d9c8b2f201db36ce581fc04fa0030fe7.ppt
- Количество слайдов: 50
 Neuronal protective agents Andrew Nataraj 1
Neuronal protective agents Andrew Nataraj 1
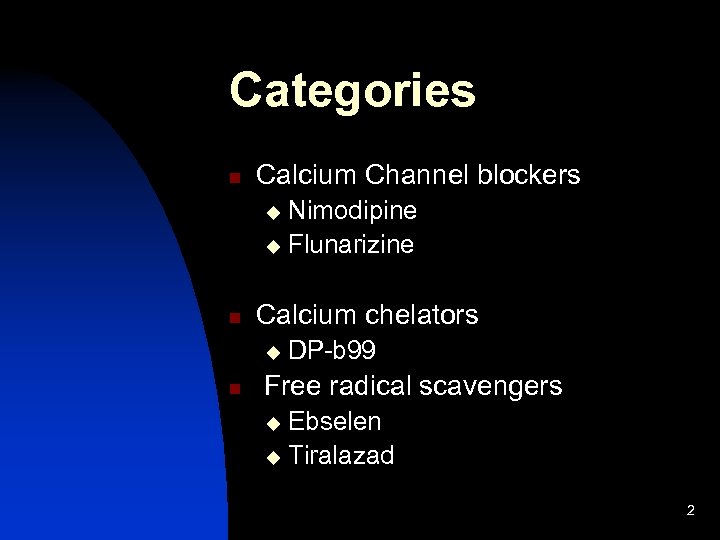 Categories n Calcium Channel blockers Nimodipine u Flunarizine u n Calcium chelators u n DP-b 99 Free radical scavengers Ebselen u Tiralazad u 2
Categories n Calcium Channel blockers Nimodipine u Flunarizine u n Calcium chelators u n DP-b 99 Free radical scavengers Ebselen u Tiralazad u 2
 Categories n GABA agonists u n Clomethiazole Glutamate antagonists u AMPA antagonists: GYKI 52466 « NBQX « YM 90 K « YM 872 « ZK-200775 « n Kainate antagonists: u SYM 2081 3
Categories n GABA agonists u n Clomethiazole Glutamate antagonists u AMPA antagonists: GYKI 52466 « NBQX « YM 90 K « YM 872 « ZK-200775 « n Kainate antagonists: u SYM 2081 3
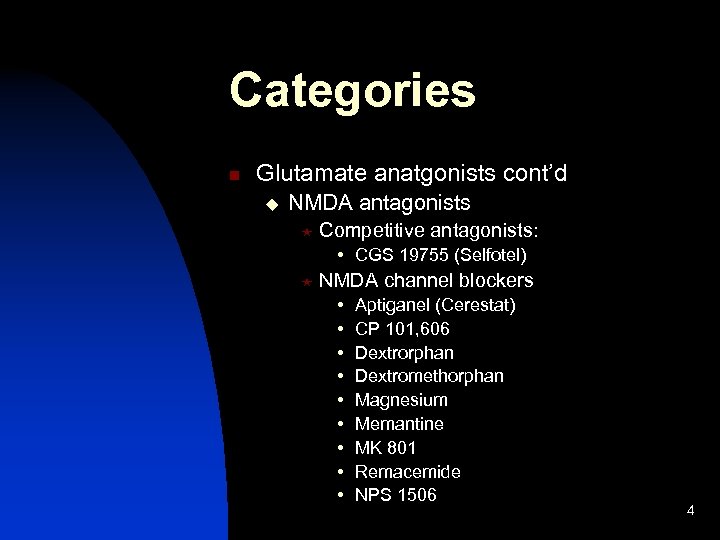 Categories n Glutamate anatgonists cont’d u NMDA antagonists « Competitive antagonists: • CGS 19755 (Selfotel) « NMDA channel blockers • • • Aptiganel (Cerestat) CP 101, 606 Dextrorphan Dextromethorphan Magnesium Memantine MK 801 Remacemide NPS 1506 4
Categories n Glutamate anatgonists cont’d u NMDA antagonists « Competitive antagonists: • CGS 19755 (Selfotel) « NMDA channel blockers • • • Aptiganel (Cerestat) CP 101, 606 Dextrorphan Dextromethorphan Magnesium Memantine MK 801 Remacemide NPS 1506 4
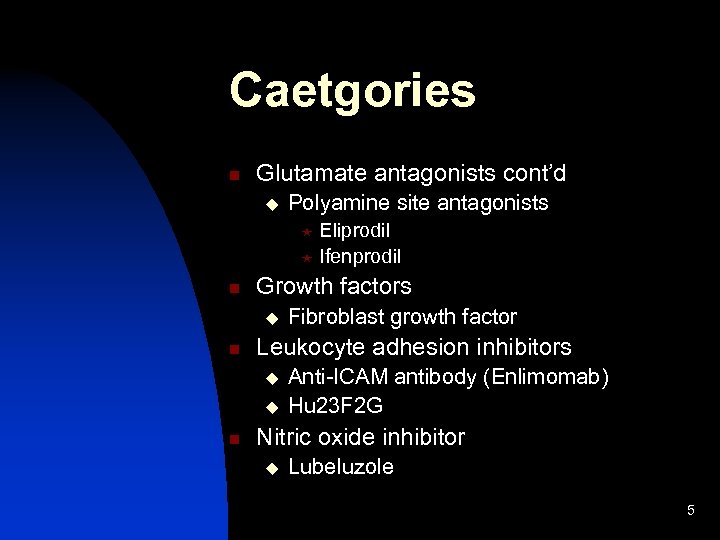 Caetgories n Glutamate antagonists cont’d u Polyamine site antagonists Eliprodil « Ifenprodil « n Growth factors u n Leukocyte adhesion inhibitors u u n Fibroblast growth factor Anti-ICAM antibody (Enlimomab) Hu 23 F 2 G Nitric oxide inhibitor u Lubeluzole 5
Caetgories n Glutamate antagonists cont’d u Polyamine site antagonists Eliprodil « Ifenprodil « n Growth factors u n Leukocyte adhesion inhibitors u u n Fibroblast growth factor Anti-ICAM antibody (Enlimomab) Hu 23 F 2 G Nitric oxide inhibitor u Lubeluzole 5
 Categories n Opioid antagonists Naloxone u Nalmefene u n Phosphatidylcholine precursor u n Citicholine (CDP-choline) Serotonin agonist u BAYX 3072 6
Categories n Opioid antagonists Naloxone u Nalmefene u n Phosphatidylcholine precursor u n Citicholine (CDP-choline) Serotonin agonist u BAYX 3072 6
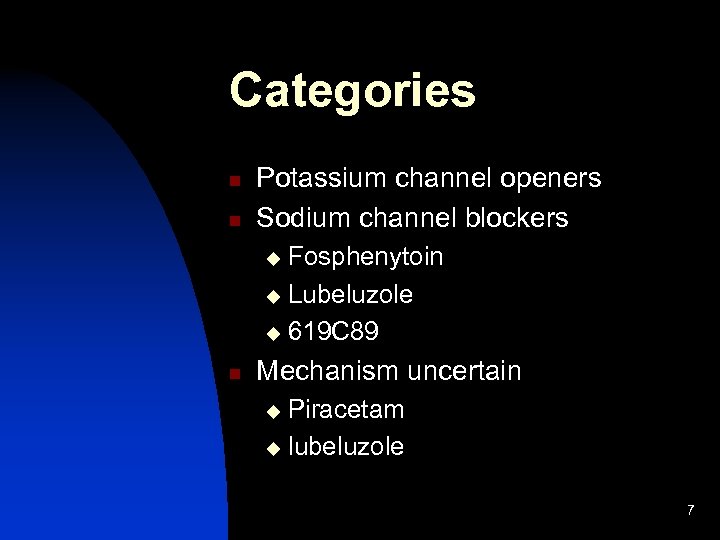 Categories n n Potassium channel openers Sodium channel blockers Fosphenytoin u Lubeluzole u 619 C 89 u n Mechanism uncertain Piracetam u lubeluzole u 7
Categories n n Potassium channel openers Sodium channel blockers Fosphenytoin u Lubeluzole u 619 C 89 u n Mechanism uncertain Piracetam u lubeluzole u 7
 Nimodipine (Nimotop) n n n Blocks L-type calcium channels Approved for treatment of subarachnoid hemorrhage Several studies regarding nimodipine in stroke, with some confliciting results 8
Nimodipine (Nimotop) n n n Blocks L-type calcium channels Approved for treatment of subarachnoid hemorrhage Several studies regarding nimodipine in stroke, with some confliciting results 8
 Nimodipine n Nimodipine and Perfusion changes after stroke (NIMPAS) u n n Stroke 1999; 30: 1417 -1423 Prospective, double blind, randomized controlled trial with 50 patients Inclusion: CT, SPECT, and nimodipine within 12 hours of symptoms 30 mg po 6 h for 14 days Primary outcome: SPECT 24 hrs and 3 months later; modified Canadian Neurological Scale; and CT and Barthel stroke index at 3 months 9
