752d0abe1f8de4a48b4c61ba44b23e49.ppt
- Количество слайдов: 22
 Neurological complica. ons a 1 er transcatheter aor. c valve implanta. on with a self‐‐‐expanding bioprosthesis or surgical aor. c valve replacement in pa. ents at intermediate‐‐‐risk for surgery A. Pieter Kappetein, Erasmus Medical Centre, Ro 9 erdam, Netherlands Nicolas M. Van Mieghem, Erasmus Medical Centre, Ro 9 erdam, Netherlands Michael J. Reardon, Methodist Debakey Heart and Vascular Center, Houston, TX, USA Patrick W. J. C. Serruys, Erasmus Medical Centre, Ro 9 erdam, Netherlands Jeffrey J. Popma, Beth Israel Deaconess Medical Center, Boston, MA, USA For the SURTAVI Inves. Ngators
Neurological complica. ons a 1 er transcatheter aor. c valve implanta. on with a self‐‐‐expanding bioprosthesis or surgical aor. c valve replacement in pa. ents at intermediate‐‐‐risk for surgery A. Pieter Kappetein, Erasmus Medical Centre, Ro 9 erdam, Netherlands Nicolas M. Van Mieghem, Erasmus Medical Centre, Ro 9 erdam, Netherlands Michael J. Reardon, Methodist Debakey Heart and Vascular Center, Houston, TX, USA Patrick W. J. C. Serruys, Erasmus Medical Centre, Ro 9 erdam, Netherlands Jeffrey J. Popma, Beth Israel Deaconess Medical Center, Boston, MA, USA For the SURTAVI Inves. Ngators
 Speaker's name: Prof. A Pieter Kappetein I do not have any poten. al conflict of interest X I have the following poten. al conflicts of interest to report: • Ins. Ntu. Nonal grant/research support: Medtronic personnel performed all sta. Ns. Ncal analyses and verified the accuracy of the data, and assisted in the graphical display of the data presented. 2
Speaker's name: Prof. A Pieter Kappetein I do not have any poten. al conflict of interest X I have the following poten. al conflicts of interest to report: • Ins. Ntu. Nonal grant/research support: Medtronic personnel performed all sta. Ns. Ncal analyses and verified the accuracy of the data, and assisted in the graphical display of the data presented. 2
 SURTAVI TRIAL Background • An increased risk for death, long‐‐‐term morbidity and poor quality of life is associated with periprocedural stroke a. Wer surgical or transcatheter aor. Nc valve implanta. Non (TAVI). • The SURTAVI Trial showed that TAVI with a self‐ ‐‐expanding Core. Valve or Evolut R bioprosthesis was noninferior to surgical aor. Nc valve replacement (SAVR) for all‐‐‐cause mortality or disabling stroke at 2 years. • As TAVI con. Nnues to be clinically evaluated in lower‐‐‐risk popula. Nons, an understanding of the rela. Nve risk for neurological complica. Nons and their clinical consequences following SAVR and TAVI is cri. Ncal. 3
