6d150cc2358c56fbdfc8a83479ec7fb5.ppt
- Количество слайдов: 117
 NDA 21 -514 DAYTRANATM (methylphenidate transdermal system) Psychopharmacologic Drugs Advisory Committee co-developed by Noven Pharmaceuticals, Inc. Shire Pharmaceuticals Douglas Hay, Ph. D Senior Vice President, Global Regulatory Affairs Shire Pharmaceuticals 1
NDA 21 -514 DAYTRANATM (methylphenidate transdermal system) Psychopharmacologic Drugs Advisory Committee co-developed by Noven Pharmaceuticals, Inc. Shire Pharmaceuticals Douglas Hay, Ph. D Senior Vice President, Global Regulatory Affairs Shire Pharmaceuticals 1
 Proposed Indication for Methylphenidate Transdermal System (MTS) TM DAYTRANA (methylphenidate transdermal system) is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) 2
Proposed Indication for Methylphenidate Transdermal System (MTS) TM DAYTRANA (methylphenidate transdermal system) is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) 2
 External Consultants • Stephen V. Faraone, Ph. D – Professor of Psychiatry and Director Child and Adolescent Psychiatric Research SUNY, Upstate Medical University, Syracuse Drug Effects on Growth in ADHD • Marc Lerner, MD – Clinical Professor of Pediatrics University of California, Irvine Developmental Pediatrics and ADHD • Judith Owens, MD, MPH – Associate Professor of Pediatrics Brown University School of Medicine, Providence, RI Sleep Disorders in ADHD • Sharon Wigal, Ph. D – Associate Clinical Professor of Pediatrics University of California, Irvine Clinical Trials in ADHD 3
External Consultants • Stephen V. Faraone, Ph. D – Professor of Psychiatry and Director Child and Adolescent Psychiatric Research SUNY, Upstate Medical University, Syracuse Drug Effects on Growth in ADHD • Marc Lerner, MD – Clinical Professor of Pediatrics University of California, Irvine Developmental Pediatrics and ADHD • Judith Owens, MD, MPH – Associate Professor of Pediatrics Brown University School of Medicine, Providence, RI Sleep Disorders in ADHD • Sharon Wigal, Ph. D – Associate Clinical Professor of Pediatrics University of California, Irvine Clinical Trials in ADHD 3
 External Consultants • David Heal, Ph. D, DSc – Director Rena. Sci Consultancy Ltd, UK Methylphenidate Pharmacology • Jack Henningfield, Ph. D – Adjunct Professor, Behavioral Biology The Johns Hopkins University School of Medicine Vice President, Pinney Associates, Bethesda, MD Risk Management 4
External Consultants • David Heal, Ph. D, DSc – Director Rena. Sci Consultancy Ltd, UK Methylphenidate Pharmacology • Jack Henningfield, Ph. D – Adjunct Professor, Behavioral Biology The Johns Hopkins University School of Medicine Vice President, Pinney Associates, Bethesda, MD Risk Management 4
 Agenda • Introduction • ADHD: Current Treatment – Marc Lerner, MD University of California, Irvine • Clinical Efficacy of MTS in the Treatment of ADHD – Liza Squires, MD Sr. Director, Clinical Research, Shire Pharmaceuticals • Safety and Tolerability of MTS in Children with ADHD – Raymond Pratt, MD Vice President, Global Clinical Medicine, Shire Pharm. • Clinical Perspective of MTS Treatment of ADHD – Sharon Wigal, Ph. D University of California, Irvine • Overall Benefit/Risk of MTS in the Treatment of ADHD – Raymond Pratt, MD 5
Agenda • Introduction • ADHD: Current Treatment – Marc Lerner, MD University of California, Irvine • Clinical Efficacy of MTS in the Treatment of ADHD – Liza Squires, MD Sr. Director, Clinical Research, Shire Pharmaceuticals • Safety and Tolerability of MTS in Children with ADHD – Raymond Pratt, MD Vice President, Global Clinical Medicine, Shire Pharm. • Clinical Perspective of MTS Treatment of ADHD – Sharon Wigal, Ph. D University of California, Irvine • Overall Benefit/Risk of MTS in the Treatment of ADHD – Raymond Pratt, MD 5
 Rationale for the Development of Transdermal Methylphenidate • Alternative for patients with difficulty swallowing pills – Caregivers indicate ~15% of children with ADHD have “extreme” or “high difficulty” in swallowing pills • Avoids any food effect – Concern with many oral ADHD medications • Opportunity to visibly monitor compliance • Sustained treatment without need for supplemental therapy • Flexible period of treatment in children requiring an altered regimen 6
Rationale for the Development of Transdermal Methylphenidate • Alternative for patients with difficulty swallowing pills – Caregivers indicate ~15% of children with ADHD have “extreme” or “high difficulty” in swallowing pills • Avoids any food effect – Concern with many oral ADHD medications • Opportunity to visibly monitor compliance • Sustained treatment without need for supplemental therapy • Flexible period of treatment in children requiring an altered regimen 6
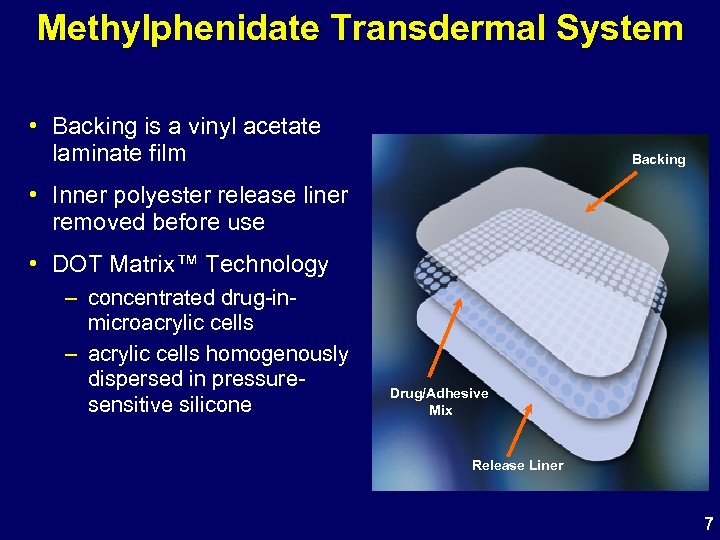 Methylphenidate Transdermal System • Backing is a vinyl acetate laminate film Backing • Inner polyester release liner removed before use • DOT Matrix™ Technology – concentrated drug-inmicroacrylic cells – acrylic cells homogenously dispersed in pressuresensitive silicone Drug/Adhesive Mix Release Liner 7
Methylphenidate Transdermal System • Backing is a vinyl acetate laminate film Backing • Inner polyester release liner removed before use • DOT Matrix™ Technology – concentrated drug-inmicroacrylic cells – acrylic cells homogenously dispersed in pressuresensitive silicone Drug/Adhesive Mix Release Liner 7
 Methylphenidate Transdermal System • Thin and transparent • Effective drug delivery over a small patch area • No need for irritating enhancers • Excellent adhesion 8
Methylphenidate Transdermal System • Thin and transparent • Effective drug delivery over a small patch area • No need for irritating enhancers • Excellent adhesion 8
 Methylphenidate Transdermal Patch Current Dose Strengths Dose (mg) delivered over 9 -h wear time 10 mg (1. 1 mg/h† x 9 h) Patch Surface Area 12. 5 cm 2 16 mg (1. 8 mg/h† x 9 h) 18. 75 cm 2 20 mg (2. 2 mg/h† x 9 h) 25 cm 2 27 mg (3. 0 mg/h† x 9 h) 37. 5 cm 2 Methylphenidate doses delivered by the MTS patch are comparable to other sustained-release methylphenidate-based products on the market †Nominal delivery rate per hour in pediatric subjects aged 6 -12 9
Methylphenidate Transdermal Patch Current Dose Strengths Dose (mg) delivered over 9 -h wear time 10 mg (1. 1 mg/h† x 9 h) Patch Surface Area 12. 5 cm 2 16 mg (1. 8 mg/h† x 9 h) 18. 75 cm 2 20 mg (2. 2 mg/h† x 9 h) 25 cm 2 27 mg (3. 0 mg/h† x 9 h) 37. 5 cm 2 Methylphenidate doses delivered by the MTS patch are comparable to other sustained-release methylphenidate-based products on the market †Nominal delivery rate per hour in pediatric subjects aged 6 -12 9
 MTS Regulatory / Development History • NDA 21 -514 submitted by Noven June 2002 – 12 -hour wear time • FDA Action Letter April 2003 – acknowledged efficacy of MTS – identified incidence of insomnia, anorexia, and weight loss as unacceptable – recommended consideration of shorter duration of wear time to potentially mitigate these, as well as dermal effects • Noven and Shire signed collaborative agreement in 2003 • Discussions with FDA regarding subsequent clinical program; 9 -hour wear time • NDA resubmission on June 28, 2005 10
MTS Regulatory / Development History • NDA 21 -514 submitted by Noven June 2002 – 12 -hour wear time • FDA Action Letter April 2003 – acknowledged efficacy of MTS – identified incidence of insomnia, anorexia, and weight loss as unacceptable – recommended consideration of shorter duration of wear time to potentially mitigate these, as well as dermal effects • Noven and Shire signed collaborative agreement in 2003 • Discussions with FDA regarding subsequent clinical program; 9 -hour wear time • NDA resubmission on June 28, 2005 10
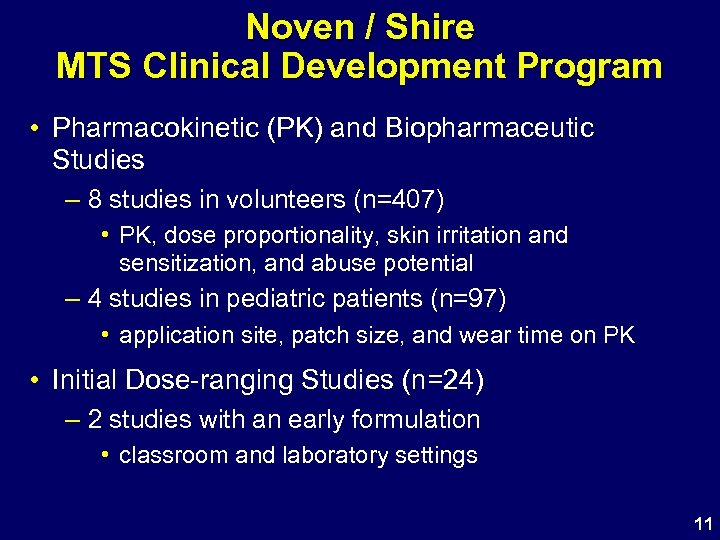 Noven / Shire MTS Clinical Development Program • Pharmacokinetic (PK) and Biopharmaceutic Studies – 8 studies in volunteers (n=407) • PK, dose proportionality, skin irritation and sensitization, and abuse potential – 4 studies in pediatric patients (n=97) • application site, patch size, and wear time on PK • Initial Dose-ranging Studies (n=24) – 2 studies with an early formulation • classroom and laboratory settings 11
Noven / Shire MTS Clinical Development Program • Pharmacokinetic (PK) and Biopharmaceutic Studies – 8 studies in volunteers (n=407) • PK, dose proportionality, skin irritation and sensitization, and abuse potential – 4 studies in pediatric patients (n=97) • application site, patch size, and wear time on PK • Initial Dose-ranging Studies (n=24) – 2 studies with an early formulation • classroom and laboratory settings 11
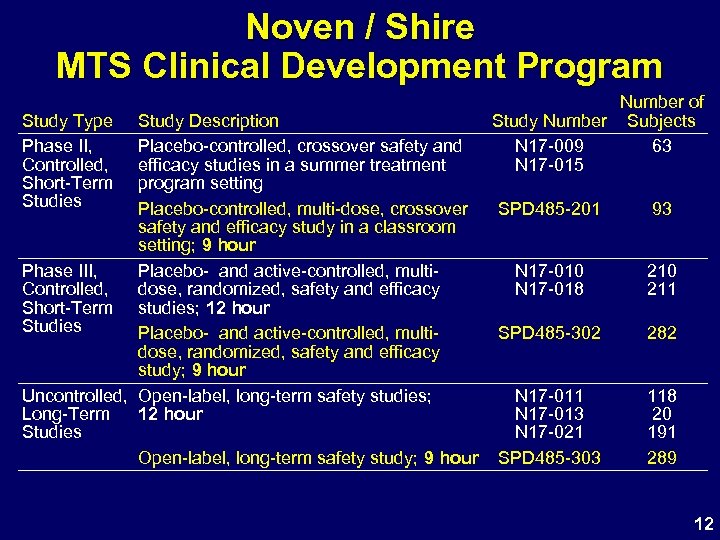 Noven / Shire MTS Clinical Development Program Study Type Phase II, Controlled, Short-Term Studies Study Description Placebo-controlled, crossover safety and efficacy studies in a summer treatment program setting Placebo-controlled, multi-dose, crossover safety and efficacy study in a classroom setting; 9 hour Phase III, Placebo- and active-controlled, multi. Controlled, dose, randomized, safety and efficacy Short-Term studies; 12 hour Studies Placebo- and active-controlled, multidose, randomized, safety and efficacy study; 9 hour Uncontrolled, Open-label, long-term safety studies; Long-Term 12 hour Studies Open-label, long-term safety study; 9 hour Number of Study Number Subjects N 17 -009 63 N 17 -015 SPD 485 -201 93 N 17 -010 N 17 -018 210 211 SPD 485 -302 282 N 17 -011 N 17 -013 N 17 -021 SPD 485 -303 118 20 191 289 12