Nimodipine n Nimodipine and Perfusion changes after stroke (NIMPAS) u n n Stroke 1999; 30: 1417 -1423 Prospective, double blind, randomized controlled trial with 50 patients Inclusion: CT, SPECT, and nimodipine within 12 hours of symptoms 30 mg po 6 h for 14 days Primary outcome: SPECT 24 hrs and 3 months later; modified Canadian Neurological Scale; and CT and Barthel stroke index at 3 months 9
 Nimodipine n Results: no change in perfusion volumes at 3 months u Non-nutritional reperfusion in nimodipine group was associated with worse functional outcome at 3 months (p=. 06) 10
Nimodipine n Results: no change in perfusion volumes at 3 months u Non-nutritional reperfusion in nimodipine group was associated with worse functional outcome at 3 months (p=. 06) 10
 Nimodipine n Randomized, Double blind, placebo-controlled trial of nimodipine in Acute Ischemic Hemispheric Stroke u n n n Stroke 1994; 25: 1348 -1353 Multicentered, n=350 Acute, hemispheric stroke, and within 48 hours of onset 120 mg nimodipine po/day 11
Nimodipine n Randomized, Double blind, placebo-controlled trial of nimodipine in Acute Ischemic Hemispheric Stroke u n n n Stroke 1994; 25: 1348 -1353 Multicentered, n=350 Acute, hemispheric stroke, and within 48 hours of onset 120 mg nimodipine po/day 11
 Nimodipine n n Outcome: Rankin score in 12 months Results: higher case fatality at 1 and 3 months in nimodipine group (p=. 004, and p=. 03), which is not statistically significant at 1 year 12
Nimodipine n n Outcome: Rankin score in 12 months Results: higher case fatality at 1 and 3 months in nimodipine group (p=. 004, and p=. 03), which is not statistically significant at 1 year 12
 Nimodipine Double-blind Study of Nimodipine in Non-Severe Stroke u n n Eur Neurol 1990; 30(1): 23 -6 N=60, presented within 48 hours, and Mathew scale 50 -75 30 mg po qid Outcome: Mathew scale at 4 months Results: no difference 13
Nimodipine Double-blind Study of Nimodipine in Non-Severe Stroke u n n Eur Neurol 1990; 30(1): 23 -6 N=60, presented within 48 hours, and Mathew scale 50 -75 30 mg po qid Outcome: Mathew scale at 4 months Results: no difference 13
 Nimodipine n Nimodipine in Acute Ischemic Stroke u n n Acta Neurol Scand 1989 Oct; 80(4): 282 -6 N=4, admitted within 12 hours 40 mg tid po Outcome: Mathew scale to day 28 Higher rate of improvement in nimodipine group 14
Nimodipine n Nimodipine in Acute Ischemic Stroke u n n Acta Neurol Scand 1989 Oct; 80(4): 282 -6 N=4, admitted within 12 hours 40 mg tid po Outcome: Mathew scale to day 28 Higher rate of improvement in nimodipine group 14
 Nimodipine n Placebo-controlled Trial of Nimodipine in the Treatment of Acute Ischemic Cerebral Infarction u n n n Stroke 1990 Jul; 21(7): 1023 -8 Multicentred, n=164 Outcome: Mathew scale and mortality at 28 days Result: no difference but post hoc subgroup analysis of patients with better baseline score (Mathew>65) had better outcome in nimodipine group 15
Nimodipine n Placebo-controlled Trial of Nimodipine in the Treatment of Acute Ischemic Cerebral Infarction u n n n Stroke 1990 Jul; 21(7): 1023 -8 Multicentred, n=164 Outcome: Mathew scale and mortality at 28 days Result: no difference but post hoc subgroup analysis of patients with better baseline score (Mathew>65) had better outcome in nimodipine group 15