SURTAVI TRIAL Background • An increased risk for death, long‐‐‐term morbidity and poor quality of life is associated with periprocedural stroke a. Wer surgical or transcatheter aor. Nc valve implanta. Non (TAVI). • The SURTAVI Trial showed that TAVI with a self‐ ‐‐expanding Core. Valve or Evolut R bioprosthesis was noninferior to surgical aor. Nc valve replacement (SAVR) for all‐‐‐cause mortality or disabling stroke at 2 years. • As TAVI con. Nnues to be clinically evaluated in lower‐‐‐risk popula. Nons, an understanding of the rela. Nve risk for neurological complica. Nons and their clinical consequences following SAVR and TAVI is cri. Ncal. 3
 SURTAVI TRIAL Methods PATIENTS: • Severe, symptoma. Nc aor. Nc stenosis at intermediate surgical risk • Risk determined by heart team at each site: – Es. Nmated surgical mortality ≥ 3% and <15% and other measures of comorbidity, frailty and disability – Interna. Nonal screening commi 9 ee confirmed pa. Nent eligibility STUDY: • Independent Clinical Events Commi 9 ee adjudicated all neurological events. • VARC‐‐‐ 2* defini. Nons of stroke • Encephalopathy included evidence of altered mental state (e. g. seizures, delirium, confusion, hallucina. Nons, demen. Na) • Neurologist or stroke specialist evaluated pa. Nents with suspected event – Imaging at discre. Non of specialist • All stroke and encephalopathy were compared between TAVI and SAVR at 30 days. *Kappetein AP, et al. Eur Heart J 2012; 33: 2403‐‐‐ 18. 4
SURTAVI TRIAL Methods PATIENTS: • Severe, symptoma. Nc aor. Nc stenosis at intermediate surgical risk • Risk determined by heart team at each site: – Es. Nmated surgical mortality ≥ 3% and <15% and other measures of comorbidity, frailty and disability – Interna. Nonal screening commi 9 ee confirmed pa. Nent eligibility STUDY: • Independent Clinical Events Commi 9 ee adjudicated all neurological events. • VARC‐‐‐ 2* defini. Nons of stroke • Encephalopathy included evidence of altered mental state (e. g. seizures, delirium, confusion, hallucina. Nons, demen. Na) • Neurologist or stroke specialist evaluated pa. Nents with suspected event – Imaging at discre. Non of specialist • All stroke and encephalopathy were compared between TAVI and SAVR at 30 days. *Kappetein AP, et al. Eur Heart J 2012; 33: 2403‐‐‐ 18. 4
