37191980aaa6023f7fe96d93f53ea6dc.ppt
- Количество слайдов: 38
 NC STATE UNIVERSITY U. A. S. L. P. Process Integration for Environmental Control in Engineering Curricula “AIR POLLUTION” I. Q. Francisco Gómez Rivera Universidad Autónoma de San Luis Potosí Dr. John Heitmann Jr. Dr. Pedro Medellín Milán Universidad Autónoma de San Luis Potosí January-May 2005 North Carolina State University
NC STATE UNIVERSITY U. A. S. L. P. Process Integration for Environmental Control in Engineering Curricula “AIR POLLUTION” I. Q. Francisco Gómez Rivera Universidad Autónoma de San Luis Potosí Dr. John Heitmann Jr. Dr. Pedro Medellín Milán Universidad Autónoma de San Luis Potosí January-May 2005 North Carolina State University
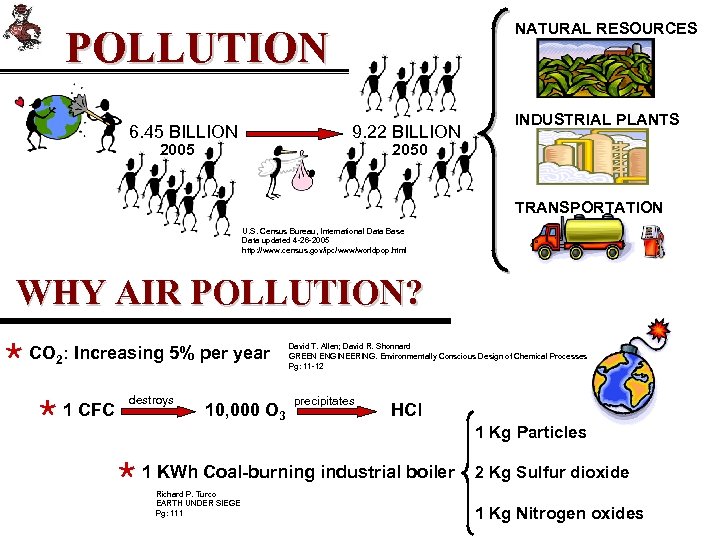 NATURAL RESOURCES POLLUTION 6. 45 BILLION 9. 22 BILLION 2005 INDUSTRIAL PLANTS 2050 TRANSPORTATION U. S. Census Bureau, International Data Base Data updated 4 -26 -2005 http: //www. census. gov/ipc/www/worldpop. html WHY AIR POLLUTION? × CO 2: Increasing 5% per year × 1 CFC destroys 10, 000 O 3 David T. Allen; David R. Shonnard GREEN ENGINEERING. Environmentally Conscious Design of Chemical Processes Pg: 11 -12 precipitates HCl × 1 KWh Coal-burning industrial boiler Richard P. Turco EARTH UNDER SIEGE Pg: 111 1 Kg Particles 2 Kg Sulfur dioxide 1 Kg Nitrogen oxides
NATURAL RESOURCES POLLUTION 6. 45 BILLION 9. 22 BILLION 2005 INDUSTRIAL PLANTS 2050 TRANSPORTATION U. S. Census Bureau, International Data Base Data updated 4 -26 -2005 http: //www. census. gov/ipc/www/worldpop. html WHY AIR POLLUTION? × CO 2: Increasing 5% per year × 1 CFC destroys 10, 000 O 3 David T. Allen; David R. Shonnard GREEN ENGINEERING. Environmentally Conscious Design of Chemical Processes Pg: 11 -12 precipitates HCl × 1 KWh Coal-burning industrial boiler Richard P. Turco EARTH UNDER SIEGE Pg: 111 1 Kg Particles 2 Kg Sulfur dioxide 1 Kg Nitrogen oxides
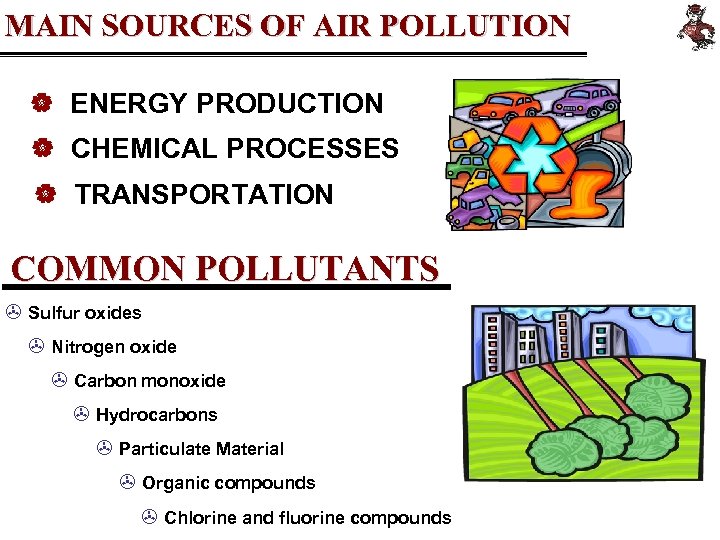 MAIN SOURCES OF AIR POLLUTION | ENERGY PRODUCTION | CHEMICAL PROCESSES | TRANSPORTATION COMMON POLLUTANTS > Sulfur oxides > Nitrogen oxide > Carbon monoxide > Hydrocarbons > Particulate Material > Organic compounds > Chlorine and fluorine compounds
MAIN SOURCES OF AIR POLLUTION | ENERGY PRODUCTION | CHEMICAL PROCESSES | TRANSPORTATION COMMON POLLUTANTS > Sulfur oxides > Nitrogen oxide > Carbon monoxide > Hydrocarbons > Particulate Material > Organic compounds > Chlorine and fluorine compounds
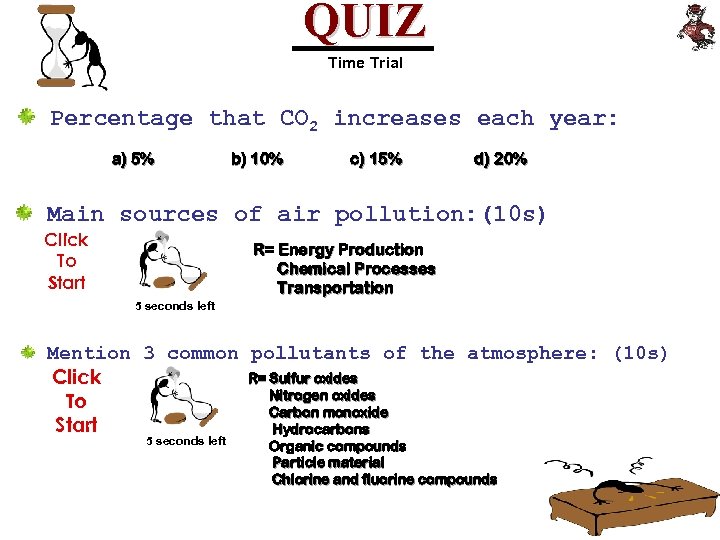 QUIZ Time Trial Percentage that CO 2 increases each year: a) 5% b) 10% c) 15% d) 20% Main sources of air pollution: (10 s) Click To Start R= Energy Production Chemical Processes Transportation 5 seconds left Mention 3 common pollutants of the atmosphere: (10 s) R= Sulfur oxides Click Nitrogen oxides To Carbon monoxide Start Hydrocarbons 5 seconds left Organic compounds Particle material Chlorine and fluorine compounds
QUIZ Time Trial Percentage that CO 2 increases each year: a) 5% b) 10% c) 15% d) 20% Main sources of air pollution: (10 s) Click To Start R= Energy Production Chemical Processes Transportation 5 seconds left Mention 3 common pollutants of the atmosphere: (10 s) R= Sulfur oxides Click Nitrogen oxides To Carbon monoxide Start Hydrocarbons 5 seconds left Organic compounds Particle material Chlorine and fluorine compounds
 ENERGY PRODUCTION SITUATION
ENERGY PRODUCTION SITUATION
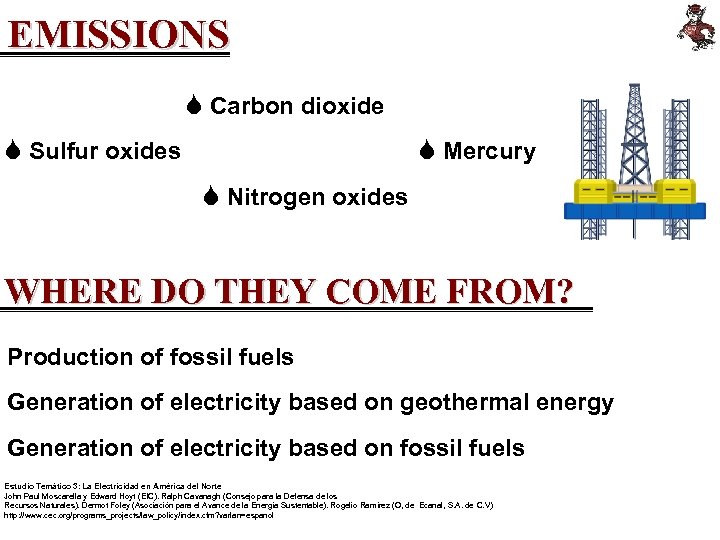 EMISSIONS S Carbon dioxide S Sulfur oxides S Mercury S Nitrogen oxides WHERE DO THEY COME FROM? Production of fossil fuels Generation of electricity based on geothermal energy Generation of electricity based on fossil fuels Estudio Temático 3: La Electricidad en América del Norte John Paul Moscarella y Edward Hoyt (EIC). Ralph Cavanagh (Consejo para la Defensa de los Recursos Naturales). Dermot Foley (Asociación para el Avance de la Energía Sustentable). Rogelio Ramírez (O, de Ecanal, S. A. de C. V) http: //www. cec. org/programs_projects/law_policy/index. cfm? varlan=espanol