Noven / Shire MTS Clinical Development Program Study Type Phase II, Controlled, Short-Term Studies Study Description Placebo-controlled, crossover safety and efficacy studies in a summer treatment program setting Placebo-controlled, multi-dose, crossover safety and efficacy study in a classroom setting; 9 hour Phase III, Placebo- and active-controlled, multi. Controlled, dose, randomized, safety and efficacy Short-Term studies; 12 hour Studies Placebo- and active-controlled, multidose, randomized, safety and efficacy study; 9 hour Uncontrolled, Open-label, long-term safety studies; Long-Term 12 hour Studies Open-label, long-term safety study; 9 hour Number of Study Number Subjects N 17 -009 63 N 17 -015 SPD 485 -201 93 N 17 -010 N 17 -018 210 211 SPD 485 -302 282 N 17 -011 N 17 -013 N 17 -021 SPD 485 -303 118 20 191 289 12
 ADHD: Current Treatment Marc Lerner, MD Clinical Professor of Pediatrics University of California, Irvine 13
ADHD: Current Treatment Marc Lerner, MD Clinical Professor of Pediatrics University of California, Irvine 13
 Prevalence and Public Health Impact of ADHD • ADHD affects 3 -5% of all school-age children 1 – Recent data suggests rates up to 7. 5% in school-aged children 2 – Diagnosed in boys at rates up to 4 times more than in girls 3, 4 • ADHD accounts for up to 50% of mental health referrals for children 5 • ADHD often persists throughout childhood into adolescence and adulthood 6, 7, 8 14
Prevalence and Public Health Impact of ADHD • ADHD affects 3 -5% of all school-age children 1 – Recent data suggests rates up to 7. 5% in school-aged children 2 – Diagnosed in boys at rates up to 4 times more than in girls 3, 4 • ADHD accounts for up to 50% of mental health referrals for children 5 • ADHD often persists throughout childhood into adolescence and adulthood 6, 7, 8 14
 Risks Associated with ADHD • Academic 1, 2, 3, 4, 5, 6 – Poor academic performance – Higher drop-out rate – Suspension / expulsion • Social 8, 9 – Interaction problems with teachers, peers and parents – Adult, more often separated, divorced, or unmarried • Work 6, 7 – More frequent job changes – quit, fired – Lower status jobs – More often unemployed • Personal 10 – Co-morbidity: ODD, Anxiety, Depression 15
Risks Associated with ADHD • Academic 1, 2, 3, 4, 5, 6 – Poor academic performance – Higher drop-out rate – Suspension / expulsion • Social 8, 9 – Interaction problems with teachers, peers and parents – Adult, more often separated, divorced, or unmarried • Work 6, 7 – More frequent job changes – quit, fired – Lower status jobs – More often unemployed • Personal 10 – Co-morbidity: ODD, Anxiety, Depression 15
 Pharmacological Treatment for Children with ADHD • Clinicians should recommend pharmacotherapy to improve target outcomes in children with ADHD 2 • Stimulant medication is a first-line treatment for ADHD 1 • Methylphenidate (MPH) reduces core symptoms of ADHD 3 • Any individual stimulant effective in ~70% of ADHD patients – Many who fail to respond to one may respond to another 3, 4 • Stimulants generally safe and well tolerated 4 • Therapeutic goal for stimulants in ADHD is to provide optimal efficacy with manageable side effects 16
Pharmacological Treatment for Children with ADHD • Clinicians should recommend pharmacotherapy to improve target outcomes in children with ADHD 2 • Stimulant medication is a first-line treatment for ADHD 1 • Methylphenidate (MPH) reduces core symptoms of ADHD 3 • Any individual stimulant effective in ~70% of ADHD patients – Many who fail to respond to one may respond to another 3, 4 • Stimulants generally safe and well tolerated 4 • Therapeutic goal for stimulants in ADHD is to provide optimal efficacy with manageable side effects 16
 References 14. 1 14. 2 14. 3 14. 4 Jensen PS. Ritalin: Theory and Practice, 2 nd ed. Larchmont, NY: Mary Ann Liebert, Inc. ; 1999: 1 -3 Barbaresi WJ, Katusic SK, Colligan RC, Pankratz VS, et al. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002 Mar; 156(3): 217 -24 US Department of Health and Human Services, 1999 Scott-Levin Inc. , Physician Drug and Diagnosis Audit (PDDA), 2001 14. 5 The MTA Cooperative Group, A 14 -month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999 Dec; 56(12): 1073 -86 14. 6 Mannuzza S, Klein RG, Bessler A, Malloy P, La. Padula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998 Apr; 155(4): 493 -8 14. 7 Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8 -year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990 Jul; 29(4): 546 -57 Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Arch Gen Psychiatry. 1985 Oct; 42(10): 937 -47 Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002; 63 Suppl 12: 10 -5. Fischer M, Barkley RA, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: II. Academic, attentional, and neuropsychological status. J Consult Clin Psychol. 1990 Oct; 58(5): 580 -8 Hechtman L, Weiss G. Long-term outcome of hyperactive children. Am J Orthopsychiatry. 1983 Jul; 53(3): 532 -41 Faraone SV, Biederman J, Lehman BK, et al. Intellectual performance and school failure in children with attention deficit hyperactivity disorder and in their siblings. J Abnorm Psychol. 1993 Nov; 102(4): 616 -23 14. 8 15. 1 15. 2 15. 3 15. 4 15. 5 Biederman J, Monuteaux MC, Doyle AE, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004 Oct; 72(5): 757 -66 15. 6 Wilens TE, Dodson WA clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004 Oct; 65(10): 1301 -13 15. 7 Barkley RA, Fischer M, Smallish L, et al. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002 May; 111(2): 279 -89 15. 8 Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child. Psychol Psychiatry. 2004 Feb; 45(2): 195 -211 15. 9 Eakin L, Minde K. Hechtman L. The marital and family functioning of adults with ADHD and their spouses. J Atten Disord. 2004 Aug; 8(1): 1 -10 15. 10 Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2004; 65 Suppl 3: 3 -7 16. 1 American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001 Oct; 108(4): 1033 -44 16. 2 Greenhill LL, Pliszka S, Dulcan MK, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002 Feb; 41(2 Suppl): 26 S-49 S 16. 3 Spencer TJ, Biederman J, Harding M, O'Donnell D, et al. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry. 1996 Nov; 35(11): 1460 -9 16. 4 AACAP. Managing ADHD. Version 1. 1. Baltimore, MD: International Guidance Center; 2003; Guidelines Pocket Card 17
References 14. 1 14. 2 14. 3 14. 4 Jensen PS. Ritalin: Theory and Practice, 2 nd ed. Larchmont, NY: Mary Ann Liebert, Inc. ; 1999: 1 -3 Barbaresi WJ, Katusic SK, Colligan RC, Pankratz VS, et al. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002 Mar; 156(3): 217 -24 US Department of Health and Human Services, 1999 Scott-Levin Inc. , Physician Drug and Diagnosis Audit (PDDA), 2001 14. 5 The MTA Cooperative Group, A 14 -month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999 Dec; 56(12): 1073 -86 14. 6 Mannuzza S, Klein RG, Bessler A, Malloy P, La. Padula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998 Apr; 155(4): 493 -8 14. 7 Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8 -year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990 Jul; 29(4): 546 -57 Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Arch Gen Psychiatry. 1985 Oct; 42(10): 937 -47 Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002; 63 Suppl 12: 10 -5. Fischer M, Barkley RA, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: II. Academic, attentional, and neuropsychological status. J Consult Clin Psychol. 1990 Oct; 58(5): 580 -8 Hechtman L, Weiss G. Long-term outcome of hyperactive children. Am J Orthopsychiatry. 1983 Jul; 53(3): 532 -41 Faraone SV, Biederman J, Lehman BK, et al. Intellectual performance and school failure in children with attention deficit hyperactivity disorder and in their siblings. J Abnorm Psychol. 1993 Nov; 102(4): 616 -23 14. 8 15. 1 15. 2 15. 3 15. 4 15. 5 Biederman J, Monuteaux MC, Doyle AE, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004 Oct; 72(5): 757 -66 15. 6 Wilens TE, Dodson WA clinical perspective of attention-deficit/hyperactivity disorder into adulthood. J Clin Psychiatry. 2004 Oct; 65(10): 1301 -13 15. 7 Barkley RA, Fischer M, Smallish L, et al. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002 May; 111(2): 279 -89 15. 8 Barkley RA, Fischer M, Smallish L, et al. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child. Psychol Psychiatry. 2004 Feb; 45(2): 195 -211 15. 9 Eakin L, Minde K. Hechtman L. The marital and family functioning of adults with ADHD and their spouses. J Atten Disord. 2004 Aug; 8(1): 1 -10 15. 10 Biederman J. Impact of comorbidity in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2004; 65 Suppl 3: 3 -7 16. 1 American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder and Committee on Quality Improvement. Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001 Oct; 108(4): 1033 -44 16. 2 Greenhill LL, Pliszka S, Dulcan MK, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002 Feb; 41(2 Suppl): 26 S-49 S 16. 3 Spencer TJ, Biederman J, Harding M, O'Donnell D, et al. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry. 1996 Nov; 35(11): 1460 -9 16. 4 AACAP. Managing ADHD. Version 1. 1. Baltimore, MD: International Guidance Center; 2003; Guidelines Pocket Card 17
 AAP Treatment Guidelines for ADHD PEDIATRICS Vol. 108 No. 4 October 2001, pp. 1033 -1044 • Stimulants are generally considered safe medications, with few contraindications to their use • Side effects occur early in treatment and tend to be mild and short-lived • The most common side effects are decreased appetite, stomachache or headache, delayed sleep onset, jitteriness, or social withdrawal • Most side effects can be successfully managed through adjustments of dosage or schedule 18
AAP Treatment Guidelines for ADHD PEDIATRICS Vol. 108 No. 4 October 2001, pp. 1033 -1044 • Stimulants are generally considered safe medications, with few contraindications to their use • Side effects occur early in treatment and tend to be mild and short-lived • The most common side effects are decreased appetite, stomachache or headache, delayed sleep onset, jitteriness, or social withdrawal • Most side effects can be successfully managed through adjustments of dosage or schedule 18
