6ef5416bc676f081dbb511abf7d31410.ppt
- Количество слайдов: 37

NCI SBIR Funding for Technology Development Presentation at DP Clinical David Beylin Program Director NCI SBIR Development Center March 26, 2010

Today’s Presentation • Program Overview • SBIR Funding Opportunities • NCI SBIR Development Center • New SBIR Bridge Award

Overview

NCI SBIR Focus: Commercializing Innovation Therapeutics Medical Devices Research Tools e. Health Cancer diagnosis, treatment, research 4

Program Descriptions Set Aside • SBIR: Set-aside Program for Small Business Concerns to engage in Federal R&D with potential for commercialization • STTR: Set-aside Program to facilitate Cooperative R&D between Small Business Concerns and U. S. Research Institutions with potential for commercialization 2. 5% 0. 3% A $108 M Program at the NCI

SBIR & STTR: Three-Phase Program PHASE I – R 41, R 43 • Feasibility Study • $100 K and 6 -month (SBIR) * • or 12 -month (STTR) Award PHASE II – R 42, R 44 • Full Research/R&D • $750 K and 2 -year Award (SBIR & STTR) * • Commercialization plan required PHASE III • Commercialization Stage • Use of non-SBIR/STTR Funds * These funding levels are guidelines. You should request the budget appropriate to accomplish the goals of the project.

Reasons to Seek SBIR & STTR Funding • Provides seed funding for innovative technology development projects • A stable and predictable source of funding • Intellectual property rights are retained by the small business concern • Not a loan – no repayment is required • Doesn’t impact stock or shares in any way (no dilution of capital) • Provides recognition, verification and visibility • Can be a leveraging tool to attract other funding (VC, etc. )

SBIR Eligibility Requirements Small Business Concern • Organized for-profit U. S. business • 500 or fewer employees, including affiliates • Must be: • At least 51% U. S. - owned by individuals and independently operated or • At least 51% owned and controlled by another (one) business concern that is at least 51% owned and controlled by one or more individuals • Principal Investigator’s primary employment must be with the Small Business Concern

STTR Eligibility Requirements • Applicant is a Small Business Concern • Formal Cooperative R&D Effort • Minimum 40% by small business • Minimum 30% by U. S. research institution • U. S. Research Institution • College or University • Other non-profit research organization • Federal R&D center • Intellectual Property Agreement • Allocation of IP rights and rights to carry out follow-on R&D and commercialization • Principal Investigator’s primary employment may be with either the Small Business Concern or the research institution

SBIR Funding Opportunities (Phase I & II)
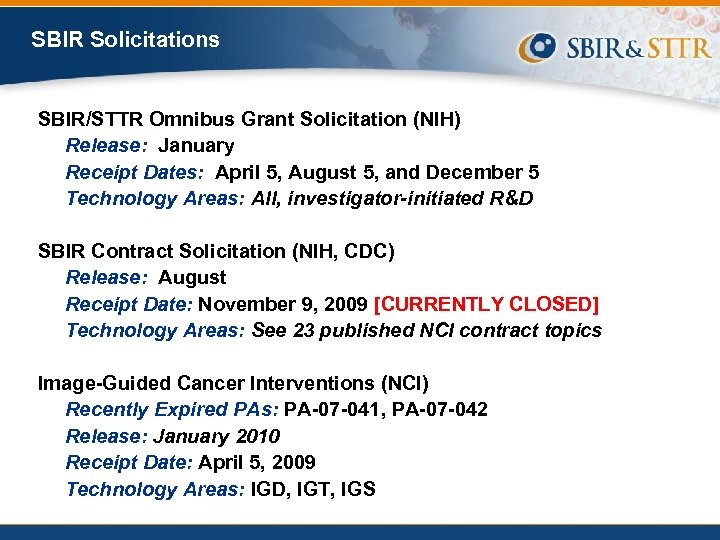
SBIR Solicitations SBIR/STTR Omnibus Grant Solicitation (NIH) Release: January Receipt Dates: April 5, August 5, and December 5 Technology Areas: All, investigator-initiated R&D SBIR Contract Solicitation (NIH, CDC) Release: August Receipt Date: November 9, 2009 [CURRENTLY CLOSED] Technology Areas: See 23 published NCI contract topics Image-Guided Cancer Interventions (NCI) Recently Expired PAs: PA-07 -041, PA-07 -042 Release: January 2010 Receipt Date: April 5, 2009 Technology Areas: IGD, IGT, IGS