 Nimodipine n Controlled Trial of Nimodipine in Acute Ischemic Stroke u n n n N Engl J Med 1988 Jan 28; 318(4): 203 -7 Multicentred, n=186, presentation within 12 hours symptom onset Outcome: Mathew scale and death at 28 days and six months Results: in nimodipine group: decreased mortality in men at 28 days and improved and better neurological status at 6 months 16
Nimodipine n Controlled Trial of Nimodipine in Acute Ischemic Stroke u n n n N Engl J Med 1988 Jan 28; 318(4): 203 -7 Multicentred, n=186, presentation within 12 hours symptom onset Outcome: Mathew scale and death at 28 days and six months Results: in nimodipine group: decreased mortality in men at 28 days and improved and better neurological status at 6 months 16
 Nimodipine n Very Early Nimodipine Use in Stroke (VENUS) u n n n Presented at 24 th International Joint Conference on Stroke and Cerebral Circulation N=434, present within 6 hours, and receive nimodipine for 10 days Outcome: death and Rankin score at 10 days and 3 months Results: no difference in outcome, and trial was terminated early 17
Nimodipine n Very Early Nimodipine Use in Stroke (VENUS) u n n n Presented at 24 th International Joint Conference on Stroke and Cerebral Circulation N=434, present within 6 hours, and receive nimodipine for 10 days Outcome: death and Rankin score at 10 days and 3 months Results: no difference in outcome, and trial was terminated early 17
 Nimodipine n Randomized, Double-Blind, Placebo. Controlled Trial of Nimodipine in Acute Stroke u n n Lancet 1990 Nov 17; 336(8725): 1205 -9 Multicentred, n=1215 Within 48 hours, and previous independent functioning Outcome: 6 months independence, as defined of over 60 on Barthel index Results: no difference at 6 months; delayed recovery in nimodipine group at 3 weeks. 18
Nimodipine n Randomized, Double-Blind, Placebo. Controlled Trial of Nimodipine in Acute Stroke u n n Lancet 1990 Nov 17; 336(8725): 1205 -9 Multicentred, n=1215 Within 48 hours, and previous independent functioning Outcome: 6 months independence, as defined of over 60 on Barthel index Results: no difference at 6 months; delayed recovery in nimodipine group at 3 weeks. 18
 Nimodipine n American Nimodipine Study u n n Stroke 1992 Apr; 23(4): 615 Multicentred, n=1064 Ischemic stroke within 48 hours Primary outcome: Toronto scale, and motor strength up to 21 days Results: no difference overall but posthoc subgroup analysis showed less worsening in nimodipine group if given within 18 hours and pretreatment scan was negative (p=. 005) 19
Nimodipine n American Nimodipine Study u n n Stroke 1992 Apr; 23(4): 615 Multicentred, n=1064 Ischemic stroke within 48 hours Primary outcome: Toronto scale, and motor strength up to 21 days Results: no difference overall but posthoc subgroup analysis showed less worsening in nimodipine group if given within 18 hours and pretreatment scan was negative (p=. 005) 19
 Calcium channel blocker - flunarizine n Flunarizine in stroke treatment (FIST) u n n Acta Neurol Scand 1996: 93(1) 56 -60 Multicentered, n=331 Ischemic stroke in MCA territory and within 24 hours, GCS>3 Outcome: modified Rankin score, mortality and modified Barthel index Result: no difference 20
Calcium channel blocker - flunarizine n Flunarizine in stroke treatment (FIST) u n n Acta Neurol Scand 1996: 93(1) 56 -60 Multicentered, n=331 Ischemic stroke in MCA territory and within 24 hours, GCS>3 Outcome: modified Rankin score, mortality and modified Barthel index Result: no difference 20
