48e18c5775e8107ee09914ef8d1ad90a.ppt
- Количество слайдов: 65
 Neurological Emergency Treatment Trials Network Overview of the new network nett. umich. edu
Neurological Emergency Treatment Trials Network Overview of the new network nett. umich. edu
 Overview 1. The Problem - Neurological Emergencies 2. Developing a Solution 3. The Nuts and Bolts - NETT 4. Impact
Overview 1. The Problem - Neurological Emergencies 2. Developing a Solution 3. The Nuts and Bolts - NETT 4. Impact
 1. Neurological Emergencies • Spectrum of pathology • High burden of disease • Importance of early treatment
1. Neurological Emergencies • Spectrum of pathology • High burden of disease • Importance of early treatment
 Neurological Emergencies Spectrum of Pathology • Neurotrauma: Brain & Spinal Cord Injury • Stroke: Ischemic & Hemorrhagic • Status Epilepticus • CNS Infections: Meningitis & Encephalitis • Anoxic Brain Injury • Others: Bell’s Palsy, Headache, etc.
Neurological Emergencies Spectrum of Pathology • Neurotrauma: Brain & Spinal Cord Injury • Stroke: Ischemic & Hemorrhagic • Status Epilepticus • CNS Infections: Meningitis & Encephalitis • Anoxic Brain Injury • Others: Bell’s Palsy, Headache, etc.
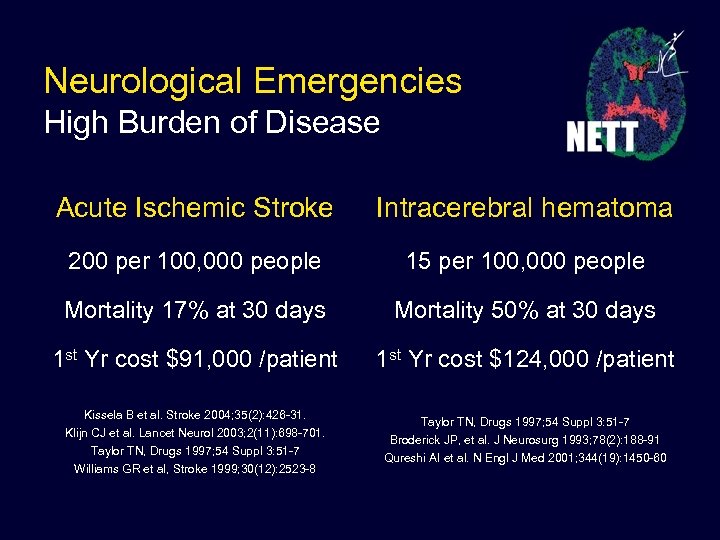 Neurological Emergencies High Burden of Disease Acute Ischemic Stroke Intracerebral hematoma 200 per 100, 000 people 15 per 100, 000 people Mortality 17% at 30 days Mortality 50% at 30 days 1 st Yr cost $91, 000 /patient 1 st Yr cost $124, 000 /patient Kissela B et al. Stroke 2004; 35(2): 426 -31. Klijn CJ et al. Lancet Neurol 2003; 2(11): 698 -701. Taylor TN, Drugs 1997; 54 Suppl 3: 51 -7 Williams GR et al, Stroke 1999; 30(12): 2523 -8 Taylor TN, Drugs 1997; 54 Suppl 3: 51 -7 Broderick JP, et al. J Neurosurg 1993; 78(2): 188 -91 Qureshi AI et al. N Engl J Med 2001; 344(19): 1450 -60
Neurological Emergencies High Burden of Disease Acute Ischemic Stroke Intracerebral hematoma 200 per 100, 000 people 15 per 100, 000 people Mortality 17% at 30 days Mortality 50% at 30 days 1 st Yr cost $91, 000 /patient 1 st Yr cost $124, 000 /patient Kissela B et al. Stroke 2004; 35(2): 426 -31. Klijn CJ et al. Lancet Neurol 2003; 2(11): 698 -701. Taylor TN, Drugs 1997; 54 Suppl 3: 51 -7 Williams GR et al, Stroke 1999; 30(12): 2523 -8 Taylor TN, Drugs 1997; 54 Suppl 3: 51 -7 Broderick JP, et al. J Neurosurg 1993; 78(2): 188 -91 Qureshi AI et al. N Engl J Med 2001; 344(19): 1450 -60
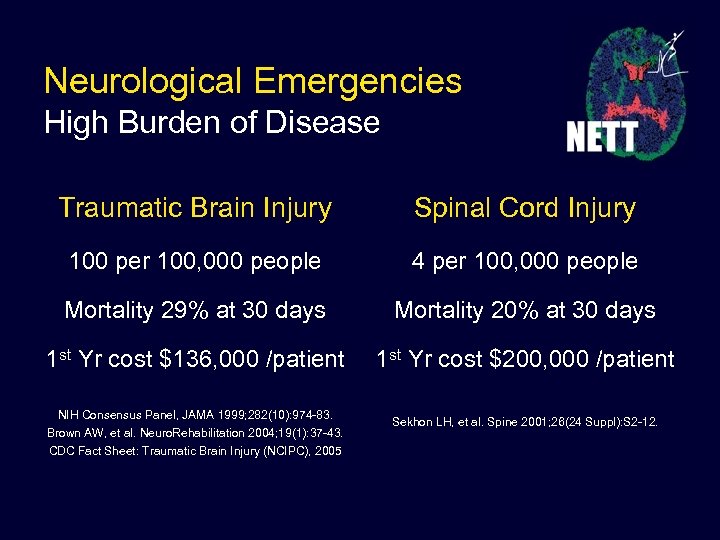 Neurological Emergencies High Burden of Disease Traumatic Brain Injury Spinal Cord Injury 100 per 100, 000 people 4 per 100, 000 people Mortality 29% at 30 days Mortality 20% at 30 days 1 st Yr cost $136, 000 /patient 1 st Yr cost $200, 000 /patient NIH Consensus Panel, JAMA 1999; 282(10): 974 -83. Brown AW, et al. Neuro. Rehabilitation 2004; 19(1): 37 -43. CDC Fact Sheet: Traumatic Brain Injury (NCIPC), 2005 Sekhon LH, et al. Spine 2001; 26(24 Suppl): S 2 -12.
