10d3eff13a80c1ab1f79f3644171016e.ppt
- Количество слайдов: 23

NATIONAL EFFORTS TO MITIGATE BIOLOGICAL THREATS – INDONESIA’S EXPERIENCE Herawati Sudoyo Eijkman Institute for Molecular Biology Jl Diponegoro 68 Jakarta 10430, Indonesia

Indonesia’s Experience q Bioterrorism prevention measures Risk assessments Biosafety laboratories infrastructures for dangerous pathogens q Systems to detect, diagnose and track outbreaks of highly infectious diseases q Systems to track the origin of the outbreak of highly infectious diseases q Emergency response systems for control and containment of infectious disease events
Indonesia – A Rapidly Developing Country with Serious Problems in Infectious Disease q q West to east 4, 500 km; North to South 2, 000 km Dry land 1. 8 million sq. km. (Borneo 0. 7, Sumatra 0. 4, Java 0. 1) More than 17, 000 islands – 3, 000 inhabited Population 220, 000 - > 500 ethnic groups
Indonesia – A Rapidly Developing Country with Serious Problems in Infectious Disease Malaria: – 15 million malaria cases and 42, 000 deaths every year (2005) – Highest case number and fatality rate in the world Tuberculosis: – ranked third in TB burden following India and China – TB is third of major causes of mortality in Indonesia – WHO estimate, Indonesia has 269 TB cases/100, 000 populations Dengue: – Most important viral borne disease in Indonesia – Outbreak in 2004: 78, 690 cases with 954 deaths (CFR- 1. 2%) – In 2007: 123, 174 cases with 1, 251 deaths Hepatitis B: – 10% (3. 4 -20. 3%) of population are HBV carriers – Moderate-to-high endemic (WHO) Avian Influenza: – June 2005 -June 2009: 262 positive cases; 115 deaths, CFR: 80% – Highest case number and fatality rate in the world
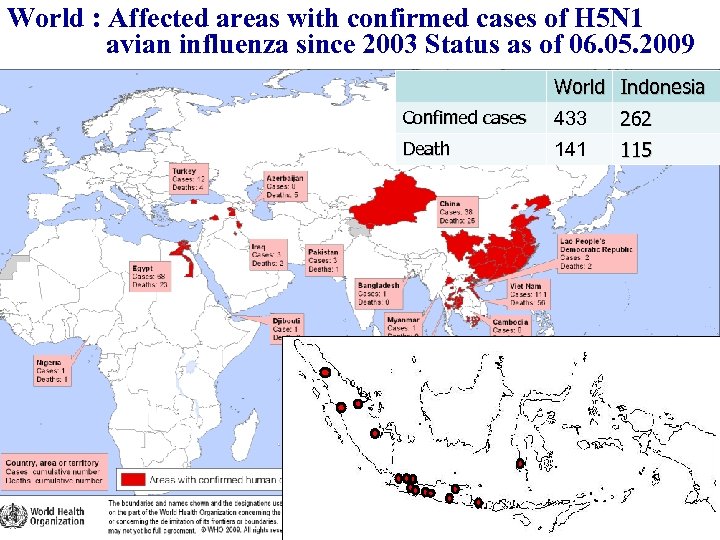
World : Affected areas with confirmed cases of H 5 N 1 avian influenza since 2003 Status as of 06. 05. 2009 Confimed cases Death World Indonesia 433 262 141 115

National Actions to Strengthen Biological Security q Building a safe, secure and sustainability capacity q Best practices on biological safety and security q Building capacity to detect, diagnose and track outbreaks of highly infectious diseases q Enhancing cooperation Jakarta EIJKMAN NIHRD UI Surabaya TDC-UNAIR Bogor BALITVET Institutions working on Avian Influenza and BSL 3 facilities

I. Building a Safe, Secure and Sustainability Capacity - Pressure • Urgent need for Avian Influenza genomic risk assessment (MOH policy) • No BSL 3 was on the horizon in Indonesia at the time • Awareness of international community watching Funds allocated by Indonesian government with support from parliament

Why Need a BSL 3 Facility? q Research is an essential component of response to emerging infectious diseases q Role in national response to Emerging Infectious Disease • To provide scientific and technological support to the national diagnostic laboratory network, including capacity building • As the leading research laboratory, in particular in genomic research (viral as well as host) and pathology • As the major back up diagnostic facility in emergency situation, such as in pandemic response q Current infectious disease research activity – e. g. culture of tuberculosis (airborne) bacteria q Prepare for future emerging infectious disease threat e. g recent experience of NOT being able to respond scientifically to threat of SARS

Building a BSL 3 Facility at Eijkman • A new wing entirely for the BSL 3 attached to the current building • Total space available: 150 sq m (15 x 10 m) • For handling pathogenic bacteria and viruses - No animal work

II. Best Practices on Biological Safety and Security - Challenges ·……The human element is the crucial part of the chain for many aspects of biosafety and biosecurity, good facilities and procedures are not sufficient if personnel are not adequately trained and do not clearly understand their roles and responsibilities…………………. . q Laboratory biosecurity training, complementary to biosafety training is provided - protection, assurance and continuity of operations q Should not be a one-time event – offered regularly and taken currently. To refresh memories and to learn about new developments and advances in different areas Most importantly, strengthening the spirits!