 Calcium chelators DP-b 99 n n Membrane activated calcium chelator Phase I trial only: no CV or CNS side effects 21
Calcium chelators DP-b 99 n n Membrane activated calcium chelator Phase I trial only: no CV or CNS side effects 21
 Antioxidants – Tirilazad (Freedox) n n Lipid peroxidation inhibitor Randomized Trial of Tirilazad Mesylate in Acute Stroke (RANTTAS) u n n Stroke 1996 Sep; 27(9): 1453 -1458 Multicentred, n=556, symptoms within 6 hours 150 mg tirilazad iv and 1. 5 mg/kg q 6 h iv for 11 more doses Outcome: GCS and Barthel index at 3 months Results: no difference 22
Antioxidants – Tirilazad (Freedox) n n Lipid peroxidation inhibitor Randomized Trial of Tirilazad Mesylate in Acute Stroke (RANTTAS) u n n Stroke 1996 Sep; 27(9): 1453 -1458 Multicentred, n=556, symptoms within 6 hours 150 mg tirilazad iv and 1. 5 mg/kg q 6 h iv for 11 more doses Outcome: GCS and Barthel index at 3 months Results: no difference 22
 Tirilazad n Randomized Trial of High Dose Tirilazad in Acute Stroke (RANTAS II) u n n n Stroke 1998; 29: 1256 -1257 Multicentred, n=126 Higher dosage than RANTTAS: males given 10 mg/kg/d for 2 days and females 15 mg/kg/d for 1 day and then 12 mg/kg/d Results: trial discontinued after 126 patients because of safety concerns arising from a trial in Europe u Analysis of these patients showed no difference 23
Tirilazad n Randomized Trial of High Dose Tirilazad in Acute Stroke (RANTAS II) u n n n Stroke 1998; 29: 1256 -1257 Multicentred, n=126 Higher dosage than RANTTAS: males given 10 mg/kg/d for 2 days and females 15 mg/kg/d for 1 day and then 12 mg/kg/d Results: trial discontinued after 126 patients because of safety concerns arising from a trial in Europe u Analysis of these patients showed no difference 23
 GABA agonists clomethiazole n Clomethiazole Acute Stroke Study (CLASS) u n n Stroke 1999; 30(1): 21 -28 N=1360, multicentred Within 12 hours, no major respiratory, renal, hepatic disorder Outcome: Barthel index at 3 months Result: no difference; may cause sedation; possible benefit in hemorrhagic stroke 24
GABA agonists clomethiazole n Clomethiazole Acute Stroke Study (CLASS) u n n Stroke 1999; 30(1): 21 -28 N=1360, multicentred Within 12 hours, no major respiratory, renal, hepatic disorder Outcome: Barthel index at 3 months Result: no difference; may cause sedation; possible benefit in hemorrhagic stroke 24
 Clomethiazole n n Clomethiazole in Acute Stroke Study in Ischemic, Hemorrhagic, and 47 t. PAtreated patients Patients within 12 hours with large ischemic infarcts (n=1200), patients who received t. PA (n=100), and with hemorrhagic infarct (n=200) Outcome: for ischemic infarcts, functional recovery as defined by >60 on Barthel index; for other groups, assess safety Ongoing trial: results not published 25
Clomethiazole n n Clomethiazole in Acute Stroke Study in Ischemic, Hemorrhagic, and 47 t. PAtreated patients Patients within 12 hours with large ischemic infarcts (n=1200), patients who received t. PA (n=100), and with hemorrhagic infarct (n=200) Outcome: for ischemic infarcts, functional recovery as defined by >60 on Barthel index; for other groups, assess safety Ongoing trial: results not published 25
 Glutamate antagonists – YM 872 n n n AMPA receptor antagonist Only Phase I completed, demonstrating safety in elderly subjects Others ongoing 26
Glutamate antagonists – YM 872 n n n AMPA receptor antagonist Only Phase I completed, demonstrating safety in elderly subjects Others ongoing 26