Selected SBIR Contract Topics in 2009 Ø Development of Anticancer Agents Ø Nanotechnology Imaging Agents or Devices for Improved Detection of Cancer Ø Biopsy Instruments and Devices that Preserve Molecular Profiles in Tumors Ø Innovative Devices to Protect Radiosensitive Organs and Structures During Radiation Therapy Ø Quantitative Cell-Based Imaging For Clinical Diagnosis and Treatment Ø … Ø Full List of 23 Topics: Ø http: //sbir. cancer. gov/funding/past/contracts/PHS 2010 -1. asp Ø Next contract solicitation will be released in August of 2010 (sign-up for updates at http: //sbir. cancer. gov)

Topic 277: Companion Diagnostics- Predictive and Prognostic Tests Enabling Personalized Medicine Ø Goal: develop companion diagnostics to select patients in whom a particular therapeutic regimen, including existing drugs and those in late clinical development, will be safe and effective Ø Fast-Track proposals allowed: yes Ø Budget: $200, 000 Phase I; $1, 500, 000 Phase II; < 4 awards anticipated Ø Phase I activities and deliverables: • Develop a working diagnostic test. • Characterize the variation, reproducibility, and accuracy of the test • Demonstrate suitability of the test for use in the clinic, conduct benchmarking studies against current tests (if available).

Topic 277: Companion Diagnostics- Predictive and Prognostic Tests Enabling Personalized Medicine, cont Ø Phase II Activities and Deliverables: • Demonstrate clinical utility and value by testing sufficient numbers of patients for statistical significance regarding patient selection for therapy. • Perform correlation studies between any animal models and treatment in human subjects, if applicable • Establish a marketing partnership or alliance, as appropriate, with the pharmaceutical company that is developing or selling therapy, unless therapy is on the open market. • Deliver the final SOP to NCI.
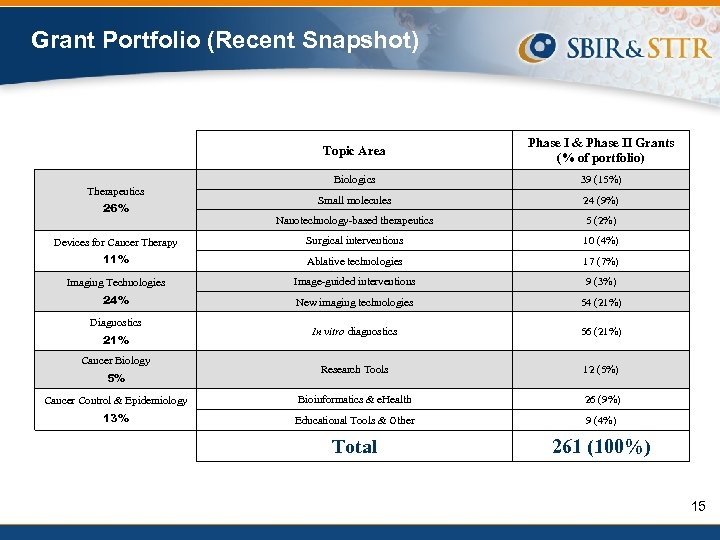
Grant Portfolio (Recent Snapshot) Topic Area Phase I & Phase II Grants (% of portfolio) Biologics 39 (15%) Small molecules 24 (9%) Nanotechnology-based therapeutics 5 (2%) Devices for Cancer Therapy Surgical interventions 10 (4%) 11% Ablative technologies 17 (7%) Imaging Technologies Image-guided interventions 9 (3%) 24% New imaging technologies 54 (21%) In vitro diagnostics 56 (21%) Research Tools 12 (5%) Cancer Control & Epidemiology Bioinformatics & e. Health 26 (9%) 13% Educational Tools & Other 9 (4%) Total 261 (100%) Therapeutics 26% Diagnostics 21% Cancer Biology 5% 15