EMISSIONS S Carbon dioxide S Sulfur oxides S Mercury S Nitrogen oxides WHERE DO THEY COME FROM? Production of fossil fuels Generation of electricity based on geothermal energy Generation of electricity based on fossil fuels Estudio Temático 3: La Electricidad en América del Norte John Paul Moscarella y Edward Hoyt (EIC). Ralph Cavanagh (Consejo para la Defensa de los Recursos Naturales). Dermot Foley (Asociación para el Avance de la Energía Sustentable). Rogelio Ramírez (O, de Ecanal, S. A. de C. V) http: //www. cec. org/programs_projects/law_policy/index. cfm? varlan=espanol
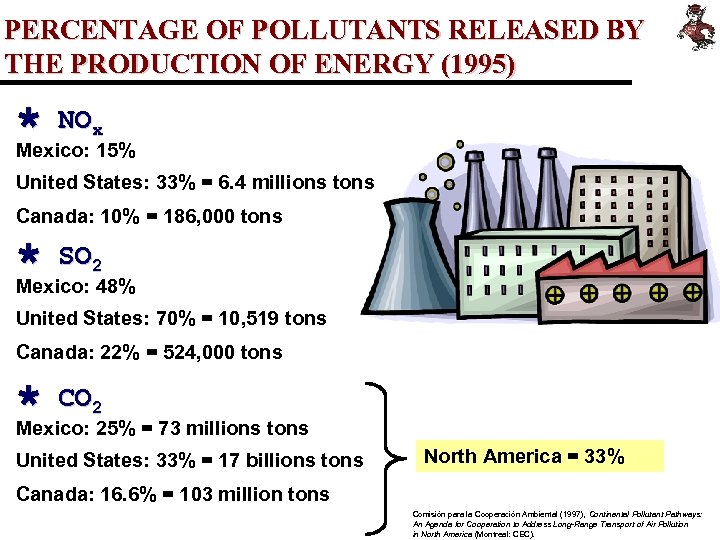 PERCENTAGE OF POLLUTANTS RELEASED BY THE PRODUCTION OF ENERGY (1995) Ù NOx Mexico: 15% United States: 33% = 6. 4 millions tons Canada: 10% = 186, 000 tons Ù SO 2 Mexico: 48% United States: 70% = 10, 519 tons Canada: 22% = 524, 000 tons Ù CO 2 Mexico: 25% = 73 millions tons United States: 33% = 17 billions tons North America = 33% Canada: 16. 6% = 103 million tons Comisión para la Cooperación Ambiental (1997), Continental Pollutant Pathways: An Agenda for Cooperation to Address Long-Range Transport of Air Pollution in North America (Montreal: CEC).
PERCENTAGE OF POLLUTANTS RELEASED BY THE PRODUCTION OF ENERGY (1995) Ù NOx Mexico: 15% United States: 33% = 6. 4 millions tons Canada: 10% = 186, 000 tons Ù SO 2 Mexico: 48% United States: 70% = 10, 519 tons Canada: 22% = 524, 000 tons Ù CO 2 Mexico: 25% = 73 millions tons United States: 33% = 17 billions tons North America = 33% Canada: 16. 6% = 103 million tons Comisión para la Cooperación Ambiental (1997), Continental Pollutant Pathways: An Agenda for Cooperation to Address Long-Range Transport of Air Pollution in North America (Montreal: CEC).
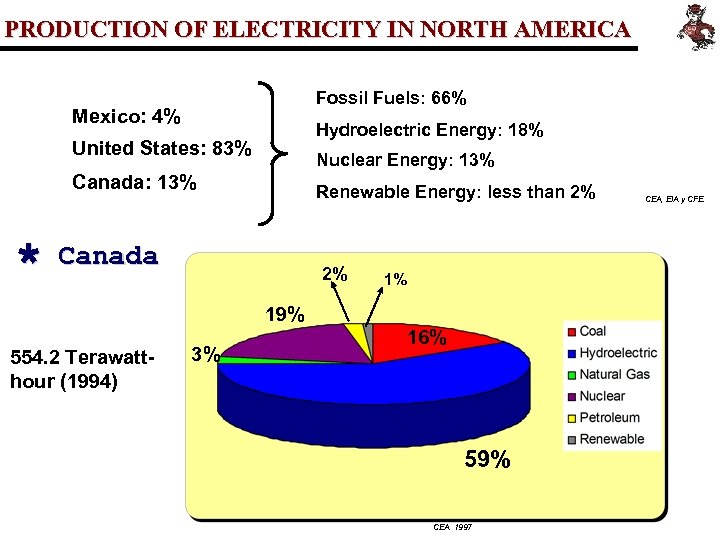 PRODUCTION OF ELECTRICITY IN NORTH AMERICA Fossil Fuels: 66% Mexico: 4% Hydroelectric Energy: 18% United States: 83% Nuclear Energy: 13% Canada: 13% Renewable Energy: less than 2% Ù Canada 2% 1% 19% 554. 2 Terawatthour (1994) 3% 16% 59% CEA. 1997 CEA, EIA y CFE.
PRODUCTION OF ELECTRICITY IN NORTH AMERICA Fossil Fuels: 66% Mexico: 4% Hydroelectric Energy: 18% United States: 83% Nuclear Energy: 13% Canada: 13% Renewable Energy: less than 2% Ù Canada 2% 1% 19% 554. 2 Terawatthour (1994) 3% 16% 59% CEA. 1997 CEA, EIA y CFE.
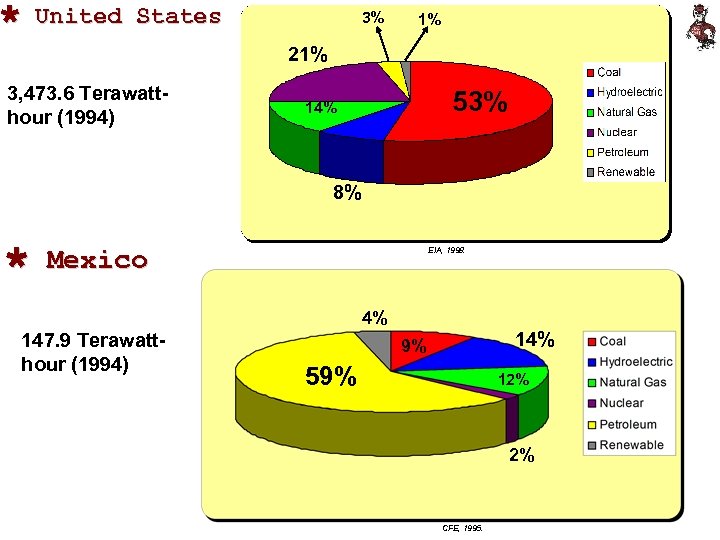 Ù United States 3% 1% 21% 3, 473. 6 Terawatthour (1994) 53% 14% 8% Ù Mexico EIA, 1998. 4% 147. 9 Terawatthour (1994) 14% 9% 59% 12% 2% CFE, 1995.
Ù United States 3% 1% 21% 3, 473. 6 Terawatthour (1994) 53% 14% 8% Ù Mexico EIA, 1998. 4% 147. 9 Terawatthour (1994) 14% 9% 59% 12% 2% CFE, 1995.
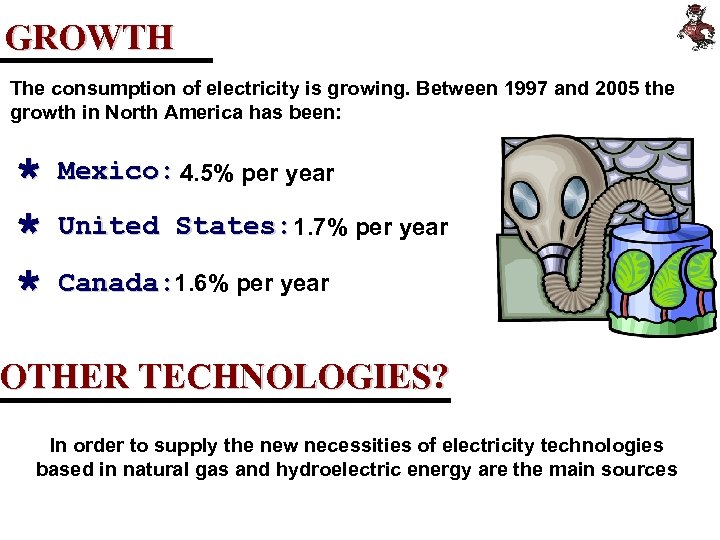 GROWTH The consumption of electricity is growing. Between 1997 and 2005 the growth in North America has been: Ù Mexico: 4. 5% per year Ù United States: 1. 7% per year Ù Canada: 1. 6% per year OTHER TECHNOLOGIES? In order to supply the new necessities of electricity technologies based in natural gas and hydroelectric energy are the main sources
GROWTH The consumption of electricity is growing. Between 1997 and 2005 the growth in North America has been: Ù Mexico: 4. 5% per year Ù United States: 1. 7% per year Ù Canada: 1. 6% per year OTHER TECHNOLOGIES? In order to supply the new necessities of electricity technologies based in natural gas and hydroelectric energy are the main sources
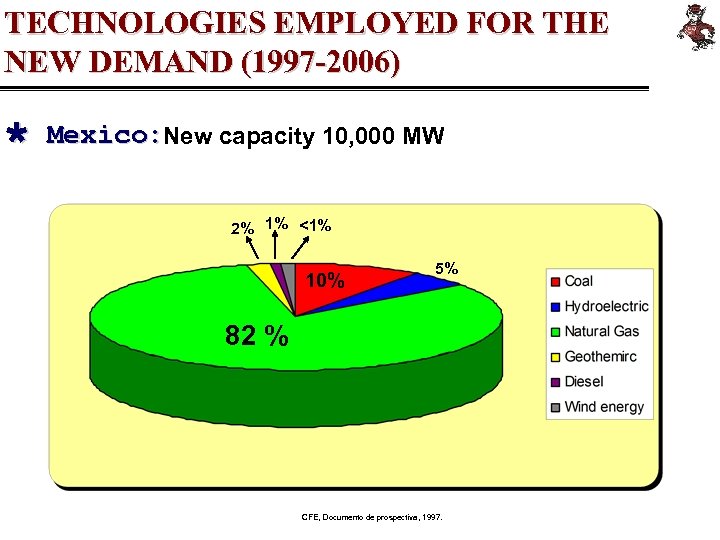 TECHNOLOGIES EMPLOYED FOR THE NEW DEMAND (1997 -2006) Ù Mexico: New capacity 10, 000 MW 2% 1% <1% 10% 5% 82 % CFE, Documento de prospectiva, 1997.