 Glutamate antagonists – ZK 200775 n n AMPA receptor antagonist Trials halted because of excessive sedation at therapeutic levels 27
Glutamate antagonists – ZK 200775 n n AMPA receptor antagonist Trials halted because of excessive sedation at therapeutic levels 27
 Glutamate antagonists – CGS 19755 (Selfotel) n n Competitive NMDA receptor antagonist Acute Stroke Studies involving Selfotel treatment (ASSIST) u n n n Stroke 2000; 31(2): 347 -54 Multicentered, RCT, n=567 Presented within 6 hours onset, and hemispheric stroke 1. 5 mg/kg iv Selfotel over 5 minutes Outcome: Barthel index at 3 months Results: no difference. Higher mortality in selfotel group at 30 days (p<. 05), and more behavioral effects 28
Glutamate antagonists – CGS 19755 (Selfotel) n n Competitive NMDA receptor antagonist Acute Stroke Studies involving Selfotel treatment (ASSIST) u n n n Stroke 2000; 31(2): 347 -54 Multicentered, RCT, n=567 Presented within 6 hours onset, and hemispheric stroke 1. 5 mg/kg iv Selfotel over 5 minutes Outcome: Barthel index at 3 months Results: no difference. Higher mortality in selfotel group at 30 days (p<. 05), and more behavioral effects 28
 Glutamate antagonists – Aptiganel (Cerestat) n n NMDA channel blocker Phase III trial of Cerestat in Acute Stroke Patients u n n n Not published Multicentred trial of ischemic stroke within 6 hours Outcome: modified Rankin at 3 months Interim analysis of 628 patients concluded that continuation was not justified. 29
Glutamate antagonists – Aptiganel (Cerestat) n n NMDA channel blocker Phase III trial of Cerestat in Acute Stroke Patients u n n n Not published Multicentred trial of ischemic stroke within 6 hours Outcome: modified Rankin at 3 months Interim analysis of 628 patients concluded that continuation was not justified. 29
 Glutamate antagonists – CP 101, 606 n n n NMDA channel blocker Selective for NR 2 B subunit An open-label study of CP-101, 606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage u n n n Ann NY Acad Sci 1999; 890: 51 -8 N=30 (20 with head injury), given iv infusion, initially. 75 mg/kg/hr; infusion given for 2, 24, and 72 hours Outcome: GCS at 6 months Patients with infusions for 24 and 72 hours better GCS at 6 months 30
Glutamate antagonists – CP 101, 606 n n n NMDA channel blocker Selective for NR 2 B subunit An open-label study of CP-101, 606 in subjects with a severe traumatic head injury or spontaneous intracerebral hemorrhage u n n n Ann NY Acad Sci 1999; 890: 51 -8 N=30 (20 with head injury), given iv infusion, initially. 75 mg/kg/hr; infusion given for 2, 24, and 72 hours Outcome: GCS at 6 months Patients with infusions for 24 and 72 hours better GCS at 6 months 30
 Glutamate antagonists 0 CP 101, 606 n A double blind, placebo controlled study of the safety, tolerability, and pharmacokinetics of CP-101, 606 in patients with a mild or moderate traumatic brain injury u n n Ann NY Acad Sci 1999; 890: 42 -50 Infusion began within 12 hours: . 75 mg/kg/hr for 2 hours then. 37 mg/kg/hr for 22 -70 hrs No major adverse reactions and tolerated well 31
Glutamate antagonists 0 CP 101, 606 n A double blind, placebo controlled study of the safety, tolerability, and pharmacokinetics of CP-101, 606 in patients with a mild or moderate traumatic brain injury u n n Ann NY Acad Sci 1999; 890: 42 -50 Infusion began within 12 hours: . 75 mg/kg/hr for 2 hours then. 37 mg/kg/hr for 22 -70 hrs No major adverse reactions and tolerated well 31