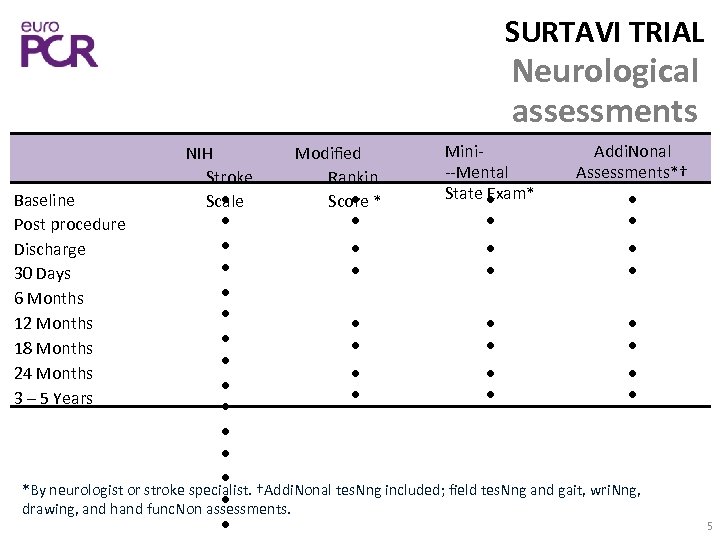 SURTAVI TRIAL Neurological assessments Mini‐ Addi. Nonal NIH Modified ‐‐Mental Assessments*† Stroke Rankin State Exam* Baseline Scale Score * Post procedure Discharge 30 Days 6 Months 12 Months 18 Months 24 Months 3 – 5 Years *By neurologist or stroke specialist. †Addi. Nonal tes. Nng included; field tes. Nng and gait, wri. Nng, drawing, and hand func. Non assessments. 5
SURTAVI TRIAL Neurological assessments Mini‐ Addi. Nonal NIH Modified ‐‐Mental Assessments*† Stroke Rankin State Exam* Baseline Scale Score * Post procedure Discharge 30 Days 6 Months 12 Months 18 Months 24 Months 3 – 5 Years *By neurologist or stroke specialist. †Addi. Nonal tes. Nng included; field tes. Nng and gait, wri. Nng, drawing, and hand func. Non assessments. 5
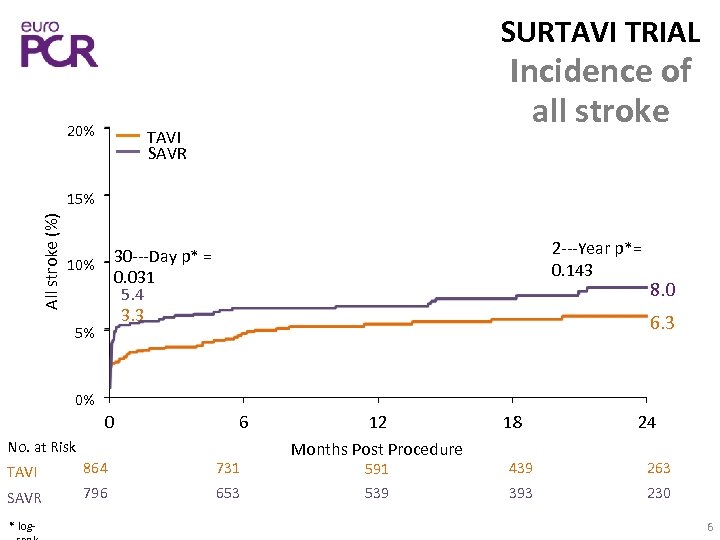 SURTAVI TRIAL 20% Incidence of all stroke TAVI SAVR All stroke (%) 15% 2‐‐‐Year p*= 0. 143 30‐‐‐Day p* = 0. 031 5. 4 3. 3 10% 5% 8. 0 6. 3 0% 0 6 No. at Risk TAVI 864 731 SAVR 796 653 * log‐ 12 Months Post Procedure 18 24 591 439 263 539 393 230 6
SURTAVI TRIAL 20% Incidence of all stroke TAVI SAVR All stroke (%) 15% 2‐‐‐Year p*= 0. 143 30‐‐‐Day p* = 0. 031 5. 4 3. 3 10% 5% 8. 0 6. 3 0% 0 6 No. at Risk TAVI 864 731 SAVR 796 653 * log‐ 12 Months Post Procedure 18 24 591 439 263 539 393 230 6
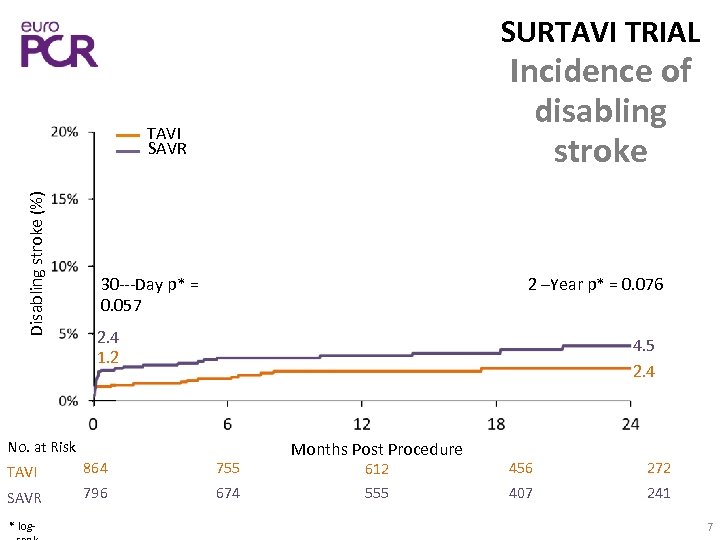 SURTAVI TRIAL Incidence of disabling stroke Disabling stroke (%) TAVI SAVR 30‐‐‐Day p* = 0. 057 2 –Year p* = 0. 076 2. 4 1. 2 4. 5 2. 4 No. at Risk TAVI 864 755 SAVR 796 674 * log‐ Months Post Procedure 612 456 272 555 407 241 7
SURTAVI TRIAL Incidence of disabling stroke Disabling stroke (%) TAVI SAVR 30‐‐‐Day p* = 0. 057 2 –Year p* = 0. 076 2. 4 1. 2 4. 5 2. 4 No. at Risk TAVI 864 755 SAVR 796 674 * log‐ Months Post Procedure 612 456 272 555 407 241 7
 SURTAVI TRIAL 20% Incidence of non‐ ‐‐disabling stroke TAVI SAVR Non‐‐‐disabling stroke (%) 15% 10% 30‐‐‐Day p* = 0. 230 3. 0 2. 1 5% 2‐‐‐Year p* = 0. 834 4. 1 4. 0 0% 0 6 No. at Risk TAVI 864 738 SAVR 796 669 * log‐ 12 Months Post Procedure 18 24 600 447 271 553 400 238 8
SURTAVI TRIAL 20% Incidence of non‐ ‐‐disabling stroke TAVI SAVR Non‐‐‐disabling stroke (%) 15% 10% 30‐‐‐Day p* = 0. 230 3. 0 2. 1 5% 2‐‐‐Year p* = 0. 834 4. 1 4. 0 0% 0 6 No. at Risk TAVI 864 738 SAVR 796 669 * log‐ 12 Months Post Procedure 18 24 600 447 271 553 400 238 8
