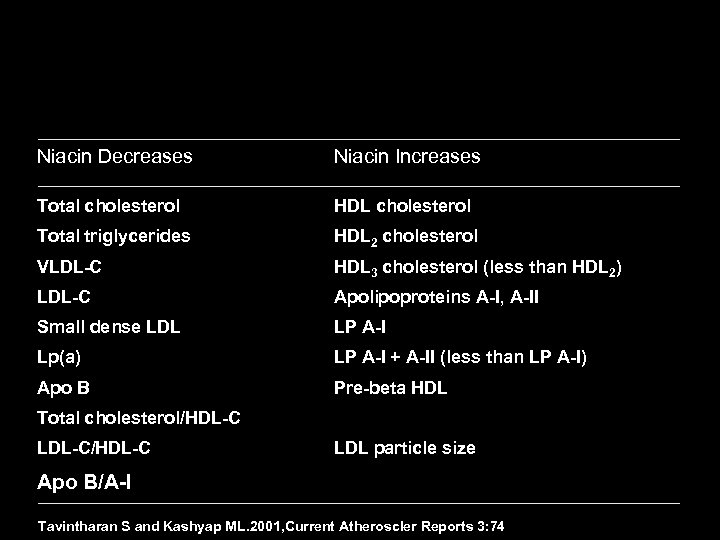 NIACIN: a broad-spectrum antidyslipidemic agent Niacin Decreases Niacin Increases Total cholesterol HDL cholesterol Total triglycerides HDL 2 cholesterol VLDL-C HDL 3 cholesterol (less than HDL 2) LDL-C Apolipoproteins A-I, A-II Small dense LDL LP A-I Lp(a) LP A-I + A-II (less than LP A-I) Apo B Pre-beta HDL Total cholesterol/HDL-C LDL-C/HDL-C LDL particle size Apo B/A-I Tavintharan S and Kashyap ML. 2001, Current Atheroscler Reports 3: 74
NIACIN: a broad-spectrum antidyslipidemic agent Niacin Decreases Niacin Increases Total cholesterol HDL cholesterol Total triglycerides HDL 2 cholesterol VLDL-C HDL 3 cholesterol (less than HDL 2) LDL-C Apolipoproteins A-I, A-II Small dense LDL LP A-I Lp(a) LP A-I + A-II (less than LP A-I) Apo B Pre-beta HDL Total cholesterol/HDL-C LDL-C/HDL-C LDL particle size Apo B/A-I Tavintharan S and Kashyap ML. 2001, Current Atheroscler Reports 3: 74
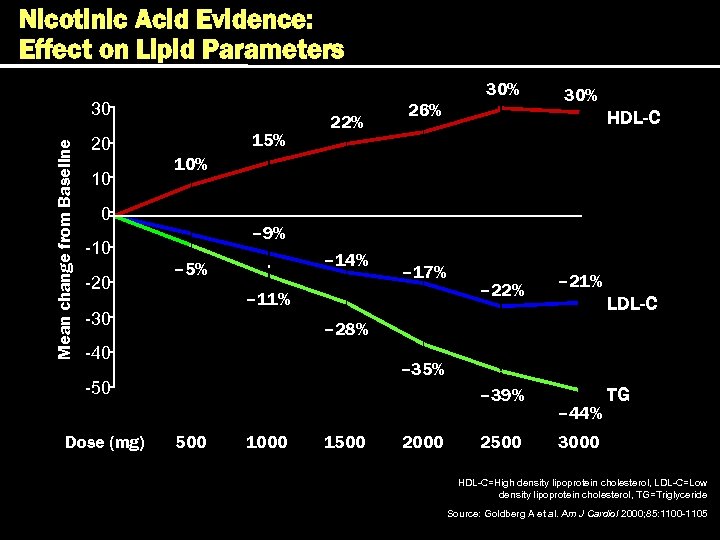 Nicotinic Acid Evidence: Effect on Lipid Parameters 30% Mean change from Baseline 30 20 10 15% HDL-C – 9% -10 – 14% – 5% – 17% – 11% -30 – 22% – 21% LDL-C – 28% -40 – 35% -50 Dose (mg) 26% 10% 0 -20 22% 30% – 39% 500 1000 1500 2000 2500 – 44% TG 3000 HDL-C=High density lipoprotein cholesterol, LDL-C=Low density lipoprotein cholesterol, TG=Triglyceride Source: Goldberg A et al. Am J Cardiol 2000; 85: 1100 -1105