NCI SBIR Development Center

SBIR Development Center Old SBIR Management Model at NCI • Awards were managed by 40 -50 people who each spent a small amount of their time on SBIR (currently the model at other NIH Institutes) • Few of these NCI program managers had significant industry experience or commercialization expertise New Development Center at NCI • Team of 8 Program Directors and the Center Director • Exclusively focused on the management of NCI’s SBIR/STTR portfolio • Program Directors have previous industry experience and professional networks to help mentor awardees in commercialization strategy and process • Center is developing a range of new activities to help small businesses 17

SBIR Development Center Staff Michael Weingarten, MA (Director) Ali Andalibi, Ph. D (Branch Chief) Previous • NASA – Program Manager, NASA Technology Commercialization Program Previous • NSF – SBIR Program Director, Medical Biotechnology • House Ear Institute – Scientist & Director, New Technology and Project Development • Trega Biosciences, Inc. – Research Scientist Greg Evans, Ph. D (Branch Chief) Natalia Kruchinin, Ph. D (Program Director) Previous • NHLBI/NIH – Program Director, Translational and Multicenter Clinical Research in Hemoglobinopathies • NHGRI/NIH – Senior Staff Fellow Previous • QIAGEN, Inc. – Molecular Diagnostics Applications Manager • Motorola, Inc. – Senior Scientist, Gene Expression Assays Patti Weber, Dr. PH (Program Director) Andrew J. Kurtz, Ph. D (Program Director) Previous • International Heart Institute of Montana – Tissue Engineering and Surgical Research • Ribi Immuno. Chem Research, Inc. – Team Leader, Cardiovascular Pharmacology Previous • NIH – AAAS Science & Technology Policy Fellow • Cedra Corporation – Research Associate, Bio. Analytical Assays and Pharmacokinetics Analysis David Beylin, MS, MBA (Program Director) Jian Lou, Ph. D (Program Director) Previous • X/Seed Capital Management, LLC, EIR • Naviscan PET Systems, Inc. , Vice President, Research Previous • Johnson & Johnson – Research Scientist, Target Validation & Biomarker Development • Lumicyte, Inc. – Director, Molecular Biology Systems Analysis Deepa Narayanan, MS (Program Director) Todd Haim, Ph. D (Program Analyst / AAAS Fellow) Previous • Naviscan PET Systems, Inc. , Director, Clinical Data Management (Oncology Imaging & Clinical Trials) • Fox Chase Cancer Center, Scientific Associate (Molecular Imaging Lab) Previous • National Academy of Sciences – Christine Mirzayan Science and Technology Policy Fellow • Pfizer Research Laboratories – Postdoctoral Fellow, Cardiac Pathogenesis & Metabolic Disorders

Mentoring and Facilitation Goal • To work closely with promising SBIR Phase II awardees in order to advance their technologies towards the clinic. Path • Mentor and guide companies throughout the award period. • Hold an annual SBIR Investor Forum to learn more about portfolio companies, encourage inter-company collaborations/partnerships, and showcase to investors. • When appropriate, act as a liaison to bring investors (VC, angels, strategic partners) and NCI SBIR companies together.

NCI SBIR Investor Forum (available to NCI Phase II grantees )

NCI SBIR Investor Forum Exclusive opportunity for 14 NCI awardees to showcase their companies to investors http: //sbir. cancer. gov/investorforum/ Featured Small Businesses • Present to and network with close to 200 top investors and strategic partners • Participate in panel discussion with successful Bridge awardees and their investors Investors • Opportunity to evaluate NCI’s top companies with innovative technologies • Exclusive one-on-one meetings • Follow-Up discussions, MTA’s and Due Diligence now underway 21

Investor Forum Participants Strategic partners Investors Other Organizations and Media