Neurological Emergencies High Burden of Disease Traumatic Brain Injury Spinal Cord Injury 100 per 100, 000 people 4 per 100, 000 people Mortality 29% at 30 days Mortality 20% at 30 days 1 st Yr cost $136, 000 /patient 1 st Yr cost $200, 000 /patient NIH Consensus Panel, JAMA 1999; 282(10): 974 -83. Brown AW, et al. Neuro. Rehabilitation 2004; 19(1): 37 -43. CDC Fact Sheet: Traumatic Brain Injury (NCIPC), 2005 Sekhon LH, et al. Spine 2001; 26(24 Suppl): S 2 -12.
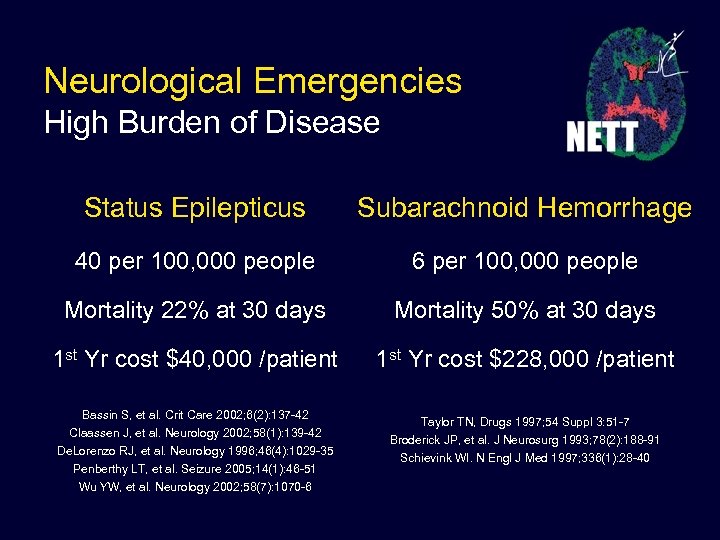 Neurological Emergencies High Burden of Disease Status Epilepticus Subarachnoid Hemorrhage 40 per 100, 000 people 6 per 100, 000 people Mortality 22% at 30 days Mortality 50% at 30 days 1 st Yr cost $40, 000 /patient 1 st Yr cost $228, 000 /patient Bassin S, et al. Crit Care 2002; 6(2): 137 -42 Claassen J, et al. Neurology 2002; 58(1): 139 -42 De. Lorenzo RJ, et al. Neurology 1996; 46(4): 1029 -35 Penberthy LT, et al. Seizure 2005; 14(1): 46 -51 Wu YW, et al. Neurology 2002; 58(7): 1070 -6 Taylor TN, Drugs 1997; 54 Suppl 3: 51 -7 Broderick JP, et al. J Neurosurg 1993; 78(2): 188 -91 Schievink WI. N Engl J Med 1997; 336(1): 28 -40
Neurological Emergencies High Burden of Disease Status Epilepticus Subarachnoid Hemorrhage 40 per 100, 000 people 6 per 100, 000 people Mortality 22% at 30 days Mortality 50% at 30 days 1 st Yr cost $40, 000 /patient 1 st Yr cost $228, 000 /patient Bassin S, et al. Crit Care 2002; 6(2): 137 -42 Claassen J, et al. Neurology 2002; 58(1): 139 -42 De. Lorenzo RJ, et al. Neurology 1996; 46(4): 1029 -35 Penberthy LT, et al. Seizure 2005; 14(1): 46 -51 Wu YW, et al. Neurology 2002; 58(7): 1070 -6 Taylor TN, Drugs 1997; 54 Suppl 3: 51 -7 Broderick JP, et al. J Neurosurg 1993; 78(2): 188 -91 Schievink WI. N Engl J Med 1997; 336(1): 28 -40
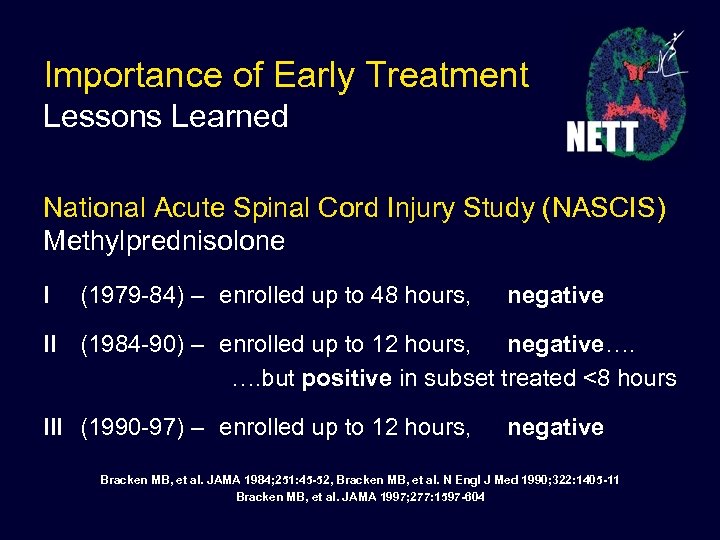 Importance of Early Treatment Lessons Learned National Acute Spinal Cord Injury Study (NASCIS) Methylprednisolone I (1979 -84) – enrolled up to 48 hours, negative II (1984 -90) – enrolled up to 12 hours, negative…. …. but positive in subset treated <8 hours III (1990 -97) – enrolled up to 12 hours, negative Bracken MB, et al. JAMA 1984; 251: 45 -52, Bracken MB, et al. N Engl J Med 1990; 322: 1405 -11 Bracken MB, et al. JAMA 1997; 277: 1597 -604
Importance of Early Treatment Lessons Learned National Acute Spinal Cord Injury Study (NASCIS) Methylprednisolone I (1979 -84) – enrolled up to 48 hours, negative II (1984 -90) – enrolled up to 12 hours, negative…. …. but positive in subset treated <8 hours III (1990 -97) – enrolled up to 12 hours, negative Bracken MB, et al. JAMA 1984; 251: 45 -52, Bracken MB, et al. N Engl J Med 1990; 322: 1405 -11 Bracken MB, et al. JAMA 1997; 277: 1597 -604
