57c582923751014cfd850acb25e1687a.ppt
- Количество слайдов: 28
 Navigating Your Inhalation Patents Through the European Patent Office Presented at: RDD Europe 2015 Respiratory Drug Delivery Date: May 7, 2015 Presented by: Richard J. Basile Member St. Onge Steward Johnston & Reens LLC Stamford, Connecticut, U. S. rbasile@ssjr. com
Navigating Your Inhalation Patents Through the European Patent Office Presented at: RDD Europe 2015 Respiratory Drug Delivery Date: May 7, 2015 Presented by: Richard J. Basile Member St. Onge Steward Johnston & Reens LLC Stamford, Connecticut, U. S. rbasile@ssjr. com
 What is the European Patent Office Created by International Treaty Went into Force in 1977 Single Examination Good for 38 Contracting States More Efficient and Predictable Than Prior System
What is the European Patent Office Created by International Treaty Went into Force in 1977 Single Examination Good for 38 Contracting States More Efficient and Predictable Than Prior System
 Filing to Obtain a European Patent Must be (1) New, (2) Involve Inventive Step, and (3) Involve Industrial Application Official Languages English French German
Filing to Obtain a European Patent Must be (1) New, (2) Involve Inventive Step, and (3) Involve Industrial Application Official Languages English French German
 Parts of European Patent Application Request for Grant of European Application Description of the Invention One or more Claims Drawings (if referenced in the description) Abstract
Parts of European Patent Application Request for Grant of European Application Description of the Invention One or more Claims Drawings (if referenced in the description) Abstract
 Parts of European Patent Application(cont. ) Description must be “clear and complete” Application shall disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person of skill in the art. Article 83 EPC
Parts of European Patent Application(cont. ) Description must be “clear and complete” Application shall disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person of skill in the art. Article 83 EPC
 Filing European Patent Applications can be physically submitted to EPO Munich, Berlin, The Hague Industrial property office of contracting state Vast majority of applications filed on line www. epo. org Less chance materials are lost or misplaced
Filing European Patent Applications can be physically submitted to EPO Munich, Berlin, The Hague Industrial property office of contracting state Vast majority of applications filed on line www. epo. org Less chance materials are lost or misplaced
 Review and Grant Procedure First Stage (a)review of file formalities (b)preparation of search report and preliminary opinion on patentability (c)publication of application with search report End of first stage is good time to assess likelihood of getting patent granted
Review and Grant Procedure First Stage (a)review of file formalities (b)preparation of search report and preliminary opinion on patentability (c)publication of application with search report End of first stage is good time to assess likelihood of getting patent granted
 Review and Grant Procedure (cont. ) Second Stage Substantive Examination by Examiner Claims must satisfy three elements of patentability May not amend claims to include subject matter beyond content of application.
Review and Grant Procedure (cont. ) Second Stage Substantive Examination by Examiner Claims must satisfy three elements of patentability May not amend claims to include subject matter beyond content of application.
 Post Grant Proceedings Opposition Proceeding Filed within 9 months of patent grant By Third party Three Grounds as Basis for opposition (1) Not patentable subject matter or inventive (2) Invention not disclosed clearly and completely (3) Claimed subject matter extends beyond content of application
Post Grant Proceedings Opposition Proceeding Filed within 9 months of patent grant By Third party Three Grounds as Basis for opposition (1) Not patentable subject matter or inventive (2) Invention not disclosed clearly and completely (3) Claimed subject matter extends beyond content of application
 Post Grant Proceedings (cont. ) Revocation or Limitation Proceeding Filed by Patent Proprietor Done to correct known problems or weaknesses with patent Often done with eye toward litigation
Post Grant Proceedings (cont. ) Revocation or Limitation Proceeding Filed by Patent Proprietor Done to correct known problems or weaknesses with patent Often done with eye toward litigation
 Boards of Appeal Decisions: Lack of Novelty Claim: Particles suitable for use in pulmonary drug delivery by inhalation, which particles are spherical and crystalline, have a rough surface and incorporate an active agent, the particles being obtainable by a method according to any one of claims 1 to 8.
Boards of Appeal Decisions: Lack of Novelty Claim: Particles suitable for use in pulmonary drug delivery by inhalation, which particles are spherical and crystalline, have a rough surface and incorporate an active agent, the particles being obtainable by a method according to any one of claims 1 to 8.
