7b14b51647e5069367e2fe80920b0cc3.ppt
- Количество слайдов: 17
 NAT Proficiency Study Program in Japan Saeko Mizusawa, Yoshiaki Okada National Institute of Infectious Diseases, Japan So. GAT XXI In Brussels, Belgium 28 -29 May 2009
NAT Proficiency Study Program in Japan Saeko Mizusawa, Yoshiaki Okada National Institute of Infectious Diseases, Japan So. GAT XXI In Brussels, Belgium 28 -29 May 2009
 Background National Standards for NAT Calibrated against WHO IS n HCV(1999 ), HBV(2002 ), HIV(2002) Guideline for Industry on NAT 2004 n n HCV, HBV, HIV Validated method with 100 IU/ml (95%DL) Guideline for Look Back Study 2005 n n n HCV: core antigen HBV: NAT HIV: antibody
Background National Standards for NAT Calibrated against WHO IS n HCV(1999 ), HBV(2002 ), HIV(2002) Guideline for Industry on NAT 2004 n n HCV, HBV, HIV Validated method with 100 IU/ml (95%DL) Guideline for Look Back Study 2005 n n n HCV: core antigen HBV: NAT HIV: antibody
 Objectives To assess the proficiency and sensitivity of the validated routine NAT implemented in plasma pool and blood screening. To assess the performance of diagnostic HBV -NAT to test recipients according to Lookback guideline. To chose the suitable HBV-NAT kits if necessary.
Objectives To assess the proficiency and sensitivity of the validated routine NAT implemented in plasma pool and blood screening. To assess the performance of diagnostic HBV -NAT to test recipients according to Lookback guideline. To chose the suitable HBV-NAT kits if necessary.
 Materials and Methods Panels : Prepared by NIID by diluting the respective national Standard. Each panel consists of 8 coded samples, 7 positive and one negative samples. Assay: Three independent assays on different days with validated routine NAT methods Data : Reported ”positive or negative” for qualitative assays and “concentration” for quantitative assays. The presented data were analyzed by NIID.
Materials and Methods Panels : Prepared by NIID by diluting the respective national Standard. Each panel consists of 8 coded samples, 7 positive and one negative samples. Assay: Three independent assays on different days with validated routine NAT methods Data : Reported ”positive or negative” for qualitative assays and “concentration” for quantitative assays. The presented data were analyzed by NIID.