 Glutamate antagonists dextrorphan n n NMDA channel blocker Safety, tolerability, and pharmacokinetics of the NMDA antagonist dextrorphan in patients with acute stroke u n n Stroke 1995; 26(2): 254 -58 N=22 given loading dose and infusion Higher doses were not tolerated, but lower doses (145 -180 loading followed by 50 -75 mg/hr for 11 hours) were better tolerated and produced plasma levels which may be neuroprotective 32
Glutamate antagonists dextrorphan n n NMDA channel blocker Safety, tolerability, and pharmacokinetics of the NMDA antagonist dextrorphan in patients with acute stroke u n n Stroke 1995; 26(2): 254 -58 N=22 given loading dose and infusion Higher doses were not tolerated, but lower doses (145 -180 loading followed by 50 -75 mg/hr for 11 hours) were better tolerated and produced plasma levels which may be neuroprotective 32
 Magnesium n n Blockers voltage gated calcium channels and NMDA receptors Intravenous magnesium efficacy in stroke (IMAGES) began after safety study revealed no incidence of adverse effects Recruiting 2700 patients, within 12 hours Multicentred, RCT 33
Magnesium n n Blockers voltage gated calcium channels and NMDA receptors Intravenous magnesium efficacy in stroke (IMAGES) began after safety study revealed no incidence of adverse effects Recruiting 2700 patients, within 12 hours Multicentred, RCT 33
 Glutamate antagonists – MK 801 (dizocilpine) n n NMDA receptor blocker No current clinical development for stroke 34
Glutamate antagonists – MK 801 (dizocilpine) n n NMDA receptor blocker No current clinical development for stroke 34
 Glutamate antagonists – NPS 1506 n n NMDA channel blocker Phase I trials are on hold for financial reasons 35
Glutamate antagonists – NPS 1506 n n NMDA channel blocker Phase I trials are on hold for financial reasons 35
 Glutamate antagonists remacemide n n NMDA receptor blocker No trials in acute ischemic stroke u n Phase 2 has demonstrated the safe dosage, but not enough power to comment on neurological status Possible neuroprotective benefit, as shown in patients neuropsychological outcome after cardiac surgery (p=. 028) u Stroke 1998; 29(11): 2357 -62 36
Glutamate antagonists remacemide n n NMDA receptor blocker No trials in acute ischemic stroke u n Phase 2 has demonstrated the safe dosage, but not enough power to comment on neurological status Possible neuroprotective benefit, as shown in patients neuropsychological outcome after cardiac surgery (p=. 028) u Stroke 1998; 29(11): 2357 -62 36
 Glutamate antagonists – ACEA 1021 (licostinel) n n Glycine site antagonist Trials halted because results from Phase I trial, revealed ACEA 1021 crystals in subjects’ urine 37
Glutamate antagonists – ACEA 1021 (licostinel) n n Glycine site antagonist Trials halted because results from Phase I trial, revealed ACEA 1021 crystals in subjects’ urine 37
 Glutamate antagonists – GV 150526 (gavestinel) Glycine site antagonist n n Glycine antagonist for Neuroprotection (GAIN 1) and GAIN 2 u n n n Presented, not published Multicentred, patients within 12 hours of symptom onset; intended to assess safety profile Outcome: Barthel index day 7 and 4 weeks Results: no increase in adverse events 38