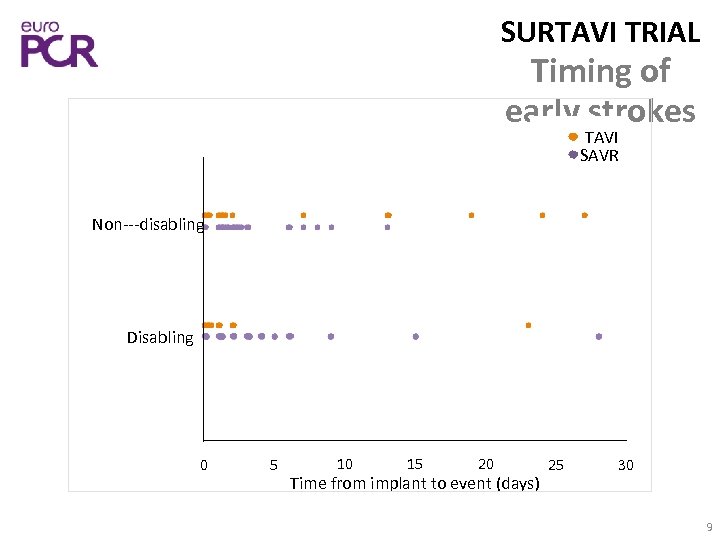 SURTAVI TRIAL Timing of early strokes TAVI SAVR Non‐‐‐disabling Disabling 0 5 10 15 20 Time from implant to event (days) 25 30 9
SURTAVI TRIAL Timing of early strokes TAVI SAVR Non‐‐‐disabling Disabling 0 5 10 15 20 Time from implant to event (days) 25 30 9
 SURTAVI TRIAL Percentage of Pa. Nents (%) Early stroke severity Modified Rankin Scores at 30 Days 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 7, 1 9, 3 42, 9 53, 4 50, 0 37, 3 TAVI N=28 0– 1 SAVR N=43 2– 6 Missing 10 I
SURTAVI TRIAL Percentage of Pa. Nents (%) Early stroke severity Modified Rankin Scores at 30 Days 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 7, 1 9, 3 42, 9 53, 4 50, 0 37, 3 TAVI N=28 0– 1 SAVR N=43 2– 6 Missing 10 I
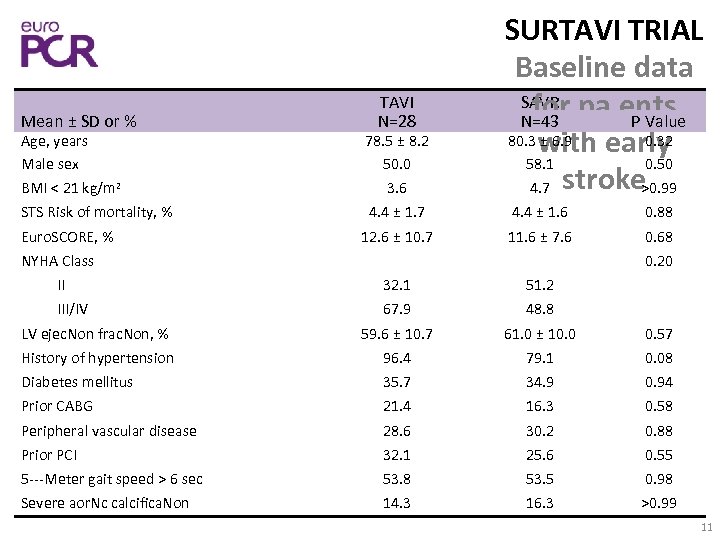 Mean ± SD or % TAVI N=28 SURTAVI TRIAL Baseline data SAVR pa. ents for N=43 P Value 80. 3 with early ± 6. 9 0. 32 58. 1 0. 50 4. 7 stroke >0. 99 Age, years Male sex BMI < 21 kg/m 2 78. 5 ± 8. 2 50. 0 3. 6 STS Risk of mortality, % 4. 4 ± 1. 7 4. 4 ± 1. 6 0. 88 12. 6 ± 10. 7 11. 6 ± 7. 6 0. 68 Euro. SCORE, % NYHA Class 0. 20 II 32. 1 51. 2 III/IV 67. 9 48. 8 LV ejec. Non frac. Non, % 59. 6 ± 10. 7 61. 0 ± 10. 0 0. 57 History of hypertension 96. 4 79. 1 0. 08 Diabetes mellitus 35. 7 34. 9 0. 94 Prior CABG 21. 4 16. 3 0. 58 Peripheral vascular disease 28. 6 30. 2 0. 88 Prior PCI 32. 1 25. 6 0. 55 5‐‐‐Meter gait speed > 6 sec 53. 8 53. 5 0. 98 Severe aor. Nc calcifica. Non 14. 3 16. 3 >0. 99 11
Mean ± SD or % TAVI N=28 SURTAVI TRIAL Baseline data SAVR pa. ents for N=43 P Value 80. 3 with early ± 6. 9 0. 32 58. 1 0. 50 4. 7 stroke >0. 99 Age, years Male sex BMI < 21 kg/m 2 78. 5 ± 8. 2 50. 0 3. 6 STS Risk of mortality, % 4. 4 ± 1. 7 4. 4 ± 1. 6 0. 88 12. 6 ± 10. 7 11. 6 ± 7. 6 0. 68 Euro. SCORE, % NYHA Class 0. 20 II 32. 1 51. 2 III/IV 67. 9 48. 8 LV ejec. Non frac. Non, % 59. 6 ± 10. 7 61. 0 ± 10. 0 0. 57 History of hypertension 96. 4 79. 1 0. 08 Diabetes mellitus 35. 7 34. 9 0. 94 Prior CABG 21. 4 16. 3 0. 58 Peripheral vascular disease 28. 6 30. 2 0. 88 Prior PCI 32. 1 25. 6 0. 55 5‐‐‐Meter gait speed > 6 sec 53. 8 53. 5 0. 98 Severe aor. Nc calcifica. Non 14. 3 16. 3 >0. 99 11
 SURTAVI TRIAL TAVI baseline data: early vs No no stroke Stroke P Value Age, years Strok e N=28 78. 5 ± 8. 2 Male sex 50. 0 57. 9 0. 41 STS Risk of mortality, % 4. 4 ± 1. 7 4. 4 ± 1. 5 0. 89 History of hypertension 96. 4 92. 6 0. 72 Diabetes mellitus 35. 7 34. 1 0. 86 Peripheral vascular disease 28. 6 30. 9 0. 80 Severe aor. Nc calcifica. Non 14. 3 12. 4 0. 77 Mean ± SD or % N=836 80. 0 ± 6. 1 0. 35 12