TECHNOLOGIES EMPLOYED FOR THE NEW DEMAND (1997 -2006) Ù Mexico: New capacity 10, 000 MW 2% 1% <1% 10% 5% 82 % CFE, Documento de prospectiva, 1997.
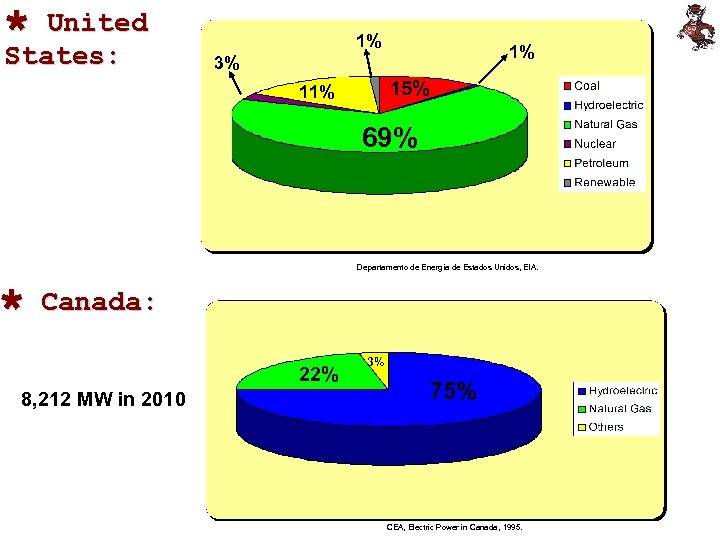 Ù United States: 1% 1% 3% 15% 11% 69% Departamento de Energía de Estados Unidos, EIA. Ù Canada: 22% 8, 212 MW in 2010 3% 75% CEA, Electric Power in Canada, 1995.
Ù United States: 1% 1% 3% 15% 11% 69% Departamento de Energía de Estados Unidos, EIA. Ù Canada: 22% 8, 212 MW in 2010 3% 75% CEA, Electric Power in Canada, 1995.
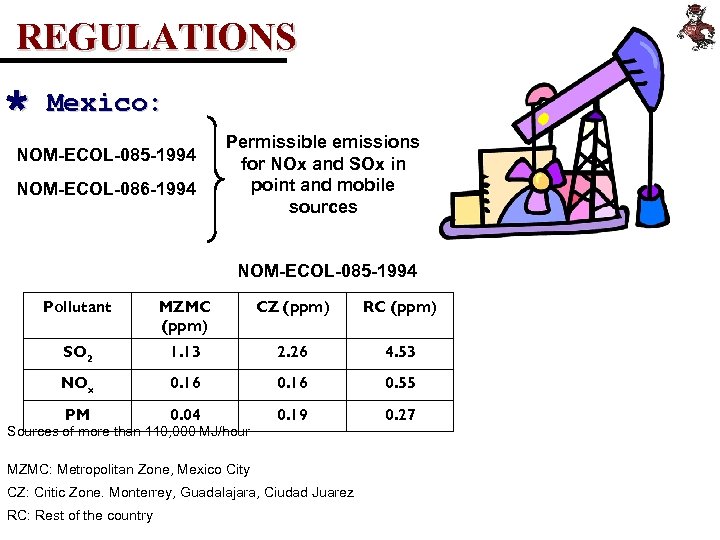 REGULATIONS Ù Mexico: NOM-ECOL-085 -1994 NOM-ECOL-086 -1994 Permissible emissions for NOx and SOx in point and mobile sources NOM-ECOL-085 -1994 Pollutant MZMC (ppm) CZ (ppm) RC (ppm) SO 2 1. 13 2. 26 4. 53 NOx 0. 16 0. 55 PM 0. 04 0. 19 0. 27 Sources of more than 110, 000 MJ/hour MZMC: Metropolitan Zone, Mexico City CZ: Critic Zone. Monterrey, Guadalajara, Ciudad Juarez RC: Rest of the country
REGULATIONS Ù Mexico: NOM-ECOL-085 -1994 NOM-ECOL-086 -1994 Permissible emissions for NOx and SOx in point and mobile sources NOM-ECOL-085 -1994 Pollutant MZMC (ppm) CZ (ppm) RC (ppm) SO 2 1. 13 2. 26 4. 53 NOx 0. 16 0. 55 PM 0. 04 0. 19 0. 27 Sources of more than 110, 000 MJ/hour MZMC: Metropolitan Zone, Mexico City CZ: Critic Zone. Monterrey, Guadalajara, Ciudad Juarez RC: Rest of the country
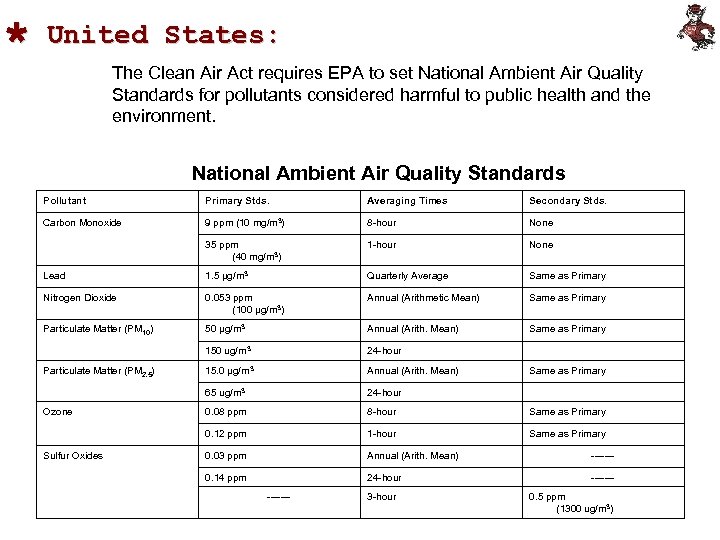 Ù United States: The Clean Air Act requires EPA to set National Ambient Air Quality Standards for pollutants considered harmful to public health and the environment. National Ambient Air Quality Standards Pollutant Primary Stds. Averaging Times Secondary Stds. Carbon Monoxide 9 ppm (10 mg/m 3) 8 -hour None 35 ppm (40 mg/m 3) 1 -hour None Lead 1. 5 µg/m 3 Quarterly Average Same as Primary Nitrogen Dioxide 0. 053 ppm (100 µg/m 3) Annual (Arithmetic Mean) Same as Primary Particulate Matter (PM 10) 50 µg/m 3 Annual (Arith. Mean) Same as Primary 150 ug/m 3 24 -hour 15. 0 µg/m 3 Annual (Arith. Mean) Same as Primary 65 ug/m 3 24 -hour 0. 08 ppm 8 -hour Same as Primary 0. 12 ppm 1 -hour Same as Primary 0. 03 ppm Annual (Arith. Mean) ------- 0. 14 ppm 24 -hour ------- Particulate Matter (PM 2. 5) Ozone Sulfur Oxides ------- 3 -hour 0. 5 ppm (1300 ug/m 3)
Ù United States: The Clean Air Act requires EPA to set National Ambient Air Quality Standards for pollutants considered harmful to public health and the environment. National Ambient Air Quality Standards Pollutant Primary Stds. Averaging Times Secondary Stds. Carbon Monoxide 9 ppm (10 mg/m 3) 8 -hour None 35 ppm (40 mg/m 3) 1 -hour None Lead 1. 5 µg/m 3 Quarterly Average Same as Primary Nitrogen Dioxide 0. 053 ppm (100 µg/m 3) Annual (Arithmetic Mean) Same as Primary Particulate Matter (PM 10) 50 µg/m 3 Annual (Arith. Mean) Same as Primary 150 ug/m 3 24 -hour 15. 0 µg/m 3 Annual (Arith. Mean) Same as Primary 65 ug/m 3 24 -hour 0. 08 ppm 8 -hour Same as Primary 0. 12 ppm 1 -hour Same as Primary 0. 03 ppm Annual (Arith. Mean) ------- 0. 14 ppm 24 -hour ------- Particulate Matter (PM 2. 5) Ozone Sulfur Oxides ------- 3 -hour 0. 5 ppm (1300 ug/m 3)
 QUIZ Time Trial Principal emissions related to energy production : (10 s) Click To Start 5 seconds left R= Carbon dioxide Sulfur oxides Nitrogen oxides Mercury Percentage of CO 2 generated by energy production in N. A. : a) 20% b) 10% c) 40% d) 30% Percentage of fossil fuel in the energy production: a) 60% b) 62% c) 64% d) 66% Name one of the 2 technologies used to meet the new demand: (10 s) Click To Start R= Hydroelectric energy Natural Gas 5 seconds left
QUIZ Time Trial Principal emissions related to energy production : (10 s) Click To Start 5 seconds left R= Carbon dioxide Sulfur oxides Nitrogen oxides Mercury Percentage of CO 2 generated by energy production in N. A. : a) 20% b) 10% c) 40% d) 30% Percentage of fossil fuel in the energy production: a) 60% b) 62% c) 64% d) 66% Name one of the 2 technologies used to meet the new demand: (10 s) Click To Start R= Hydroelectric energy Natural Gas 5 seconds left