Nicotinic Acid Evidence: Effect on Lipid Parameters 30% Mean change from Baseline 30 20 10 15% HDL-C – 9% -10 – 14% – 5% – 17% – 11% -30 – 22% – 21% LDL-C – 28% -40 – 35% -50 Dose (mg) 26% 10% 0 -20 22% 30% – 39% 500 1000 1500 2000 2500 – 44% TG 3000 HDL-C=High density lipoprotein cholesterol, LDL-C=Low density lipoprotein cholesterol, TG=Triglyceride Source: Goldberg A et al. Am J Cardiol 2000; 85: 1100 -1105
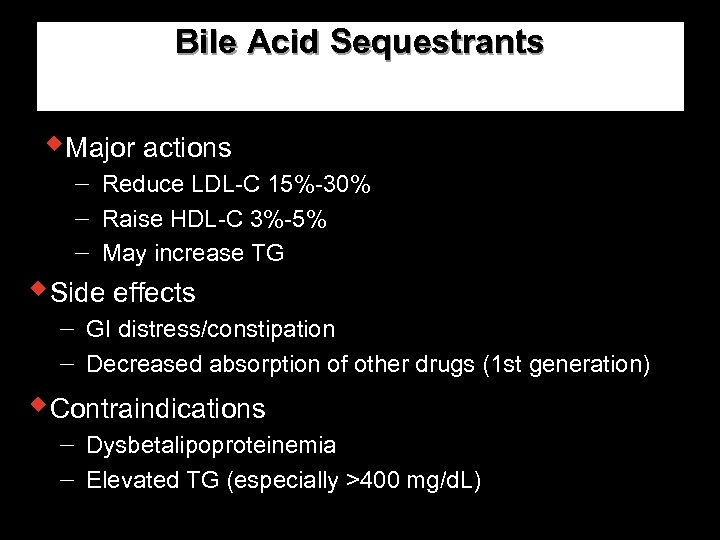 Bile Acid Sequestrants w. Major actions Reduce LDL-C 15%-30% Raise HDL-C 3%-5% May increase TG w. Side effects GI distress/constipation Decreased absorption of other drugs (1 st generation) w. Contraindications Dysbetalipoproteinemia Elevated TG (especially >400 mg/d. L)
Bile Acid Sequestrants w. Major actions Reduce LDL-C 15%-30% Raise HDL-C 3%-5% May increase TG w. Side effects GI distress/constipation Decreased absorption of other drugs (1 st generation) w. Contraindications Dysbetalipoproteinemia Elevated TG (especially >400 mg/d. L)
 New Bile Acid Sequestrant: Colesevelam w. Lower dose for effect w. Fewer GI complaints than with other bile acid sequestrants w. Reduces absorption of -carotene w. Requires 4 -6 tablets/day Davidson et al. Expert Opin Investig Drugs. 2000; 9: 2663.
New Bile Acid Sequestrant: Colesevelam w. Lower dose for effect w. Fewer GI complaints than with other bile acid sequestrants w. Reduces absorption of -carotene w. Requires 4 -6 tablets/day Davidson et al. Expert Opin Investig Drugs. 2000; 9: 2663.