Regulatory Assistance Program (available to NCI Phase II grantees )
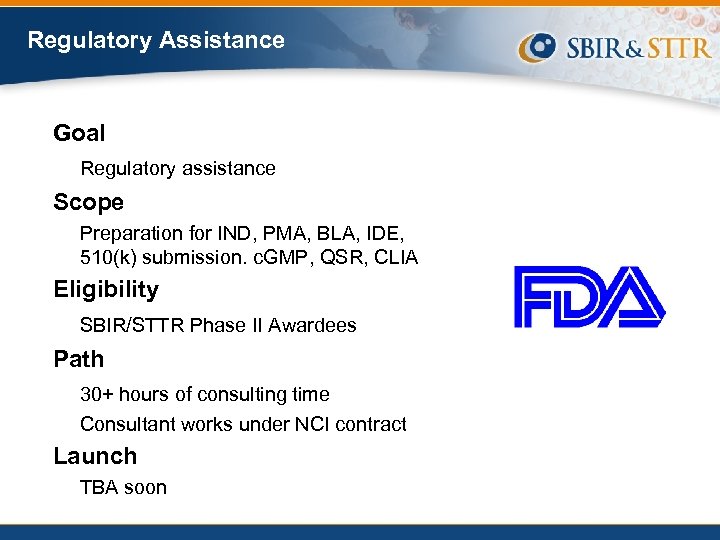
Regulatory Assistance Goal Regulatory assistance Scope Preparation for IND, PMA, BLA, IDE, 510(k) submission. c. GMP, QSR, CLIA Eligibility SBIR/STTR Phase II Awardees Path 30+ hours of consulting time Consultant works under NCI contract Launch TBA soon

New SBIR Bridge Award

Phase II SBIR and Commercialization Success Today, many awardees complete the SBIR Phase II award without advancing the technology far enough to attract private investment • Significant resources are required for getting through the FDA approval process • This funding gap is known as the “Valley of Death”
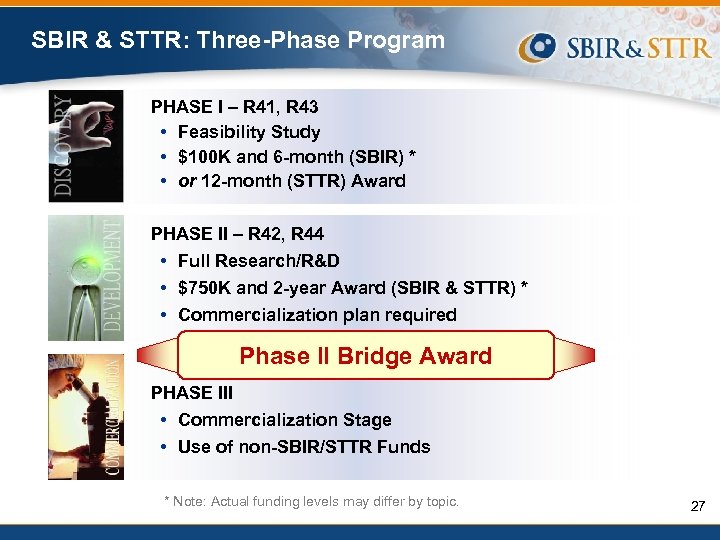
SBIR & STTR: Three-Phase Program PHASE I – R 41, R 43 • Feasibility Study • $100 K and 6 -month (SBIR) * • or 12 -month (STTR) Award PHASE II – R 42, R 44 • Full Research/R&D • $750 K and 2 -year Award (SBIR & STTR) * • Commercialization plan required Phase II Bridge Award PHASE III • Commercialization Stage • Use of non-SBIR/STTR Funds * Note: Actual funding levels may differ by topic. 27

SBIR Phase II Bridge Award Follow-on to SBIR Phase II • Goal to help early-stage companies cross the “Valley of Death” by: • Incentivizing third-party investment earlier in the development process by Ø Sharing in the investment risk with third-party investors RFA Incentive Structure • Gives competitive preference and funding priority to applicants that can raise substantial third-party funds (i. e. , minimum 1: 1 match) • Affords NIH the opportunity to leverage millions in external resources • Third-party investors are also expected to provide valuable input in several ways: 1. Rigorous commercialization due diligence prior to award 2. Commercialization guidance during the award 3. Additional financing beyond the Bridge Award project period 28