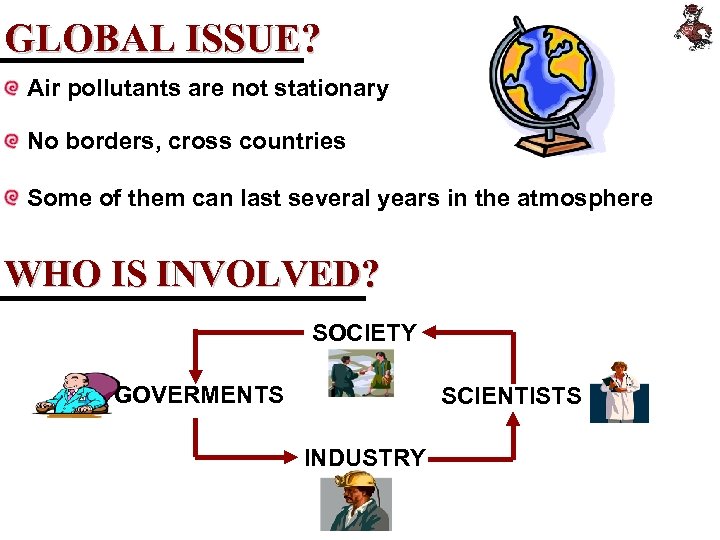 GLOBAL ISSUE? Air pollutants are not stationary No borders, cross countries Some of them can last several years in the atmosphere WHO IS INVOLVED? SOCIETY GOVERMENTS SCIENTISTS INDUSTRY
GLOBAL ISSUE? Air pollutants are not stationary No borders, cross countries Some of them can last several years in the atmosphere WHO IS INVOLVED? SOCIETY GOVERMENTS SCIENTISTS INDUSTRY
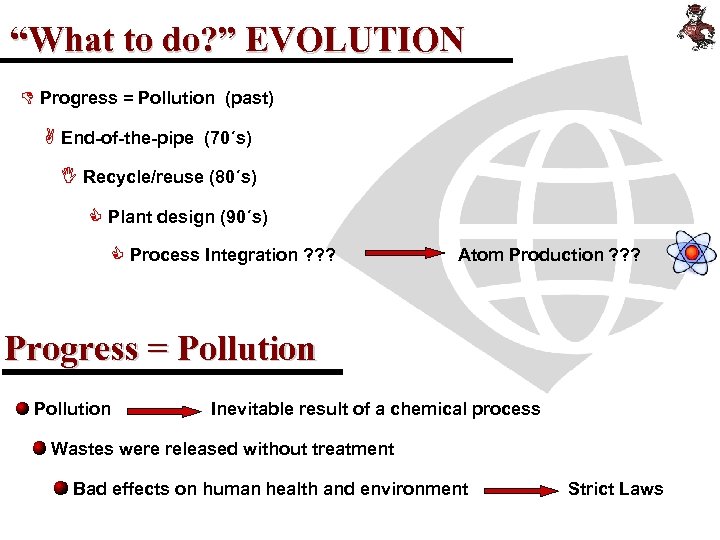 “What to do? ” EVOLUTION D Progress = Pollution (past) A End-of-the-pipe (70´s) I Recycle/reuse (80´s) C Plant design (90´s) C Process Integration ? ? ? Atom Production ? ? ? Progress = Pollution Inevitable result of a chemical process Wastes were released without treatment Bad effects on human health and environment Strict Laws
“What to do? ” EVOLUTION D Progress = Pollution (past) A End-of-the-pipe (70´s) I Recycle/reuse (80´s) C Plant design (90´s) C Process Integration ? ? ? Atom Production ? ? ? Progress = Pollution Inevitable result of a chemical process Wastes were released without treatment Bad effects on human health and environment Strict Laws
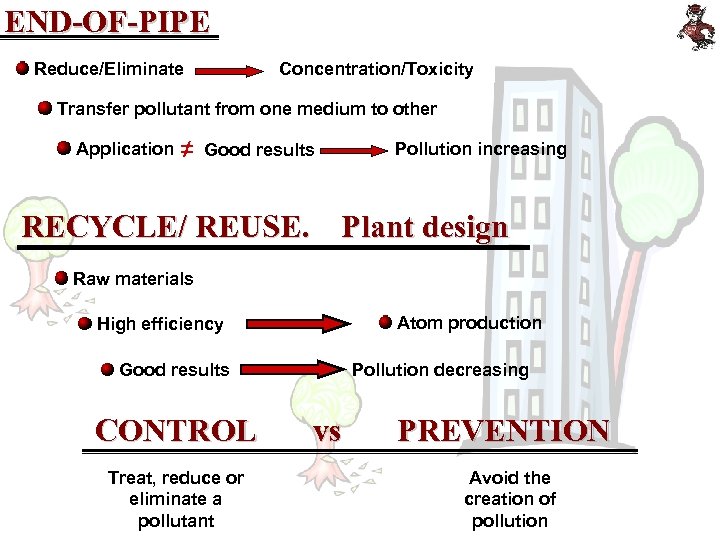 END-OF-PIPE Reduce/Eliminate Concentration/Toxicity Transfer pollutant from one medium to other Application ≠ Good results Pollution increasing RECYCLE/ REUSE. Plant design Raw materials Atom production High efficiency Good results CONTROL Treat, reduce or eliminate a pollutant Pollution decreasing vs PREVENTION Avoid the creation of pollution
END-OF-PIPE Reduce/Eliminate Concentration/Toxicity Transfer pollutant from one medium to other Application ≠ Good results Pollution increasing RECYCLE/ REUSE. Plant design Raw materials Atom production High efficiency Good results CONTROL Treat, reduce or eliminate a pollutant Pollution decreasing vs PREVENTION Avoid the creation of pollution
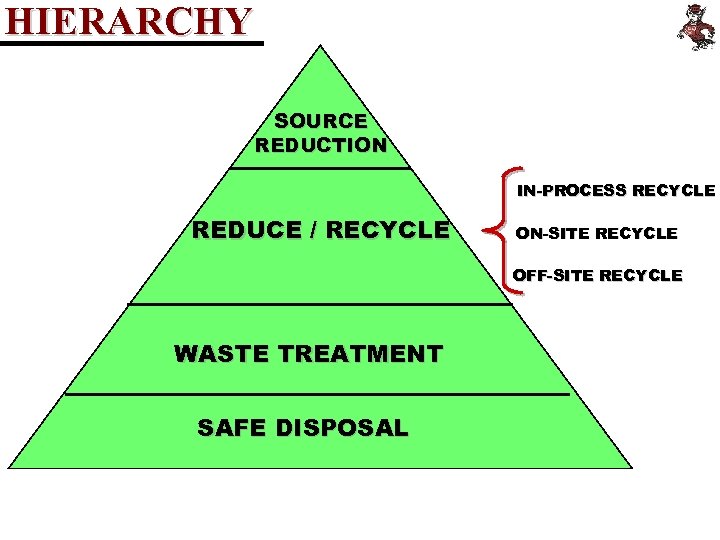 HIERARCHY SOURCE REDUCTION IN-PROCESS RECYCLE REDUCE / RECYCLE ON-SITE RECYCLE OFF-SITE RECYCLE WASTE TREATMENT SAFE DISPOSAL
HIERARCHY SOURCE REDUCTION IN-PROCESS RECYCLE REDUCE / RECYCLE ON-SITE RECYCLE OFF-SITE RECYCLE WASTE TREATMENT SAFE DISPOSAL
 QUIZ Time Trial Name the “what to do? ” evolution: (10 s) Click To Start R= Progress = Pollution End-of-pipe (70’s) 5 seconds left Recycle/Reuse (80’s) Process integration? ? ? Plant design (90’s) What is pollution control? : (10 s) Click To Start R= Treat, reduce or eliminate a pollutant 5 seconds left What is pollution prevention? : (10 s) Click To Start R= Avoid the creation of pollution 5 seconds left Name the hierarchy pyramid: (10 s) Click To Start R= Source reduction Reduce/recycle: in-process, on-site, off-site 5 seconds left Waste treatment Safe disposal
QUIZ Time Trial Name the “what to do? ” evolution: (10 s) Click To Start R= Progress = Pollution End-of-pipe (70’s) 5 seconds left Recycle/Reuse (80’s) Process integration? ? ? Plant design (90’s) What is pollution control? : (10 s) Click To Start R= Treat, reduce or eliminate a pollutant 5 seconds left What is pollution prevention? : (10 s) Click To Start R= Avoid the creation of pollution 5 seconds left Name the hierarchy pyramid: (10 s) Click To Start R= Source reduction Reduce/recycle: in-process, on-site, off-site 5 seconds left Waste treatment Safe disposal
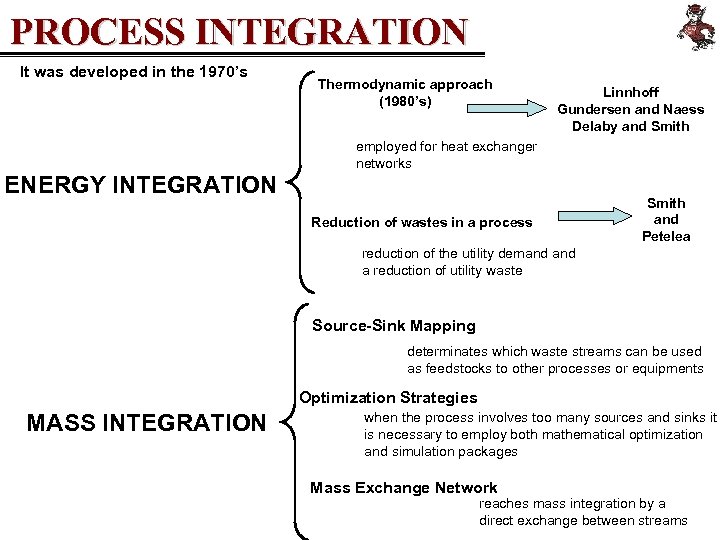 PROCESS INTEGRATION It was developed in the 1970’s Thermodynamic approach (1980’s) Linnhoff Gundersen and Naess Delaby and Smith employed for heat exchanger networks ENERGY INTEGRATION Reduction of wastes in a process Smith and Petelea reduction of the utility demand a reduction of utility waste Source-Sink Mapping determinates which waste streams can be used as feedstocks to other processes or equipments Optimization Strategies MASS INTEGRATION when the process involves too many sources and sinks it is necessary to employ both mathematical optimization and simulation packages Mass Exchange Network reaches mass integration by a direct exchange between streams