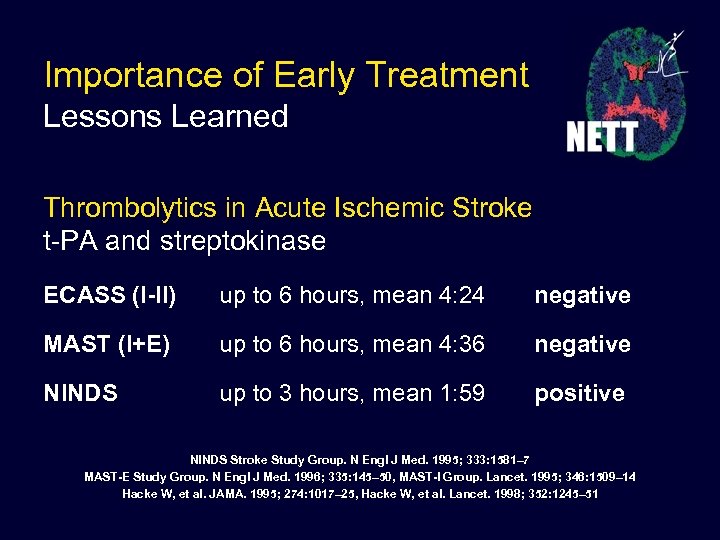 Importance of Early Treatment Lessons Learned Thrombolytics in Acute Ischemic Stroke t-PA and streptokinase ECASS (I-II) up to 6 hours, mean 4: 24 negative MAST (I+E) up to 6 hours, mean 4: 36 negative NINDS up to 3 hours, mean 1: 59 positive NINDS Stroke Study Group. N Engl J Med. 1995; 333: 1581– 7 MAST-E Study Group. N Engl J Med. 1996; 335: 145– 50, MAST-I Group. Lancet. 1995; 346: 1509– 14 Hacke W, et al. JAMA. 1995; 274: 1017– 25, Hacke W, et al. Lancet. 1998; 352: 1245– 51
Importance of Early Treatment Lessons Learned Thrombolytics in Acute Ischemic Stroke t-PA and streptokinase ECASS (I-II) up to 6 hours, mean 4: 24 negative MAST (I+E) up to 6 hours, mean 4: 36 negative NINDS up to 3 hours, mean 1: 59 positive NINDS Stroke Study Group. N Engl J Med. 1995; 333: 1581– 7 MAST-E Study Group. N Engl J Med. 1996; 335: 145– 50, MAST-I Group. Lancet. 1995; 346: 1509– 14 Hacke W, et al. JAMA. 1995; 274: 1017– 25, Hacke W, et al. Lancet. 1998; 352: 1245– 51
 2. Developing a solution • Boots on the ground • Multi-disciplinary composition • Emergence of a network • Design for the future
2. Developing a solution • Boots on the ground • Multi-disciplinary composition • Emergence of a network • Design for the future
 Boots on the ground Emergency Medicine driven • Neurological emergencies are treated in the initial minutes and hours after arrival mainly by emergency physicians. • The ED is a challenging and chaotic environment in which to conduct research. • Emergency physicians represent the “boots on the ground”, those on the front line with the manpower and expertise to conduct research in the ED.
Boots on the ground Emergency Medicine driven • Neurological emergencies are treated in the initial minutes and hours after arrival mainly by emergency physicians. • The ED is a challenging and chaotic environment in which to conduct research. • Emergency physicians represent the “boots on the ground”, those on the front line with the manpower and expertise to conduct research in the ED.
 Multi-disciplinary composition Neurology, Neurosurgery, EMS, Neuro Critical Care, and Trauma • Research encompassing a continuum of care that starts in the ambulance or in the emergency department and continues in the ICU, in the OR, on the stroke unit, or in the clinic. • Network leadership, Hub PI’s, and Trial PI’s represent a range of specialties.
Multi-disciplinary composition Neurology, Neurosurgery, EMS, Neuro Critical Care, and Trauma • Research encompassing a continuum of care that starts in the ambulance or in the emergency department and continues in the ICU, in the OR, on the stroke unit, or in the clinic. • Network leadership, Hub PI’s, and Trial PI’s represent a range of specialties.