SURTAVI TRIAL TAVI baseline data: early vs No no stroke Stroke P Value Age, years Strok e N=28 78. 5 ± 8. 2 Male sex 50. 0 57. 9 0. 41 STS Risk of mortality, % 4. 4 ± 1. 7 4. 4 ± 1. 5 0. 89 History of hypertension 96. 4 92. 6 0. 72 Diabetes mellitus 35. 7 34. 1 0. 86 Peripheral vascular disease 28. 6 30. 9 0. 80 Severe aor. Nc calcifica. Non 14. 3 12. 4 0. 77 Mean ± SD or % N=836 80. 0 ± 6. 1 0. 35 12
 SURTAVI TRIAL SAVR baseline data: No early vs. P no Stroke Value N=753 79. 7 ± stroke 6. 0 0. 54 Age, years Strok e N=43 80. 3 ± 6. 9 Male sex 58. 1 54. 8 0. 67 STS Risk of mortality, % 4. 4 ± 1. 6 4. 5 ± 1. 6 0. 55 History of hypertension 79. 1 91. 0 0. 01 Diabetes mellitus 34. 9 34. 8 0. 99 Peripheral vascular disease 30. 2 29. 9 0. 96 Severe aor. Nc calcifica. Non 16. 3 10. 5 0. 23 Mean ± SD or % 13
SURTAVI TRIAL SAVR baseline data: No early vs. P no Stroke Value N=753 79. 7 ± stroke 6. 0 0. 54 Age, years Strok e N=43 80. 3 ± 6. 9 Male sex 58. 1 54. 8 0. 67 STS Risk of mortality, % 4. 4 ± 1. 6 4. 5 ± 1. 6 0. 55 History of hypertension 79. 1 91. 0 0. 01 Diabetes mellitus 34. 9 34. 8 0. 99 Peripheral vascular disease 30. 2 29. 9 0. 96 Severe aor. Nc calcifica. Non 16. 3 10. 5 0. 23 Mean ± SD or % 13
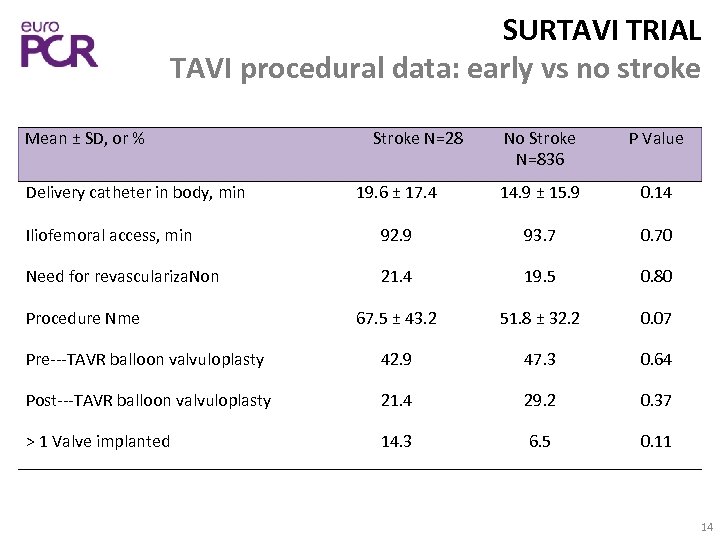 SURTAVI TRIAL TAVI procedural data: early vs no stroke No Stroke N=836 P Value 19. 6 ± 17. 4 14. 9 ± 15. 9 0. 14 Iliofemoral access, min 92. 9 93. 7 0. 70 Need for revasculariza. Non 21. 4 19. 5 0. 80 67. 5 ± 43. 2 51. 8 ± 32. 2 0. 07 Pre‐‐‐TAVR balloon valvuloplasty 42. 9 47. 3 0. 64 Post‐‐‐TAVR balloon valvuloplasty 21. 4 29. 2 0. 37 > 1 Valve implanted 14. 3 6. 5 0. 11 Mean ± SD, or % Delivery catheter in body, min Procedure Nme Stroke N=28 14
SURTAVI TRIAL TAVI procedural data: early vs no stroke No Stroke N=836 P Value 19. 6 ± 17. 4 14. 9 ± 15. 9 0. 14 Iliofemoral access, min 92. 9 93. 7 0. 70 Need for revasculariza. Non 21. 4 19. 5 0. 80 67. 5 ± 43. 2 51. 8 ± 32. 2 0. 07 Pre‐‐‐TAVR balloon valvuloplasty 42. 9 47. 3 0. 64 Post‐‐‐TAVR balloon valvuloplasty 21. 4 29. 2 0. 37 > 1 Valve implanted 14. 3 6. 5 0. 11 Mean ± SD, or % Delivery catheter in body, min Procedure Nme Stroke N=28 14