RFA Highlights Mechanism & Budgets • Uses the SBIR Phase II (R 44) competing renewal mechanism • Provides up to $1 M per year for up to 3 years • Available to current Phase II grant awards, and those that ended within last 2 years Preferred Third-Party Matching Funds • Cash, liquid assets, convertible debt Sources of Funds • Another company, venture capital firm, individual “angel” investor, foundation, university, state or local government, or any combination 29
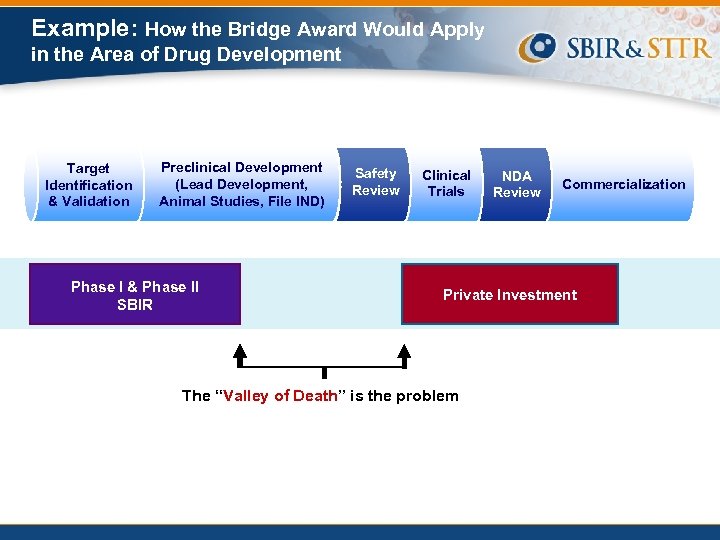
Example: How the Bridge Award Would Apply in the Area of Drug Development Target Identification & Validation Preclinical Development (Lead Development, Animal Studies, File IND) Phase I & Phase II SBIR Safety Review Clinical Trials NDA Review Commercialization Private Investment The “Valley of Death” is the problem
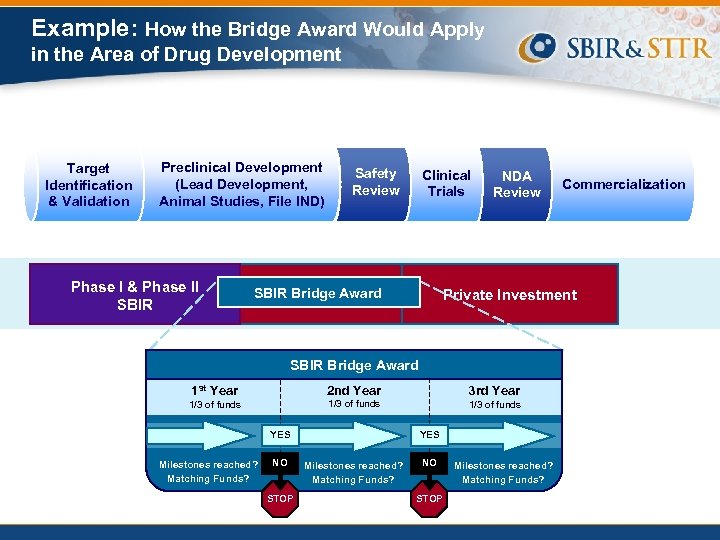
Example: How the Bridge Award Would Apply in the Area of Drug Development Target Identification & Validation Preclinical Development (Lead Development, Animal Studies, File IND) Phase I & Phase II SBIR Safety Review Clinical Trials SBIR Bridge Award NDA Review Private Investment SBIR Bridge Award 1 st Year 2 nd Year 3 rd Year 1/3 of funds YES Milestones reached? Matching Funds? NO STOP YES Milestones reached? Matching Funds? Commercialization NO STOP Milestones reached? Matching Funds?