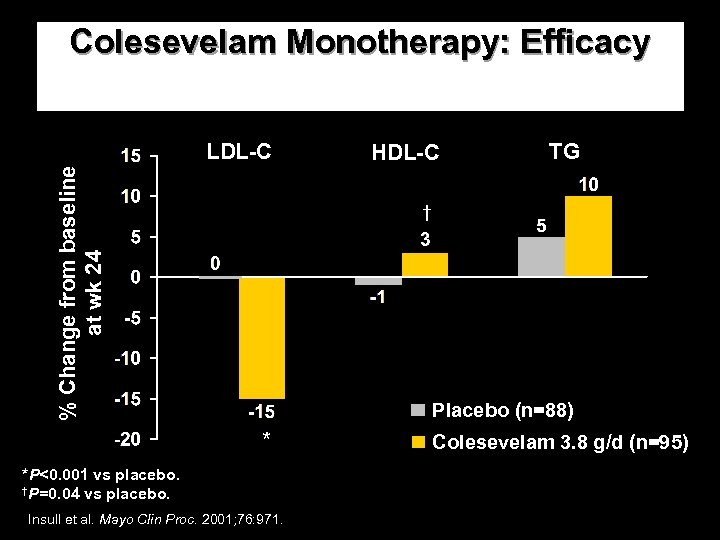 Colesevelam Monotherapy: Efficacy % Change from baseline at wk 24 LDL-C HDL-C TG † Placebo (n=88) * *P<0. 001 vs placebo. †P=0. 04 vs placebo. Insull et al. Mayo Clin Proc. 2001; 76: 971. Colesevelam 3. 8 g/d (n=95)
Colesevelam Monotherapy: Efficacy % Change from baseline at wk 24 LDL-C HDL-C TG † Placebo (n=88) * *P<0. 001 vs placebo. †P=0. 04 vs placebo. Insull et al. Mayo Clin Proc. 2001; 76: 971. Colesevelam 3. 8 g/d (n=95)
 Pharmacologic Therapy: Fibrates w Inhibit hepatic TG production and increase HDL production w Used to treat elevated TG (20%-50% ) and low HDL-C (10%-20% ) w Variable effect on LDL-C w Side effects Dyspepsia, gallstones, myopathy Increased with statins w Contraindicated in patients with severe renal or hepatic disease Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
Pharmacologic Therapy: Fibrates w Inhibit hepatic TG production and increase HDL production w Used to treat elevated TG (20%-50% ) and low HDL-C (10%-20% ) w Variable effect on LDL-C w Side effects Dyspepsia, gallstones, myopathy Increased with statins w Contraindicated in patients with severe renal or hepatic disease Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001; 285: 2486.
 Limitations of Current Intestinal-Acting Agents w. Bile acid sequestrants Noncompliance GI tolerability Reduced absorption of lipid-soluble vitamins May increase TG in patients with hypertriglyceridemia w. Plant stanol and sterol esters Lack of selectivity Some patients may find difficult to incorporate into diet May reduce absorption of lipid-soluble vitamins
Limitations of Current Intestinal-Acting Agents w. Bile acid sequestrants Noncompliance GI tolerability Reduced absorption of lipid-soluble vitamins May increase TG in patients with hypertriglyceridemia w. Plant stanol and sterol esters Lack of selectivity Some patients may find difficult to incorporate into diet May reduce absorption of lipid-soluble vitamins
 Ezetimibe — Localizes at Brush Border of Small Intestine w. Ezetimibe, a selective cholesterol absorption inhibitor, localizes and appears to act at the brush border of the small intestine and inhibits cholesterol absorption w. This results in A decrease in the delivery of intestinal cholesterol to the liver A reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood
Ezetimibe — Localizes at Brush Border of Small Intestine w. Ezetimibe, a selective cholesterol absorption inhibitor, localizes and appears to act at the brush border of the small intestine and inhibits cholesterol absorption w. This results in A decrease in the delivery of intestinal cholesterol to the liver A reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood
 Ezetimibe and Statins Complementary Mechanisms w Ezetimibe reduces the delivery of cholesterol to the liver w Statins reduce cholesterol synthesis in the liver w The distinct mechanism of ezetimibe is complementary to that of statins w The effects of ezetimibe, either alone or in addition to a statin, on cardiovascular morbidity or mortality have not been established Knopp RH. N Engl J Med. 1999; 341: 498– 511.
Ezetimibe and Statins Complementary Mechanisms w Ezetimibe reduces the delivery of cholesterol to the liver w Statins reduce cholesterol synthesis in the liver w The distinct mechanism of ezetimibe is complementary to that of statins w The effects of ezetimibe, either alone or in addition to a statin, on cardiovascular morbidity or mortality have not been established Knopp RH. N Engl J Med. 1999; 341: 498– 511.