Glutamate antagonists – GV 150526 (gavestinel) Glycine site antagonist n n Glycine antagonist for Neuroprotection (GAIN 1) and GAIN 2 u n n n Presented, not published Multicentred, patients within 12 hours of symptom onset; intended to assess safety profile Outcome: Barthel index day 7 and 4 weeks Results: no increase in adverse events 38
 Glutamate antagonists – GV 150526 n GAIN International u n n Not published N=1804; within 6 hours of moderate stroke. Patients functionally independent before stroke RCT, multicentred Outcome: Barthel index 3 months and mortality Results: no significant difference 39
Glutamate antagonists – GV 150526 n GAIN International u n n Not published N=1804; within 6 hours of moderate stroke. Patients functionally independent before stroke RCT, multicentred Outcome: Barthel index 3 months and mortality Results: no significant difference 39
 Glutamate antagonists – GV 150526 n n GAIN Americas Same criteria as International N=561 Preliminary result: no effect 40
Glutamate antagonists – GV 150526 n n GAIN Americas Same criteria as International N=561 Preliminary result: no effect 40
 Glutamate antagonists – SL 820715 (eliprodil) n n n Polyamine site antagonist No demonstrated efficacy Phase 3 trial discontinued u Results not reported 41
Glutamate antagonists – SL 820715 (eliprodil) n n n Polyamine site antagonist No demonstrated efficacy Phase 3 trial discontinued u Results not reported 41
 Growth factors – fibroblast growth factor n n Phase III trial halted because of safety issues after interim analysis Patients received 5 -10 mg of fibroblast growth factor, n=302 Outcome: Rankin scale 3 months Significantly worse outcome in growth factor group 42
Growth factors – fibroblast growth factor n n Phase III trial halted because of safety issues after interim analysis Patients received 5 -10 mg of fibroblast growth factor, n=302 Outcome: Rankin scale 3 months Significantly worse outcome in growth factor group 42
 Leukocyte adhesion inhibitor – Anti ICAM 1 antibody (enlimomab) intercellular Monoclonal antibody against n n adhesion molecule ICAM 1, which is required for leukoctye attachment and migration Enlimomab Actue Stroke Trial (EAST) u n n n Neurology 1997; 48(Supp) A 270 N=625, within 6 hours ischemic stroke Outcome: modified Rankin scale 90 days Results: mortality and Rankin score worse in enlimomab group (p=. 004) 43
Leukocyte adhesion inhibitor – Anti ICAM 1 antibody (enlimomab) intercellular Monoclonal antibody against n n adhesion molecule ICAM 1, which is required for leukoctye attachment and migration Enlimomab Actue Stroke Trial (EAST) u n n n Neurology 1997; 48(Supp) A 270 N=625, within 6 hours ischemic stroke Outcome: modified Rankin scale 90 days Results: mortality and Rankin score worse in enlimomab group (p=. 004) 43
 Leukoctye adhesion inhibitor – Hu 23 F 2 G n n n Leukarrest Monoclonal antibody against neutrophil CD 11/CD 18 adhesion molecule Hu 23 F 2 G Phase 3 stroke trial (HALT) stopped because interim analysis showed no success 44
Leukoctye adhesion inhibitor – Hu 23 F 2 G n n n Leukarrest Monoclonal antibody against neutrophil CD 11/CD 18 adhesion molecule Hu 23 F 2 G Phase 3 stroke trial (HALT) stopped because interim analysis showed no success 44
 Nitric oxide inhibitor - lubeluzole n n n Lubeluzole in ischemic stroke Phase III – multicentred RCT done after Phase II trial suggested benefit (Stroke 1997; 28: 2338 -2346) 0 -8 hours from onset, exclude severe stroke Outcome: Barthel index at 12 weeks Results: no difference 45