 Lack of Novelty Patent owner argued novelty based on (a) rough surface and (b) particle size distribution based on manufacturing process. Board Finds No Novelty Roughness not defined No mention of particle size distribution in claim
Lack of Novelty Patent owner argued novelty based on (a) rough surface and (b) particle size distribution based on manufacturing process. Board Finds No Novelty Roughness not defined No mention of particle size distribution in claim
 Lack of Clarity Claim: Particles suitable for use in pulmonary drug delivery by inhalation, which particles are spherical and crystalline, the relative degree of crystallinity being 90% or higher, have a rough surface and incorporate an active agent, the particles being obtainable by a method according to any one of claims 1 to 8
Lack of Clarity Claim: Particles suitable for use in pulmonary drug delivery by inhalation, which particles are spherical and crystalline, the relative degree of crystallinity being 90% or higher, have a rough surface and incorporate an active agent, the particles being obtainable by a method according to any one of claims 1 to 8
 Lack of Clarity To determine crystallinity description mentions Use of x-ray powder diffraction Use of reference powder, beclomethasone, having crystallinity of 79%
Lack of Clarity To determine crystallinity description mentions Use of x-ray powder diffraction Use of reference powder, beclomethasone, having crystallinity of 79%
 Lack of Clarity Board rejects for lack of clarity because among other things, methodology for determining “relative degree of crystallinity” was not fully described in patent. Missing: how relevant diffraction maxima selected; way estimation based on broadening of diffraction maxima is to be carried out; how reference sample is chosen
Lack of Clarity Board rejects for lack of clarity because among other things, methodology for determining “relative degree of crystallinity” was not fully described in patent. Missing: how relevant diffraction maxima selected; way estimation based on broadening of diffraction maxima is to be carried out; how reference sample is chosen
 Lack of Clarity “Under these circumstances, the skilled person is not in a position to determine whether a given sample meets the requirement ‘the relative degree of crystallinity being 90% or higher’”
Lack of Clarity “Under these circumstances, the skilled person is not in a position to determine whether a given sample meets the requirement ‘the relative degree of crystallinity being 90% or higher’”
 Insufficiency of Disclosure Claim: Particles for use as a carrier in the preparation of pharmaceutical formulations for the pulmonary administration of micronized active ingredients by means of a powder inhaler, wherein the median diameter of said particles is greater than 90µm, the surface rugosity is less or equal to 1. 1 upon determination of the fractal dimension as described on page 14, line 15 -page 15, line 11 and their surface is coated with an additive selected from lubricants, anti-adherents and soluble polymers.
Insufficiency of Disclosure Claim: Particles for use as a carrier in the preparation of pharmaceutical formulations for the pulmonary administration of micronized active ingredients by means of a powder inhaler, wherein the median diameter of said particles is greater than 90µm, the surface rugosity is less or equal to 1. 1 upon determination of the fractal dimension as described on page 14, line 15 -page 15, line 11 and their surface is coated with an additive selected from lubricants, anti-adherents and soluble polymers.
 Insufficiency of Disclosure Claim included the location in description of methodology of how to measure rugosity BUT, described a new method, adapted by inventors using SEM. PROBLEM, Same particle could or could not meet claim requirement based on magnification used to acquire image of particle surface
Insufficiency of Disclosure Claim included the location in description of methodology of how to measure rugosity BUT, described a new method, adapted by inventors using SEM. PROBLEM, Same particle could or could not meet claim requirement based on magnification used to acquire image of particle surface
 Insufficiency of Disclosure Board blamed proprietor for deliberately deciding to use own uncommon method “In general terms, when the issue of sufficiency concerns the description of a method for determining a parameter, the less common the method the more accurate the information provided in the description should be. ”
Insufficiency of Disclosure Board blamed proprietor for deliberately deciding to use own uncommon method “In general terms, when the issue of sufficiency concerns the description of a method for determining a parameter, the less common the method the more accurate the information provided in the description should be. ”
 No Inventive Step Claim: A medication delivery apparatus (50) comprising an antistatic component made of a material having surface resistivity of between about 10 E 10 and 10 E 12 ohm/sq, wherein at least a portion of said component is seethrough Prior art device had see-through spacer for MDI made of antistatic material.