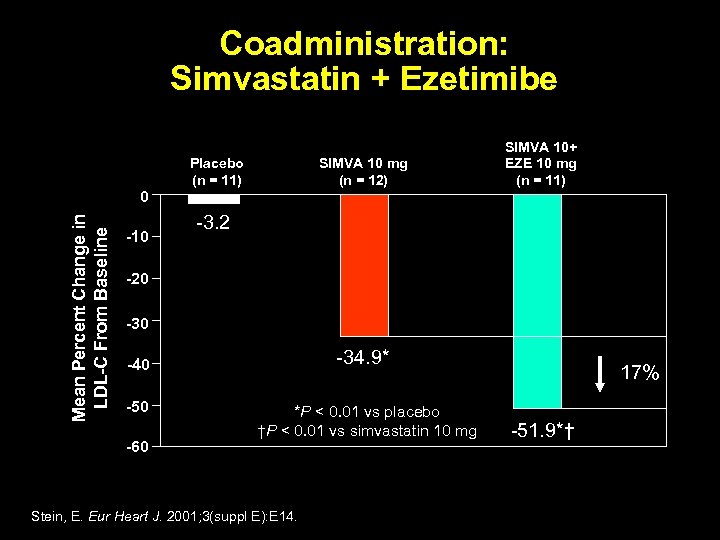 Coadministration: Simvastatin + Ezetimibe Placebo (n = 11) SIMVA 10 mg (n = 12) Mean Percent Change in LDL-C From Baseline 0 -10 SIMVA 10+ EZE 10 mg (n = 11) -3. 2 -20 -34. 9* -40 -50 -60 *P < 0. 01 vs placebo †P < 0. 01 vs simvastatin 10 mg Stein, E. Eur Heart J. 2001; 3(suppl E): E 14. 17% -51. 9*†
Coadministration: Simvastatin + Ezetimibe Placebo (n = 11) SIMVA 10 mg (n = 12) Mean Percent Change in LDL-C From Baseline 0 -10 SIMVA 10+ EZE 10 mg (n = 11) -3. 2 -20 -34. 9* -40 -50 -60 *P < 0. 01 vs placebo †P < 0. 01 vs simvastatin 10 mg Stein, E. Eur Heart J. 2001; 3(suppl E): E 14. 17% -51. 9*†
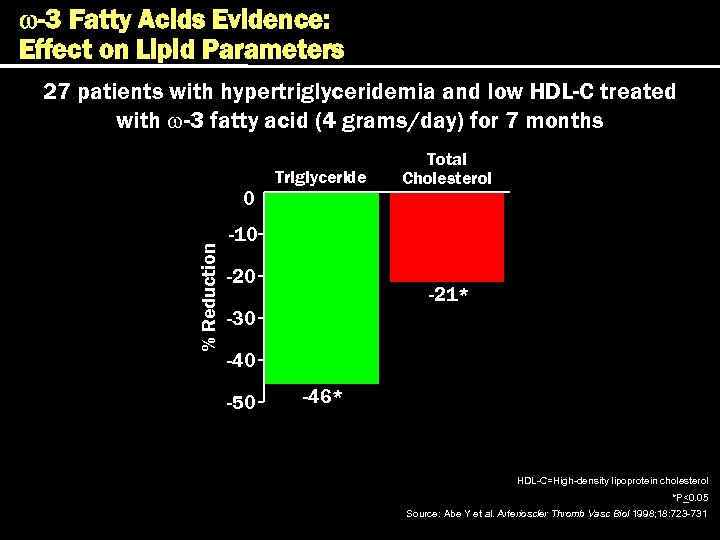 w-3 Fatty Acids Evidence: Effect on Lipid Parameters 27 patients with hypertriglyceridemia and low HDL-C treated with w-3 fatty acid (4 grams/day) for 7 months % Reduction 0 Triglyceride Total Cholesterol -10 -21* -30 -40 -50 -46* HDL-C=High-density lipoprotein cholesterol *P<0. 05 Source: Abe Y et al. Arterioscler Thromb Vasc Biol 1998; 18: 723 -731
w-3 Fatty Acids Evidence: Effect on Lipid Parameters 27 patients with hypertriglyceridemia and low HDL-C treated with w-3 fatty acid (4 grams/day) for 7 months % Reduction 0 Triglyceride Total Cholesterol -10 -21* -30 -40 -50 -46* HDL-C=High-density lipoprotein cholesterol *P<0. 05 Source: Abe Y et al. Arterioscler Thromb Vasc Biol 1998; 18: 723 -731
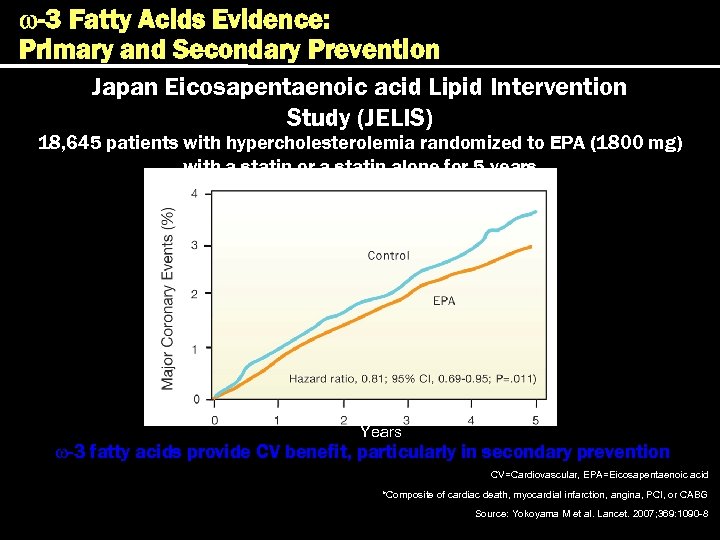 w-3 Fatty Acids Evidence: Primary and Secondary Prevention Japan Eicosapentaenoic acid Lipid Intervention Study (JELIS) 18, 645 patients with hypercholesterolemia randomized to EPA (1800 mg) with a statin or a statin alone for 5 years Years w-3 fatty acids provide CV benefit, particularly in secondary prevention CV=Cardiovascular, EPA=Eicosapentaenoic acid *Composite of cardiac death, myocardial infarction, angina, PCI, or CABG Source: Yokoyama M et al. Lancet. 2007; 369: 1090 -8