Nitric oxide inhibitor - lubeluzole n n n Lubeluzole in ischemic stroke Phase III – multicentred RCT done after Phase II trial suggested benefit (Stroke 1997; 28: 2338 -2346) 0 -8 hours from onset, exclude severe stroke Outcome: Barthel index at 12 weeks Results: no difference 45
 Opioid antagonists – nalmefene (Cervene) n n Selective kappa opiate receptor antagonist Cervene in acute ischemic stroke u n n Stroke 2000; 31(6): 1234 -9 Multicentred, n=368. This Phase 3 trial followed Phase 2, which suggested benefit in people <70. Presenting within 6 hours Outcome: Barthel scale and GCS at 12 weeks Results: no difference 46
Opioid antagonists – nalmefene (Cervene) n n Selective kappa opiate receptor antagonist Cervene in acute ischemic stroke u n n Stroke 2000; 31(6): 1234 -9 Multicentred, n=368. This Phase 3 trial followed Phase 2, which suggested benefit in people <70. Presenting within 6 hours Outcome: Barthel scale and GCS at 12 weeks Results: no difference 46
 Phosphatidylcholin e precursor citicoline n n Acts as membrane stabilizer Citicoline stroke study u n n Neurology 1997; 49(3): 671 -8 Multicentered, n=259 Randomized to doses of 500 mg/d, 1000 mg/d or 2000 mg/d Outcome: Barthel index 12 weeks Result: better outcome in the 500 mg and 2000 mg groups. 47
Phosphatidylcholin e precursor citicoline n n Acts as membrane stabilizer Citicoline stroke study u n n Neurology 1997; 49(3): 671 -8 Multicentered, n=259 Randomized to doses of 500 mg/d, 1000 mg/d or 2000 mg/d Outcome: Barthel index 12 weeks Result: better outcome in the 500 mg and 2000 mg groups. 47
 Phosphatidylycholi ne precursors citicoline n n n Phase III trial of citicoline 2000 mg vs. placebo Multicentred trial, n=899 Patients present within 24 hours, and receive drug for 6 wks. Outcome: gain of 7 points on NIHSS in 12 weeks Result: no difference in primary endpoint, but the secondary endpoint of complete/near-complete recovery was higher (p<. 05) in citicoline group 48
Phosphatidylycholi ne precursors citicoline n n n Phase III trial of citicoline 2000 mg vs. placebo Multicentred trial, n=899 Patients present within 24 hours, and receive drug for 6 wks. Outcome: gain of 7 points on NIHSS in 12 weeks Result: no difference in primary endpoint, but the secondary endpoint of complete/near-complete recovery was higher (p<. 05) in citicoline group 48
 Serotonin agonist – Bay x 3072 (repinotan) n n n Bayer Randomized Acute Ischemia Neuroprotectant Study Multicentre, n=120 (Phase 2) Within 6 hours Outcome: NIHSS at 4 weeks Results not published Phase 3 ongoing 49
Serotonin agonist – Bay x 3072 (repinotan) n n n Bayer Randomized Acute Ischemia Neuroprotectant Study Multicentre, n=120 (Phase 2) Within 6 hours Outcome: NIHSS at 4 weeks Results not published Phase 3 ongoing 49
 Sodium channel blockers fosphenytoin n n Pro-drug of phenytoin Phase 3 trial: Multicentre study to evaluate the safety and efficacy of iv fosphenytoin in patients with acute ischemic stroke N=462, treatment within 4 hours Outcome: Rankin scale 3 months Results: no difference 50
Sodium channel blockers fosphenytoin n n Pro-drug of phenytoin Phase 3 trial: Multicentre study to evaluate the safety and efficacy of iv fosphenytoin in patients with acute ischemic stroke N=462, treatment within 4 hours Outcome: Rankin scale 3 months Results: no difference 50