PROCESS INTEGRATION It was developed in the 1970’s Thermodynamic approach (1980’s) Linnhoff Gundersen and Naess Delaby and Smith employed for heat exchanger networks ENERGY INTEGRATION Reduction of wastes in a process Smith and Petelea reduction of the utility demand a reduction of utility waste Source-Sink Mapping determinates which waste streams can be used as feedstocks to other processes or equipments Optimization Strategies MASS INTEGRATION when the process involves too many sources and sinks it is necessary to employ both mathematical optimization and simulation packages Mass Exchange Network reaches mass integration by a direct exchange between streams
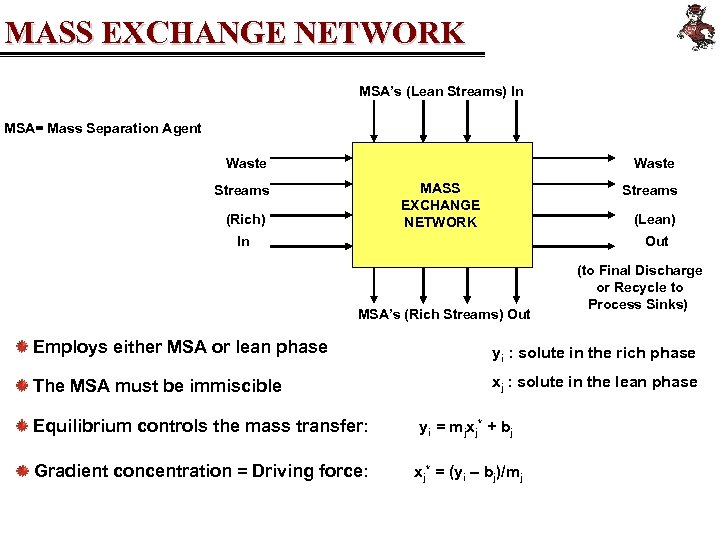 MASS EXCHANGE NETWORK MSA’s (Lean Streams) In MSA= Mass Separation Agent Waste MASS EXCHANGE NETWORK Streams (Rich) Streams (Lean) In Out MSA’s (Rich Streams) Out (to Final Discharge or Recycle to Process Sinks) Employs either MSA or lean phase yi : solute in the rich phase The MSA must be immiscible xj : solute in the lean phase Equilibrium controls the mass transfer: yi = mjxj* + bj Gradient concentration = Driving force: xj* = (yi – bj)/mj
MASS EXCHANGE NETWORK MSA’s (Lean Streams) In MSA= Mass Separation Agent Waste MASS EXCHANGE NETWORK Streams (Rich) Streams (Lean) In Out MSA’s (Rich Streams) Out (to Final Discharge or Recycle to Process Sinks) Employs either MSA or lean phase yi : solute in the rich phase The MSA must be immiscible xj : solute in the lean phase Equilibrium controls the mass transfer: yi = mjxj* + bj Gradient concentration = Driving force: xj* = (yi – bj)/mj
 REGULATIONS COMISSION FOR ENVIRONMENTAL COOPERATION http: //www. cec. org/programs_projects/law_policy/index. cfm? varlan=english CANADA: ENVIRONMENT CANADA http: //www. ec. gc. ca UNITED STATES: ENVIRONMENTAL PROTECTION AGENCY http: //www. epa. com MEXICO: SECRETARIA DEL MEDIO AMBIENTE Y RECURSOS NATURALES http: //www. semarnat. gob. mx
REGULATIONS COMISSION FOR ENVIRONMENTAL COOPERATION http: //www. cec. org/programs_projects/law_policy/index. cfm? varlan=english CANADA: ENVIRONMENT CANADA http: //www. ec. gc. ca UNITED STATES: ENVIRONMENTAL PROTECTION AGENCY http: //www. epa. com MEXICO: SECRETARIA DEL MEDIO AMBIENTE Y RECURSOS NATURALES http: //www. semarnat. gob. mx
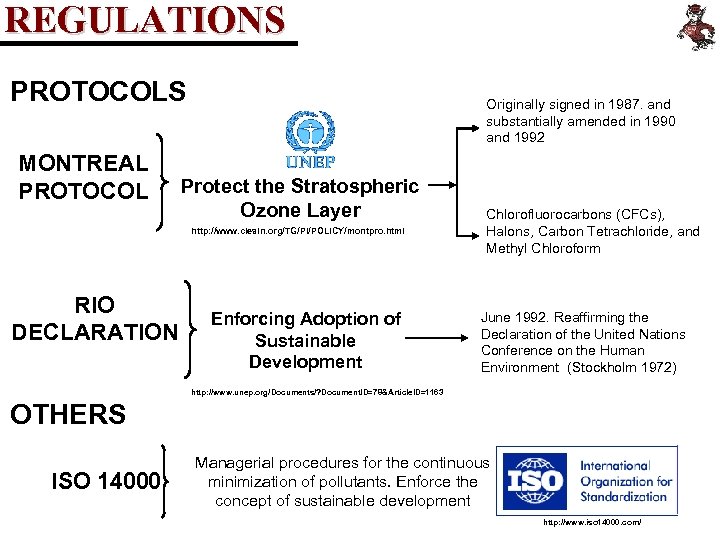 REGULATIONS PROTOCOLS MONTREAL PROTOCOL Originally signed in 1987. and substantially amended in 1990 and 1992 Protect the Stratospheric Ozone Layer http: //www. ciesin. org/TG/PI/POLICY/montpro. html RIO DECLARATION Enforcing Adoption of Sustainable Development Chlorofluorocarbons (CFCs), Halons, Carbon Tetrachloride, and Methyl Chloroform June 1992. Reaffirming the Declaration of the United Nations Conference on the Human Environment (Stockholm 1972) http: //www. unep. org/Documents/? Document. ID=78&Article. ID=1163 OTHERS ISO 14000 Managerial procedures for the continuous minimization of pollutants. Enforce the concept of sustainable development http: //www. iso 14000. com/
REGULATIONS PROTOCOLS MONTREAL PROTOCOL Originally signed in 1987. and substantially amended in 1990 and 1992 Protect the Stratospheric Ozone Layer http: //www. ciesin. org/TG/PI/POLICY/montpro. html RIO DECLARATION Enforcing Adoption of Sustainable Development Chlorofluorocarbons (CFCs), Halons, Carbon Tetrachloride, and Methyl Chloroform June 1992. Reaffirming the Declaration of the United Nations Conference on the Human Environment (Stockholm 1972) http: //www. unep. org/Documents/? Document. ID=78&Article. ID=1163 OTHERS ISO 14000 Managerial procedures for the continuous minimization of pollutants. Enforce the concept of sustainable development http: //www. iso 14000. com/