 AAP Treatment Guidelines for ADHD PEDIATRICS Vol. 108 No. 4 October 2001, pp. 1033 -1044 • Children who fail to show positive effects or who experience intolerable side effects on one stimulant medication should be tried on another • Children who fail 2 stimulant medications can be tried on a third type or formulation of stimulant medication • 15% to 30% of children experience motor tics, most of which are transient, while on stimulant medications • The presence of tics before or during medical management of ADHD is not an absolute contraindication to the use of stimulant medications 19
AAP Treatment Guidelines for ADHD PEDIATRICS Vol. 108 No. 4 October 2001, pp. 1033 -1044 • Children who fail to show positive effects or who experience intolerable side effects on one stimulant medication should be tried on another • Children who fail 2 stimulant medications can be tried on a third type or formulation of stimulant medication • 15% to 30% of children experience motor tics, most of which are transient, while on stimulant medications • The presence of tics before or during medical management of ADHD is not an absolute contraindication to the use of stimulant medications 19
 Gaps with Available ADHD Treatments • Provide clinicians with greater flexibility for ADHD treatment • Provide families with the opportunity to individualize the child’s treatment to meet the needs of the day – Options to support the child’s functioning early in the day – Ability to shorten PM medication impact – Reduction in multiple prescriptions & co-pays 20
Gaps with Available ADHD Treatments • Provide clinicians with greater flexibility for ADHD treatment • Provide families with the opportunity to individualize the child’s treatment to meet the needs of the day – Options to support the child’s functioning early in the day – Ability to shorten PM medication impact – Reduction in multiple prescriptions & co-pays 20
 “Physicians should be alert for problems with adherence” Hippocrates, 200 B. C. 21
“Physicians should be alert for problems with adherence” Hippocrates, 200 B. C. 21
 Issues Related to Treatment Compliance in Children with ADHD • Doesn’t like the taste • Doesn’t like taking medications • Refuses to take the medication • Gags when taking medication • Parent gives up due to stress 22
Issues Related to Treatment Compliance in Children with ADHD • Doesn’t like the taste • Doesn’t like taking medications • Refuses to take the medication • Gags when taking medication • Parent gives up due to stress 22
 Conclusions • ADHD is a significant, impairing disorder with potential long-term implications • MPH is a first-line treatment for ADHD • Some patients respond preferentially to one ADHD treatment over another, AND • Different treatments offer different advantages There is a group of patients that is not receiving optimal therapy Families need additional therapeutic options 23
Conclusions • ADHD is a significant, impairing disorder with potential long-term implications • MPH is a first-line treatment for ADHD • Some patients respond preferentially to one ADHD treatment over another, AND • Different treatments offer different advantages There is a group of patients that is not receiving optimal therapy Families need additional therapeutic options 23
 Clinical Efficacy of MTS in Children with ADHD Liza Squires, MD Senior Director, Global Clinical Medicine Shire Pharmaceuticals 24
Clinical Efficacy of MTS in Children with ADHD Liza Squires, MD Senior Director, Global Clinical Medicine Shire Pharmaceuticals 24
 MTS Clinical Development Program Efficacy Objectives • Establish efficacy with a 9 -hour wear time – Study 201: Laboratory Classroom Study • Onset and duration – Study 302: Outpatient Study • Efficacy in an outpatient setting 25
MTS Clinical Development Program Efficacy Objectives • Establish efficacy with a 9 -hour wear time – Study 201: Laboratory Classroom Study • Onset and duration – Study 302: Outpatient Study • Efficacy in an outpatient setting 25
 Study 101 • Identify clinically optimal wear time based upon PK profile • Four arm, single-dose, crossover, PK study • MTS 25 cm 2, 6 -, 8 -, and 10 -hour patch wear time and Concerta 36 mg • 24 children with ADHD age 6 -12 years 26
Study 101 • Identify clinically optimal wear time based upon PK profile • Four arm, single-dose, crossover, PK study • MTS 25 cm 2, 6 -, 8 -, and 10 -hour patch wear time and Concerta 36 mg • 24 children with ADHD age 6 -12 years 26
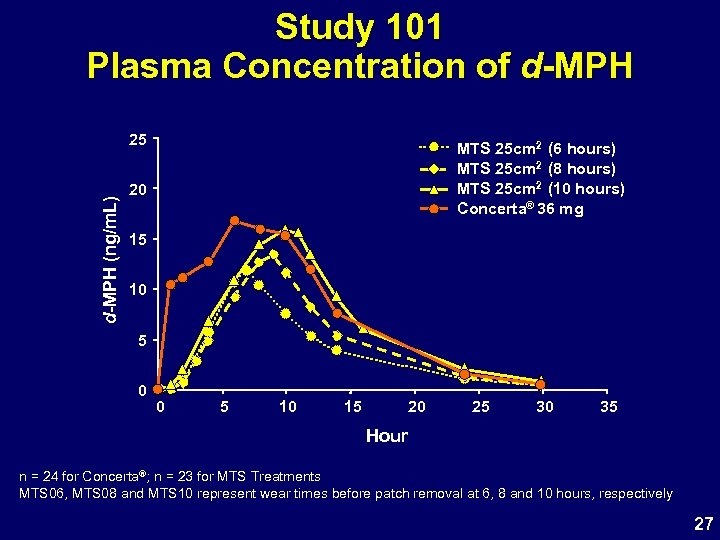 Study 101 Plasma Concentration of d-MPH (ng/m. L) 25 MTS 25 cm 2 (6 hours) MTS 25 cm 2 (8 hours) MTS 25 cm 2 (10 hours) Concerta® 36 mg 20 15 10 5 0 0 5 10 15 20 25 30 35 Hour n = 24 for Concerta®; n = 23 for MTS Treatments MTS 06, MTS 08 and MTS 10 represent wear times before patch removal at 6, 8 and 10 hours, respectively 27
Study 101 Plasma Concentration of d-MPH (ng/m. L) 25 MTS 25 cm 2 (6 hours) MTS 25 cm 2 (8 hours) MTS 25 cm 2 (10 hours) Concerta® 36 mg 20 15 10 5 0 0 5 10 15 20 25 30 35 Hour n = 24 for Concerta®; n = 23 for MTS Treatments MTS 06, MTS 08 and MTS 10 represent wear times before patch removal at 6, 8 and 10 hours, respectively 27
 Study 201 MTS Laboratory Classroom Study • Assess efficacy and time course of treatment effect, tolerability and safety of MTS – SKAMP Deportment scale (SKAMP-D) scores – PERMP (Math Productivity) Scores – Multiple time points during 2 classroom days • Double-blind, multi-center, placebo-controlled, crossover design laboratory classroom study • Pediatric patients age 6 -12 years diagnosed with ADHD by DSM-IV-TR criteria 28
Study 201 MTS Laboratory Classroom Study • Assess efficacy and time course of treatment effect, tolerability and safety of MTS – SKAMP Deportment scale (SKAMP-D) scores – PERMP (Math Productivity) Scores – Multiple time points during 2 classroom days • Double-blind, multi-center, placebo-controlled, crossover design laboratory classroom study • Pediatric patients age 6 -12 years diagnosed with ADHD by DSM-IV-TR criteria 28
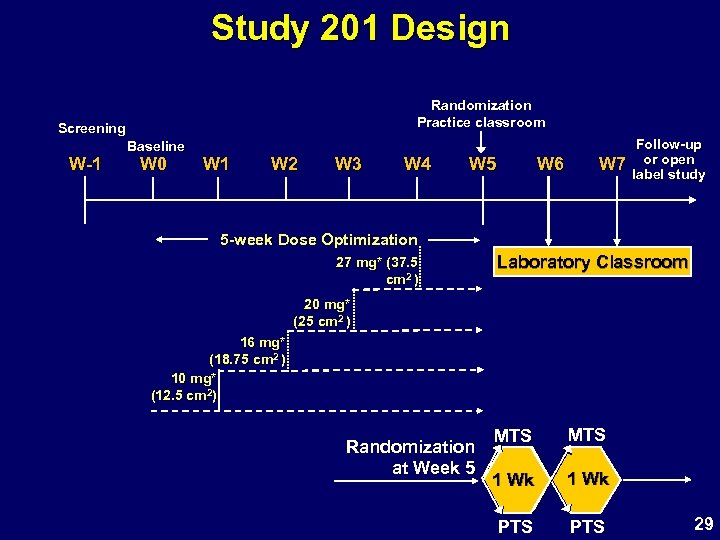 Study 201 Design Randomization Practice classroom Screening W-1 Baseline W 0 W 1 W 2 W 3 W 4 W 5 W 6 W 7 Follow-up or open label study 5 -week Dose Optimization 27 mg* (37. 5 cm 2 ) Laboratory Classroom 20 mg* (25 cm 2 ) 16 mg* (18. 75 cm 2 ) 10 mg* (12. 5 cm 2) Randomization at Week 5 MTS 1 Wk PTS 29
Study 201 Design Randomization Practice classroom Screening W-1 Baseline W 0 W 1 W 2 W 3 W 4 W 5 W 6 W 7 Follow-up or open label study 5 -week Dose Optimization 27 mg* (37. 5 cm 2 ) Laboratory Classroom 20 mg* (25 cm 2 ) 16 mg* (18. 75 cm 2 ) 10 mg* (12. 5 cm 2) Randomization at Week 5 MTS 1 Wk PTS 29
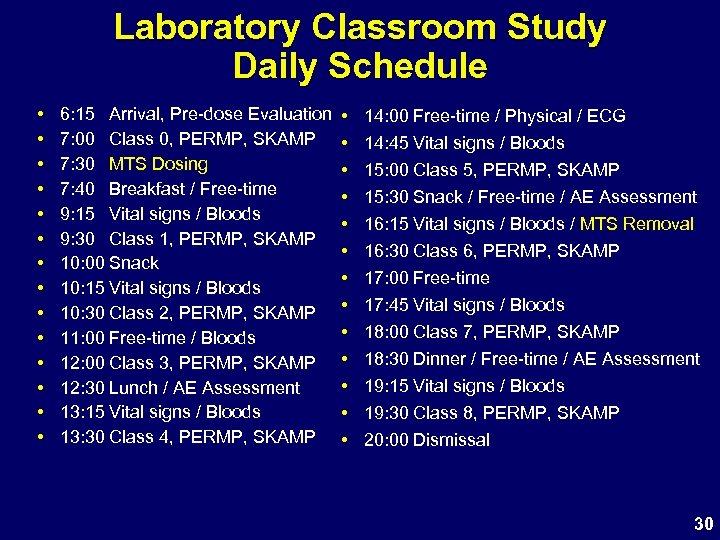 Laboratory Classroom Study Daily Schedule • • • • 6: 15 Arrival, Pre-dose Evaluation 7: 00 Class 0, PERMP, SKAMP 7: 30 MTS Dosing 7: 40 Breakfast / Free-time 9: 15 Vital signs / Bloods 9: 30 Class 1, PERMP, SKAMP 10: 00 Snack 10: 15 Vital signs / Bloods 10: 30 Class 2, PERMP, SKAMP 11: 00 Free-time / Bloods 12: 00 Class 3, PERMP, SKAMP 12: 30 Lunch / AE Assessment 13: 15 Vital signs / Bloods 13: 30 Class 4, PERMP, SKAMP • • • • 14: 00 Free-time / Physical / ECG 14: 45 Vital signs / Bloods 15: 00 Class 5, PERMP, SKAMP 15: 30 Snack / Free-time / AE Assessment 16: 15 Vital signs / Bloods / MTS Removal 16: 30 Class 6, PERMP, SKAMP 17: 00 Free-time 17: 45 Vital signs / Bloods 18: 00 Class 7, PERMP, SKAMP 18: 30 Dinner / Free-time / AE Assessment 19: 15 Vital signs / Bloods 19: 30 Class 8, PERMP, SKAMP 20: 00 Dismissal 30
Laboratory Classroom Study Daily Schedule • • • • 6: 15 Arrival, Pre-dose Evaluation 7: 00 Class 0, PERMP, SKAMP 7: 30 MTS Dosing 7: 40 Breakfast / Free-time 9: 15 Vital signs / Bloods 9: 30 Class 1, PERMP, SKAMP 10: 00 Snack 10: 15 Vital signs / Bloods 10: 30 Class 2, PERMP, SKAMP 11: 00 Free-time / Bloods 12: 00 Class 3, PERMP, SKAMP 12: 30 Lunch / AE Assessment 13: 15 Vital signs / Bloods 13: 30 Class 4, PERMP, SKAMP • • • • 14: 00 Free-time / Physical / ECG 14: 45 Vital signs / Bloods 15: 00 Class 5, PERMP, SKAMP 15: 30 Snack / Free-time / AE Assessment 16: 15 Vital signs / Bloods / MTS Removal 16: 30 Class 6, PERMP, SKAMP 17: 00 Free-time 17: 45 Vital signs / Bloods 18: 00 Class 7, PERMP, SKAMP 18: 30 Dinner / Free-time / AE Assessment 19: 15 Vital signs / Bloods 19: 30 Class 8, PERMP, SKAMP 20: 00 Dismissal 30
 Study 201 Primary Analysis SKAMP Deportment Score (0 -9 Hours) Mean (SD) Median Range LS Mean (SE) Difference and 95% CI of LS Mean (MTS-PTS) p-value MTS N=79 3. 2 3. 6 2. 2 0 to 17 3. 2 0. 6 PTS N=79 8. 0 6. 3 7. 3 0 to 29 8. 0 0. 6 -4. 8 95% CI -5. 9 to -3. 6 <0. 0001 31
Study 201 Primary Analysis SKAMP Deportment Score (0 -9 Hours) Mean (SD) Median Range LS Mean (SE) Difference and 95% CI of LS Mean (MTS-PTS) p-value MTS N=79 3. 2 3. 6 2. 2 0 to 17 3. 2 0. 6 PTS N=79 8. 0 6. 3 7. 3 0 to 29 8. 0 0. 6 -4. 8 95% CI -5. 9 to -3. 6 <0. 0001 31
 Study 201 SKAMP-D Onset & Duration 12. 0 Mean SKAMP-D (SEM) MTS 10. 0 Placebo 8. 0 6. 0 4. 0 * * 2. 0 * * 0. 0 -1 0 1 Patch Application 2 3 4 5 6 Time (hr) 7 * * * 8 9 * 10 11 12 Patch Removal *p<0. 05 vs Placebo 32
Study 201 SKAMP-D Onset & Duration 12. 0 Mean SKAMP-D (SEM) MTS 10. 0 Placebo 8. 0 6. 0 4. 0 * * 2. 0 * * 0. 0 -1 0 1 Patch Application 2 3 4 5 6 Time (hr) 7 * * * 8 9 * 10 11 12 Patch Removal *p<0. 05 vs Placebo 32
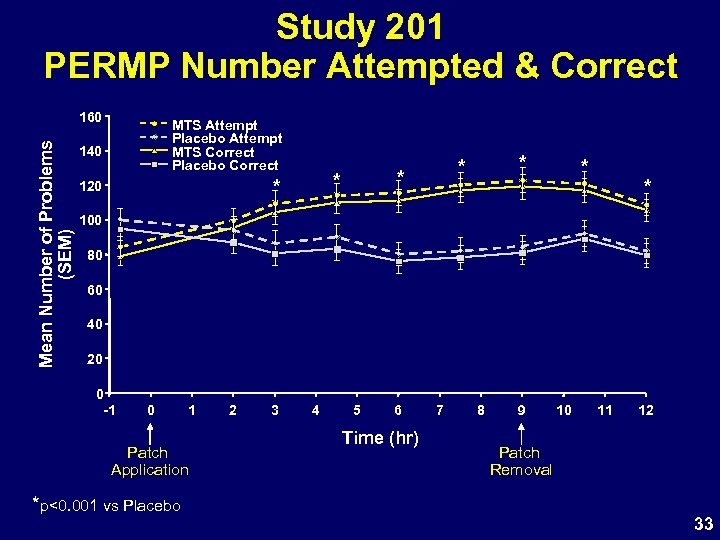 Study 201 PERMP Number Attempted & Correct Mean Number of Problems (SEM) 160 MTS Attempt Placebo Attempt MTS Correct Placebo Correct 140 * 120 * * * 100 80 60 40 20 0 -1 0 1 Patch Application *p<0. 001 vs Placebo 2 3 4 5 6 Time (hr) 7 8 9 10 11 12 Patch Removal 33