w-3 Fatty Acids Evidence: Primary and Secondary Prevention Japan Eicosapentaenoic acid Lipid Intervention Study (JELIS) 18, 645 patients with hypercholesterolemia randomized to EPA (1800 mg) with a statin or a statin alone for 5 years Years w-3 fatty acids provide CV benefit, particularly in secondary prevention CV=Cardiovascular, EPA=Eicosapentaenoic acid *Composite of cardiac death, myocardial infarction, angina, PCI, or CABG Source: Yokoyama M et al. Lancet. 2007; 369: 1090 -8
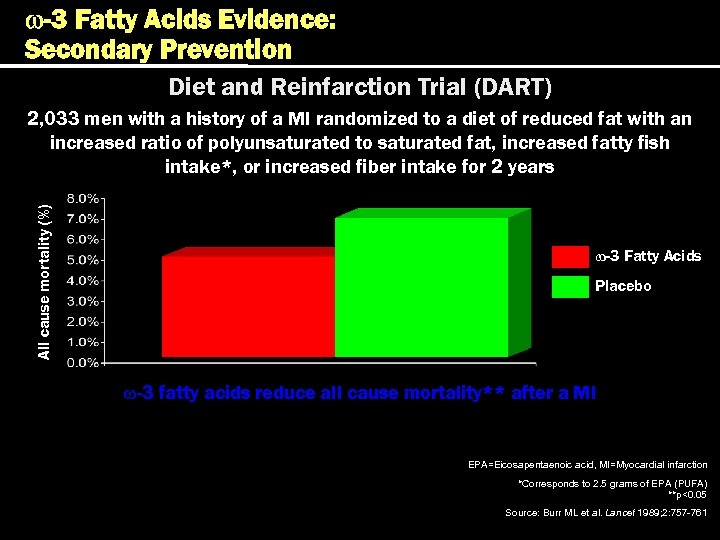 w-3 Fatty Acids Evidence: Secondary Prevention Diet and Reinfarction Trial (DART) All cause mortality (%) 2, 033 men with a history of a MI randomized to a diet of reduced fat with an increased ratio of polyunsaturated to saturated fat, increased fatty fish intake*, or increased fiber intake for 2 years w-3 Fatty Acids Placebo w-3 fatty acids reduce all cause mortality** after a MI EPA=Eicosapentaenoic acid, MI=Myocardial infarction *Corresponds to 2. 5 grams of EPA (PUFA) **p<0. 05 Source: Burr ML et al. Lancet 1989; 2: 757 -761
w-3 Fatty Acids Evidence: Secondary Prevention Diet and Reinfarction Trial (DART) All cause mortality (%) 2, 033 men with a history of a MI randomized to a diet of reduced fat with an increased ratio of polyunsaturated to saturated fat, increased fatty fish intake*, or increased fiber intake for 2 years w-3 Fatty Acids Placebo w-3 fatty acids reduce all cause mortality** after a MI EPA=Eicosapentaenoic acid, MI=Myocardial infarction *Corresponds to 2. 5 grams of EPA (PUFA) **p<0. 05 Source: Burr ML et al. Lancet 1989; 2: 757 -761