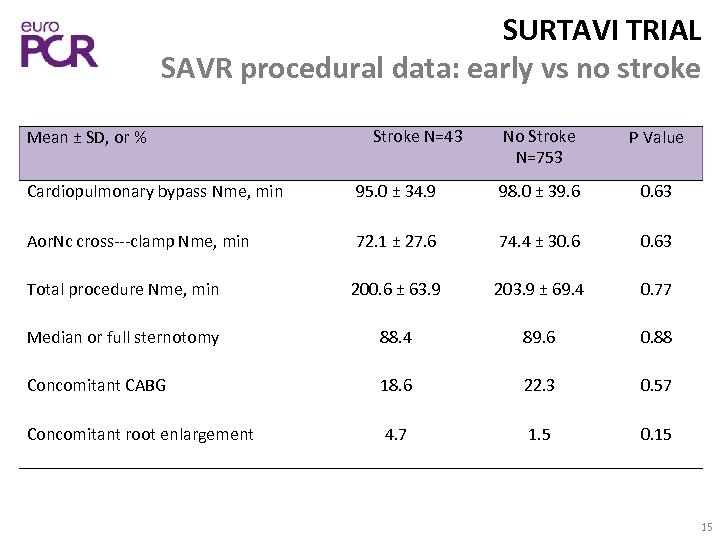 SURTAVI TRIAL SAVR procedural data: early vs no stroke Mean ± SD, or % Stroke N=43 No Stroke N=753 P Value Cardiopulmonary bypass Nme, min 95. 0 ± 34. 9 98. 0 ± 39. 6 0. 63 Aor. Nc cross‐‐‐clamp Nme, min 72. 1 ± 27. 6 74. 4 ± 30. 63 Total procedure Nme, min 200. 6 ± 63. 9 203. 9 ± 69. 4 0. 77 Median or full sternotomy 88. 4 89. 6 0. 88 Concomitant CABG 18. 6 22. 3 0. 57 Concomitant root enlargement 4. 7 1. 5 0. 15 15
SURTAVI TRIAL SAVR procedural data: early vs no stroke Mean ± SD, or % Stroke N=43 No Stroke N=753 P Value Cardiopulmonary bypass Nme, min 95. 0 ± 34. 9 98. 0 ± 39. 6 0. 63 Aor. Nc cross‐‐‐clamp Nme, min 72. 1 ± 27. 6 74. 4 ± 30. 63 Total procedure Nme, min 200. 6 ± 63. 9 203. 9 ± 69. 4 0. 77 Median or full sternotomy 88. 4 89. 6 0. 88 Concomitant CABG 18. 6 22. 3 0. 57 Concomitant root enlargement 4. 7 1. 5 0. 15 15
 Mean ± SD, or % ICU dura. Non, hours Length of stay, days Strok e N=28 88. 0 ± 88. 6 8. 9 ± 5. 1 SURTAVI TRIAL TAVI hospitalisa. on No data: early vs Stroke P Value no N=836 stroke 47. 2 ± 41. 1 0. 03 5. 6 ± 4. 8 <0. 001 Discharge Loca. Non Home Another hospital Rehabilita. Non clinic Skilled nursing facility Other Pa. Nent died in hospital <0. 001 35. 7 86. 7 3. 6 1. 2 32. 1 5. 9 14. 3 3. 6 1. 4 10. 7 1. 2 16
Mean ± SD, or % ICU dura. Non, hours Length of stay, days Strok e N=28 88. 0 ± 88. 6 8. 9 ± 5. 1 SURTAVI TRIAL TAVI hospitalisa. on No data: early vs Stroke P Value no N=836 stroke 47. 2 ± 41. 1 0. 03 5. 6 ± 4. 8 <0. 001 Discharge Loca. Non Home Another hospital Rehabilita. Non clinic Skilled nursing facility Other Pa. Nent died in hospital <0. 001 35. 7 86. 7 3. 6 1. 2 32. 1 5. 9 14. 3 3. 6 1. 4 10. 7 1. 2 16
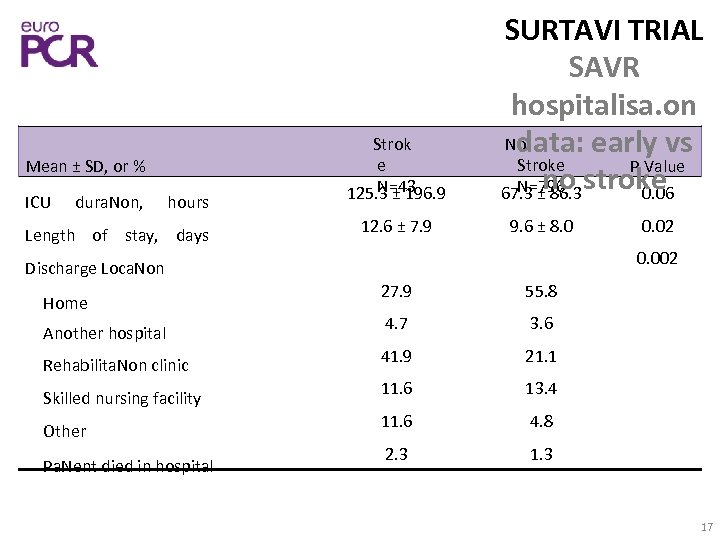 Mean ± SD, or % ICU dura. Non, hours Length of stay, days Strok e N=43 125. 3 ± 196. 9 12. 6 ± 7. 9 SURTAVI TRIAL SAVR hospitalisa. on No data: early vs Stroke P Value no N=796 stroke 67. 3 ± 86. 3 0. 06 9. 6 ± 8. 0 0. 002 Discharge Loca. Non Home Another hospital Rehabilita. Non clinic Skilled nursing facility Other Pa. Nent died in hospital 0. 02 27. 9 55. 8 4. 7 3. 6 41. 9 21. 1 11. 6 13. 4 11. 6 4. 8 2. 3 17
Mean ± SD, or % ICU dura. Non, hours Length of stay, days Strok e N=43 125. 3 ± 196. 9 12. 6 ± 7. 9 SURTAVI TRIAL SAVR hospitalisa. on No data: early vs Stroke P Value no N=796 stroke 67. 3 ± 86. 3 0. 06 9. 6 ± 8. 0 0. 002 Discharge Loca. Non Home Another hospital Rehabilita. Non clinic Skilled nursing facility Other Pa. Nent died in hospital 0. 02 27. 9 55. 8 4. 7 3. 6 41. 9 21. 1 11. 6 13. 4 11. 6 4. 8 2. 3 17