Study 201 PERMP Number Attempted & Correct Mean Number of Problems (SEM) 160 MTS Attempt Placebo Attempt MTS Correct Placebo Correct 140 * 120 * * * 100 80 60 40 20 0 -1 0 1 Patch Application *p<0. 001 vs Placebo 2 3 4 5 6 Time (hr) 7 8 9 10 11 12 Patch Removal 33
![Study 201 Secondary Efficacy Endpoints Scale p-value MTS-PTS Difference [95% CI] SKAMP (Total) p<0. Study 201 Secondary Efficacy Endpoints Scale p-value MTS-PTS Difference [95% CI] SKAMP (Total) p<0.](https://present5.com/presentation/6d150cc2358c56fbdfc8a83479ec7fb5/image-34.jpg) Study 201 Secondary Efficacy Endpoints Scale p-value MTS-PTS Difference [95% CI] SKAMP (Total) p<0. 0001 -8. 5 [-10. 2 to -6. 8] ADHD-RS-IV (Total) p<0. 0001 -16. 5 [-19. 8 to -13. 1] CPRS (Total) p<0. 0001 PGA p<0. 0001 CGI p<0. 0001 -15. 1 [-20. 5 to -9. 7] Improved (1 or 2) 65 -76% Improved (1 or 2) 78 -80% 34
Study 201 Secondary Efficacy Endpoints Scale p-value MTS-PTS Difference [95% CI] SKAMP (Total) p<0. 0001 -8. 5 [-10. 2 to -6. 8] ADHD-RS-IV (Total) p<0. 0001 -16. 5 [-19. 8 to -13. 1] CPRS (Total) p<0. 0001 PGA p<0. 0001 CGI p<0. 0001 -15. 1 [-20. 5 to -9. 7] Improved (1 or 2) 65 -76% Improved (1 or 2) 78 -80% 34
 Study 201 Efficacy Summary • Overall efficacy of MTS in reducing ADHD symptoms apparent to trained observers, clinicians and parents – Statistically significant improvement in all primary and secondary efficacy endpoints • 9 -hour target wear time – Onset of effect within 2 hours of application – Duration of effect through 12 hours 35
Study 201 Efficacy Summary • Overall efficacy of MTS in reducing ADHD symptoms apparent to trained observers, clinicians and parents – Statistically significant improvement in all primary and secondary efficacy endpoints • 9 -hour target wear time – Onset of effect within 2 hours of application – Duration of effect through 12 hours 35
 Study 302 Design • Evaluate the safety and efficacy of methylphenidate transdermal system (MTS) compared to placebo with reference to Concerta® • A phase III, randomized, double-blind, multi-center, parallel-group, placebo-controlled, doseoptimization study • 38 study centers in the US – 274 subjects • Pediatric patients age 6 -12 years with ADHD by DSM-IV-TR criteria 36
Study 302 Design • Evaluate the safety and efficacy of methylphenidate transdermal system (MTS) compared to placebo with reference to Concerta® • A phase III, randomized, double-blind, multi-center, parallel-group, placebo-controlled, doseoptimization study • 38 study centers in the US – 274 subjects • Pediatric patients age 6 -12 years with ADHD by DSM-IV-TR criteria 36
 Study 302 Design Baseline Screening Randomization W-1 W 0 W 1 W 2 W 3 W 4 5 -Week Dose-Optimization 27 mg (37. 5 cm 2) 20 mg (25 cm 2) MTS and Placebo Patch Optimization W 5 W 6 W 7 Follow-up or openlabel study Dose Maintenance 16 mg (18. 75 cm 2) 10 mg (12. 5 cm 2) Concerta® and Placebo Capsule Optimization 54 mg 36 mg 27 mg 18 mg 37
Study 302 Design Baseline Screening Randomization W-1 W 0 W 1 W 2 W 3 W 4 5 -Week Dose-Optimization 27 mg (37. 5 cm 2) 20 mg (25 cm 2) MTS and Placebo Patch Optimization W 5 W 6 W 7 Follow-up or openlabel study Dose Maintenance 16 mg (18. 75 cm 2) 10 mg (12. 5 cm 2) Concerta® and Placebo Capsule Optimization 54 mg 36 mg 27 mg 18 mg 37
 Study 302 Clinical Outcome Measures • Primary Outcome – Clinician-rated ADHD-RS-IV • Secondary Outcomes – Conners’ Teacher Rating Scale Revised SF (CTRS-R) – Conners’ Parent Rating Scale Revised SF (CPRS-R) – Clinical Global Impressions (CGI-I) – Parent Global Assessment (PGA) 38
Study 302 Clinical Outcome Measures • Primary Outcome – Clinician-rated ADHD-RS-IV • Secondary Outcomes – Conners’ Teacher Rating Scale Revised SF (CTRS-R) – Conners’ Parent Rating Scale Revised SF (CPRS-R) – Clinical Global Impressions (CGI-I) – Parent Global Assessment (PGA) 38
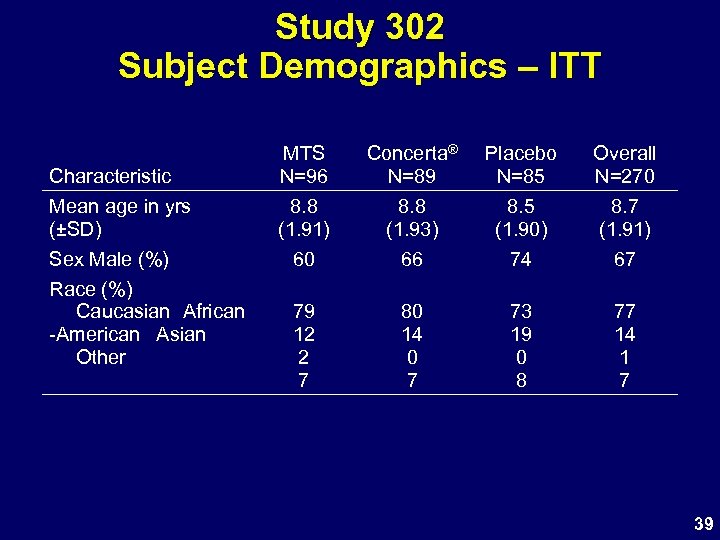 Study 302 Subject Demographics – ITT Characteristic Mean age in yrs (±SD) Sex Male (%) Race (%) Caucasian African -American Asian Other MTS N=96 8. 8 (1. 91) 60 Concerta® N=89 8. 8 (1. 93) 66 Placebo N=85 8. 5 (1. 90) 74 Overall N=270 8. 7 (1. 91) 67 79 12 2 7 80 14 0 7 73 19 0 8 77 14 1 7 39
Study 302 Subject Demographics – ITT Characteristic Mean age in yrs (±SD) Sex Male (%) Race (%) Caucasian African -American Asian Other MTS N=96 8. 8 (1. 91) 60 Concerta® N=89 8. 8 (1. 93) 66 Placebo N=85 8. 5 (1. 90) 74 Overall N=270 8. 7 (1. 91) 67 79 12 2 7 80 14 0 7 73 19 0 8 77 14 1 7 39
 Study 302: ADHD-RS-IV Change from Baseline to Endpoint MTS N=96 ADHD-RS-IV Baseline Score Concerta® N=89 Placebo N=85 43. 0 7. 5 43. 8 6. 7 41. 9 7. 4 Change From Baseline (LOCF) LS Mean SE Difference Active – Placebo [95% CI] -21. 6 1. 5 -11. 3 [-15. 6 to -7. 06] p<0. 0001 †Reference -24. 2 1. 5 -13. 9 [-18. 06 to -9. 7] -10. 3 1. 5 p<0. 0001† only not a primary comparison 40
Study 302: ADHD-RS-IV Change from Baseline to Endpoint MTS N=96 ADHD-RS-IV Baseline Score Concerta® N=89 Placebo N=85 43. 0 7. 5 43. 8 6. 7 41. 9 7. 4 Change From Baseline (LOCF) LS Mean SE Difference Active – Placebo [95% CI] -21. 6 1. 5 -11. 3 [-15. 6 to -7. 06] p<0. 0001 †Reference -24. 2 1. 5 -13. 9 [-18. 06 to -9. 7] -10. 3 1. 5 p<0. 0001† only not a primary comparison 40
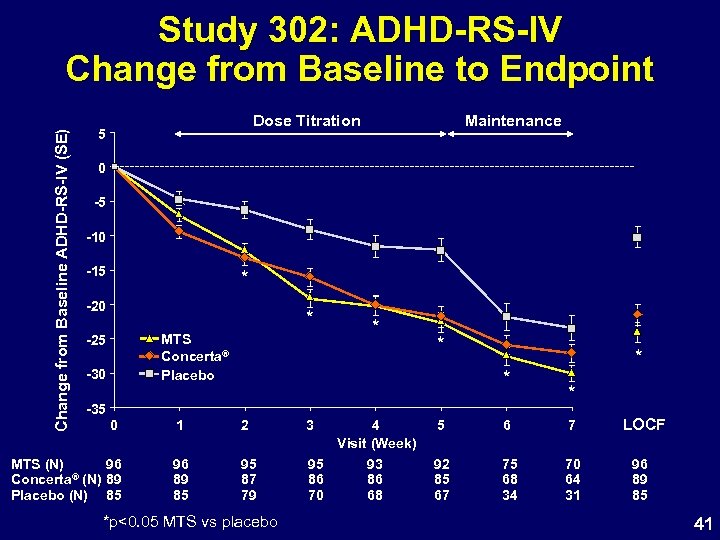 Change from Baseline ADHD-RS-IV (SE) Study 302: ADHD-RS-IV Change from Baseline to Endpoint Dose Titration 5 Maintenance 0 -5 -10 -15 * -20 * MTS Concerta® Placebo -25 -30 * * * -35 0 MTS (N) 96 ® (N) 89 Concerta Placebo (N) 85 1 2 3 96 89 85 95 87 79 95 86 70 *p<0. 05 MTS vs placebo * * 4 Visit (Week) 5 6 7 93 86 68 92 85 67 75 68 34 70 64 31 LOCF 96 89 85 41
Change from Baseline ADHD-RS-IV (SE) Study 302: ADHD-RS-IV Change from Baseline to Endpoint Dose Titration 5 Maintenance 0 -5 -10 -15 * -20 * MTS Concerta® Placebo -25 -30 * * * -35 0 MTS (N) 96 ® (N) 89 Concerta Placebo (N) 85 1 2 3 96 89 85 95 87 79 95 86 70 *p<0. 05 MTS vs placebo * * 4 Visit (Week) 5 6 7 93 86 68 92 85 67 75 68 34 70 64 31 LOCF 96 89 85 41
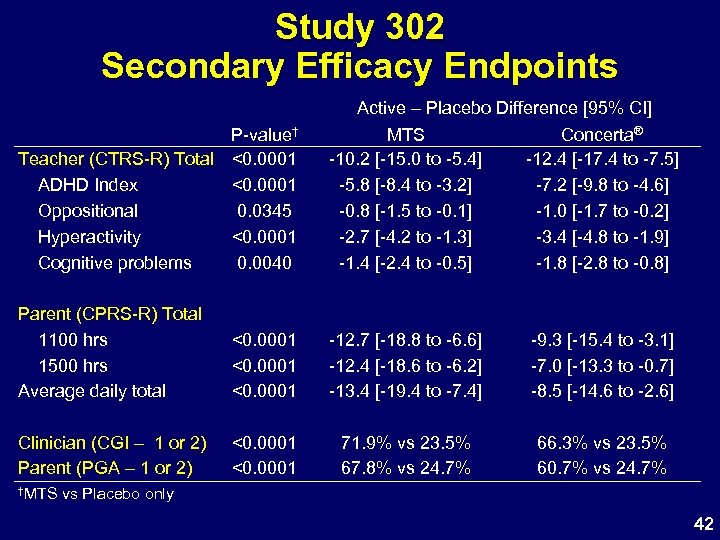 Study 302 Secondary Efficacy Endpoints P-value† Teacher (CTRS-R) Total <0. 0001 ADHD Index <0. 0001 Oppositional 0. 0345 Hyperactivity <0. 0001 Cognitive problems 0. 0040 Active – Placebo Difference [95% CI] MTS Concerta® -10. 2 [-15. 0 to -5. 4] -12. 4 [-17. 4 to -7. 5] -5. 8 [-8. 4 to -3. 2] -7. 2 [-9. 8 to -4. 6] -0. 8 [-1. 5 to -0. 1] -1. 0 [-1. 7 to -0. 2] -2. 7 [-4. 2 to -1. 3] -3. 4 [-4. 8 to -1. 9] -1. 4 [-2. 4 to -0. 5] -1. 8 [-2. 8 to -0. 8] Parent (CPRS-R) Total 1100 hrs 1500 hrs Average daily total <0. 0001 -12. 7 [-18. 8 to -6. 6] -12. 4 [-18. 6 to -6. 2] -13. 4 [-19. 4 to -7. 4] -9. 3 [-15. 4 to -3. 1] -7. 0 [-13. 3 to -0. 7] -8. 5 [-14. 6 to -2. 6] Clinician (CGI – 1 or 2) Parent (PGA – 1 or 2) <0. 0001 71. 9% vs 23. 5% 67. 8% vs 24. 7% 66. 3% vs 23. 5% 60. 7% vs 24. 7% †MTS vs Placebo only 42
Study 302 Secondary Efficacy Endpoints P-value† Teacher (CTRS-R) Total <0. 0001 ADHD Index <0. 0001 Oppositional 0. 0345 Hyperactivity <0. 0001 Cognitive problems 0. 0040 Active – Placebo Difference [95% CI] MTS Concerta® -10. 2 [-15. 0 to -5. 4] -12. 4 [-17. 4 to -7. 5] -5. 8 [-8. 4 to -3. 2] -7. 2 [-9. 8 to -4. 6] -0. 8 [-1. 5 to -0. 1] -1. 0 [-1. 7 to -0. 2] -2. 7 [-4. 2 to -1. 3] -3. 4 [-4. 8 to -1. 9] -1. 4 [-2. 4 to -0. 5] -1. 8 [-2. 8 to -0. 8] Parent (CPRS-R) Total 1100 hrs 1500 hrs Average daily total <0. 0001 -12. 7 [-18. 8 to -6. 6] -12. 4 [-18. 6 to -6. 2] -13. 4 [-19. 4 to -7. 4] -9. 3 [-15. 4 to -3. 1] -7. 0 [-13. 3 to -0. 7] -8. 5 [-14. 6 to -2. 6] Clinician (CGI – 1 or 2) Parent (PGA – 1 or 2) <0. 0001 71. 9% vs 23. 5% 67. 8% vs 24. 7% 66. 3% vs 23. 5% 60. 7% vs 24. 7% †MTS vs Placebo only 42
 Study 302 Efficacy Summary • MTS, worn for 9 hours, reduces symptoms of ADHD based on assessments by – clinicians – teachers – parents • Statistically significant improvement in all primary and secondary efficacy endpoints 43
Study 302 Efficacy Summary • MTS, worn for 9 hours, reduces symptoms of ADHD based on assessments by – clinicians – teachers – parents • Statistically significant improvement in all primary and secondary efficacy endpoints 43
 MTS Clinical Development Program Efficacy Conclusions • MTS with a 9 -hour target wear time demonstrated significant efficacy in laboratory classroom and outpatient settings • Improvements in behavior are present within 2 hours of patch application and persist for 3 hours after patch removal • Improvements ADHD symptoms and behavior reported by – – Trained observers Teachers Parents Clinicians 44
MTS Clinical Development Program Efficacy Conclusions • MTS with a 9 -hour target wear time demonstrated significant efficacy in laboratory classroom and outpatient settings • Improvements in behavior are present within 2 hours of patch application and persist for 3 hours after patch removal • Improvements ADHD symptoms and behavior reported by – – Trained observers Teachers Parents Clinicians 44
 MTS Safety Evaluations Raymond D. Pratt, MD Vice President, Clinical Development Shire Pharmaceuticals 45
MTS Safety Evaluations Raymond D. Pratt, MD Vice President, Clinical Development Shire Pharmaceuticals 45
 Safety Evaluations MTS Program • Spontaneous and Elicited AEs – No Deaths • Clinical Laboratory Evaluations – No Clinically Relevant Lab Abnormalities • Physical Examinations, Vital Signs, ECGs – No Clinically Relevant Abnormalities • AE pattern similar in studies 201 and 302 – MPH-related – Study 302 - double-blind, placebo controlled with reference to Concerta® • Long-term study N-021 – Growth Effects 46