 QUIZ Time Trial Governmental Offices in charge of environmental quality on each country : (10 s) Click To Start 5 seconds left R= Canada: Environment Canada United States: EPA Mexico: Semarnat Protocols that protect the environment: (10 s) Click To Start R= Montreal Protocol Rio de Janeiro Convention 5 seconds left Branches of Process Integration: (10 s) Click To Start R= Energy Integration Mass Integration 5 seconds left Principal driving force on mass exchange: (10 s) Click To Start R= Gradient of concentration 5 seconds left
QUIZ Time Trial Governmental Offices in charge of environmental quality on each country : (10 s) Click To Start 5 seconds left R= Canada: Environment Canada United States: EPA Mexico: Semarnat Protocols that protect the environment: (10 s) Click To Start R= Montreal Protocol Rio de Janeiro Convention 5 seconds left Branches of Process Integration: (10 s) Click To Start R= Energy Integration Mass Integration 5 seconds left Principal driving force on mass exchange: (10 s) Click To Start R= Gradient of concentration 5 seconds left
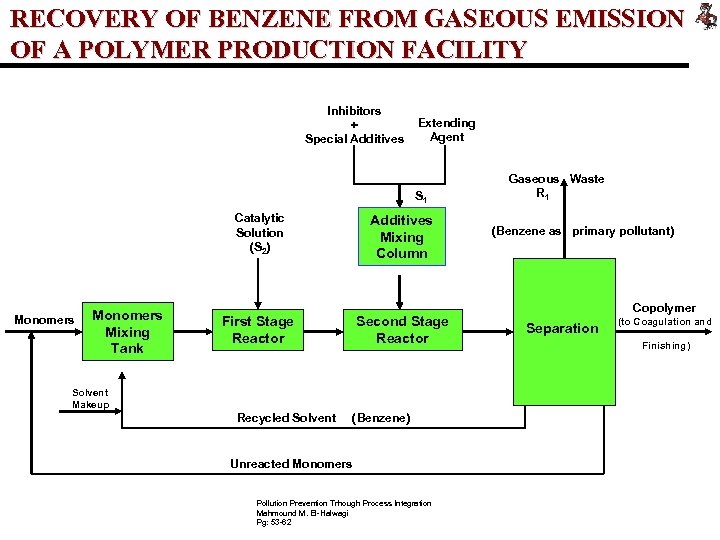 RECOVERY OF BENZENE FROM GASEOUS EMISSION OF A POLYMER PRODUCTION FACILITY Inhibitors + Special Additives Extending Agent S 1 Catalytic Solution (S 2) Monomers Mixing Tank Additives Mixing Column First Stage Reactor Second Stage Reactor Solvent Makeup Recycled Solvent (Benzene) Unreacted Monomers Pollution Prevention Trhough Process Integration Mahmound M. El-Halwagi Pg: 53 -62 Gaseous Waste R 1 (Benzene as primary pollutant) Copolymer Separation (to Coagulation and Finishing)
RECOVERY OF BENZENE FROM GASEOUS EMISSION OF A POLYMER PRODUCTION FACILITY Inhibitors + Special Additives Extending Agent S 1 Catalytic Solution (S 2) Monomers Mixing Tank Additives Mixing Column First Stage Reactor Second Stage Reactor Solvent Makeup Recycled Solvent (Benzene) Unreacted Monomers Pollution Prevention Trhough Process Integration Mahmound M. El-Halwagi Pg: 53 -62 Gaseous Waste R 1 (Benzene as primary pollutant) Copolymer Separation (to Coagulation and Finishing)
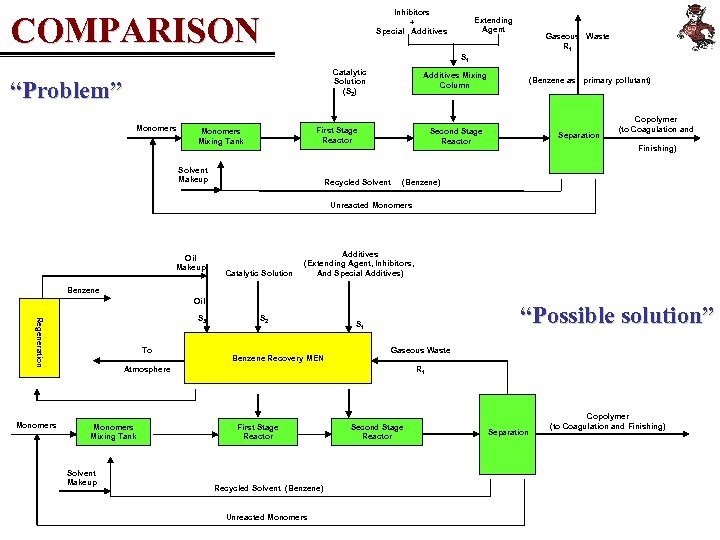 Inhibitors + Special Additives COMPARISON Extending Agent Gaseous Waste R 1 S 1 Catalytic Solution (S 2) “Problem” Monomers Additives Mixing Column First Stage Reactor Monomers Mixing Tank Solvent Makeup (Benzene as primary pollutant) Second Stage Reactor Recycled Solvent Separation Copolymer (to Coagulation and Finishing) (Benzene) Unreacted Monomers Oil Makeup Catalytic Solution Additives (Extending Agent, Inhibitors, And Special Additives) Benzene Oil Regeneration S 3 Monomers To S 2 Benzene Recovery MEN “Possible solution” S 1 Gaseous Waste Atmosphere Monomers Mixing Tank Solvent Makeup R 1 First Stage Reactor Recycled Solvent (Benzene) Unreacted Monomers Second Stage Reactor Separation Copolymer (to Coagulation and Finishing)
Inhibitors + Special Additives COMPARISON Extending Agent Gaseous Waste R 1 S 1 Catalytic Solution (S 2) “Problem” Monomers Additives Mixing Column First Stage Reactor Monomers Mixing Tank Solvent Makeup (Benzene as primary pollutant) Second Stage Reactor Recycled Solvent Separation Copolymer (to Coagulation and Finishing) (Benzene) Unreacted Monomers Oil Makeup Catalytic Solution Additives (Extending Agent, Inhibitors, And Special Additives) Benzene Oil Regeneration S 3 Monomers To S 2 Benzene Recovery MEN “Possible solution” S 1 Gaseous Waste Atmosphere Monomers Mixing Tank Solvent Makeup R 1 First Stage Reactor Recycled Solvent (Benzene) Unreacted Monomers Second Stage Reactor Separation Copolymer (to Coagulation and Finishing)
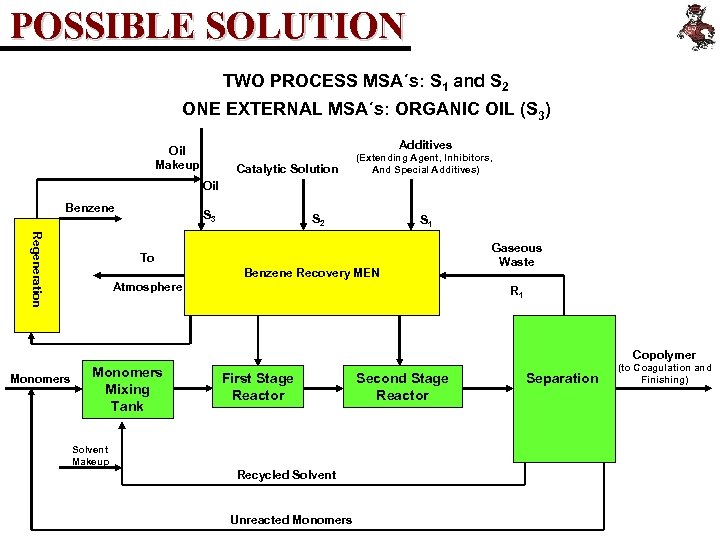 POSSIBLE SOLUTION TWO PROCESS MSA´s: S 1 and S 2 ONE EXTERNAL MSA´s: ORGANIC OIL (S 3) Additives Oil Makeup Catalytic Solution (Extending Agent, Inhibitors, And Special Additives) Oil Benzene S 3 S 2 S 1 Regeneration To Benzene Recovery MEN Atmosphere Gaseous Waste R 1 Copolymer Monomers Mixing Tank First Stage Reactor Solvent Makeup Recycled Solvent Unreacted Monomers Second Stage Reactor Separation (to Coagulation and Finishing)