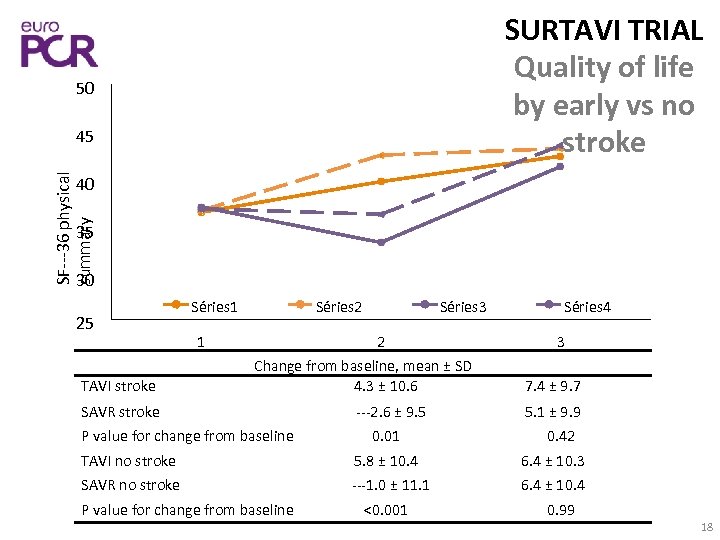 SURTAVI TRIAL Quality of life by early vs no stroke 50 SF‐‐‐ 36 physical summary 45 40 35 30 25 TAVI stroke Séries 1 1 Séries 2 Séries 3 2 Change from baseline, mean ± SD 4. 3 ± 10. 6 SAVR stroke P value for change from baseline ‐‐‐ 2. 6 ± 9. 5 0. 01 Séries 4 3 7. 4 ± 9. 7 5. 1 ± 9. 9 0. 42 TAVI no stroke 5. 8 ± 10. 4 6. 4 ± 10. 3 SAVR no stroke ‐‐‐ 1. 0 ± 11. 1 6. 4 ± 10. 4 P value for change from baseline <0. 001 0. 99 18
SURTAVI TRIAL Quality of life by early vs no stroke 50 SF‐‐‐ 36 physical summary 45 40 35 30 25 TAVI stroke Séries 1 1 Séries 2 Séries 3 2 Change from baseline, mean ± SD 4. 3 ± 10. 6 SAVR stroke P value for change from baseline ‐‐‐ 2. 6 ± 9. 5 0. 01 Séries 4 3 7. 4 ± 9. 7 5. 1 ± 9. 9 0. 42 TAVI no stroke 5. 8 ± 10. 4 6. 4 ± 10. 3 SAVR no stroke ‐‐‐ 1. 0 ± 11. 1 6. 4 ± 10. 4 P value for change from baseline <0. 001 0. 99 18
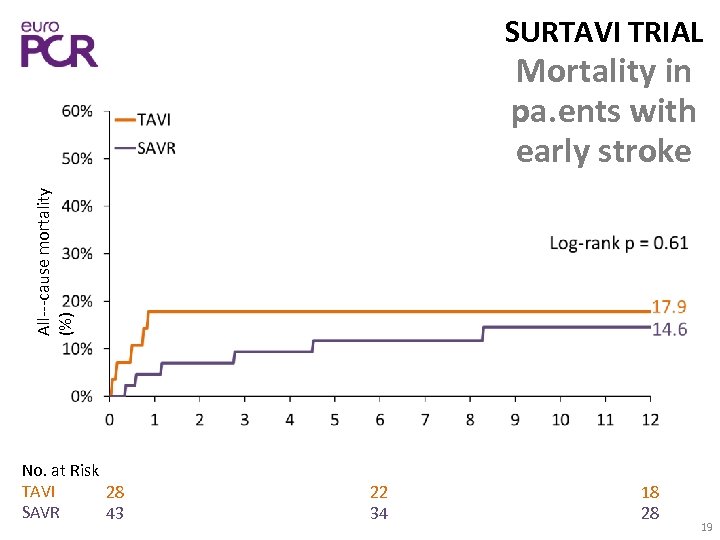 SURTAVI TRIAL All‐‐‐cause mortality (%) Mortality in pa. ents with early stroke No. at Risk TAVI 28 SAVR 43 22 34 18 28 19
SURTAVI TRIAL All‐‐‐cause mortality (%) Mortality in pa. ents with early stroke No. at Risk TAVI 28 SAVR 43 22 34 18 28 19
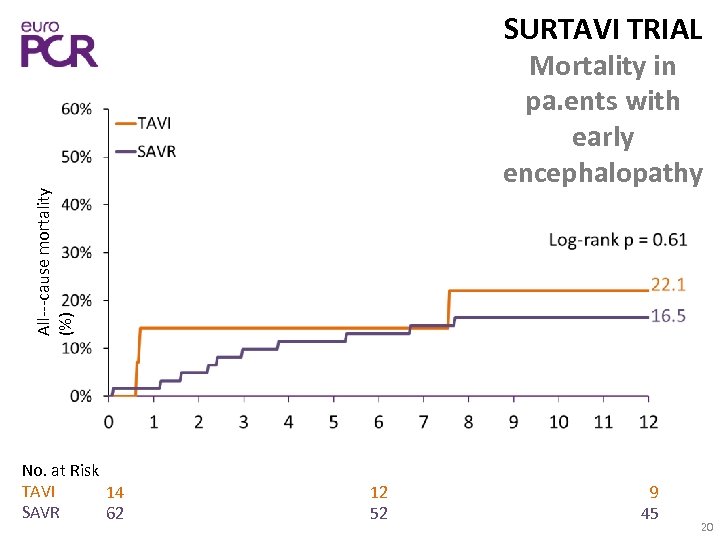 SURTAVI TRIAL All‐‐‐cause mortality (%) Mortality in pa. ents with early encephalopathy No. at Risk TAVI 14 SAVR 62 12 52 9 45 20
SURTAVI TRIAL All‐‐‐cause mortality (%) Mortality in pa. ents with early encephalopathy No. at Risk TAVI 14 SAVR 62 12 52 9 45 20
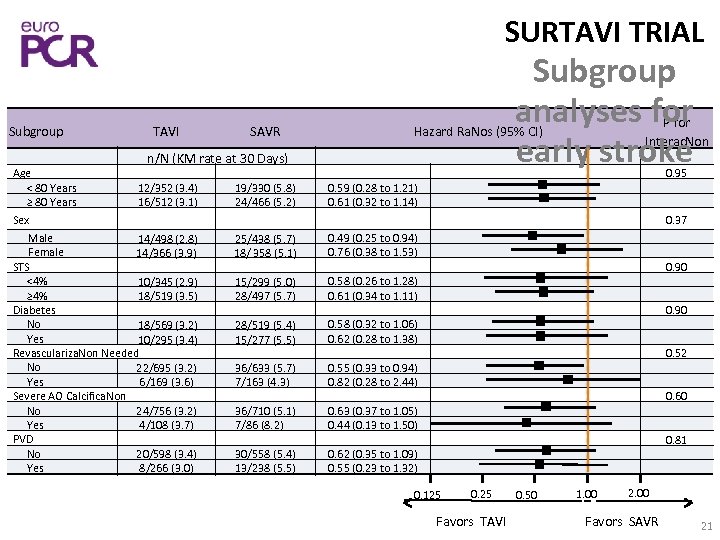 SURTAVI TRIAL Subgroup Age < 80 Years ≥ 80 Years TAVI SAVR Subgroup analyses for early stroke n/N (KM rate at 30 Days) 12/352 (3. 4) 16/512 (3. 1) Sex Male 14/498 (2. 8) Female 14/366 (3. 9) STS <4% 10/345 (2. 9) ≥ 4% 18/519 (3. 5) Diabetes No 18/569 (3. 2) Yes 10/295 (3. 4) Revasculariza. Non Needed No 22/695 (3. 2) Yes 6/169 (3. 6) Severe AO Calcifica. Non 24/756 (3. 2) No Yes 4/108 (3. 7) PVD 20/598 (3. 4) No Yes 8/266 (3. 0) 19/330 (5. 8) 24/466 (5. 2) P for Interac. Non Hazard Ra. Nos (95% CI) 0. 95 0. 59 (0. 28 to 1. 21) 0. 61 (0. 32 to 1. 14) 0. 37 25/438 (5. 7) 18/ 358 (5. 1) 0. 49 (0. 25 to 0. 94) 0. 76 (0. 38 to 1. 53) 15/299 (5. 0) 28/497 (5. 7) 0. 58 (0. 26 to 1. 28) 0. 61 (0. 34 to 1. 11) 28/519 (5. 4) 15/277 (5. 5) 0. 58 (0. 32 to 1. 06) 0. 62 (0. 28 to 1. 38) 36/633 (5. 7) 7/163 (4. 3) 0. 55 (0. 33 to 0. 94) 0. 82 (0. 28 to 2. 44) 36/710 (5. 1) 7/86 (8. 2) 0. 63 (0. 37 to 1. 05) 0. 44 (0. 13 to 1. 50) 30/558 (5. 4) 13/238 (5. 5) 0. 62 (0. 35 to 1. 09) 0. 55 (0. 23 to 1. 32) 0. 90 0. 52 0. 60 0. 81 0. 125 0. 25 Favors TAVI 0. 50 1. 00 2. 00 Favors SAVR 21
