7c9c345dc2e16b4d8a188a93302a39a2.ppt
- Количество слайдов: 14
 NCI/NIST Perspective on IGI Research and Related Standards Laurence P. Clarke Ph. D, FAAPM; FISMRM NCI, DCTD, Cancer Imaging Program Detail (NIBIB) Guest Scientist ( NIST) “NCI GT” and NA-MIC Workshop Oct 19 th st 2006
NCI/NIST Perspective on IGI Research and Related Standards Laurence P. Clarke Ph. D, FAAPM; FISMRM NCI, DCTD, Cancer Imaging Program Detail (NIBIB) Guest Scientist ( NIST) “NCI GT” and NA-MIC Workshop Oct 19 th st 2006
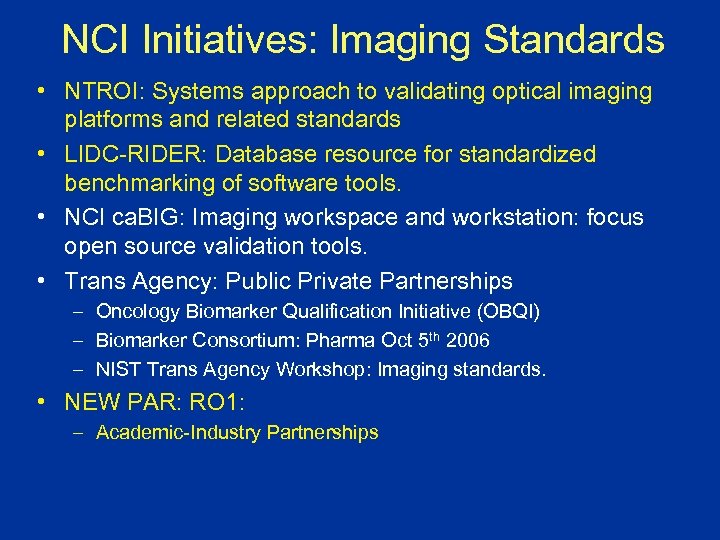 NCI Initiatives: Imaging Standards • NTROI: Systems approach to validating optical imaging platforms and related standards • LIDC-RIDER: Database resource for standardized benchmarking of software tools. • NCI ca. BIG: Imaging workspace and workstation: focus open source validation tools. • Trans Agency: Public Private Partnerships – Oncology Biomarker Qualification Initiative (OBQI) – Biomarker Consortium: Pharma Oct 5 th 2006 – NIST Trans Agency Workshop: Imaging standards. • NEW PAR: RO 1: – Academic-Industry Partnerships
NCI Initiatives: Imaging Standards • NTROI: Systems approach to validating optical imaging platforms and related standards • LIDC-RIDER: Database resource for standardized benchmarking of software tools. • NCI ca. BIG: Imaging workspace and workstation: focus open source validation tools. • Trans Agency: Public Private Partnerships – Oncology Biomarker Qualification Initiative (OBQI) – Biomarker Consortium: Pharma Oct 5 th 2006 – NIST Trans Agency Workshop: Imaging standards. • NEW PAR: RO 1: – Academic-Industry Partnerships
 NTROI Objectives: U 54: 2003 -08 Build an international research network to develop a systems approach for molecular imaging for drug discovery, drug response and IG delivery • Bridge disciplines: Oversight by a steering committee: Funded activity. – Connect instrumentation development teams to biologists, chemists, and clinical teams – Sharing of optical and molecular imaging probes and cross validation studies – Sharing common IT and open source tools – Engage scientists with different federal agencies and industry: FDA, NIST, NIH and over 30 cooperate partners, to develop broad consensus on validation methods that may lead to imaging standards.
NTROI Objectives: U 54: 2003 -08 Build an international research network to develop a systems approach for molecular imaging for drug discovery, drug response and IG delivery • Bridge disciplines: Oversight by a steering committee: Funded activity. – Connect instrumentation development teams to biologists, chemists, and clinical teams – Sharing of optical and molecular imaging probes and cross validation studies – Sharing common IT and open source tools – Engage scientists with different federal agencies and industry: FDA, NIST, NIH and over 30 cooperate partners, to develop broad consensus on validation methods that may lead to imaging standards.