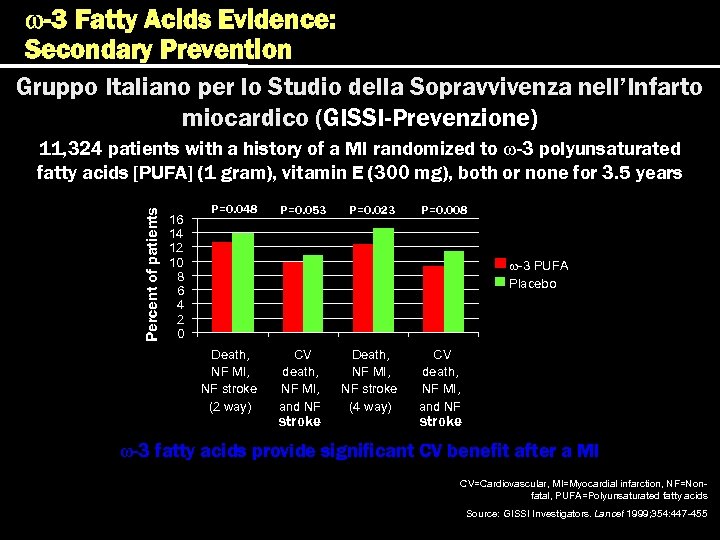 w-3 Fatty Acids Evidence: Secondary Prevention Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI-Prevenzione) Percent of patients 11, 324 patients with a history of a MI randomized to w-3 polyunsaturated fatty acids [PUFA] (1 gram), vitamin E (300 mg), both or none for 3. 5 years 16 14 12 10 8 6 4 2 0 P=0. 048 P=0. 053 P=0. 023 P=0. 008 w-3 PUFA Placebo Death, NF MI, NF stroke (2 way) CV death, NF MI, and NF stroke Death, NF MI, NF stroke (4 way) CV death, NF MI, and NF stroke w-3 fatty acids provide significant CV benefit after a MI CV=Cardiovascular, MI=Myocardial infarction, NF=Nonfatal, PUFA=Polyunsaturated fatty acids Source: GISSI Investigators. Lancet 1999; 354: 447 -455
w-3 Fatty Acids Evidence: Secondary Prevention Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI-Prevenzione) Percent of patients 11, 324 patients with a history of a MI randomized to w-3 polyunsaturated fatty acids [PUFA] (1 gram), vitamin E (300 mg), both or none for 3. 5 years 16 14 12 10 8 6 4 2 0 P=0. 048 P=0. 053 P=0. 023 P=0. 008 w-3 PUFA Placebo Death, NF MI, NF stroke (2 way) CV death, NF MI, and NF stroke Death, NF MI, NF stroke (4 way) CV death, NF MI, and NF stroke w-3 fatty acids provide significant CV benefit after a MI CV=Cardiovascular, MI=Myocardial infarction, NF=Nonfatal, PUFA=Polyunsaturated fatty acids Source: GISSI Investigators. Lancet 1999; 354: 447 -455
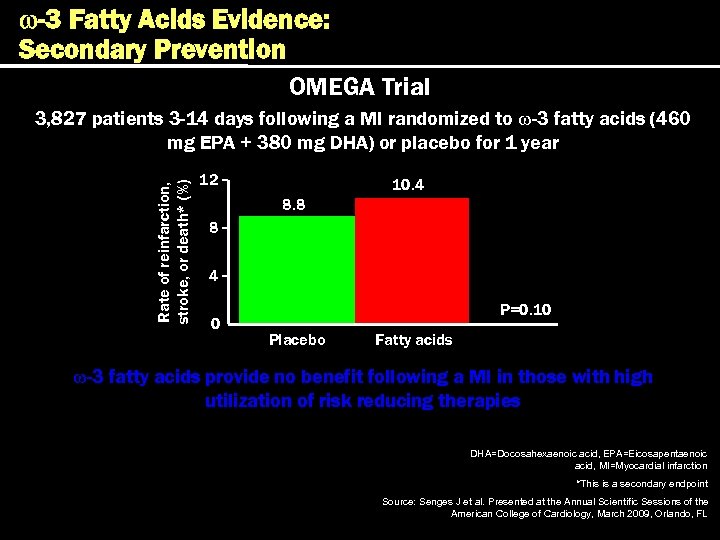 w-3 Fatty Acids Evidence: Secondary Prevention OMEGA Trial Rate of reinfarction, stroke, or death* (%) 3, 827 patients 3 -14 days following a MI randomized to w-3 fatty acids (460 mg EPA + 380 mg DHA) or placebo for 1 year 12 10. 4 8. 8 8 4 0 P=0. 10 Placebo Fatty acids w-3 fatty acids provide no benefit following a MI in those with high utilization of risk reducing therapies DHA=Docosahexaenoic acid, EPA=Eicosapentaenoic acid, MI=Myocardial infarction *This is a secondary endpoint Source: Senges J et al. Presented at the Annual Scientific Sessions of the American College of Cardiology, March 2009, Orlando, FL