POSSIBLE SOLUTION TWO PROCESS MSA´s: S 1 and S 2 ONE EXTERNAL MSA´s: ORGANIC OIL (S 3) Additives Oil Makeup Catalytic Solution (Extending Agent, Inhibitors, And Special Additives) Oil Benzene S 3 S 2 S 1 Regeneration To Benzene Recovery MEN Atmosphere Gaseous Waste R 1 Copolymer Monomers Mixing Tank First Stage Reactor Solvent Makeup Recycled Solvent Unreacted Monomers Second Stage Reactor Separation (to Coagulation and Finishing)
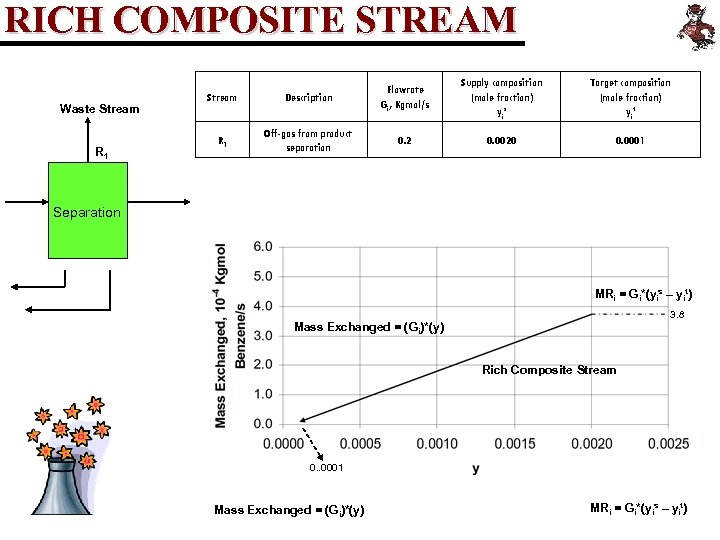 RICH COMPOSITE STREAM Waste Stream R 1 Stream Description Flowrate Gi, Kgmol/s R 1 Off-gas from product separation 0. 2 Supply composition (mole fraction) yi s Target composition (mole fraction) yi t 0. 0020 0. 0001 Separation MRi = Gi*(yis – yit) 3. 8 Mass Exchanged = (Gi)*(y) Rich Composite Stream 0. . 0001 Mass Exchanged = (Gi)*(y) MRi = Gi*(yis – yit)
RICH COMPOSITE STREAM Waste Stream R 1 Stream Description Flowrate Gi, Kgmol/s R 1 Off-gas from product separation 0. 2 Supply composition (mole fraction) yi s Target composition (mole fraction) yi t 0. 0020 0. 0001 Separation MRi = Gi*(yis – yit) 3. 8 Mass Exchanged = (Gi)*(y) Rich Composite Stream 0. . 0001 Mass Exchanged = (Gi)*(y) MRi = Gi*(yis – yit)
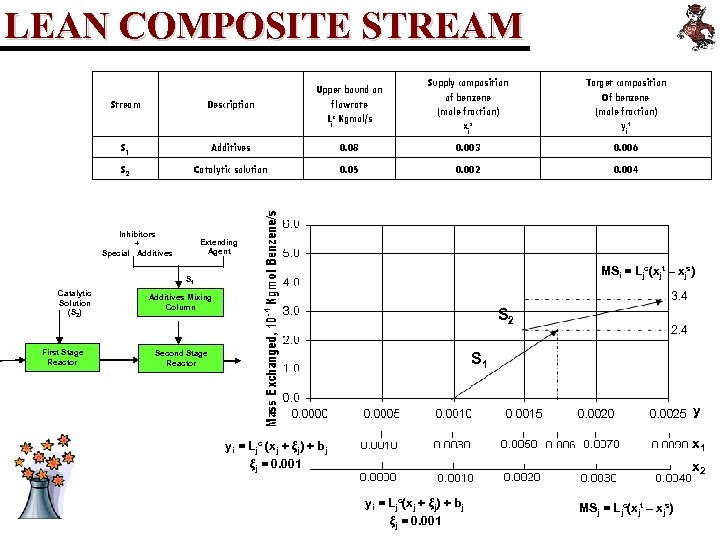 LEAN COMPOSITE STREAM Supply composition of benzene (mole fraction) xj s Target composition Of benzene (mole fraction) yj t Stream Description Upper bound on flowrate Ljc Kgmol/s S 1 Additives 0. 08 0. 003 0. 006 S 2 Catalytic solution 0. 05 0. 002 0. 004 Inhibitors + Special Additives Extending Agent MSi = Ljc(xjt – xjs) S 1 Catalytic Solution (S 2) First Stage Reactor 3. 4 Additives Mixing Column S 2 Second Stage Reactor 2. 4 S 1 y x 1 yi = Ljc (xj + ξj) + bj ξj = 0. 001 x 2 yi = Ljc(xj + ξj) + bj ξj = 0. 001 MSj = Ljc(xjt – xjs)
LEAN COMPOSITE STREAM Supply composition of benzene (mole fraction) xj s Target composition Of benzene (mole fraction) yj t Stream Description Upper bound on flowrate Ljc Kgmol/s S 1 Additives 0. 08 0. 003 0. 006 S 2 Catalytic solution 0. 05 0. 002 0. 004 Inhibitors + Special Additives Extending Agent MSi = Ljc(xjt – xjs) S 1 Catalytic Solution (S 2) First Stage Reactor 3. 4 Additives Mixing Column S 2 Second Stage Reactor 2. 4 S 1 y x 1 yi = Ljc (xj + ξj) + bj ξj = 0. 001 x 2 yi = Ljc(xj + ξj) + bj ξj = 0. 001 MSj = Ljc(xjt – xjs)
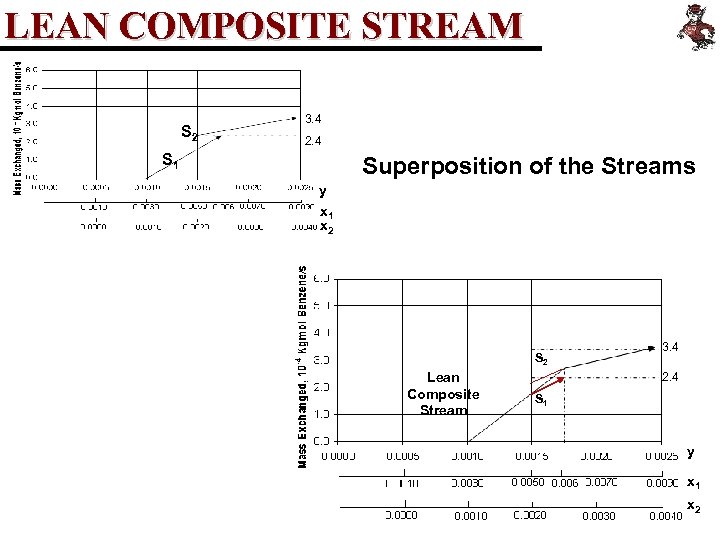 LEAN COMPOSITE STREAM S 2 3. 4 2. 4 S 1 Superposition of the Streams y x 1 x 2 S 2 Lean Composite Stream 3. 4 2. 4 S 1 y x 1 x 2
LEAN COMPOSITE STREAM S 2 3. 4 2. 4 S 1 Superposition of the Streams y x 1 x 2 S 2 Lean Composite Stream 3. 4 2. 4 S 1 y x 1 x 2
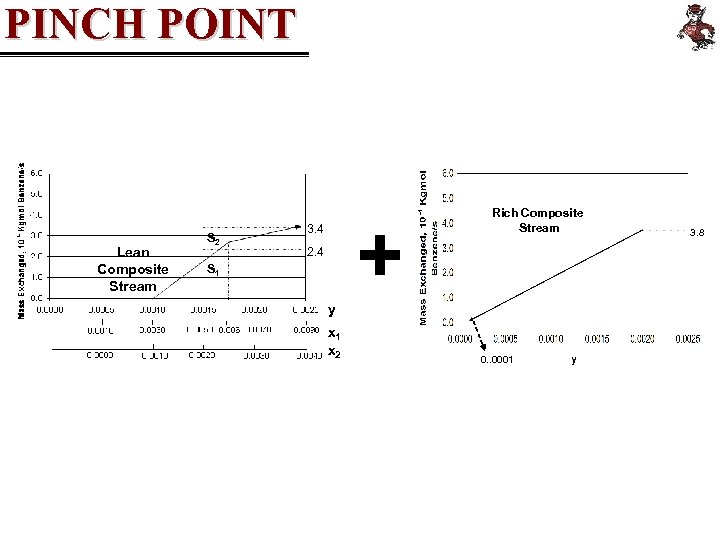 PINCH POINT Lean Composite Stream S 2 + 3. 4 2. 4 S 1 Rich Composite Stream y x 1 x 2 0. . 0001 3. 8
PINCH POINT Lean Composite Stream S 2 + 3. 4 2. 4 S 1 Rich Composite Stream y x 1 x 2 0. . 0001 3. 8
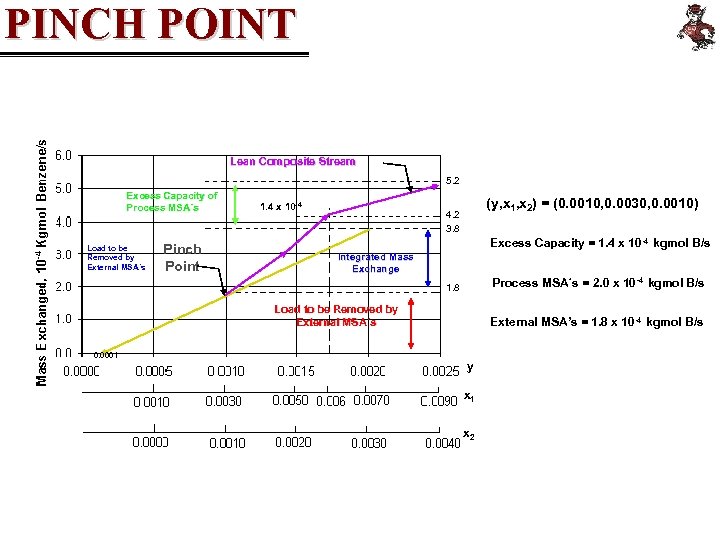 PINCH POINT Lean Composite Stream 5. 2 Excess Capacity of Process MSA´s Load to be Removed by External MSA´s Pinch Point 1. 4 x 10 -4 (y, x 1, x 2) = (0. 0010, 0. 0030, 0. 0010) 4. 2 3. 8 Excess Capacity = 1. 4 x 10 -4 kgmol B/s Integrated Mass Exchange Process MSA´s = 2. 0 x 10 -4 kgmol B/s 1. 8 Load to be Removed by External MSA´s 0. 0001 External MSA’s = 1. 8 x 10 -4 kgmol B/s y x 1 x 2
PINCH POINT Lean Composite Stream 5. 2 Excess Capacity of Process MSA´s Load to be Removed by External MSA´s Pinch Point 1. 4 x 10 -4 (y, x 1, x 2) = (0. 0010, 0. 0030, 0. 0010) 4. 2 3. 8 Excess Capacity = 1. 4 x 10 -4 kgmol B/s Integrated Mass Exchange Process MSA´s = 2. 0 x 10 -4 kgmol B/s 1. 8 Load to be Removed by External MSA´s 0. 0001 External MSA’s = 1. 8 x 10 -4 kgmol B/s y x 1 x 2