Safety Evaluations MTS Program • Spontaneous and Elicited AEs – No Deaths • Clinical Laboratory Evaluations – No Clinically Relevant Lab Abnormalities • Physical Examinations, Vital Signs, ECGs – No Clinically Relevant Abnormalities • AE pattern similar in studies 201 and 302 – MPH-related – Study 302 - double-blind, placebo controlled with reference to Concerta® • Long-term study N-021 – Growth Effects 46
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI – Headache – Tics – Appetite AEs – Weight and Growth – Sleep Effects – Dermal Evaluations 47
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI – Headache – Tics – Appetite AEs – Weight and Growth – Sleep Effects – Dermal Evaluations 47
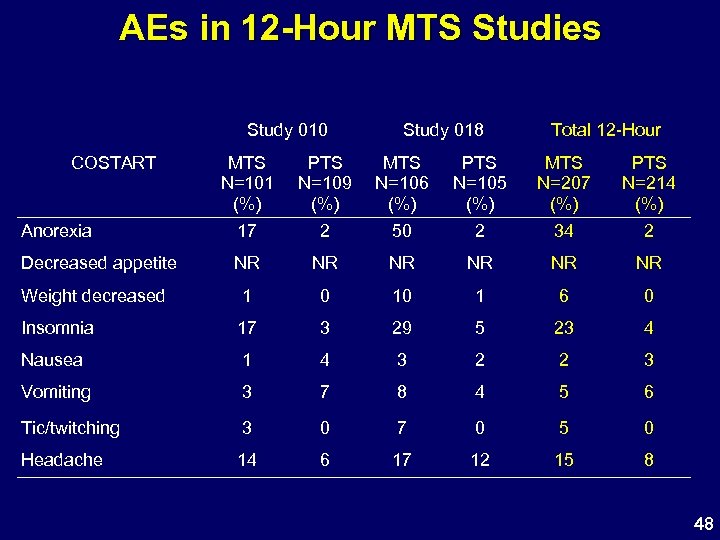 AEs in 12 -Hour MTS Studies Study 010 COSTART Study 018 Total 12 -Hour MTS N=101 (%) PTS N=109 (%) MTS N=106 (%) PTS N=105 (%) MTS N=207 (%) PTS N=214 (%) Anorexia 17 2 50 2 34 2 Decreased appetite NR NR NR Weight decreased 1 0 10 1 6 0 Insomnia 17 3 29 5 23 4 Nausea 1 4 3 2 2 3 Vomiting 3 7 8 4 5 6 Tic/twitching 3 0 7 0 5 0 Headache 14 6 17 12 15 8 48
AEs in 12 -Hour MTS Studies Study 010 COSTART Study 018 Total 12 -Hour MTS N=101 (%) PTS N=109 (%) MTS N=106 (%) PTS N=105 (%) MTS N=207 (%) PTS N=214 (%) Anorexia 17 2 50 2 34 2 Decreased appetite NR NR NR Weight decreased 1 0 10 1 6 0 Insomnia 17 3 29 5 23 4 Nausea 1 4 3 2 2 3 Vomiting 3 7 8 4 5 6 Tic/twitching 3 0 7 0 5 0 Headache 14 6 17 12 15 8 48
 AEs in 12 -Hour MTS Studies Study 010 Med. DRA Study 018 Total 12 -Hour MTS N=101 (%) PTS N=109 (%) MTS N=106 (%) PTS N=105 (%) MTS N=207 (%) PTS N=214 (%) Anorexia 3 1 15 0 9 1 Decreased appetite 14 1 36 2 25 2 Weight decreased 1 0 10 1 6 0 Insomnia 17 3 29 5 23 4 Nausea 1 4 3 2 2 3 Vomiting 3 7 8 4 5 6 Tic 3 0 7 0 5 0 Headache 14 6 17 12 15 8 49
AEs in 12 -Hour MTS Studies Study 010 Med. DRA Study 018 Total 12 -Hour MTS N=101 (%) PTS N=109 (%) MTS N=106 (%) PTS N=105 (%) MTS N=207 (%) PTS N=214 (%) Anorexia 3 1 15 0 9 1 Decreased appetite 14 1 36 2 25 2 Weight decreased 1 0 10 1 6 0 Insomnia 17 3 29 5 23 4 Nausea 1 4 3 2 2 3 Vomiting 3 7 8 4 5 6 Tic 3 0 7 0 5 0 Headache 14 6 17 12 15 8 49
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI – Headache – Tics – Appetite AEs – Weight and Growth – Sleep Effects – Dermal Evaluations 50
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI – Headache – Tics – Appetite AEs – Weight and Growth – Sleep Effects – Dermal Evaluations 50
 Most Commonly Reported Treatment. Emergent AEs (≥ 5% and 2 x Placebo) Adverse Event (Preferred Term) Med. DRA 7. 0 % of subjects with any AE Decreased appetite Insomnia Nausea Vomiting Weight decreased Tic Affect lability Nasal congestion Anorexia Nasopharyngitis Percent of subjects reporting AEs MTS Concerta Placebo N=98 N=91 N=85 75 26 69 19 58 5 13 12 10 9 7 6 6 5 5 8 8 10 8 1 3 3 3 4 5 2 5 0 0 0 1 1 2 51
Most Commonly Reported Treatment. Emergent AEs (≥ 5% and 2 x Placebo) Adverse Event (Preferred Term) Med. DRA 7. 0 % of subjects with any AE Decreased appetite Insomnia Nausea Vomiting Weight decreased Tic Affect lability Nasal congestion Anorexia Nasopharyngitis Percent of subjects reporting AEs MTS Concerta Placebo N=98 N=91 N=85 75 26 69 19 58 5 13 12 10 9 7 6 6 5 5 8 8 10 8 1 3 3 3 4 5 2 5 0 0 0 1 1 2 51
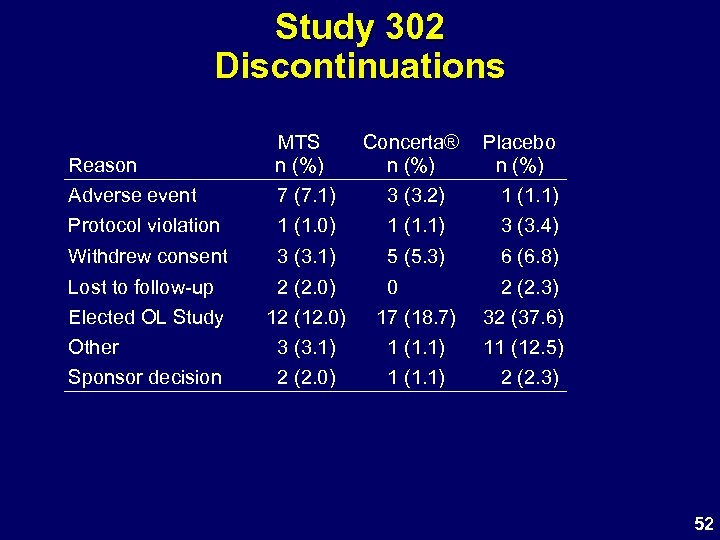 Study 302 Discontinuations Reason MTS n (%) Concerta® n (%) Placebo n (%) Adverse event 7 (7. 1) 3 (3. 2) 1 (1. 1) Protocol violation 1 (1. 0) 1 (1. 1) 3 (3. 4) Withdrew consent 3 (3. 1) 5 (5. 3) 6 (6. 8) Lost to follow-up Elected OL Study Other Sponsor decision 2 (2. 0) 12 (12. 0) 3 (3. 1) 2 (2. 0) 0 17 (18. 7) 1 (1. 1) 2 (2. 3) 32 (37. 6) 11 (12. 5) 2 (2. 3) 52
Study 302 Discontinuations Reason MTS n (%) Concerta® n (%) Placebo n (%) Adverse event 7 (7. 1) 3 (3. 2) 1 (1. 1) Protocol violation 1 (1. 0) 1 (1. 1) 3 (3. 4) Withdrew consent 3 (3. 1) 5 (5. 3) 6 (6. 8) Lost to follow-up Elected OL Study Other Sponsor decision 2 (2. 0) 12 (12. 0) 3 (3. 1) 2 (2. 0) 0 17 (18. 7) 1 (1. 1) 2 (2. 3) 32 (37. 6) 11 (12. 5) 2 (2. 3) 52
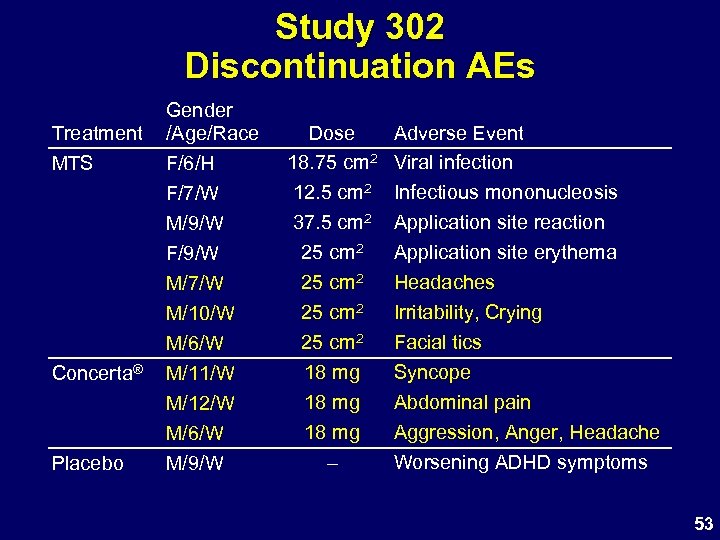 Study 302 Discontinuation AEs Treatment MTS Concerta® Placebo Gender /Age/Race F/6/H F/7/W M/9/W F/9/W M/7/W M/10/W M/6/W M/11/W M/12/W M/6/W M/9/W Dose 18. 75 cm 2 12. 5 cm 2 37. 5 cm 2 25 cm 2 18 mg – Adverse Event Viral infection Infectious mononucleosis Application site reaction Application site erythema Headaches Irritability, Crying Facial tics Syncope Abdominal pain Aggression, Anger, Headache Worsening ADHD symptoms 53
Study 302 Discontinuation AEs Treatment MTS Concerta® Placebo Gender /Age/Race F/6/H F/7/W M/9/W F/9/W M/7/W M/10/W M/6/W M/11/W M/12/W M/6/W M/9/W Dose 18. 75 cm 2 12. 5 cm 2 37. 5 cm 2 25 cm 2 18 mg – Adverse Event Viral infection Infectious mononucleosis Application site reaction Application site erythema Headaches Irritability, Crying Facial tics Syncope Abdominal pain Aggression, Anger, Headache Worsening ADHD symptoms 53
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth – Sleep Effects – Dermal Evaluations 54
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth – Sleep Effects – Dermal Evaluations 54
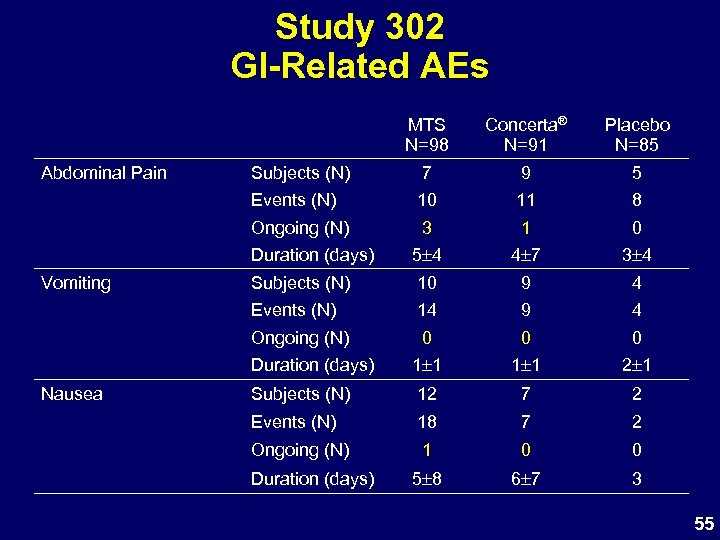 Study 302 GI-Related AEs MTS N=98 7 9 5 10 11 8 3 1 0 Duration (days) 5 4 4 7 3 4 Subjects (N) 10 9 4 Events (N) 14 9 4 Ongoing (N) 0 0 0 Duration (days) Nausea Subjects (N) Ongoing (N) Vomiting Placebo N=85 Events (N) Abdominal Pain Concerta® N=91 1 1 2 1 Subjects (N) 12 7 2 Events (N) 18 7 2 Ongoing (N) 1 0 0 5 8 6 7 3 Duration (days) 55
Study 302 GI-Related AEs MTS N=98 7 9 5 10 11 8 3 1 0 Duration (days) 5 4 4 7 3 4 Subjects (N) 10 9 4 Events (N) 14 9 4 Ongoing (N) 0 0 0 Duration (days) Nausea Subjects (N) Ongoing (N) Vomiting Placebo N=85 Events (N) Abdominal Pain Concerta® N=91 1 1 2 1 Subjects (N) 12 7 2 Events (N) 18 7 2 Ongoing (N) 1 0 0 5 8 6 7 3 Duration (days) 55
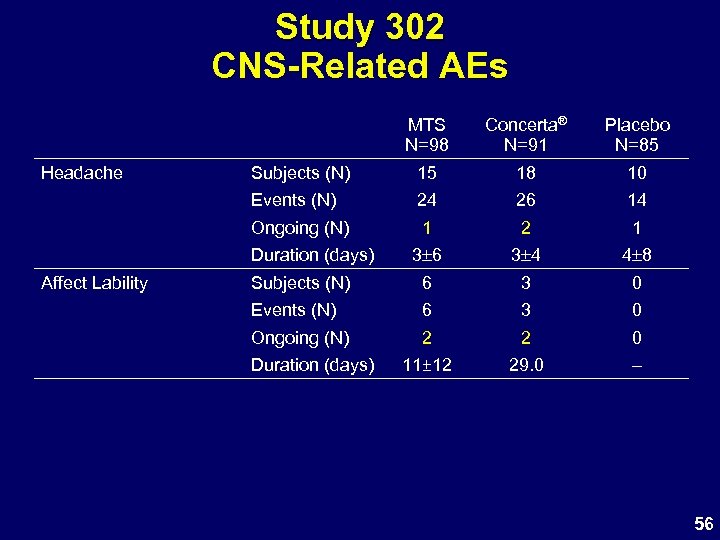 Study 302 CNS-Related AEs MTS N=98 Placebo N=85 Subjects (N) 15 18 10 Events (N) 24 26 14 Ongoing (N) Headache Concerta® N=91 1 2 1 3 6 3 4 4 8 Subjects (N) 6 3 0 Events (N) 6 3 0 Ongoing (N) 2 2 0 11± 12 29. 0 – Duration (days) Affect Lability Duration (days) 56
Study 302 CNS-Related AEs MTS N=98 Placebo N=85 Subjects (N) 15 18 10 Events (N) 24 26 14 Ongoing (N) Headache Concerta® N=91 1 2 1 3 6 3 4 4 8 Subjects (N) 6 3 0 Events (N) 6 3 0 Ongoing (N) 2 2 0 11± 12 29. 0 – Duration (days) Affect Lability Duration (days) 56
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 57
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 57
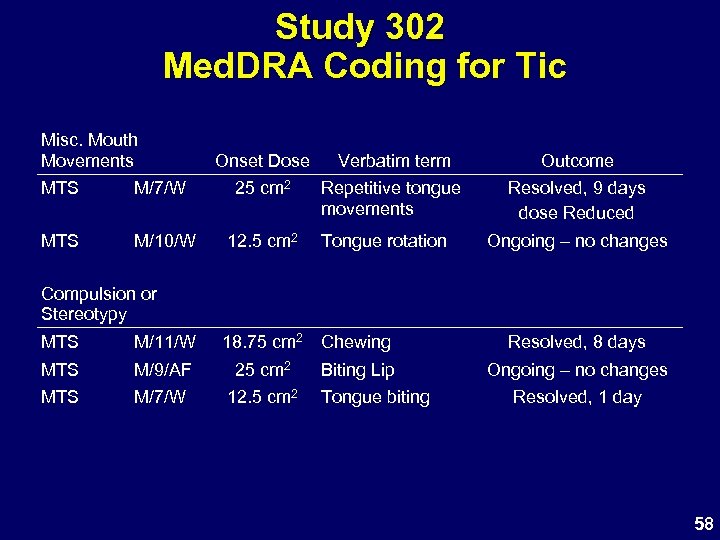 Study 302 Med. DRA Coding for Tic Misc. Mouth Movements Onset Dose Verbatim term Outcome Repetitive tongue movements Resolved, 9 days dose Reduced MTS M/7/W 25 cm 2 MTS M/10/W 12. 5 cm 2 Tongue rotation Ongoing – no changes Compulsion or Stereotypy MTS M/11/W 18. 75 cm 2 Chewing MTS M/9/AF 25 cm 2 MTS M/7/W 12. 5 cm 2 Biting Lip Tongue biting Resolved, 8 days Ongoing – no changes Resolved, 1 day 58
Study 302 Med. DRA Coding for Tic Misc. Mouth Movements Onset Dose Verbatim term Outcome Repetitive tongue movements Resolved, 9 days dose Reduced MTS M/7/W 25 cm 2 MTS M/10/W 12. 5 cm 2 Tongue rotation Ongoing – no changes Compulsion or Stereotypy MTS M/11/W 18. 75 cm 2 Chewing MTS M/9/AF 25 cm 2 MTS M/7/W 12. 5 cm 2 Biting Lip Tongue biting Resolved, 8 days Ongoing – no changes Resolved, 1 day 58
 Study 302 Med. DRA Coding for Tic Onset Dose Verbatim term MTS M/6/W 25 cm 2 Tic MTS M/8/W 25 cm 2 Tongue Facial Tic Concerta® M/12/W 54 mg Tongue Tic Outcome D/C Ongoing @ 30 days Resolved, 9 day Ongoing – no changes 59
Study 302 Med. DRA Coding for Tic Onset Dose Verbatim term MTS M/6/W 25 cm 2 Tic MTS M/8/W 25 cm 2 Tongue Facial Tic Concerta® M/12/W 54 mg Tongue Tic Outcome D/C Ongoing @ 30 days Resolved, 9 day Ongoing – no changes 59
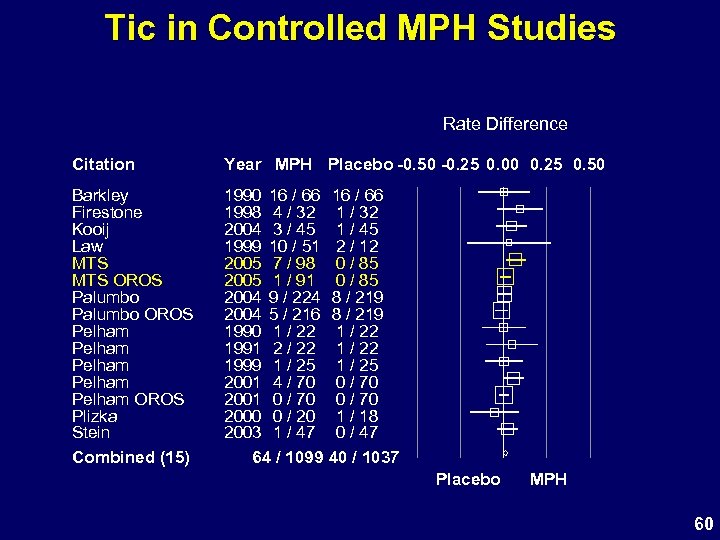 Tic in Controlled MPH Studies Rate Difference Citation Year MPH Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 Barkley Firestone Kooij Law MTS OROS Palumbo OROS Pelham Pelham OROS Plizka Stein Combined (15) 1990 16 / 66 1998 4 / 32 2004 3 / 45 1999 10 / 51 2005 7 / 98 2005 1 / 91 2004 9 / 224 2004 5 / 216 1990 1 / 22 1991 2 / 22 1999 1 / 25 2001 4 / 70 2001 0 / 70 2000 0 / 20 2003 1 / 47 64 / 1099 16 / 66 1 / 32 1 / 45 2 / 12 0 / 85 8 / 219 1 / 22 1 / 25 0 / 70 1 / 18 0 / 47 40 / 1037 Placebo MPH 60
Tic in Controlled MPH Studies Rate Difference Citation Year MPH Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 Barkley Firestone Kooij Law MTS OROS Palumbo OROS Pelham Pelham OROS Plizka Stein Combined (15) 1990 16 / 66 1998 4 / 32 2004 3 / 45 1999 10 / 51 2005 7 / 98 2005 1 / 91 2004 9 / 224 2004 5 / 216 1990 1 / 22 1991 2 / 22 1999 1 / 25 2001 4 / 70 2001 0 / 70 2000 0 / 20 2003 1 / 47 64 / 1099 16 / 66 1 / 32 1 / 45 2 / 12 0 / 85 8 / 219 1 / 22 1 / 25 0 / 70 1 / 18 0 / 47 40 / 1037 Placebo MPH 60
 Tic in Open-Label Studies Events (N) Total (N) Events (%) Concerta® PI Study 1 39 432 8. 0 Concerta® PI Study 2 9 682 1. 3 Wilens 2005: Concerta® 40 408 9. 8 MTS N-021 2 191 1. 0 MTS 303 5 255 2. 0 Study 61
Tic in Open-Label Studies Events (N) Total (N) Events (%) Concerta® PI Study 1 39 432 8. 0 Concerta® PI Study 2 9 682 1. 3 Wilens 2005: Concerta® 40 408 9. 8 MTS N-021 2 191 1. 0 MTS 303 5 255 2. 0 Study 61
 Study 302 AEs Coded as Tics • Most MTS events were transient – 4 of 7 resolved with continuing MTS – Only 1 discontinuation – Symptoms mild, do not interfere with activity • Verbatim descriptions not all tics – Abnormal tongue and mouth movements – Compulsions and stereotypy are common in ADHD • Overall frequency of tics in MTS studies consistent with published data with stimulants • Tic not a contraindication to stimulant Rx 62