FY 2009 Bridge Awards San Diego, CA $3. 0 M for the commercialization of ASONEP™, a firstin-class monoclonal antibody against the angiogenic growth factor S 1 P Oriental, NC $3. 0 M for the development of a photoacoustic computed tomography (CT) scanner for preclinical molecular imaging Norcross, GA $2. 5 M for the development of Light. Touch®, a point-ofcare device for cervical cancer screening Northridge, CA $3. 0 M for the development of a novel molecular breast imaging technique to guide early-stage patient care Miramar, FL $3. 0 M for the development of ALT-801, a fusion protein consisting of IL-2 coupled with a soluble T-cell receptor fragment that recognizes a specific form of processed p 53 antigen West Henrietta, NY $3. 0 M for the development of a cone beam breast CT scanner 32
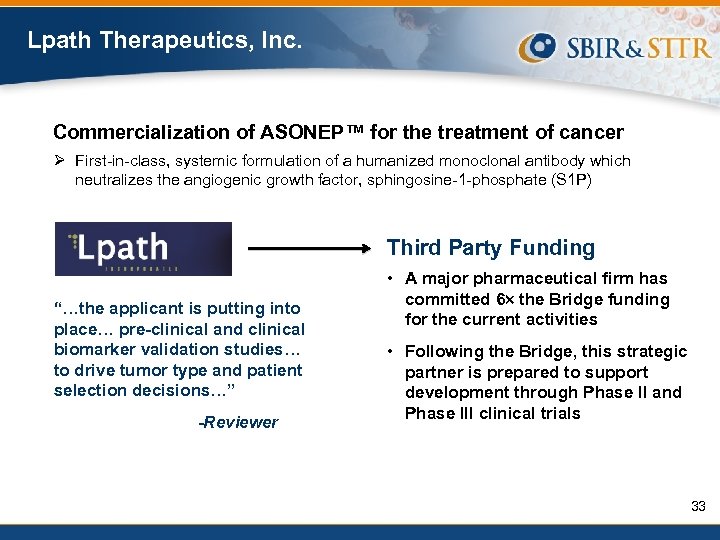
Lpath Therapeutics, Inc. Commercialization of ASONEP™ for the treatment of cancer Ø First-in-class, systemic formulation of a humanized monoclonal antibody which neutralizes the angiogenic growth factor, sphingosine-1 -phosphate (S 1 P) Third Party Funding “…the applicant is putting into place… pre-clinical and clinical biomarker validation studies… to drive tumor type and patient selection decisions…” -Reviewer • A major pharmaceutical firm has committed 6 the Bridge funding for the current activities • Following the Bridge, this strategic partner is prepared to support development through Phase II and Phase III clinical trials 33
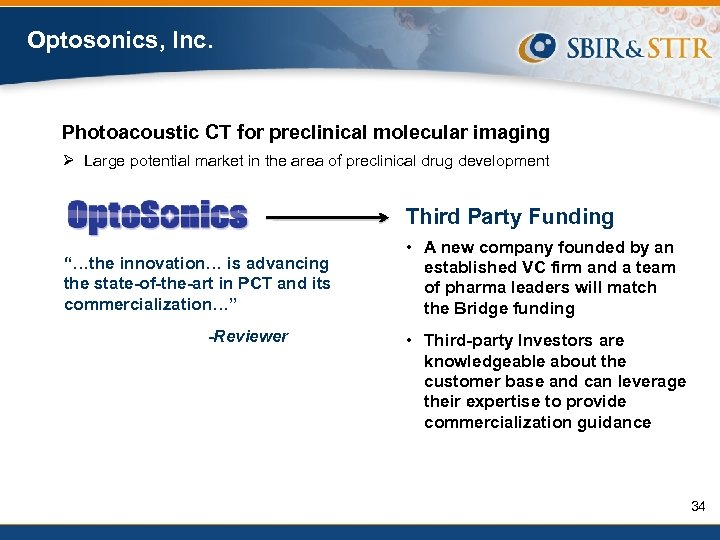
Optosonics, Inc. Photoacoustic CT for preclinical molecular imaging Ø Large potential market in the area of preclinical drug development Third Party Funding “…the innovation… is advancing the state-of-the-art in PCT and its commercialization…” -Reviewer • A new company founded by an established VC firm and a team of pharma leaders will match the Bridge funding • Third-party Investors are knowledgeable about the customer base and can leverage their expertise to provide commercialization guidance 34
Expanded Technical Scope (FY 10 reissuance) Therapeutics • Anticancer drugs and drug delivery systems • Small molecules, biologics and vaccines Devices for Cancer Therapy • Devices for therapeutic (anticancer) use of ionizing radiation • Other ablative techniques Imaging Technologies • Medical devices for in vivo cancer imaging • Image-guided interventions (e. g. , biopsy, surgery, drug delivery) • Imaging agents Cancer Diagnostics • In vitro diagnostics • Any other diagnostic modality 35

More Information on NCI SBIR & STTR Website http: //sbir. cancer. gov

http: //sbir. cancer. gov David Beylin, MS, MBA Program Director Phone: 301 -496 -0079 beylind@mail. nih. gov Register on web site for funding opportunity updates http: //sbir. cancer. gov
6ef5416bc676f081dbb511abf7d31410.ppt