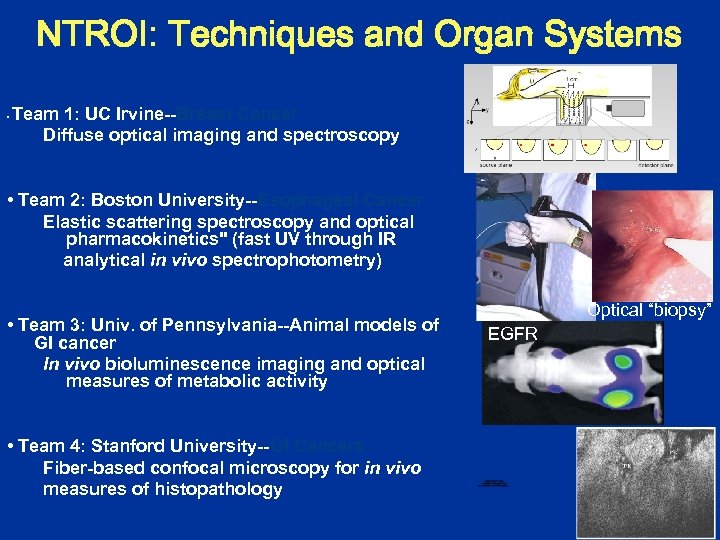 NTROI: Techniques and Organ Systems • Team 1: UC Irvine--Breast Cancer Diffuse optical imaging and spectroscopy • Team 2: Boston University--Esophageal Cancer Elastic scattering spectroscopy and optical pharmacokinetics" (fast UV through IR analytical in vivo spectrophotometry) • Team 3: Univ. of Pennsylvania--Animal models of GI cancer In vivo bioluminescence imaging and optical measures of metabolic activity • Team 4: Stanford University--GI Cancers Fiber-based confocal microscopy for in vivo measures of histopathology Optical “biopsy” EGFR
NTROI: Techniques and Organ Systems • Team 1: UC Irvine--Breast Cancer Diffuse optical imaging and spectroscopy • Team 2: Boston University--Esophageal Cancer Elastic scattering spectroscopy and optical pharmacokinetics" (fast UV through IR analytical in vivo spectrophotometry) • Team 3: Univ. of Pennsylvania--Animal models of GI cancer In vivo bioluminescence imaging and optical measures of metabolic activity • Team 4: Stanford University--GI Cancers Fiber-based confocal microscopy for in vivo measures of histopathology Optical “biopsy” EGFR
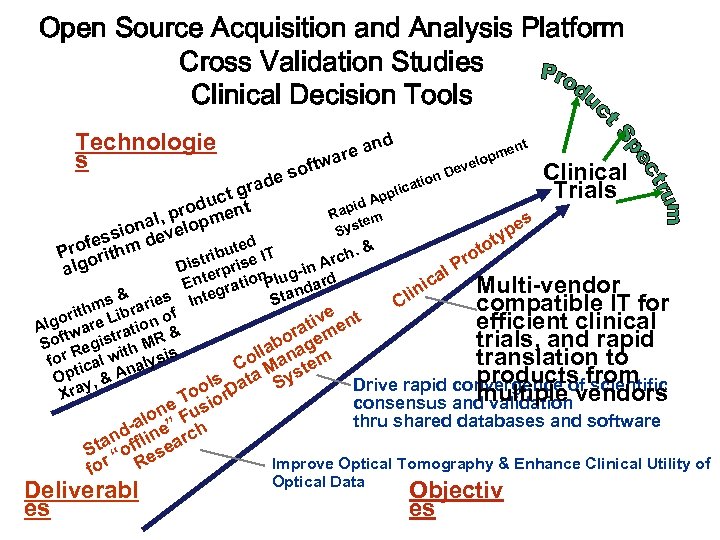 Open Source Acquisition and Analysis Platform Cross Validation Studies Clinical Decision Tools Technologie s d e an r ftwa ent pm velo o De Clinical de s tion a a lic gr Trials App uct t id d o Rap em l, prlopmen s t Sys pe iona eve ss ty d & roferithm d to ute IT P o h. ib o Arc istr prise g Pr D in al l ger Ent gration. Plu ndard ica Multi-vendor lin Sta s & aries Inte C compatible IT for m e t rith Libr n of o iv n efficient clinical Alg tware ratio & rat eme t f o g trials, and rapid b So Regis th MR i s lla ana m translation to for ical w alysi Co M ste pt & An a y products scientific O y, Drive rapid convergence of from ols n at S a D o Xr multiple consensus and validation vendors e T usio n thru shared databases and software alo e” F h d- lin rc tan off sea S “ e Improve Optical Tomography & Enhance Clinical Utility of for R Deliverabl es Optical Data Objectiv es
Open Source Acquisition and Analysis Platform Cross Validation Studies Clinical Decision Tools Technologie s d e an r ftwa ent pm velo o De Clinical de s tion a a lic gr Trials App uct t id d o Rap em l, prlopmen s t Sys pe iona eve ss ty d & roferithm d to ute IT P o h. ib o Arc istr prise g Pr D in al l ger Ent gration. Plu ndard ica Multi-vendor lin Sta s & aries Inte C compatible IT for m e t rith Libr n of o iv n efficient clinical Alg tware ratio & rat eme t f o g trials, and rapid b So Regis th MR i s lla ana m translation to for ical w alysi Co M ste pt & An a y products scientific O y, Drive rapid convergence of from ols n at S a D o Xr multiple consensus and validation vendors e T usio n thru shared databases and software alo e” F h d- lin rc tan off sea S “ e Improve Optical Tomography & Enhance Clinical Utility of for R Deliverabl es Optical Data Objectiv es