Management System q No history of running containment facilities q Management system had to be started from basics – Policies – Biosafety manual – SOPs – Risk assessments – Etc. q New personnel – Laboratory manager – Staff – Maintenance q Some documentation supplied from other institutes and support to get program started q Need own system which fits individual institute and culture

Regional Need to Promote and Implement Biosafety and Biosecurity Management More than 70 representatives from 17 countries Contributions by the WHO, APBSA and academia. q Need to enhance capabilities in addressing challenges such as emerging and reemerging diseases q Capabilities must be adapted to local needs - complexities involved in setting up new laboratories, many challenges associated with construction, on-going maintenance and running costs q Increased co-operation between countries – make a use of existing capabilities and resources

Summary of Regional Seminar on Promoting and Implementing Biosafety and Biosecurity Management, Jakarta, Juni 2008 q Adequate systems to address biosafety and biosecurity are critical in overcoming these challenges - involve physical structures, strong commitment by senior management, development of safety and security procedures and training q It was affirmed that countries in the region should learn from each other’s experiences Experience from the Eijkman Institute is of particular relevance Indonesia and Norway will co-host “International Seminar on the Biological weapons Convention Supporting Global Health: Reducing Biological Risk buy Building Capacity in Health Security – Oslo, 18 -19 June 2009
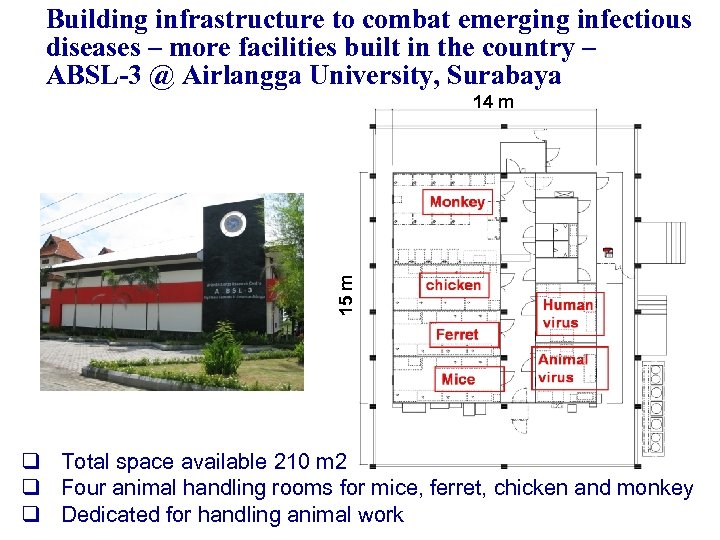
Building infrastructure to combat emerging infectious diseases – more facilities built in the country – ABSL-3 @ Airlangga University, Surabaya 15 m 14 m q Total space available 210 m 2 q Four animal handling rooms for mice, ferret, chicken and monkey q Dedicated for handling animal work

Manpower and Capacity Building Through Training, Specific Workshops and Conferences • Laboratory Biosecurity and Biosafety for BSL 3 Laboratories, Bogor, 2006 • APBA Biosafety Management Course, Singapore, July 2006 • Lab for the 21 st Century, High performance, Low Energy Design Course, Scottsdale, USA, April 2007 • Safe BSL 3 Work Practices and Procedures, Scottsdale, 2007 • ABSA 50 th Biological Safety Conference, October, 2007 • 3 rd APBA Conference, Bangkok, 2008 • 3 rd Annual Conference SEA Influenza Clinical Research Network, Bali, 2008 • Regional Seminar on Promoting and Implementing Biosafety and Biosecurity Management, Jakarta, June, 2008 • Laboratory Biorisk Management System Workshop, Jakarta, August 2008 • Advanced Topics in Managing BSL 3 Laboratories, CDC Atlanta, January, 2009 • BSL 3 Science and Safety Training Course, Emory, Atlanta, March, 2009 • 4 th APB A Conference, Manila, April 2009