w-3 Fatty Acids Evidence: Secondary Prevention OMEGA Trial Rate of reinfarction, stroke, or death* (%) 3, 827 patients 3 -14 days following a MI randomized to w-3 fatty acids (460 mg EPA + 380 mg DHA) or placebo for 1 year 12 10. 4 8. 8 8 4 0 P=0. 10 Placebo Fatty acids w-3 fatty acids provide no benefit following a MI in those with high utilization of risk reducing therapies DHA=Docosahexaenoic acid, EPA=Eicosapentaenoic acid, MI=Myocardial infarction *This is a secondary endpoint Source: Senges J et al. Presented at the Annual Scientific Sessions of the American College of Cardiology, March 2009, Orlando, FL
 CONCLUSIONS w Many persons with normal total or LDL-C levels still suffer CHD events. w While statin-based clinical trials significantly reduce risk of CHD, residual risk still exists. w Non-HDL-C, which reflects all the atherogenic lipid fractions, appears to be a stronger predictor of CHD events than LDL-C. w The measurement of non-HDL-C and its use as a secondary therapeutic target is warranted to better address residual CHD risk. w Lifestyle therapies as well as pharmacologic approaches, particular combination therapy with statins and other agents, are important for optimizing the entire lipid profile.
CONCLUSIONS w Many persons with normal total or LDL-C levels still suffer CHD events. w While statin-based clinical trials significantly reduce risk of CHD, residual risk still exists. w Non-HDL-C, which reflects all the atherogenic lipid fractions, appears to be a stronger predictor of CHD events than LDL-C. w The measurement of non-HDL-C and its use as a secondary therapeutic target is warranted to better address residual CHD risk. w Lifestyle therapies as well as pharmacologic approaches, particular combination therapy with statins and other agents, are important for optimizing the entire lipid profile.