 Reference Image Database for Drug Response (RIDER)
Reference Image Database for Drug Response (RIDER)
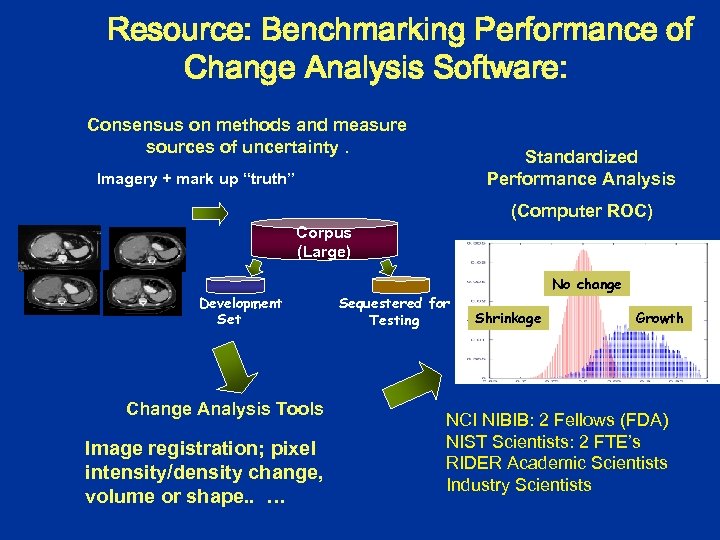 Resource: Benchmarking Performance of Change Analysis Software: Consensus on methods and measure sources of uncertainty. Standardized Performance Analysis Imagery + mark up “truth” (Computer ROC) Corpus (Large) Development Set Change Analysis Tools Image registration; pixel intensity/density change, volume or shape. . … Sequestered for Testing No change Shrinkage Growth NCI NIBIB: 2 Fellows (FDA) NIST Scientists: 2 FTE’s RIDER Academic Scientists Industry Scientists
Resource: Benchmarking Performance of Change Analysis Software: Consensus on methods and measure sources of uncertainty. Standardized Performance Analysis Imagery + mark up “truth” (Computer ROC) Corpus (Large) Development Set Change Analysis Tools Image registration; pixel intensity/density change, volume or shape. . … Sequestered for Testing No change Shrinkage Growth NCI NIBIB: 2 Fellows (FDA) NIST Scientists: 2 FTE’s RIDER Academic Scientists Industry Scientists
 One potential method to engage stakeholders for IGI standards
One potential method to engage stakeholders for IGI standards
 NIST Workshop Report • The NIST meeting was a stakeholders meeting attended by 260 scientists: – Imaging and Pharma Companies, – CRO’s and Trade Organizations • (NEMA, Ph. RMA Consortium), – RSNA, ACR, AAPM, SNM, ISMRM – Federal Government • (NIH: NCI, NIBIB, NIAMS, NIGM, NCRR) • FDA (CDRH, CDER) • NIST (CSTL, EEEL, PL, MSEL, MEL ).
NIST Workshop Report • The NIST meeting was a stakeholders meeting attended by 260 scientists: – Imaging and Pharma Companies, – CRO’s and Trade Organizations • (NEMA, Ph. RMA Consortium), – RSNA, ACR, AAPM, SNM, ISMRM – Federal Government • (NIH: NCI, NIBIB, NIAMS, NIGM, NCRR) • FDA (CDRH, CDER) • NIST (CSTL, EEEL, PL, MSEL, MEL ).
 NIST Workshop Report • There was a very strong agreement across all stakeholders that: – uncertainty in data collection and analysis across single and different imaging platforms was an important problem for quantitative measurements as applied to drug trials – i. e. . , in addition to the biological uncertainty, which was not a focus of this workshop.
NIST Workshop Report • There was a very strong agreement across all stakeholders that: – uncertainty in data collection and analysis across single and different imaging platforms was an important problem for quantitative measurements as applied to drug trials – i. e. . , in addition to the biological uncertainty, which was not a focus of this workshop.