 Multi-disciplinary collaborations Workforce by Specialty in the US • 12, 000 adult neurologists* • 1, 500 pediatric neurologists • 3, 500 neurosurgeons • 4, 000 hospital emergency departments • 22, 000 emergency physicians *30% in solo private practice
Multi-disciplinary collaborations Workforce by Specialty in the US • 12, 000 adult neurologists* • 1, 500 pediatric neurologists • 3, 500 neurosurgeons • 4, 000 hospital emergency departments • 22, 000 emergency physicians *30% in solo private practice
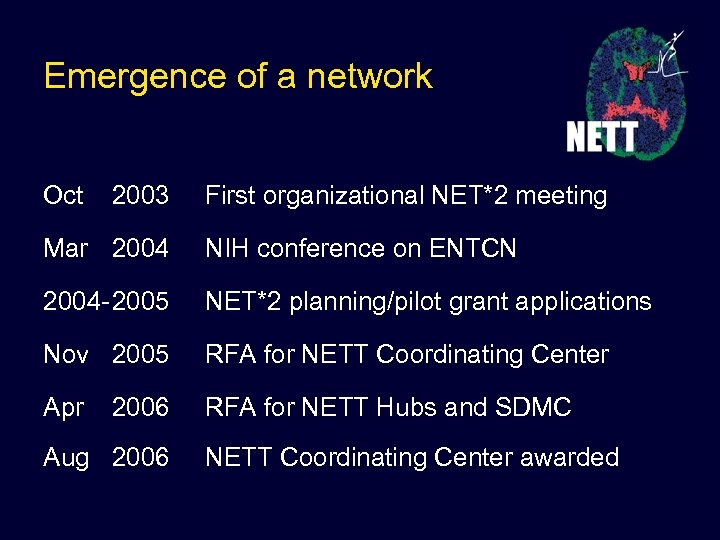 Emergence of a network Oct 2003 First organizational NET*2 meeting Mar 2004 NIH conference on ENTCN 2004 -2005 NET*2 planning/pilot grant applications Nov 2005 RFA for NETT Coordinating Center Apr 2006 RFA for NETT Hubs and SDMC Aug 2006 NETT Coordinating Center awarded
Emergence of a network Oct 2003 First organizational NET*2 meeting Mar 2004 NIH conference on ENTCN 2004 -2005 NET*2 planning/pilot grant applications Nov 2005 RFA for NETT Coordinating Center Apr 2006 RFA for NETT Hubs and SDMC Aug 2006 NETT Coordinating Center awarded
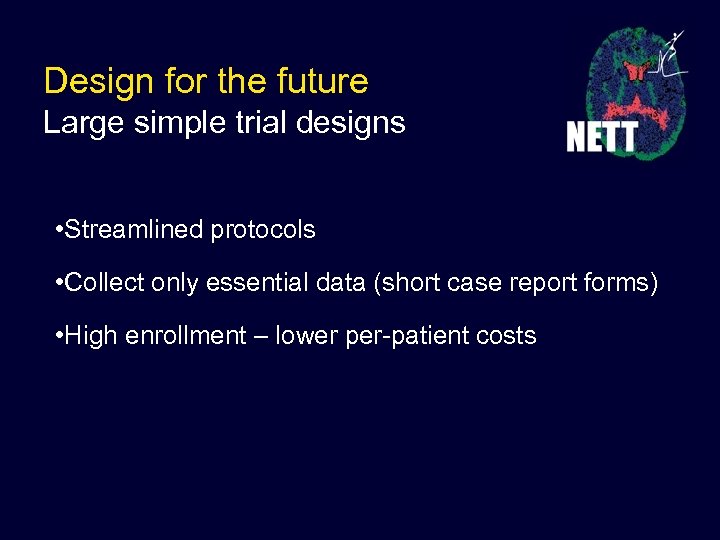 Design for the future Large simple trial designs • Streamlined protocols • Collect only essential data (short case report forms) • High enrollment – lower per-patient costs
Design for the future Large simple trial designs • Streamlined protocols • Collect only essential data (short case report forms) • High enrollment – lower per-patient costs
 Design for the future Emphasis on intervention • Focus on phase III intervention trials • Patient-oriented readily-applicable results • Diverse enrollment (patients & practice environments)
Design for the future Emphasis on intervention • Focus on phase III intervention trials • Patient-oriented readily-applicable results • Diverse enrollment (patients & practice environments)
 Design for the future Consent issues • Exception to informed consent for emergency research • Optimize methods that respect human subjects • Dedicate network resources to facilitate local efforts • Help develop centralized IRB review
Design for the future Consent issues • Exception to informed consent for emergency research • Optimize methods that respect human subjects • Dedicate network resources to facilitate local efforts • Help develop centralized IRB review
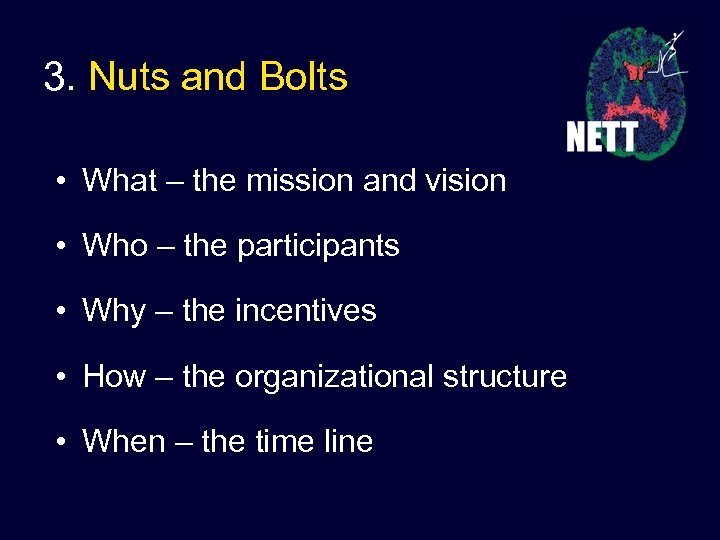 3. Nuts and Bolts • What – the mission and vision • Who – the participants • Why – the incentives • How – the organizational structure • When – the time line
3. Nuts and Bolts • What – the mission and vision • Who – the participants • Why – the incentives • How – the organizational structure • When – the time line
 Mission The mission of the Neurological Emergencies Treatment Trials (NETT) Network is to improve outcomes of patients with acute neurological problems through innovative research focused on the emergent phase of patient care.