Laboratory Capacity and Capability Building to Overcome Deficiencies in Management System Funding by the Norwegian Ministry of Foreign Affairs q Establishing an effective, best practice management system, incorporating safety and security management process and associated procedures q Devising necessary document templates q Developing the training programs and material q Developing a generic model of the system which can be applied in other Institutes in Indonesia and elsewhere q Enhancing communication around biorisk management and capacity building at all levels within SEA and beyond q Concept and practices based on CWA 15793: 2008 Laboratory Biorisk Management Standard
III. Building capacity to detect, diagnose and track outbreaks of highly infectious diseases q Development of diagnostic tests § Diagnosed by virus isolation – hemaglutinating activity indicates the presence of influenza virus § Reverse Transcriptation-PCR assay for molecular identification § Positive test by RT-PCR should be confirmed by the second Institution § RT-PCR and antigen testing carried out in BSL 2 q Tracking outbreaks § BSL 3 laboratory conditions are required for HPAI viruses culture - detection of viral sequence changes (infection with other subtypes have been associated with outbreaks in other species)
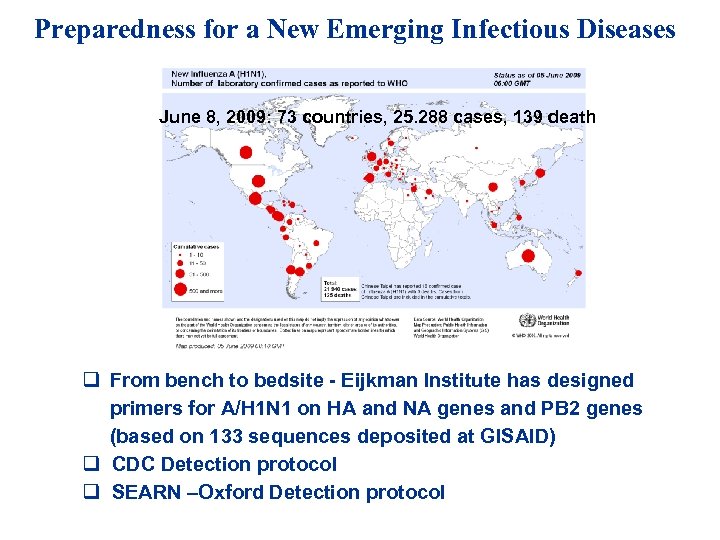
Preparedness for a New Emerging Infectious Diseases June 8, 2009: 73 countries, 25. 288 cases, 139 death q From bench to bedsite - Eijkman Institute has designed primers for A/H 1 N 1 on HA and NA genes and PB 2 genes (based on 133 sequences deposited at GISAID) q CDC Detection protocol q SEARN –Oxford Detection protocol
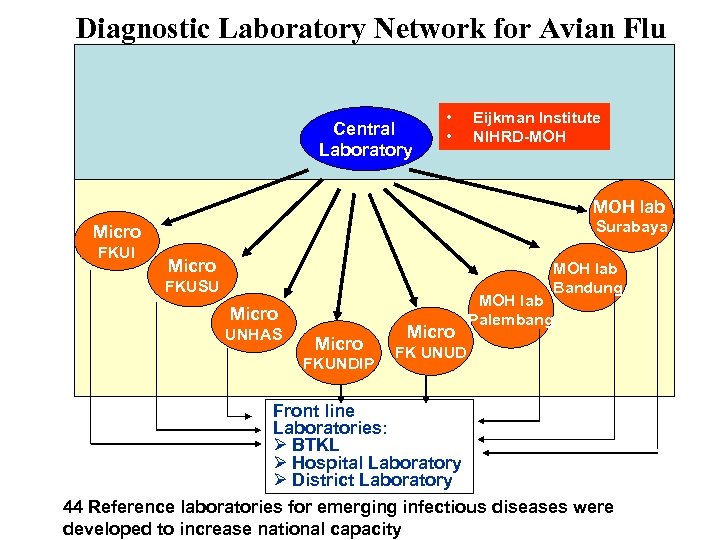
Diagnostic Laboratory Network for Avian Flu Central Laboratory • • Eijkman Institute NIHRD-MOH lab Surabaya Micro FKUI Micro MOH lab Bandung FKUSU Micro UNHAS Micro FKUNDIP Micro MOH lab Palembang FK UNUD Front line Laboratories: Ø BTKL Ø Hospital Laboratory Ø District Laboratory 44 Reference laboratories for emerging infectious diseases were developed to increase national capacity
III. Building capacity to detect, diagnose and track outbreaks of highly infectious diseases Risk Assessments q Epidemiology: • Grouping of H 5 N 1: Virological clades or subclades - H 5 N 1 of Indonesian isolates clustered together – no new strain • Surveilance - tracing sources of infection q Characteristics of Virus • Alteration of interaction with host receptors - Pandemic need changing in specificity of avian type receptor ( 2, 3) into human-type ( 2, 6) - showed the presence of avian-type receptor • Change of virulence • Drug resistance –no sign

IV. Enhancing Cooperation q Risk asessments need huge amount of materials of the virus which has to be cultured in a certified BSL 3 equipped with bio-safety SOP and well trained staff q Active participation in the region is necessary not only in the field of biosafety and biosecurity but also in infectious diseases research and surveillance - APSBA, ABSA, SEA Network of Influenza, q Active participation in Meeting of Experts of the BWC

Conclusion - National Efforts to Mitigate Biological Threats q Raising awareness of biologically threats globally – BWC, BTR q Strengthening laboratory biosafety and biosecurity to protect laboratory capacity and safely combat infectious diseases – Training, SOP q Expanding the use of safe and modern diagnostics – National Capacity Building q Participation in infectious disease surveillance networks – SEA Influenza Clinical Research Network, q Developing emergency response, recovery and mitigation plans for biological events – National Committee on Avian Flu Control and Pandemic Influenze Preparedness, MOH Expert Committee on Avain flu,

Thank you and greetings from Indonesia
10d3eff13a80c1ab1f79f3644171016e.ppt