SURTAVI TRIAL Subgroup Age < 80 Years ≥ 80 Years TAVI SAVR Subgroup analyses for early stroke n/N (KM rate at 30 Days) 12/352 (3. 4) 16/512 (3. 1) Sex Male 14/498 (2. 8) Female 14/366 (3. 9) STS <4% 10/345 (2. 9) ≥ 4% 18/519 (3. 5) Diabetes No 18/569 (3. 2) Yes 10/295 (3. 4) Revasculariza. Non Needed No 22/695 (3. 2) Yes 6/169 (3. 6) Severe AO Calcifica. Non 24/756 (3. 2) No Yes 4/108 (3. 7) PVD 20/598 (3. 4) No Yes 8/266 (3. 0) 19/330 (5. 8) 24/466 (5. 2) P for Interac. Non Hazard Ra. Nos (95% CI) 0. 95 0. 59 (0. 28 to 1. 21) 0. 61 (0. 32 to 1. 14) 0. 37 25/438 (5. 7) 18/ 358 (5. 1) 0. 49 (0. 25 to 0. 94) 0. 76 (0. 38 to 1. 53) 15/299 (5. 0) 28/497 (5. 7) 0. 58 (0. 26 to 1. 28) 0. 61 (0. 34 to 1. 11) 28/519 (5. 4) 15/277 (5. 5) 0. 58 (0. 32 to 1. 06) 0. 62 (0. 28 to 1. 38) 36/633 (5. 7) 7/163 (4. 3) 0. 55 (0. 33 to 0. 94) 0. 82 (0. 28 to 2. 44) 36/710 (5. 1) 7/86 (8. 2) 0. 63 (0. 37 to 1. 05) 0. 44 (0. 13 to 1. 50) 30/558 (5. 4) 13/238 (5. 5) 0. 62 (0. 35 to 1. 09) 0. 55 (0. 23 to 1. 32) 0. 90 0. 52 0. 60 0. 81 0. 125 0. 25 Favors TAVI 0. 50 1. 00 2. 00 Favors SAVR 21
 SURTAVI TRIAL Summary • The incidence of early (30‐‐‐day) stroke was significantly lower in pa. Nents a. Wer TAVI than a. Wer SAVR. • Early stroke pa. Nents experienced longer ICU Nmes, more days in hospital and were more frequently discharged to an alternate care facility regardless of treatment group. • With or without stroke, TAVI pa. Nents recovered quality of life sooner than SAVR pa. Nents. • All‐‐‐cause mortality at 1 year was similar for TAVI and SAVR pa. Nents with stroke or with encephalopathy at 30 days. • There were no differences in early stroke rates among TAVI and SAVR pa. Nents for select subgroups. 22
SURTAVI TRIAL Summary • The incidence of early (30‐‐‐day) stroke was significantly lower in pa. Nents a. Wer TAVI than a. Wer SAVR. • Early stroke pa. Nents experienced longer ICU Nmes, more days in hospital and were more frequently discharged to an alternate care facility regardless of treatment group. • With or without stroke, TAVI pa. Nents recovered quality of life sooner than SAVR pa. Nents. • All‐‐‐cause mortality at 1 year was similar for TAVI and SAVR pa. Nents with stroke or with encephalopathy at 30 days. • There were no differences in early stroke rates among TAVI and SAVR pa. Nents for select subgroups. 22