Mission The mission of the Neurological Emergencies Treatment Trials (NETT) Network is to improve outcomes of patients with acute neurological problems through innovative research focused on the emergent phase of patient care.
 Vision NETT will engage clinicians and providers at the front lines of emergency care to conduct large, simple multi-center clinical trials to answer research questions of clinical importance. The NETT structure will be utilized to achieve economies of scale enabling cost effective, high quality research.
Vision NETT will engage clinicians and providers at the front lines of emergency care to conduct large, simple multi-center clinical trials to answer research questions of clinical importance. The NETT structure will be utilized to achieve economies of scale enabling cost effective, high quality research.
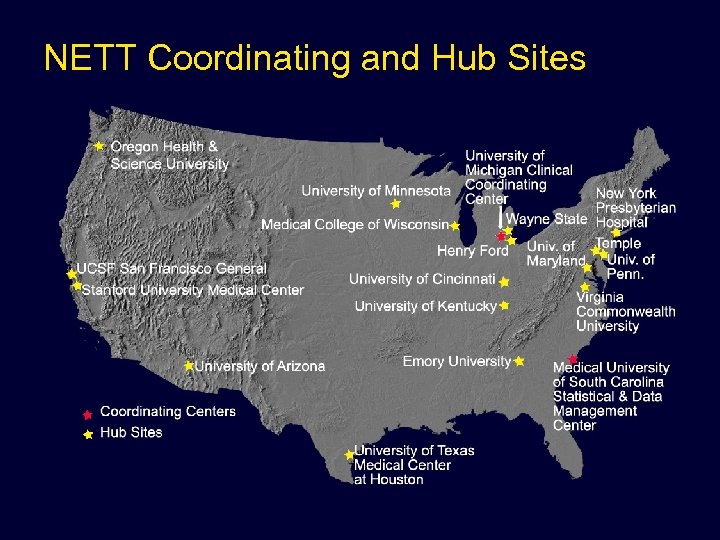 NETT Coordinating and Hub Sites
NETT Coordinating and Hub Sites
 Study Selection Investigator Initiated Studies • Investigators Initiated Studies – Incentives and Limitations – Application Process • Industry Sponsored Studies – Network / Investigator Design
Study Selection Investigator Initiated Studies • Investigators Initiated Studies – Incentives and Limitations – Application Process • Industry Sponsored Studies – Network / Investigator Design
 Study Selection Investigator Initiated Studies • Incentives – Investigator receives the trial award – Scientific control, credit, authorship preserved – Infrastructure already established • Limitations – Fewer funds stay at investigators institution – Commitment to stay within the network
Study Selection Investigator Initiated Studies • Incentives – Investigator receives the trial award – Scientific control, credit, authorship preserved – Infrastructure already established • Limitations – Fewer funds stay at investigators institution – Commitment to stay within the network
 Study Selection Investigator Initiated Studies • Process – NETT Trial Guidelines – Clinical Trial Subcommittee & NETT-AG – Administrative Consultation – Submission for Scientific Review
Study Selection Investigator Initiated Studies • Process – NETT Trial Guidelines – Clinical Trial Subcommittee & NETT-AG – Administrative Consultation – Submission for Scientific Review
 Study Selection Industry Sponsored Studies • Network / Investigator Design – Scientific Control – Shared Economies of Scale – No Direct Subsidy – NETT-AG solicits scientific review
Study Selection Industry Sponsored Studies • Network / Investigator Design – Scientific Control – Shared Economies of Scale – No Direct Subsidy – NETT-AG solicits scientific review
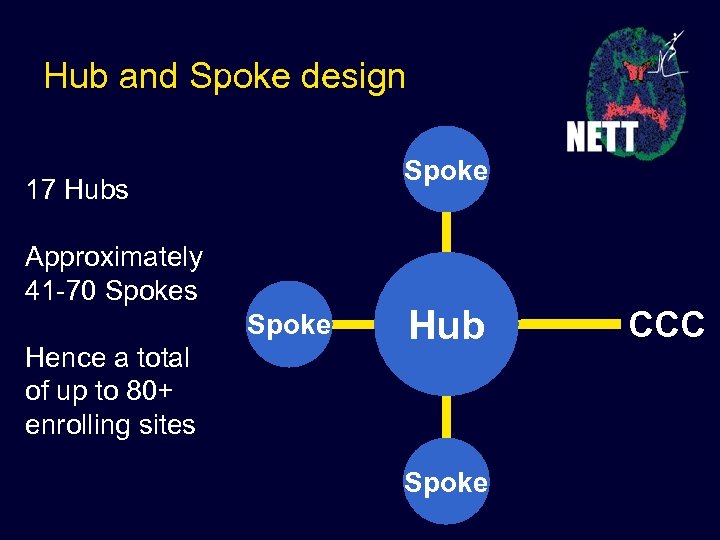 Hub and Spoke design Spoke 17 Hubs Approximately 41 -70 Spokes Spoke Hence a total of up to 80+ enrolling sites Hub Spoke CCC
Hub and Spoke design Spoke 17 Hubs Approximately 41 -70 Spokes Spoke Hence a total of up to 80+ enrolling sites Hub Spoke CCC
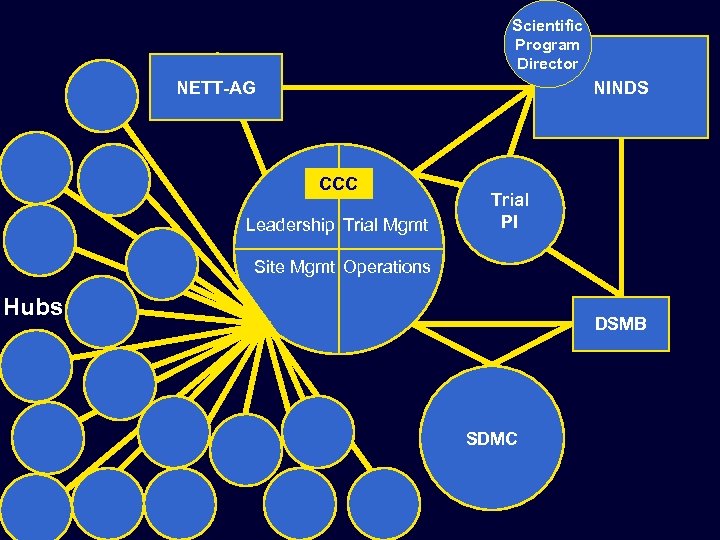 Scientific Program Director NETT-AG NINDS CCC Leadership Trial Mgmt Trial PI Site Mgmt Operations Hubs DSMB SDMC
Scientific Program Director NETT-AG NINDS CCC Leadership Trial Mgmt Trial PI Site Mgmt Operations Hubs DSMB SDMC
 Timeline • Several simultaneous trials • Staggered planning / enrollment
Timeline • Several simultaneous trials • Staggered planning / enrollment
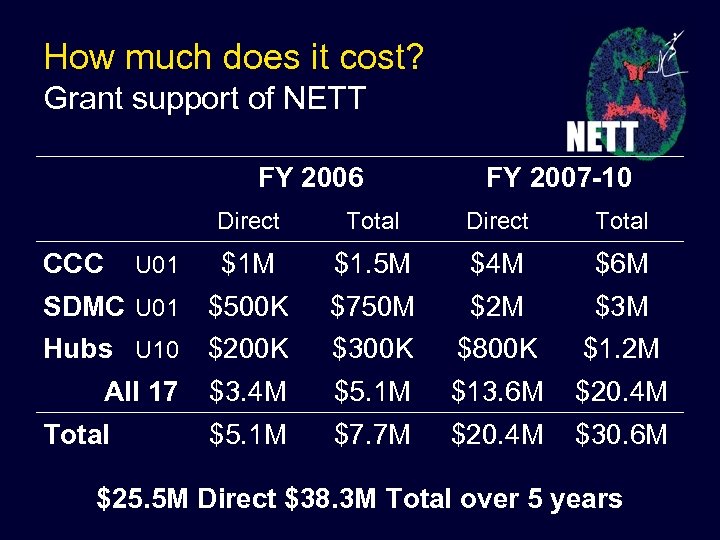 How much does it cost? Grant support of NETT FY 2006 FY 2007 -10 Direct Total $1 M $1. 5 M $4 M $6 M SDMC U 01 $500 K $750 M $2 M $3 M Hubs U 10 $200 K $300 K $800 K $1. 2 M All 17 $3. 4 M $5. 1 M $13. 6 M $20. 4 M $5. 1 M $7. 7 M $20. 4 M $30. 6 M CCC U 01 Total $25. 5 M Direct $38. 3 M Total over 5 years
How much does it cost? Grant support of NETT FY 2006 FY 2007 -10 Direct Total $1 M $1. 5 M $4 M $6 M SDMC U 01 $500 K $750 M $2 M $3 M Hubs U 10 $200 K $300 K $800 K $1. 2 M All 17 $3. 4 M $5. 1 M $13. 6 M $20. 4 M $5. 1 M $7. 7 M $20. 4 M $30. 6 M CCC U 01 Total $25. 5 M Direct $38. 3 M Total over 5 years