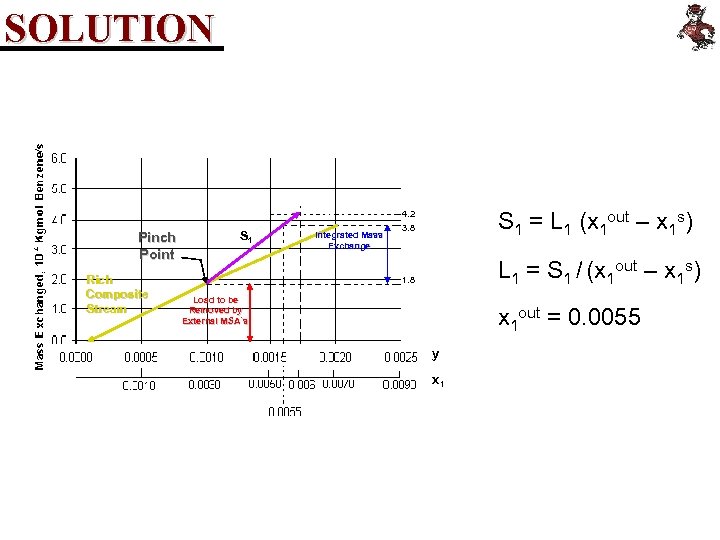 SOLUTION S 1 = L 1 (x 1 out – x 1 s) 4. 2 Pinch Point Rich Composite Stream S 1 Integrated Mass Exchange 3. 8 L 1 = S 1 / (x 1 out – x 1 s) 1. 8 Load to be Removed by External MSA´s x 1 out = 0. 0055 y x 1
SOLUTION S 1 = L 1 (x 1 out – x 1 s) 4. 2 Pinch Point Rich Composite Stream S 1 Integrated Mass Exchange 3. 8 L 1 = S 1 / (x 1 out – x 1 s) 1. 8 Load to be Removed by External MSA´s x 1 out = 0. 0055 y x 1
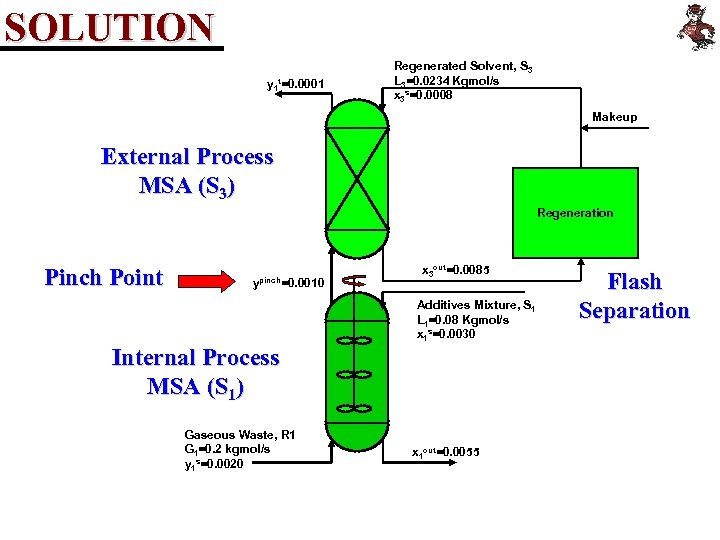 SOLUTION y 1 t=0. 0001 Regenerated Solvent, S 3 L 3=0. 0234 Kgmol/s x 3 s=0. 0008 Makeup External Process MSA (S 3) Regeneration Pinch Point ypinch=0. 0010 x 3 out=0. 0085 Additives Mixture, S 1 L 1=0. 08 Kgmol/s x 1 s=0. 0030 Internal Process MSA (S 1) Gaseous Waste, R 1 G 1=0. 2 kgmol/s y 1 s=0. 0020 x 1 out=0. 0055 Flash Separation
SOLUTION y 1 t=0. 0001 Regenerated Solvent, S 3 L 3=0. 0234 Kgmol/s x 3 s=0. 0008 Makeup External Process MSA (S 3) Regeneration Pinch Point ypinch=0. 0010 x 3 out=0. 0085 Additives Mixture, S 1 L 1=0. 08 Kgmol/s x 1 s=0. 0030 Internal Process MSA (S 1) Gaseous Waste, R 1 G 1=0. 2 kgmol/s y 1 s=0. 0020 x 1 out=0. 0055 Flash Separation
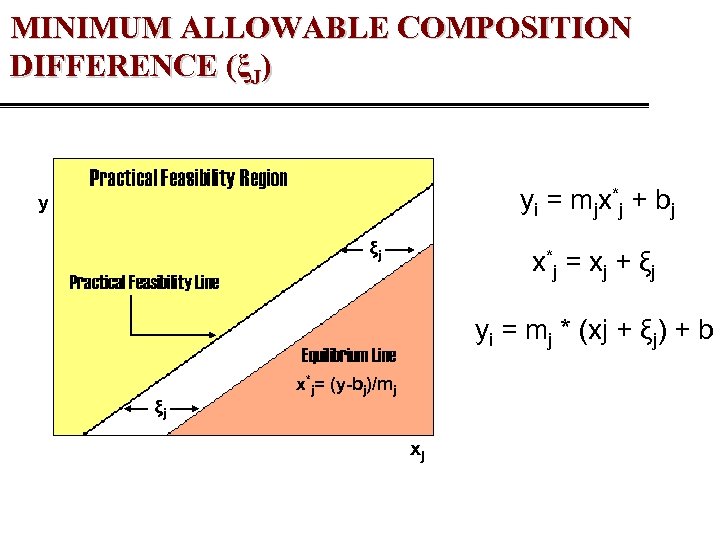 MINIMUM ALLOWABLE COMPOSITION DIFFERENCE (ξJ) Practical Feasibility Region yi = mjx*j + bj y ξj x*j = xj + ξj Practical Feasibility Line yi = mj * (xj + ξj) + b Equilibrium Line ξj x*j= (y-bj)/mj xj
MINIMUM ALLOWABLE COMPOSITION DIFFERENCE (ξJ) Practical Feasibility Region yi = mjx*j + bj y ξj x*j = xj + ξj Practical Feasibility Line yi = mj * (xj + ξj) + b Equilibrium Line ξj x*j= (y-bj)/mj xj
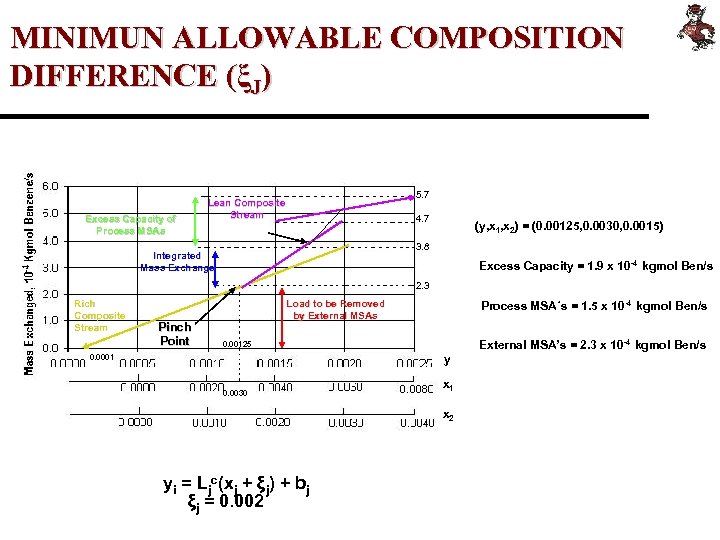 MINIMUN ALLOWABLE COMPOSITION DIFFERENCE (ξJ) Excess Capacity of Process MSAs 5. 7 Lean Composite Stream 4. 7 (y, x 1, x 2) = (0. 00125, 0. 0030, 0. 0015) 3. 8 Integrated Mass Exchange Excess Capacity = 1. 9 x 10 -4 kgmol Ben/s 2. 3 Rich Composite Stream Pinch Point Load to be Removed by External MSAs Process MSA´s = 1. 5 x 10 -4 kgmol Ben/s External MSA’s = 2. 3 x 10 -4 kgmol Ben/s 0. 00125 0. 0001 y 0. 0030 x 1 x 2 yi = Ljc(xj + ξj) + bj ξj = 0. 002
MINIMUN ALLOWABLE COMPOSITION DIFFERENCE (ξJ) Excess Capacity of Process MSAs 5. 7 Lean Composite Stream 4. 7 (y, x 1, x 2) = (0. 00125, 0. 0030, 0. 0015) 3. 8 Integrated Mass Exchange Excess Capacity = 1. 9 x 10 -4 kgmol Ben/s 2. 3 Rich Composite Stream Pinch Point Load to be Removed by External MSAs Process MSA´s = 1. 5 x 10 -4 kgmol Ben/s External MSA’s = 2. 3 x 10 -4 kgmol Ben/s 0. 00125 0. 0001 y 0. 0030 x 1 x 2 yi = Ljc(xj + ξj) + bj ξj = 0. 002
 SUMMARY Air pollution is a serious problem which will continue to become more critical in the future due to increasing Population Energy needs Transportation needs Industrial and chemical manufacturing Efforts to reduce and control air pollution have evolved over time, but require further development to meet the increasing need. Currently the best approach may be process integration to optimize plant design to minimize pollutants. “Atom production”, manufacturing with zero waste and byproducts, is a future goal not generally achieve now. Process integration for plant design centers around pinch analysis of mass exchange network (MEN) to minimize waste streams, recycle them in the process, or recover them external to the process.
SUMMARY Air pollution is a serious problem which will continue to become more critical in the future due to increasing Population Energy needs Transportation needs Industrial and chemical manufacturing Efforts to reduce and control air pollution have evolved over time, but require further development to meet the increasing need. Currently the best approach may be process integration to optimize plant design to minimize pollutants. “Atom production”, manufacturing with zero waste and byproducts, is a future goal not generally achieve now. Process integration for plant design centers around pinch analysis of mass exchange network (MEN) to minimize waste streams, recycle them in the process, or recover them external to the process.