Study 302 AEs Coded as Tics • Most MTS events were transient – 4 of 7 resolved with continuing MTS – Only 1 discontinuation – Symptoms mild, do not interfere with activity • Verbatim descriptions not all tics – Abnormal tongue and mouth movements – Compulsions and stereotypy are common in ADHD • Overall frequency of tics in MTS studies consistent with published data with stimulants • Tic not a contraindication to stimulant Rx 62
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 63
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 63
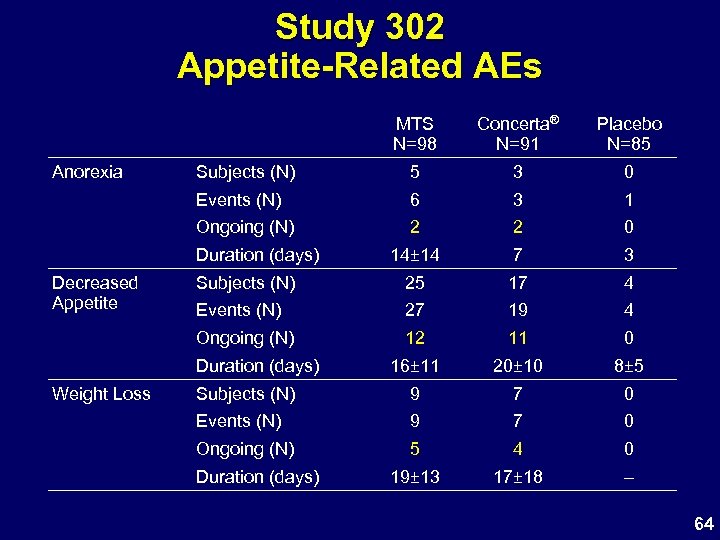 Study 302 Appetite-Related AEs MTS N=98 Placebo N=85 Subjects (N) 5 3 0 Events (N) 6 3 1 Ongoing (N) 2 2 0 14± 14 7 3 Subjects (N) 25 17 4 Events (N) 27 19 4 Ongoing (N) Anorexia Concerta® N=91 12 11 0 16± 11 20± 10 8± 5 Subjects (N) 9 7 0 Events (N) 9 7 0 Ongoing (N) 5 4 0 19± 13 17± 18 – Duration (days) Decreased Appetite Duration (days) Weight Loss Duration (days) 64
Study 302 Appetite-Related AEs MTS N=98 Placebo N=85 Subjects (N) 5 3 0 Events (N) 6 3 1 Ongoing (N) 2 2 0 14± 14 7 3 Subjects (N) 25 17 4 Events (N) 27 19 4 Ongoing (N) Anorexia Concerta® N=91 12 11 0 16± 11 20± 10 8± 5 Subjects (N) 9 7 0 Events (N) 9 7 0 Ongoing (N) 5 4 0 19± 13 17± 18 – Duration (days) Decreased Appetite Duration (days) Weight Loss Duration (days) 64
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 65
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 65
 Study 302 Weight Loss 66
Study 302 Weight Loss 66
 Study 302 Weight Changes 67
Study 302 Weight Changes 67
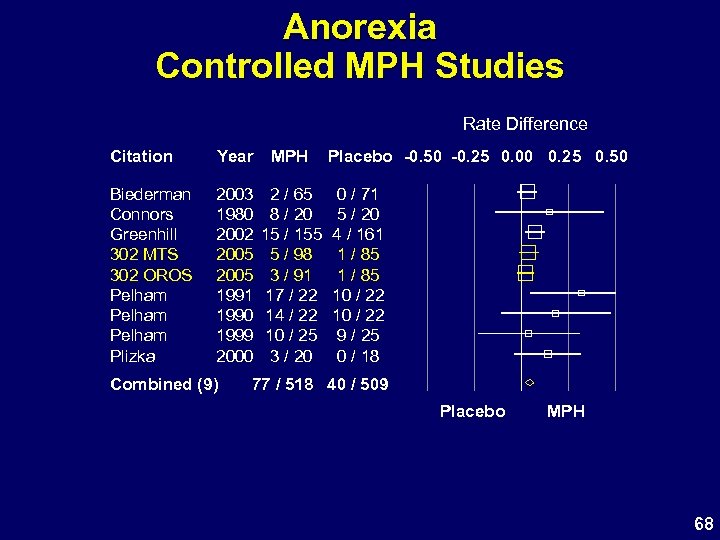 Anorexia Controlled MPH Studies Rate Difference Citation Year MPH Biederman Connors Greenhill 302 MTS 302 OROS Pelham Plizka 2003 1980 2002 2005 1991 1990 1999 2000 2 / 65 8 / 20 15 / 155 5 / 98 3 / 91 17 / 22 14 / 22 10 / 25 3 / 20 Combined (9) Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 0 / 71 5 / 20 4 / 161 1 / 85 10 / 22 9 / 25 0 / 18 77 / 518 40 / 509 Placebo MPH 68
Anorexia Controlled MPH Studies Rate Difference Citation Year MPH Biederman Connors Greenhill 302 MTS 302 OROS Pelham Plizka 2003 1980 2002 2005 1991 1990 1999 2000 2 / 65 8 / 20 15 / 155 5 / 98 3 / 91 17 / 22 14 / 22 10 / 25 3 / 20 Combined (9) Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 0 / 71 5 / 20 4 / 161 1 / 85 10 / 22 9 / 25 0 / 18 77 / 518 40 / 509 Placebo MPH 68
 Decreased Appetite Controlled MPH Studies Rate Difference Citation Year Ahmann Barkley Firestone Kooij 302 MTS 302 OROS Pelham Spencer Stein 1993 124 / 206 1990 27 / 82 1998 22 / 32 2004 10 / 45 2005 25 / 98 2005 17 / 91 2005 8 / 27 2005 28 / 104 2003 34 / 47 Combined (9) MPH Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 53 / 206 11 / 82 8 / 32 2 / 45 4 / 85 2 / 27 3 / 42 16 / 47 295 / 732 103 / 651 Placebo MPH 69
Decreased Appetite Controlled MPH Studies Rate Difference Citation Year Ahmann Barkley Firestone Kooij 302 MTS 302 OROS Pelham Spencer Stein 1993 124 / 206 1990 27 / 82 1998 22 / 32 2004 10 / 45 2005 25 / 98 2005 17 / 91 2005 8 / 27 2005 28 / 104 2003 34 / 47 Combined (9) MPH Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 53 / 206 11 / 82 8 / 32 2 / 45 4 / 85 2 / 27 3 / 42 16 / 47 295 / 732 103 / 651 Placebo MPH 69
 Study 302 Appetite and Weight • MTS: higher observed N of appetite-related AEs – Most are transient and mild – Number of ongoing AEs similar in both MPH groups • Actual weight loss similar in MPH groups • Effects are typical for oral MPH products 70
Study 302 Appetite and Weight • MTS: higher observed N of appetite-related AEs – Most are transient and mild – Number of ongoing AEs similar in both MPH groups • Actual weight loss similar in MPH groups • Effects are typical for oral MPH products 70
 Growth Effects Long-Term Evaluation • Study N-021 – 191 patients, followed for up to 3 years – 12 -hour target wear time – Includes 50 cm 2 patch • Important to assess effects of continuous use of MTS on growth parameters 71
Growth Effects Long-Term Evaluation • Study N-021 – 191 patients, followed for up to 3 years – 12 -hour target wear time – Includes 50 cm 2 patch • Important to assess effects of continuous use of MTS on growth parameters 71
 Long-Term Growth Effects 12 -Hour Wear Time • Subjects treated with MTS continue to grow during treatment • Growth deficits (observed/expected) in weight and height present with MTS (~12 hour wear time) – Deficits are small after 3 years • Short-term, weight deficits related to dose • Prior stimulant therapy predicts smaller deficits • Results similar to those reported for other stimulants 72
Long-Term Growth Effects 12 -Hour Wear Time • Subjects treated with MTS continue to grow during treatment • Growth deficits (observed/expected) in weight and height present with MTS (~12 hour wear time) – Deficits are small after 3 years • Short-term, weight deficits related to dose • Prior stimulant therapy predicts smaller deficits • Results similar to those reported for other stimulants 72
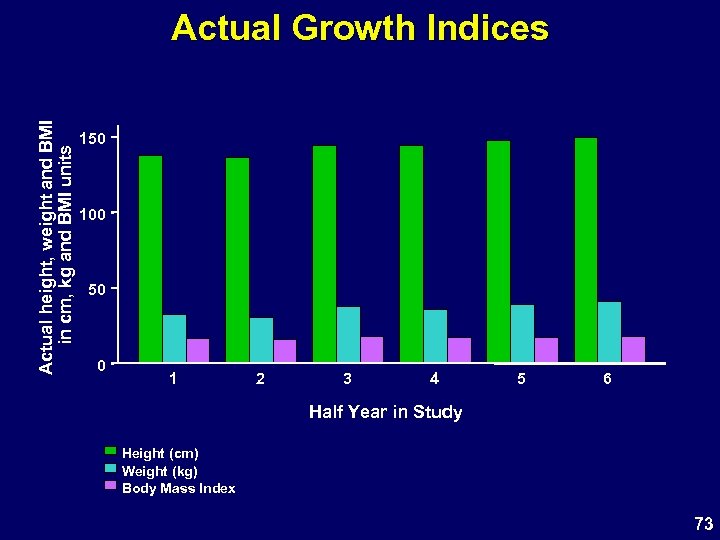 Actual height, weight and BMI in cm, kg and BMI units Actual Growth Indices 150 100 50 0 1 2 3 4 5 6 Half Year in Study Height (cm) Weight (kg) Body Mass Index 73
Actual height, weight and BMI in cm, kg and BMI units Actual Growth Indices 150 100 50 0 1 2 3 4 5 6 Half Year in Study Height (cm) Weight (kg) Body Mass Index 73
 Yearly Velocity in Z-Score Units Growth Velocity as Z-Score. 5 0 -. 5 -1 -1. 5 1 2 3 4 5 6 Half Year in Study Height Weight Body Mass Index 74
Yearly Velocity in Z-Score Units Growth Velocity as Z-Score. 5 0 -. 5 -1 -1. 5 1 2 3 4 5 6 Half Year in Study Height Weight Body Mass Index 74
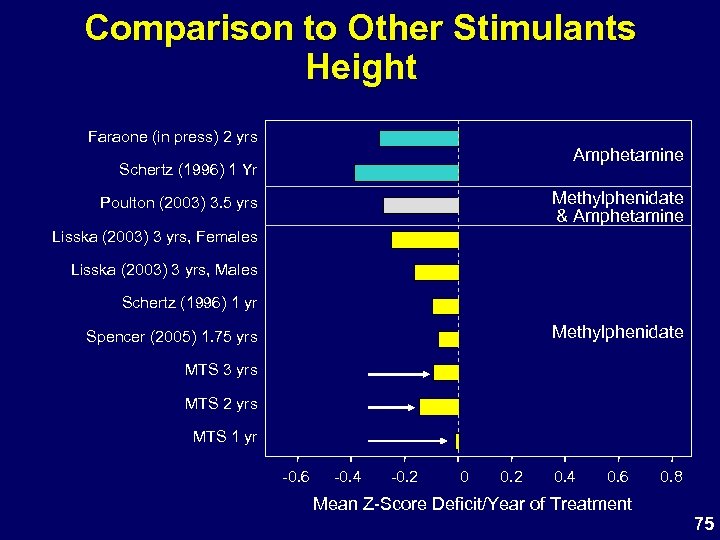 Comparison to Other Stimulants Height Faraone (in press) 2 yrs Amphetamine Schertz (1996) 1 Yr Methylphenidate & Amphetamine Poulton (2003) 3. 5 yrs Lisska (2003) 3 yrs, Females Lisska (2003) 3 yrs, Males Schertz (1996) 1 yr Methylphenidate Spencer (2005) 1. 75 yrs MTS 3 yrs MTS 2 yrs MTS 1 yr -0. 6 -0. 4 -0. 2 0. 4 0. 6 0. 8 Mean Z-Score Deficit/Year of Treatment 75
Comparison to Other Stimulants Height Faraone (in press) 2 yrs Amphetamine Schertz (1996) 1 Yr Methylphenidate & Amphetamine Poulton (2003) 3. 5 yrs Lisska (2003) 3 yrs, Females Lisska (2003) 3 yrs, Males Schertz (1996) 1 yr Methylphenidate Spencer (2005) 1. 75 yrs MTS 3 yrs MTS 2 yrs MTS 1 yr -0. 6 -0. 4 -0. 2 0. 4 0. 6 0. 8 Mean Z-Score Deficit/Year of Treatment 75
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 76
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 76
 Study 302 Sleep-Related AEs MTS N=98 Insomnia Concerta® N=91 Placebo N=85 Subjects (N) 13 7 4 Events (N) 18 12 4 Ongoing (N) 5 6 1 13± 10 11± 8 16± 14 Duration (days) 77
Study 302 Sleep-Related AEs MTS N=98 Insomnia Concerta® N=91 Placebo N=85 Subjects (N) 13 7 4 Events (N) 18 12 4 Ongoing (N) 5 6 1 13± 10 11± 8 16± 14 Duration (days) 77
 Insomnia Controlled MPH Studies Rate Difference Citation Year Ahmann Barkley Biederman Connors Firestone Greenhill Kooij 302 MTS 302 OROS Pelham Spencer Stein 1993 116 / 206 1990 28 / 82 2003 2 / 65 1980 13 / 20 1998 17 / 32 2002 11 / 155 2004 15 / 45 2005 13 / 98 2005 7 / 91 1999 7 / 25 1991 4 / 22 1990 2 / 22 2005 9 / 36 2005 25 / 104 2003 32 / 47 Combined (15) MPH Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 75 / 206 18 / 82 0 / 71 5 / 20 19 / 32 4 / 161 10 / 45 4 / 85 3 / 25 1 / 22 3 / 36 7 / 42 21 / 47 301 / 1050 175 / 981 Placebo MPH 78
Insomnia Controlled MPH Studies Rate Difference Citation Year Ahmann Barkley Biederman Connors Firestone Greenhill Kooij 302 MTS 302 OROS Pelham Spencer Stein 1993 116 / 206 1990 28 / 82 2003 2 / 65 1980 13 / 20 1998 17 / 32 2002 11 / 155 2004 15 / 45 2005 13 / 98 2005 7 / 91 1999 7 / 25 1991 4 / 22 1990 2 / 22 2005 9 / 36 2005 25 / 104 2003 32 / 47 Combined (15) MPH Placebo -0. 50 -0. 25 0. 00 0. 25 0. 50 75 / 206 18 / 82 0 / 71 5 / 20 19 / 32 4 / 161 10 / 45 4 / 85 3 / 25 1 / 22 3 / 36 7 / 42 21 / 47 301 / 1050 175 / 981 Placebo MPH 78
 Children’s Sleep Habits Questionnaire (CSHQ) • CSHQ for children aged 4 -12 years – Parents assess the previous week • Assesses quality, latency, duration, habitual efficiency, disturbances and daytime dysfunction • 33 questions: Scored as 1 to 3 – 1 (rarely occurring; never or 1 time in the week) – 2 (sometimes occurring; 2 to 4 times in the week) – 3 (usually occurring; 5 or more times in the week) • Asks if the behavior is considered a specific problem 79
Children’s Sleep Habits Questionnaire (CSHQ) • CSHQ for children aged 4 -12 years – Parents assess the previous week • Assesses quality, latency, duration, habitual efficiency, disturbances and daytime dysfunction • 33 questions: Scored as 1 to 3 – 1 (rarely occurring; never or 1 time in the week) – 2 (sometimes occurring; 2 to 4 times in the week) – 3 (usually occurring; 5 or more times in the week) • Asks if the behavior is considered a specific problem 79
 CSHQ Total Score Study 302 CSHQ Scores 80
CSHQ Total Score Study 302 CSHQ Scores 80
 Study 302 Sleep-Related Events • Insomnia associated with MPH – Observed incidence higher in MTS group • Most events resolve on continuing treatment – Transient and mild in severity – No discontinuations or dose changes • MTS and Concerta® have little effect on sleep habits 81
Study 302 Sleep-Related Events • Insomnia associated with MPH – Observed incidence higher in MTS group • Most events resolve on continuing treatment – Transient and mild in severity – No discontinuations or dose changes • MTS and Concerta® have little effect on sleep habits 81
 MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 82
MTS Safety • Overview of AEs in 12 hour wear time studies • Overview of AEs in 9 hour wear time study – GI and Headache – Tics – Appetite AEs – Weight and Growth, short and long term – Sleep Effects – Dermal Evaluations 82