 4. Impact • Opportunity to advance care of patients with neuro-emergencies • Large NIH investment in emergency medicine clinical research • Re-engineering the clinical research enterprise
4. Impact • Opportunity to advance care of patients with neuro-emergencies • Large NIH investment in emergency medicine clinical research • Re-engineering the clinical research enterprise
 nett. umich. edu
nett. umich. edu
 Priming the pipeline • RAMPART • INTERACT • Pro. TECT • NABPS
Priming the pipeline • RAMPART • INTERACT • Pro. TECT • NABPS
 Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) • • • Paramedic treatment of status epilepticus Standard treatment is IV benzodiazepine IV starts difficult / dangerous in the convulsing patient Best IV agent, lorazepam, impractical for EMS IM treatment is faster and easier Best IM agent, midazolam, is practical for EMS
Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) • • • Paramedic treatment of status epilepticus Standard treatment is IV benzodiazepine IV starts difficult / dangerous in the convulsing patient Best IV agent, lorazepam, impractical for EMS IM treatment is faster and easier Best IM agent, midazolam, is practical for EMS
 Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) • • • IM midazolam autoinjector v. IV lorazepam Double dummy blinded design Exception to consent for emergency research Outcome: termination of seizure prior to ED arrival Sample 800 patients (400 per group) Intention to treat, non-inferiority analysis
Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) • • • IM midazolam autoinjector v. IV lorazepam Double dummy blinded design Exception to consent for emergency research Outcome: termination of seizure prior to ED arrival Sample 800 patients (400 per group) Intention to treat, non-inferiority analysis
 US-Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-US) • Hematoma expansion is associated with worse outcomes in patients with ICH • Very early elevated BP may contribute to acute hematoma expansion • Acute hypertension is common with ICH • Optimal BP targets in patients with ICH are unknown
US-Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-US) • Hematoma expansion is associated with worse outcomes in patients with ICH • Very early elevated BP may contribute to acute hematoma expansion • Acute hypertension is common with ICH • Optimal BP targets in patients with ICH are unknown
 US-Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-US) • Compare systolic target of 140 vs. 180 mm. Hg • US modification of study originally designed in Austraila by our current collaborators • Phase II Trial, feasibility / safety primary outcomes • Sample 400 patients (200 per arm)
US-Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial (INTERACT-US) • Compare systolic target of 140 vs. 180 mm. Hg • US modification of study originally designed in Austraila by our current collaborators • Phase II Trial, feasibility / safety primary outcomes • Sample 400 patients (200 per arm)
 Hub pre-RFA slides
Hub pre-RFA slides
 What does an application need? • We don’t really know • Enrollment • Experience • Collaboration
What does an application need? • We don’t really know • Enrollment • Experience • Collaboration
 Enrollment • Sufficient patient volume • Access to diverse diagnoses – Adults and children – Neurotrauma, TBI and SCI – Stroke, ischemic and hemorrhagic – Seizure, meningitis, anoxic injury • Local infrastructure
Enrollment • Sufficient patient volume • Access to diverse diagnoses – Adults and children – Neurotrauma, TBI and SCI – Stroke, ischemic and hemorrhagic – Seizure, meningitis, anoxic injury • Local infrastructure
 Experience • ED clinical trials (any disease) • Institutional track record • Cross disciplinary research
Experience • ED clinical trials (any disease) • Institutional track record • Cross disciplinary research
 Collaboration • Emergency Medical Services • Spokes – Diversity – Buy in • Cross disciplinary – Emergency Medicine – Neuro-Critical Care – Neurology / Neurosurgery – Trauma surgery
Collaboration • Emergency Medical Services • Spokes – Diversity – Buy in • Cross disciplinary – Emergency Medicine – Neuro-Critical Care – Neurology / Neurosurgery – Trauma surgery