 NIST Workshop Report • There was a strong interest by all stakeholders to address these measurement problems and proposed several short, mid- and long-term recommendations to implement standards: – Several academic societies are willing to both support and collaborate with Gov. agencies , leveraging the volunteer time of their members. – NEMA expressed interest in representing the imaging industry in any interagency or NIST effort, and partner with PHRMA – RSNA expressed an interest in helping to facilitate follow up stakeholders meetings, IHE was posed as an example, that was industry lead.
NIST Workshop Report • There was a strong interest by all stakeholders to address these measurement problems and proposed several short, mid- and long-term recommendations to implement standards: – Several academic societies are willing to both support and collaborate with Gov. agencies , leveraging the volunteer time of their members. – NEMA expressed interest in representing the imaging industry in any interagency or NIST effort, and partner with PHRMA – RSNA expressed an interest in helping to facilitate follow up stakeholders meetings, IHE was posed as an example, that was industry lead.
 Examples of Areas Mentioned • Define the physical performance of different imaging platforms required to measure change analysis. • Design phantoms that may better characterize the time related physical performance of imaging systems, and the performance for specific functional and molecular based measurements. • Develop and share open source tools to analyze phantom or simulated data. • Develop and share open source tools for validation and image mark up of clinical data, including statistical methods. • Develop public resources to help optimize and validate imaging methods prior to their implementation in drug trials. • Develop scalable resources to archive de-identified image and meta data, in collaboration with industry.
Examples of Areas Mentioned • Define the physical performance of different imaging platforms required to measure change analysis. • Design phantoms that may better characterize the time related physical performance of imaging systems, and the performance for specific functional and molecular based measurements. • Develop and share open source tools to analyze phantom or simulated data. • Develop and share open source tools for validation and image mark up of clinical data, including statistical methods. • Develop public resources to help optimize and validate imaging methods prior to their implementation in drug trials. • Develop scalable resources to archive de-identified image and meta data, in collaboration with industry.
 NCI PAR: Academic-Industry Partnerships • R 01: Goal: Accelerate translational research, encourage delivery of technologies and methods for multi site clinical trials. April 1 st 2007. • Partnerships Required or encouraged. – Industry Device and/ or Pharma required. – Collaboration with NIH intramural, FDA, NIST scientists, through the use of visiting scientists – Multi PI’s and linked R 01’s permitted – Collaboration with other cooperative agreements • Research emphasis: – Imaging Platforms: Human and Pre Clinical – Validation of multi-modality imaging platforms – Open source architecture and software tools – Development of public resource for Q/C, validation of imaging methods- software tools
NCI PAR: Academic-Industry Partnerships • R 01: Goal: Accelerate translational research, encourage delivery of technologies and methods for multi site clinical trials. April 1 st 2007. • Partnerships Required or encouraged. – Industry Device and/ or Pharma required. – Collaboration with NIH intramural, FDA, NIST scientists, through the use of visiting scientists – Multi PI’s and linked R 01’s permitted – Collaboration with other cooperative agreements • Research emphasis: – Imaging Platforms: Human and Pre Clinical – Validation of multi-modality imaging platforms – Open source architecture and software tools – Development of public resource for Q/C, validation of imaging methods- software tools
 URL’s for reference • NCI CIP Program: : http: //imaging. cancer. gov/ • NCI FDA IOTF: http: //iotftraining. nci. nih. gov/ • OBQI: http: //ww. cancer. gov/newscenter/pressreleases/OBQI • FDA new pathways report: Drug Response: http: //www. fda. gov/oc/initiatives/criticalpath/ • RIDER Database resource for software evaluation: • http: //imaging. nci. nih. gov/i 3/ • NIST Workshop: http: //usms. nist. gov/workshops/bioimaging. htm • NIH Road Map: http: //nihroadmap. nih. gov/bioinformatics/grants. asp
URL’s for reference • NCI CIP Program: : http: //imaging. cancer. gov/ • NCI FDA IOTF: http: //iotftraining. nci. nih. gov/ • OBQI: http: //ww. cancer. gov/newscenter/pressreleases/OBQI • FDA new pathways report: Drug Response: http: //www. fda. gov/oc/initiatives/criticalpath/ • RIDER Database resource for software evaluation: • http: //imaging. nci. nih. gov/i 3/ • NIST Workshop: http: //usms. nist. gov/workshops/bioimaging. htm • NIH Road Map: http: //nihroadmap. nih. gov/bioinformatics/grants. asp