 Dermal Evaluations • Subjects evaluated for dermal reactions at all post-baseline visits • Adhesion Scale – Adhesion performance of transdermal system • Dermal Response Scale – Primary skin reactions and evidence of skin irritation • Dermal Discomfort Scale – Experience of discomfort and pruritus 83
Dermal Evaluations • Subjects evaluated for dermal reactions at all post-baseline visits • Adhesion Scale – Adhesion performance of transdermal system • Dermal Response Scale – Primary skin reactions and evidence of skin irritation • Dermal Discomfort Scale – Experience of discomfort and pruritus 83
 Week 7 Transdermal System Adhesion Scale (surface attached) 0: 90% 1: 75% but < 90% 2: 50% but < 75% 3: <50% 4: MTS/PTS detached 84
Week 7 Transdermal System Adhesion Scale (surface attached) 0: 90% 1: 75% but < 90% 2: 50% but < 75% 3: <50% 4: MTS/PTS detached 84
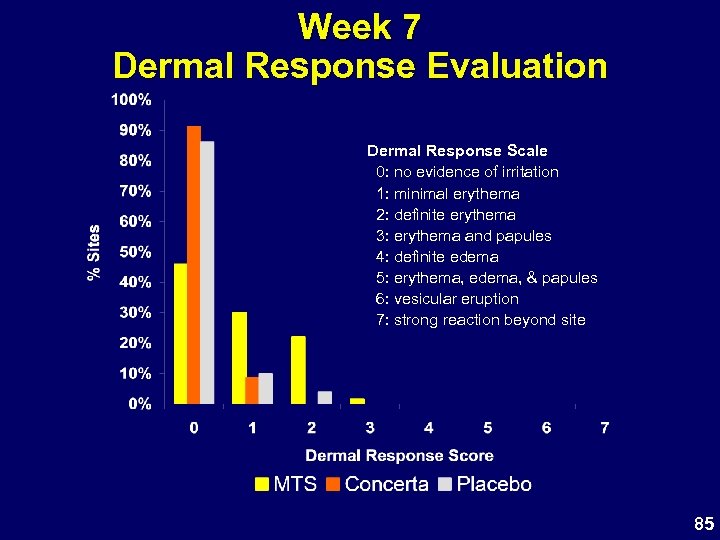 Week 7 Dermal Response Evaluation Dermal Response Scale 0: no evidence of irritation 1: minimal erythema 2: definite erythema 3: erythema and papules 4: definite edema 5: erythema, edema, & papules 6: vesicular eruption 7: strong reaction beyond site 85
Week 7 Dermal Response Evaluation Dermal Response Scale 0: no evidence of irritation 1: minimal erythema 2: definite erythema 3: erythema and papules 4: definite edema 5: erythema, edema, & papules 6: vesicular eruption 7: strong reaction beyond site 85
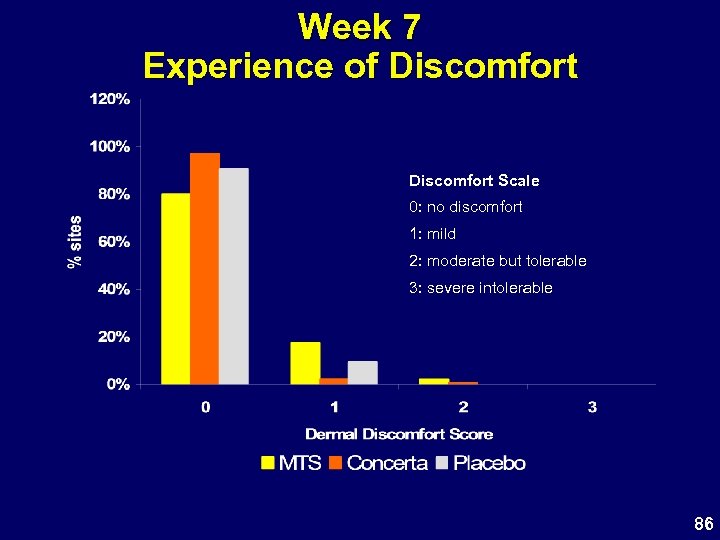 Week 7 Experience of Discomfort Scale 0: no discomfort 1: mild 2: moderate but tolerable 3: severe intolerable 86
Week 7 Experience of Discomfort Scale 0: no discomfort 1: mild 2: moderate but tolerable 3: severe intolerable 86
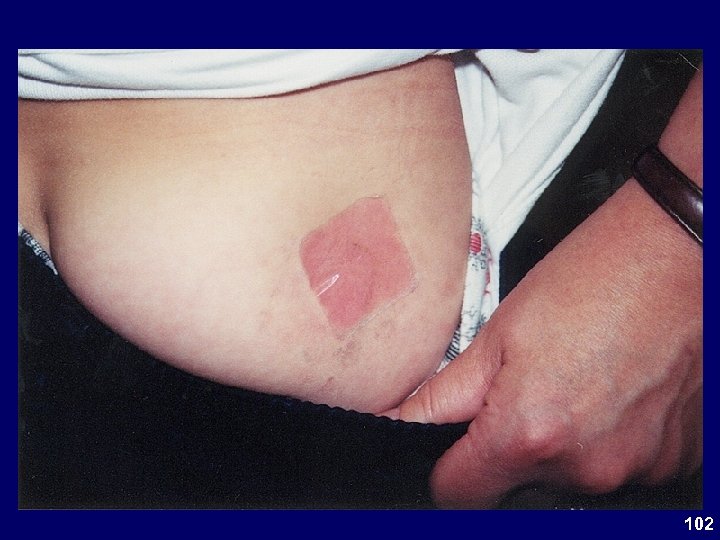
 Study 302 Dermal Assessment • MTS typically associated with slight to minimal erythema – MPH a mild irritant • Most subjects experienced no discomfort – Few cases of mild discomfort, typically itching • Excellent adhesion • Few discontinuations due to application site reactions 87
Study 302 Dermal Assessment • MTS typically associated with slight to minimal erythema – MPH a mild irritant • Most subjects experienced no discomfort – Few cases of mild discomfort, typically itching • Excellent adhesion • Few discontinuations due to application site reactions 87
 MTS Safety Conclusions • • Generally well tolerated No related serious AEs Few discontinuations due to AEs Common AEs related to stimulant effects – Mild, transient and resolve on continued treatment – Persisting AE incidence similar to Concerta® Target wear time of 9 hours reduced the incidence of anorexia and insomnia Skin reactions are mild Short and Long-term growth effects are similar to all stimulants Results consistent with other approved MPH products 88
MTS Safety Conclusions • • Generally well tolerated No related serious AEs Few discontinuations due to AEs Common AEs related to stimulant effects – Mild, transient and resolve on continued treatment – Persisting AE incidence similar to Concerta® Target wear time of 9 hours reduced the incidence of anorexia and insomnia Skin reactions are mild Short and Long-term growth effects are similar to all stimulants Results consistent with other approved MPH products 88
 MTS Clinical Perspective Sharon B. Wigal, Ph. D Associate Clinical Professor of Pediatrics University of California, Irvine 89
MTS Clinical Perspective Sharon B. Wigal, Ph. D Associate Clinical Professor of Pediatrics University of California, Irvine 89
 MTS Patients in Clinical Trials “ … an investigational medicinal patch to treat ADHD…” • Treatment-naïve children • Children with prior stimulant exposure 90
MTS Patients in Clinical Trials “ … an investigational medicinal patch to treat ADHD…” • Treatment-naïve children • Children with prior stimulant exposure 90
 MTS Experience in Laboratory School Setting • Ease in administration • Ease of removal • Level of patient/family satisfaction 91
MTS Experience in Laboratory School Setting • Ease in administration • Ease of removal • Level of patient/family satisfaction 91
 MTS Parent Comments: School • “Teacher reports the change is like night and day” • “His teacher noticed a big difference … like a different kid” • “ … much more focused and accomplishes work” 92
MTS Parent Comments: School • “Teacher reports the change is like night and day” • “His teacher noticed a big difference … like a different kid” • “ … much more focused and accomplishes work” 92
 MTS Parent Comments: Home • “Everybody comments on her improvement” • “…my child listens, follows through on directions” • “I can tell when the meds wear off because [child] pesters her brother” 93
MTS Parent Comments: Home • “Everybody comments on her improvement” • “…my child listens, follows through on directions” • “I can tell when the meds wear off because [child] pesters her brother” 93
 MTS Parents Comments: Long-term Use • “The three side effects that I have noted (loss of appetite, sleeplessness and skin irritation) have all been acceptable trade offs. . . After several weeks, his appetite returned in the evening either by acclimation or by modifying our removal time to 3: 30 PM… the earlier removal time also assisted in eradicating sleeplessness at bedtime. Lastly, I have noticed far less reddening of skin (barely any) as time has passed and have not had any complaints of skin irritation for some time. ” 94
MTS Parents Comments: Long-term Use • “The three side effects that I have noted (loss of appetite, sleeplessness and skin irritation) have all been acceptable trade offs. . . After several weeks, his appetite returned in the evening either by acclimation or by modifying our removal time to 3: 30 PM… the earlier removal time also assisted in eradicating sleeplessness at bedtime. Lastly, I have noticed far less reddening of skin (barely any) as time has passed and have not had any complaints of skin irritation for some time. ” 94
 MTS Clinical Utility Conclusions • MTS was a viable treatment option for children with ADHD – Ease of use – Compliance to treatment – Positive parent perception – Flexibility of treatment period – Manageable side effects 95
MTS Clinical Utility Conclusions • MTS was a viable treatment option for children with ADHD – Ease of use – Compliance to treatment – Positive parent perception – Flexibility of treatment period – Manageable side effects 95
 Benefit / Risk Summary Raymond D. Pratt, MD 96
Benefit / Risk Summary Raymond D. Pratt, MD 96
 Benefits of MTS Once-daily application • Behavioral response within 2 hours • Clinical benefits persist for at least 3 hours after patch removal Transdermal • No oral dosing • Excellent adhesion; minimal irritation • 9 -hour MPH exposure similar to oral MPH products • Exposure controlled by patch size and wear time • Drug delivery stops on patch removal • Decreased risk of accidental ingestion or overdose 97
Benefits of MTS Once-daily application • Behavioral response within 2 hours • Clinical benefits persist for at least 3 hours after patch removal Transdermal • No oral dosing • Excellent adhesion; minimal irritation • 9 -hour MPH exposure similar to oral MPH products • Exposure controlled by patch size and wear time • Drug delivery stops on patch removal • Decreased risk of accidental ingestion or overdose 97
 Risks of MTS • Adverse events with MTS are same as oral MPH • MTS wear times > 9 -hours might lead to a higher incidence of anorexia and insomnia • Longer-term effects with MTS on growth parameters are similar to other psychostimulants • Potential for sensitization and irritation with MTS • CII substance: potential for abuse and/or diversion 98
Risks of MTS • Adverse events with MTS are same as oral MPH • MTS wear times > 9 -hours might lead to a higher incidence of anorexia and insomnia • Longer-term effects with MTS on growth parameters are similar to other psychostimulants • Potential for sensitization and irritation with MTS • CII substance: potential for abuse and/or diversion 98
 Conclusions • MTS is a new effective delivery system for oncedaily treatment of ADHD with MPH • Onset of effect within 2 hours of application • Duration of effect covers the school day • Potential for customized treatment • Positive benefit-risk balance • Incidence of AEs similar to other MPH products 99
Conclusions • MTS is a new effective delivery system for oncedaily treatment of ADHD with MPH • Onset of effect within 2 hours of application • Duration of effect covers the school day • Potential for customized treatment • Positive benefit-risk balance • Incidence of AEs similar to other MPH products 99
 100
100
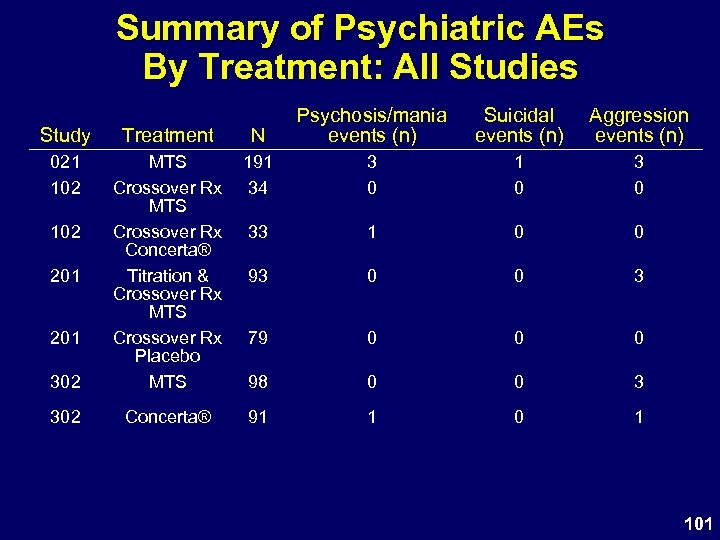 Summary of Psychiatric AEs By Treatment: All Studies Study Treatment N Psychosis/mania events (n) 021 102 191 34 3 0 1 0 33 1 0 0 93 0 0 3 79 0 0 0 302 MTS Crossover Rx Concerta® Titration & Crossover Rx MTS Crossover Rx Placebo MTS 98 0 0 3 302 Concerta® 91 1 0 1 102 201 Suicidal events (n) Aggression events (n) 101
Summary of Psychiatric AEs By Treatment: All Studies Study Treatment N Psychosis/mania events (n) 021 102 191 34 3 0 1 0 33 1 0 0 93 0 0 3 79 0 0 0 302 MTS Crossover Rx Concerta® Titration & Crossover Rx MTS Crossover Rx Placebo MTS 98 0 0 3 302 Concerta® 91 1 0 1 102 201 Suicidal events (n) Aggression events (n) 101
 102
102
 302: Summary of Patch Wear Time by Visit – Safety Population Hours of Wear Time 10 12. 5 cm 2 18. 75 cm 2 25 cm 2 37. 5 cm 2 9 8 7 Visit 3 Visit 4 Visit 5 Visit 6 Visit 7 Visit 8 Visit 9 103
302: Summary of Patch Wear Time by Visit – Safety Population Hours of Wear Time 10 12. 5 cm 2 18. 75 cm 2 25 cm 2 37. 5 cm 2 9 8 7 Visit 3 Visit 4 Visit 5 Visit 6 Visit 7 Visit 8 Visit 9 103
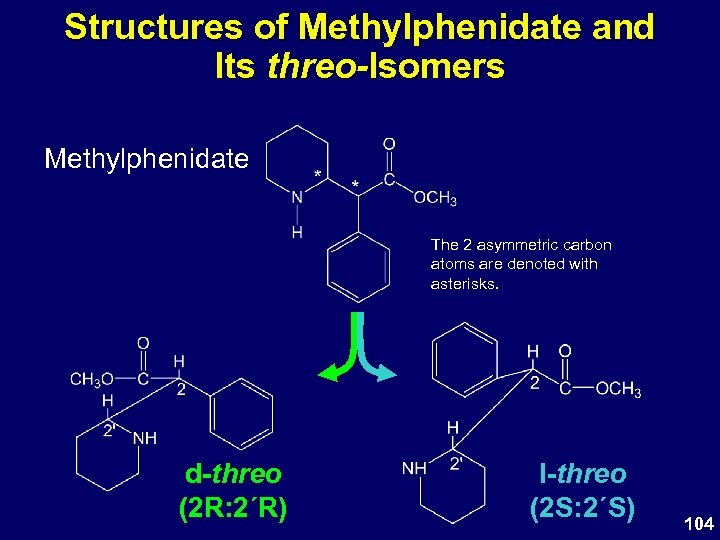 Structures of Methylphenidate and Its threo-Isomers Methylphenidate The 2 asymmetric carbon atoms are denoted with asterisks. d-threo (2 R: 2΄R) l-threo (2 S: 2΄S) 104