 Spokes • Don’t have to use all spokes for all trials • Look for areas of concentration – Trauma – Stroke – EMS expertise
Spokes • Don’t have to use all spokes for all trials • Look for areas of concentration – Trauma – Stroke – EMS expertise
 Budget suggestions • Include all effort needed to: – Set up the program – Prepare potentially complex IRB apps – Enroll subjects in two trials, best guess – Collect and report data – Provide informatics support • Include – Travel to investigator meetings
Budget suggestions • Include all effort needed to: – Set up the program – Prepare potentially complex IRB apps – Enroll subjects in two trials, best guess – Collect and report data – Provide informatics support • Include – Travel to investigator meetings
 Resources • RFA for the 3 components • ENCTN final report • UM CCC application • Links to all available at http: //sitemaker. umich. edu/NETT
Resources • RFA for the 3 components • ENCTN final report • UM CCC application • Links to all available at http: //sitemaker. umich. edu/NETT
 Simple Version
Simple Version
 What is NETT? Neurological Emergencies Treatment Trials A new clinical trials network dedicated to: – Cross-disciplinary cooperation – Interventions in minutes not hours – Large simple trial streamlined trial designs
What is NETT? Neurological Emergencies Treatment Trials A new clinical trials network dedicated to: – Cross-disciplinary cooperation – Interventions in minutes not hours – Large simple trial streamlined trial designs
 How will NETT work? • Hub and Spoke Design – Large – Scalable • Public Utility Model – Open – Economical
How will NETT work? • Hub and Spoke Design – Large – Scalable • Public Utility Model – Open – Economical
 What kinds of questions? Does very early intensive blood pressure lowering prevent hematoma expansion and improve outcome in patients with ICH? The INTERACT trial
What kinds of questions? Does very early intensive blood pressure lowering prevent hematoma expansion and improve outcome in patients with ICH? The INTERACT trial
 What kinds of questions? Does a lower dose of thrombolytic plus a glycoprotein inhibitor improve efficacy and reduce bleeding complications compared to standard dose thrombolysis? The CLEAR trial
What kinds of questions? Does a lower dose of thrombolytic plus a glycoprotein inhibitor improve efficacy and reduce bleeding complications compared to standard dose thrombolysis? The CLEAR trial
 What kinds of questions? Can progesterone infusion improve survival and neurological outcome in patients with traumatic brain injury? The Pro. TECT trial
What kinds of questions? Can progesterone infusion improve survival and neurological outcome in patients with traumatic brain injury? The Pro. TECT trial
 What kinds of questions? Can IM midazolam stop seizures as effectively as IV lorazepam in the prehospital care of status epilepticus? The RAMPART trial
What kinds of questions? Can IM midazolam stop seizures as effectively as IV lorazepam in the prehospital care of status epilepticus? The RAMPART trial
 What kinds of questions? Whatever question you want to ask…
What kinds of questions? Whatever question you want to ask…
 What’s the impact? • Opportunity to advance care of patients with neuro-emergencies • Large NIH investment in emergency medicine clinical research • Re-engineering the clinical research enterprise
What’s the impact? • Opportunity to advance care of patients with neuro-emergencies • Large NIH investment in emergency medicine clinical research • Re-engineering the clinical research enterprise
 How will you be involved? • As a Practitioner • As a Hub co-investigator • As a Trial investigator
How will you be involved? • As a Practitioner • As a Hub co-investigator • As a Trial investigator
 Alternate Slides
Alternate Slides
 NETT Impact • High level of enthusiasm by the academic emergency medicine community for highquality, non-pharma driven clinical research. • High public visibility of treatment-oriented clinical research.