No Inventive Step Claim: A medication delivery apparatus (50) comprising an antistatic component made of a material having surface resistivity of between about 10 E 10 and 10 E 12 ohm/sq, wherein at least a portion of said component is seethrough Prior art device had see-through spacer for MDI made of antistatic material.
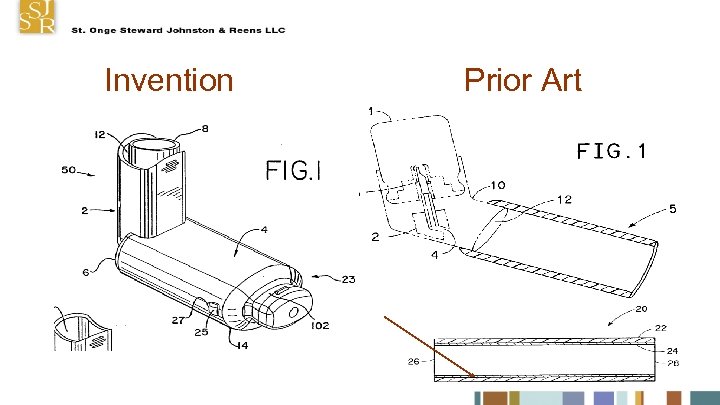 Invention Prior Art
Invention Prior Art
 No Inventive Step Auxiliary claim: A medication delivery apparatus (50) comprising an antistatic component made of a material having surface resistivity of between about 10 E 10 and 10 E 12 ohm/sq, wherein at least a portion of said component is see-through and wherein the antistatic property of the component is permanent.
No Inventive Step Auxiliary claim: A medication delivery apparatus (50) comprising an antistatic component made of a material having surface resistivity of between about 10 E 10 and 10 E 12 ohm/sq, wherein at least a portion of said component is see-through and wherein the antistatic property of the component is permanent.
 No Inventive Step Only feature missing from prior art was antistatic property was “permanent” Proprietor argued “permanent” meant about one year Board found that “permanent” meant for the useful/functional life of device and MDI’s are disposable
No Inventive Step Only feature missing from prior art was antistatic property was “permanent” Proprietor argued “permanent” meant about one year Board found that “permanent” meant for the useful/functional life of device and MDI’s are disposable
 Extending Beyond Content of Application Claim: A gaseous mixture containing nitric oxide, oxygen and less than 1 ppm NO 2, for use in therapy. Patent description limited to preventing or reversing acute pulmonary vasoconstriction Claim as drafted covers ANY therapeutic use of nitric oxide
Extending Beyond Content of Application Claim: A gaseous mixture containing nitric oxide, oxygen and less than 1 ppm NO 2, for use in therapy. Patent description limited to preventing or reversing acute pulmonary vasoconstriction Claim as drafted covers ANY therapeutic use of nitric oxide
 Extending Beyond Content of Application Claim: Use of a gaseous mixture consisting of NO and N 2 for the production of an inhalable medicament for treating pulmonary hypertension in a patient with persistent pulmonary hypertension of the newborn. Description linked therapeutic treatment of PPHN to specific effect of pulmonary vasodilation.
Extending Beyond Content of Application Claim: Use of a gaseous mixture consisting of NO and N 2 for the production of an inhalable medicament for treating pulmonary hypertension in a patient with persistent pulmonary hypertension of the newborn. Description linked therapeutic treatment of PPHN to specific effect of pulmonary vasodilation.
 Extending Beyond Content of Application Effect of pulmonary vasodilation absent from claim so it would improperly encompass forms of treatment of PPHN not disclosed in application
Extending Beyond Content of Application Effect of pulmonary vasodilation absent from claim so it would improperly encompass forms of treatment of PPHN not disclosed in application
 Extending Beyond Content of Application Third claim: Use of a gaseous mixture consisting of NO and N 2 for the production of an inhalable medicament for reversing acute pulmonary vasoconstriction resulting from persistent pulmonary hypertension of the newborn. Allowed
Extending Beyond Content of Application Third claim: Use of a gaseous mixture consisting of NO and N 2 for the production of an inhalable medicament for reversing acute pulmonary vasoconstriction resulting from persistent pulmonary hypertension of the newborn. Allowed
 Conclusion Applications must include detailed descriptions of the invention that are aligned with the scope of the claims.
Conclusion Applications must include detailed descriptions of the invention that are aligned with the scope of the claims.