Structures of Methylphenidate and Its threo-Isomers Methylphenidate The 2 asymmetric carbon atoms are denoted with asterisks. d-threo (2 R: 2΄R) l-threo (2 S: 2΄S) 104
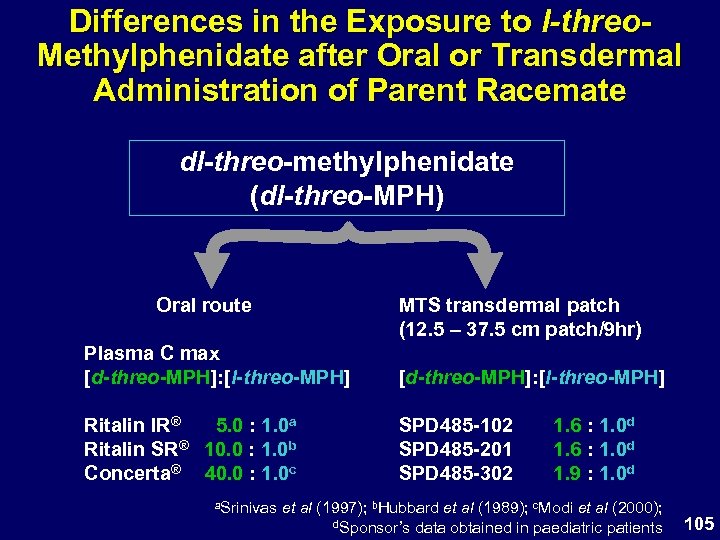 Differences in the Exposure to l-threo. Methylphenidate after Oral or Transdermal Administration of Parent Racemate dl-threo-methylphenidate (dl-threo-MPH) MTS transdermal patch (12. 5 – 37. 5 cm patch/9 hr) Oral route Plasma C max [d-threo-MPH]: [l-threo-MPH] Ritalin IR® 5. 0 : 1. 0 a Ritalin SR® 10. 0 : 1. 0 b Concerta® 40. 0 : 1. 0 c SPD 485 -102 SPD 485 -201 SPD 485 -302 a. Srinivas 1. 6 : 1. 0 d 1. 9 : 1. 0 d et al (1997); b. Hubbard et al (1989); c. Modi et al (2000); d. Sponsor’s data obtained in paediatric patients 105
Differences in the Exposure to l-threo. Methylphenidate after Oral or Transdermal Administration of Parent Racemate dl-threo-methylphenidate (dl-threo-MPH) MTS transdermal patch (12. 5 – 37. 5 cm patch/9 hr) Oral route Plasma C max [d-threo-MPH]: [l-threo-MPH] Ritalin IR® 5. 0 : 1. 0 a Ritalin SR® 10. 0 : 1. 0 b Concerta® 40. 0 : 1. 0 c SPD 485 -102 SPD 485 -201 SPD 485 -302 a. Srinivas 1. 6 : 1. 0 d 1. 9 : 1. 0 d et al (1997); b. Hubbard et al (1989); c. Modi et al (2000); d. Sponsor’s data obtained in paediatric patients 105
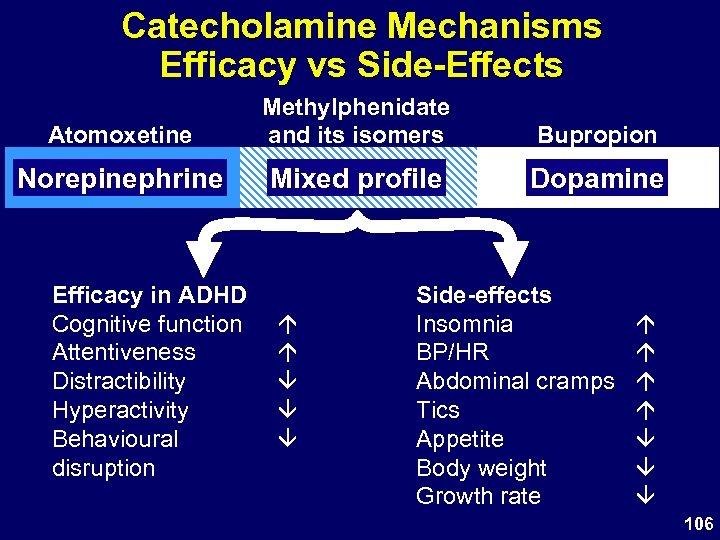 Catecholamine Mechanisms Efficacy vs Side-Effects Atomoxetine Methylphenidate and its isomers Bupropion Norepinephrine Mixed profile Dopamine Efficacy in ADHD Cognitive function Attentiveness Distractibility Hyperactivity Behavioural disruption Side-effects Insomnia BP/HR Abdominal cramps Tics Appetite Body weight Growth rate 106
Catecholamine Mechanisms Efficacy vs Side-Effects Atomoxetine Methylphenidate and its isomers Bupropion Norepinephrine Mixed profile Dopamine Efficacy in ADHD Cognitive function Attentiveness Distractibility Hyperactivity Behavioural disruption Side-effects Insomnia BP/HR Abdominal cramps Tics Appetite Body weight Growth rate 106
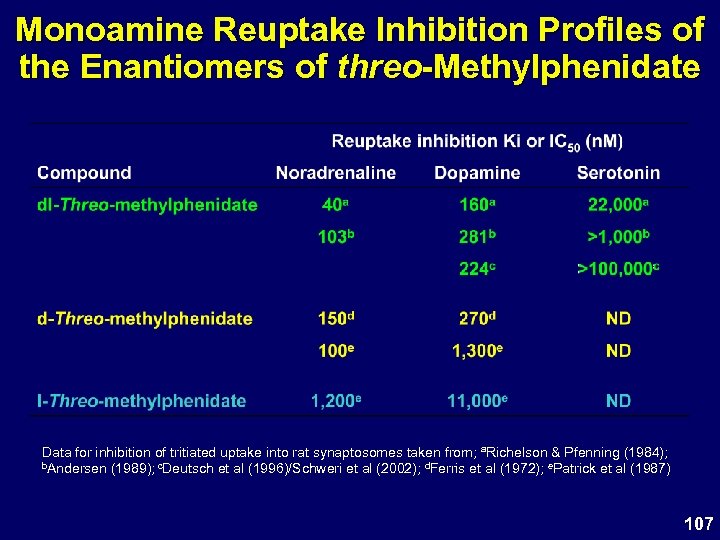 Monoamine Reuptake Inhibition Profiles of the Enantiomers of threo-Methylphenidate Data for inhibition of tritiated uptake into rat synaptosomes taken from; a. Richelson & Pfenning (1984); b. Andersen (1989); c. Deutsch et al (1996)/Schweri et al (2002); d. Ferris et al (1972); e. Patrick et al (1987) 107
Monoamine Reuptake Inhibition Profiles of the Enantiomers of threo-Methylphenidate Data for inhibition of tritiated uptake into rat synaptosomes taken from; a. Richelson & Pfenning (1984); b. Andersen (1989); c. Deutsch et al (1996)/Schweri et al (2002); d. Ferris et al (1972); e. Patrick et al (1987) 107
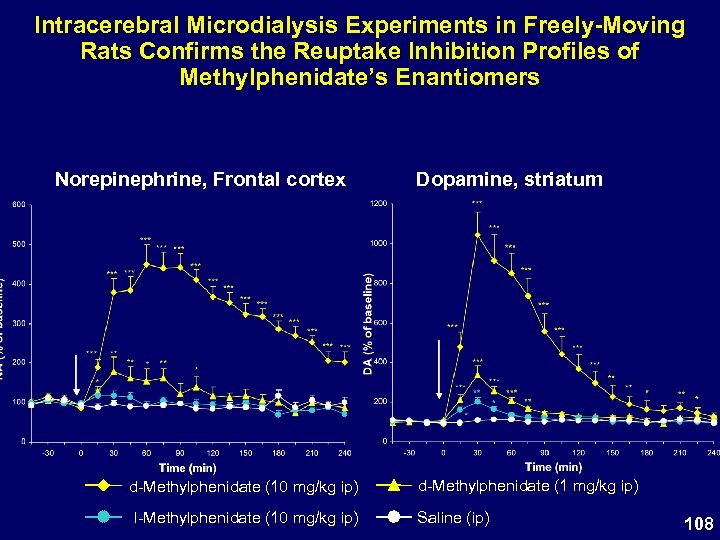 Intracerebral Microdialysis Experiments in Freely-Moving Rats Confirms the Reuptake Inhibition Profiles of Methylphenidate’s Enantiomers Norepinephrine, Frontal cortex d-Methylphenidate (10 mg/kg ip) l-Methylphenidate (10 mg/kg ip) Dopamine, striatum d-Methylphenidate (1 mg/kg ip) Saline (ip) 108
Intracerebral Microdialysis Experiments in Freely-Moving Rats Confirms the Reuptake Inhibition Profiles of Methylphenidate’s Enantiomers Norepinephrine, Frontal cortex d-Methylphenidate (10 mg/kg ip) l-Methylphenidate (10 mg/kg ip) Dopamine, striatum d-Methylphenidate (1 mg/kg ip) Saline (ip) 108
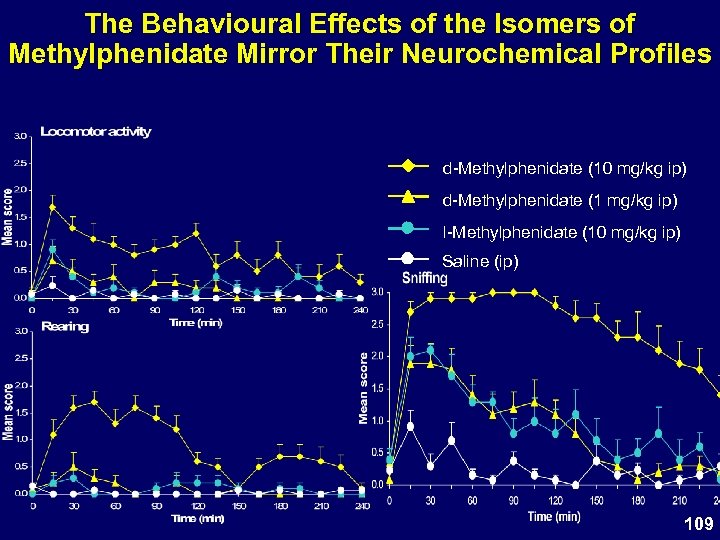 The Behavioural Effects of the Isomers of Methylphenidate Mirror Their Neurochemical Profiles d-Methylphenidate (10 mg/kg ip) d-Methylphenidate (1 mg/kg ip) l-Methylphenidate (10 mg/kg ip) Saline (ip) 109
The Behavioural Effects of the Isomers of Methylphenidate Mirror Their Neurochemical Profiles d-Methylphenidate (10 mg/kg ip) d-Methylphenidate (1 mg/kg ip) l-Methylphenidate (10 mg/kg ip) Saline (ip) 109
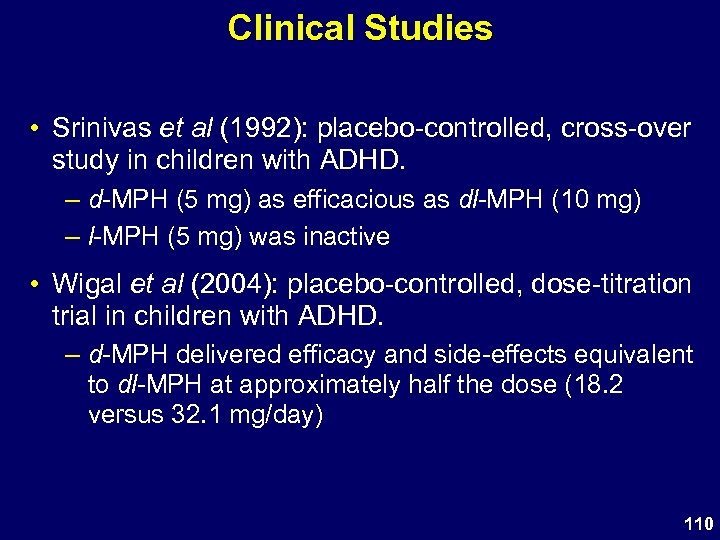 Clinical Studies • Srinivas et al (1992): placebo-controlled, cross-over study in children with ADHD. – d-MPH (5 mg) as efficacious as dl-MPH (10 mg) – l-MPH (5 mg) was inactive • Wigal et al (2004): placebo-controlled, dose-titration trial in children with ADHD. – d-MPH delivered efficacy and side-effects equivalent to dl-MPH at approximately half the dose (18. 2 versus 32. 1 mg/day) 110
Clinical Studies • Srinivas et al (1992): placebo-controlled, cross-over study in children with ADHD. – d-MPH (5 mg) as efficacious as dl-MPH (10 mg) – l-MPH (5 mg) was inactive • Wigal et al (2004): placebo-controlled, dose-titration trial in children with ADHD. – d-MPH delivered efficacy and side-effects equivalent to dl-MPH at approximately half the dose (18. 2 versus 32. 1 mg/day) 110
 Summary and Conclusions • d-Threo-methylphenidate (d-MPH) ≥ 10 x potent than the l-isomer (l-MPH) • d-MPH is the predominant driver of efficacy and side-effects • The lower potency and plasma concentrations of l-MPH dictate that it contributes at most 5 -10% of the efficacy and side-effects of dl-MPH in the MTS patch • Clinical data support the hypothesis that whilst l-MPH delivers no discernable clinical benefit, its presence in racemic MPH preparations does not adversely impact on the side-effect liability of such products 111
Summary and Conclusions • d-Threo-methylphenidate (d-MPH) ≥ 10 x potent than the l-isomer (l-MPH) • d-MPH is the predominant driver of efficacy and side-effects • The lower potency and plasma concentrations of l-MPH dictate that it contributes at most 5 -10% of the efficacy and side-effects of dl-MPH in the MTS patch • Clinical data support the hypothesis that whilst l-MPH delivers no discernable clinical benefit, its presence in racemic MPH preparations does not adversely impact on the side-effect liability of such products 111
 Dosing Diary 112
Dosing Diary 112
 Mean Plasma d-MPH Concentration vs Time Plots for Caucasians 25 MTS 06 25 cm 2 MTS 08 25 cm 2 MTS 10 25 cm 2 Concerta® 36 mg d-MPH (ng/m. L) 20 15 10 5 0 0 5 10 15 20 25 30 Time after dosing (hours) 35 Study 101: n=9 MTS; n = 10 Concerta® 113
Mean Plasma d-MPH Concentration vs Time Plots for Caucasians 25 MTS 06 25 cm 2 MTS 08 25 cm 2 MTS 10 25 cm 2 Concerta® 36 mg d-MPH (ng/m. L) 20 15 10 5 0 0 5 10 15 20 25 30 Time after dosing (hours) 35 Study 101: n=9 MTS; n = 10 Concerta® 113
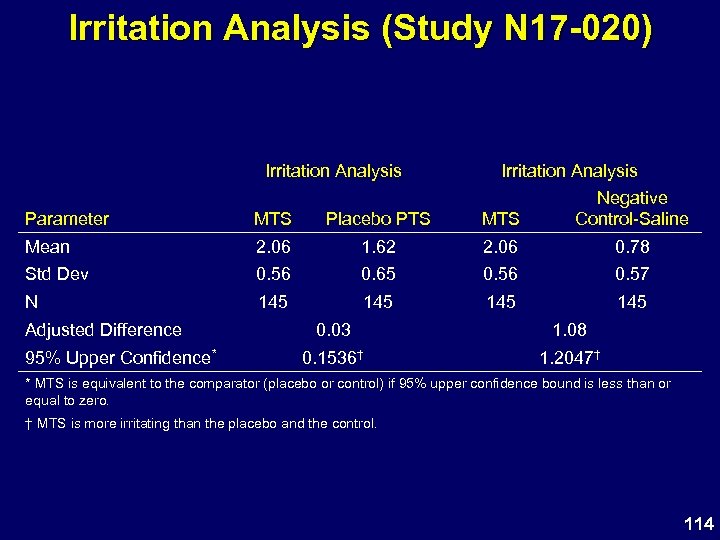 Irritation Analysis (Study N 17 -020) Irritation Analysis Parameter MTS Placebo PTS MTS Negative Control-Saline Mean 2. 06 1. 62 2. 06 0. 78 Std Dev 0. 56 0. 65 0. 56 0. 57 N 145 145 Adjusted Difference 95% Upper Confidence* 0. 03 1. 08 0. 1536† 1. 2047† * MTS is equivalent to the comparator (placebo or control) if 95% upper confidence bound is less than or equal to zero. † MTS is more irritating than the placebo and the control. 114
Irritation Analysis (Study N 17 -020) Irritation Analysis Parameter MTS Placebo PTS MTS Negative Control-Saline Mean 2. 06 1. 62 2. 06 0. 78 Std Dev 0. 56 0. 65 0. 56 0. 57 N 145 145 Adjusted Difference 95% Upper Confidence* 0. 03 1. 08 0. 1536† 1. 2047† * MTS is equivalent to the comparator (placebo or control) if 95% upper confidence bound is less than or equal to zero. † MTS is more irritating than the placebo and the control. 114
 Number of Subjects Who Underwent Challenge and Rechallenge Reactions (Study N 17 -020) Category Participating in Challenge Period N 133* Participating in Rechallenge Period 36 With Irritation at Rechallenge Period 16 With Sensitization based on Challenge and Rechallenge Periods 17 With Sensitization based on Challenge Alone 1 With Potential Sensitization to MTS, but Rechallenge Needed 8 With Sensitization based on Incomplete Challenge Period 1 *One subject (No. 331) only had a 48 -hour evaluation 115
Number of Subjects Who Underwent Challenge and Rechallenge Reactions (Study N 17 -020) Category Participating in Challenge Period N 133* Participating in Rechallenge Period 36 With Irritation at Rechallenge Period 16 With Sensitization based on Challenge and Rechallenge Periods 17 With Sensitization based on Challenge Alone 1 With Potential Sensitization to MTS, but Rechallenge Needed 8 With Sensitization based on Incomplete Challenge Period 1 *One subject (No. 331) only had a 48 -hour evaluation 115
 Treatment-Emergent Adverse Events in 5% of All Subjects Long-Term Pediatric Population by Time of Event Onset in Days Body System COSTART Term* Any Adverse Event Headache Abdominal Pain Anorexia Vomiting Weight Loss Insomnia Nervousness Application Site Reaction 1 -29 (n=322) 288 (89) 35 (11) 26 (8) 66 (20) 16 (5) 25 (8) 54 (17) 19 (6) 255 (79) 30 -59 (n=286) 209 (73) 13 (5) 9 (3) 13 (5) 4 (1) 10 (3) 5 (2) 174 (61) 60 -89 (n=255) 194 (76) 9 (4) 4 (2) 12 (5) 6 (2) 2 (1) 5 (2) 2 (1) 165 (65) 90 -119 120 -149 150 -179 180 -209 (n=171) (n=143) (n=128) (n=117) 127 82 (57) 50 (39) 36 (31) (74) 10 (6) 8 (6) 5 (4) 3 (3) 5 (3) 0 2 (2) 5 (3) 3 (2) 2 (2) 1 (1) 3 (2) 1 (1) 2 (2) 0 3 (2) 0 0 1 (1) 2 (1) 0 2 (2) 0 109 66 (46) 32 (25) 18 (15) (64) * COSTART Version 5, 1995, was used to code the reported AEs by Body System and COSTART Term 116
Treatment-Emergent Adverse Events in 5% of All Subjects Long-Term Pediatric Population by Time of Event Onset in Days Body System COSTART Term* Any Adverse Event Headache Abdominal Pain Anorexia Vomiting Weight Loss Insomnia Nervousness Application Site Reaction 1 -29 (n=322) 288 (89) 35 (11) 26 (8) 66 (20) 16 (5) 25 (8) 54 (17) 19 (6) 255 (79) 30 -59 (n=286) 209 (73) 13 (5) 9 (3) 13 (5) 4 (1) 10 (3) 5 (2) 174 (61) 60 -89 (n=255) 194 (76) 9 (4) 4 (2) 12 (5) 6 (2) 2 (1) 5 (2) 2 (1) 165 (65) 90 -119 120 -149 150 -179 180 -209 (n=171) (n=143) (n=128) (n=117) 127 82 (57) 50 (39) 36 (31) (74) 10 (6) 8 (6) 5 (4) 3 (3) 5 (3) 0 2 (2) 5 (3) 3 (2) 2 (2) 1 (1) 3 (2) 1 (1) 2 (2) 0 3 (2) 0 0 1 (1) 2 (1) 0 2 (2) 0 109 66 (46) 32 (25) 18 (15) (64) * COSTART Version 5, 1995, was used to code the reported AEs by Body System and COSTART Term 116
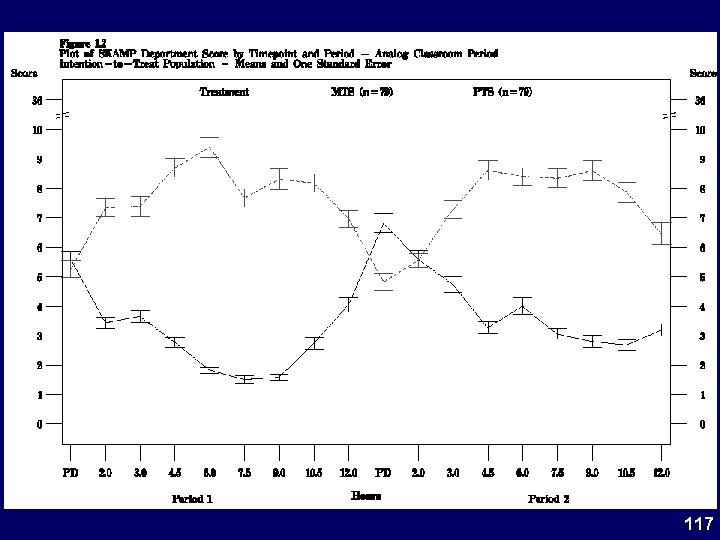 117
117