NETT Impact • High level of enthusiasm by the academic emergency medicine community for highquality, non-pharma driven clinical research. • High public visibility of treatment-oriented clinical research.
 NETT Benefits and Risks • Immediate invigoration of neurologic community • Broader involvement of trainees in research • Large number of trials in the pipeline • NETT will lead to efficient research in many diseases • Tight budget • Small numbers of Hubs • Scientific review committee tough and less interested in practical trials
NETT Benefits and Risks • Immediate invigoration of neurologic community • Broader involvement of trainees in research • Large number of trials in the pipeline • NETT will lead to efficient research in many diseases • Tight budget • Small numbers of Hubs • Scientific review committee tough and less interested in practical trials
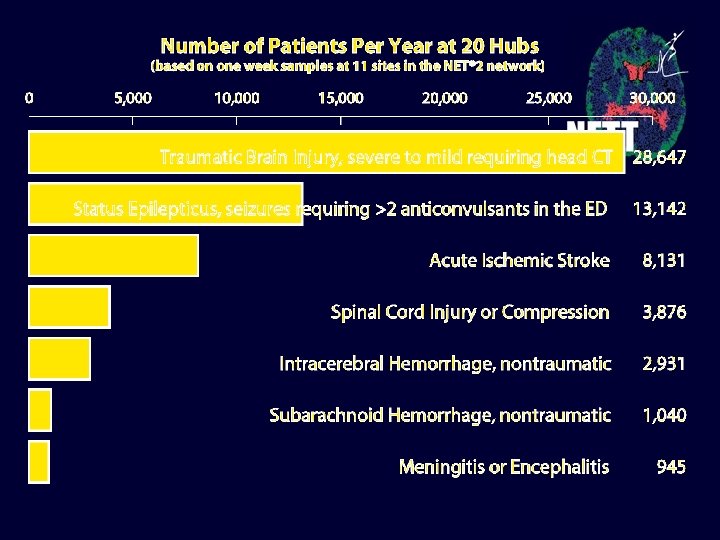
 Special Challenges to studying Neurological Emergencies • Urgency: recruitment in minutes not hours • Multiple disciplinary involvement: EMS, emergency medicine, neurology, pediatrics, neurosurgery, radiology, traumatology, rehabilitation, others • Conditions complicate informed consent
Special Challenges to studying Neurological Emergencies • Urgency: recruitment in minutes not hours • Multiple disciplinary involvement: EMS, emergency medicine, neurology, pediatrics, neurosurgery, radiology, traumatology, rehabilitation, others • Conditions complicate informed consent
 Defining Principals • Very early enrollment • Diverse enrollment, hub and spoke design • Large simple trials
Defining Principals • Very early enrollment • Diverse enrollment, hub and spoke design • Large simple trials
 Operational Principals • • Streamlined operations Technological efficiencies when possible Centralized outcome assessments Clinical translation
Operational Principals • • Streamlined operations Technological efficiencies when possible Centralized outcome assessments Clinical translation

 Neurological Emergency Treatment Trials Network
Neurological Emergency Treatment Trials Network
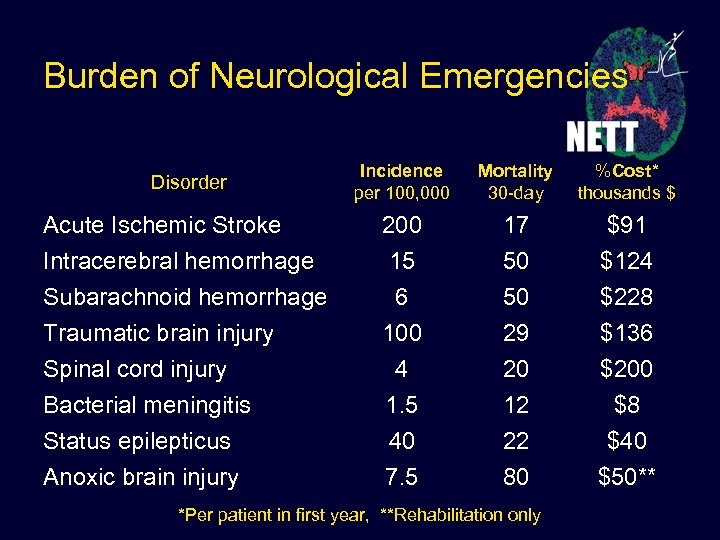 Burden of Neurological Emergencies Disorder Incidence per 100, 000 Mortality 30 -day %Cost* thousands $ Acute Ischemic Stroke Intracerebral hemorrhage Subarachnoid hemorrhage Traumatic brain injury Spinal cord injury Bacterial meningitis Status epilepticus Anoxic brain injury 200 15 6 100 4 1. 5 40 7. 5 17 50 50 29 20 12 22 80 $91 $124 $228 $136 $200 $8 $40 $50** *Per patient in first year, **Rehabilitation only
Burden of Neurological Emergencies Disorder Incidence per 100, 000 Mortality 30 -day %Cost* thousands $ Acute Ischemic Stroke Intracerebral hemorrhage Subarachnoid hemorrhage Traumatic brain injury Spinal cord injury Bacterial meningitis Status epilepticus Anoxic brain injury 200 15 6 100 4 1. 5 40 7. 5 17 50 50 29 20 12 22 80 $91 $124 $228 $136 $200 $8 $40 $50** *Per patient in first year, **Rehabilitation only