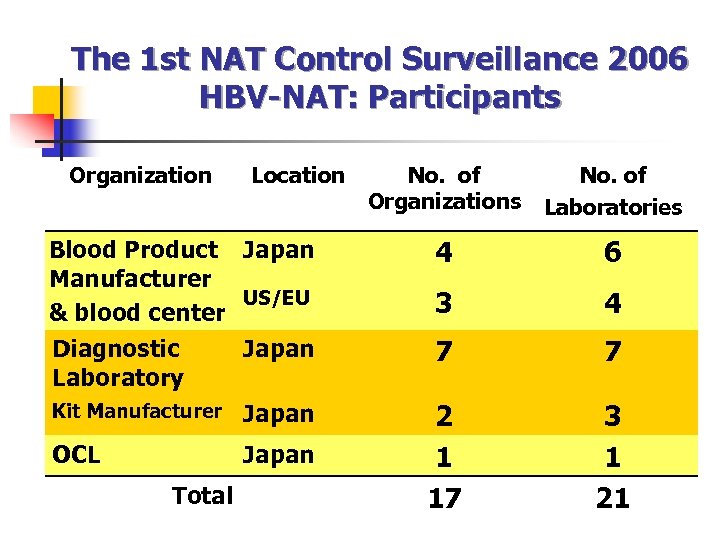 The 1 st NAT Control Surveillance 2006 HBV-NAT: Participants Organization Location Blood Product Japan Manufacturer US/EU & blood center Diagnostic Japan Laboratory Kit Manufacturer Japan OCL Japan Total No. of Organizations No. of Laboratories 4 6 3 4 7 7 2 1 17 3 1 21
The 1 st NAT Control Surveillance 2006 HBV-NAT: Participants Organization Location Blood Product Japan Manufacturer US/EU & blood center Diagnostic Japan Laboratory Kit Manufacturer Japan OCL Japan Total No. of Organizations No. of Laboratories 4 6 3 4 7 7 2 1 17 3 1 21
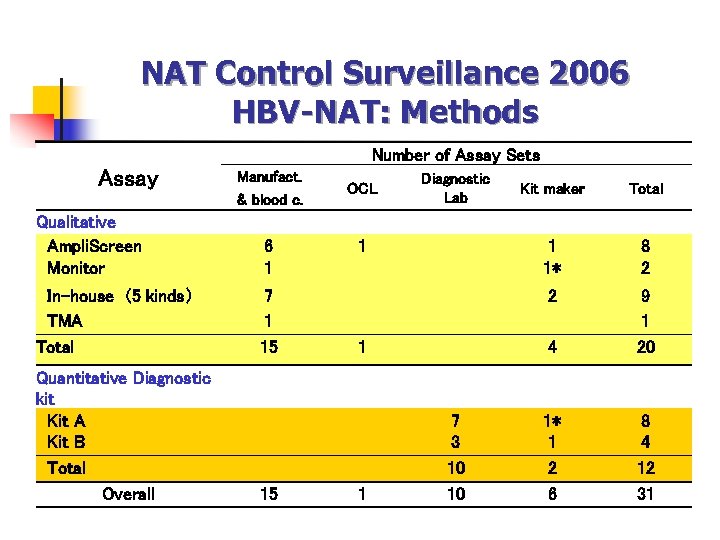 NAT Control Surveillance 2006 HBV-NAT: Methods Number of Assay Sets Assay Manufact. & blood c. Qualitative Ampli. Screen Monitor 6 1 In-house (5 kinds) TMA Total 7 1 15 Quantitative Diagnostic kit Kit A Kit B Total Overall 15 OCL Diagnostic Lab 4 1 7 3 10 10 8 2 2 1 Total 1 1* 1 Kit maker 9 1 20 1* 1 2 6 8 4 12 31
NAT Control Surveillance 2006 HBV-NAT: Methods Number of Assay Sets Assay Manufact. & blood c. Qualitative Ampli. Screen Monitor 6 1 In-house (5 kinds) TMA Total 7 1 15 Quantitative Diagnostic kit Kit A Kit B Total Overall 15 OCL Diagnostic Lab 4 1 7 3 10 10 8 2 2 1 Total 1 1* 1 Kit maker 9 1 20 1* 1 2 6 8 4 12 31
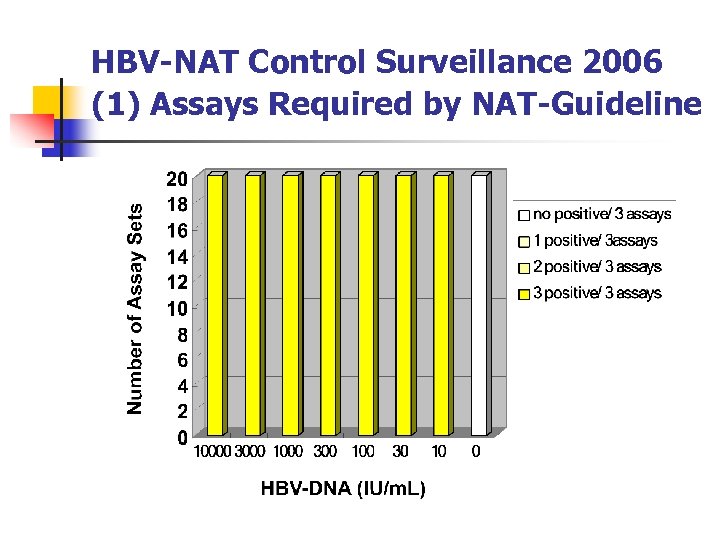 HBV-NAT Control Surveillance 2006 (1) Assays Required by NAT-Guideline
HBV-NAT Control Surveillance 2006 (1) Assays Required by NAT-Guideline
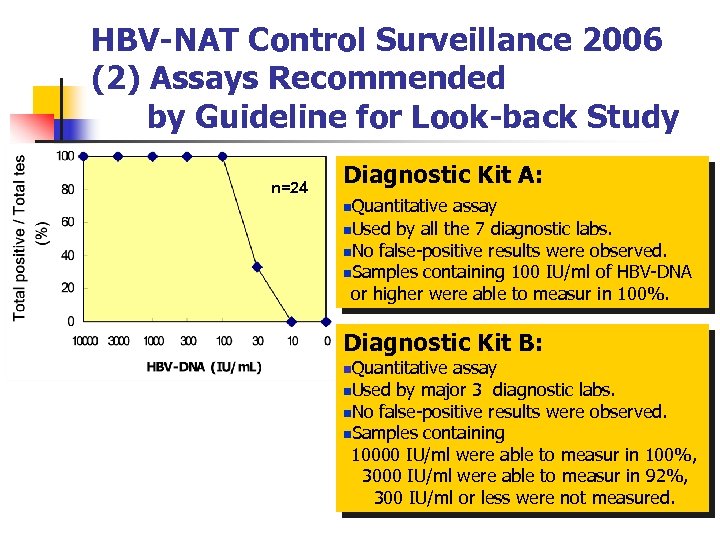 HBV-NAT Control Surveillance 2006 (2) Assays Recommended by Guideline for Look-back Study n=24 Diagnostic Kit A: n. Quantitative assay n. Used by all the 7 diagnostic labs. n. No false-positive results were observed. n. Samples containing 100 IU/ml of HBV-DNA or higher were able to measur in 100%. Diagnostic Kit B: n. Quantitative assay n. Used by major 3 diagnostic labs. n. No false-positive results were observed. n. Samples containing 10000 IU/ml were able to measur in 100%, 3000 IU/ml were able to measur in 92%, 300 IU/ml or less were not measured.
HBV-NAT Control Surveillance 2006 (2) Assays Recommended by Guideline for Look-back Study n=24 Diagnostic Kit A: n. Quantitative assay n. Used by all the 7 diagnostic labs. n. No false-positive results were observed. n. Samples containing 100 IU/ml of HBV-DNA or higher were able to measur in 100%. Diagnostic Kit B: n. Quantitative assay n. Used by major 3 diagnostic labs. n. No false-positive results were observed. n. Samples containing 10000 IU/ml were able to measur in 100%, 3000 IU/ml were able to measur in 92%, 300 IU/ml or less were not measured.
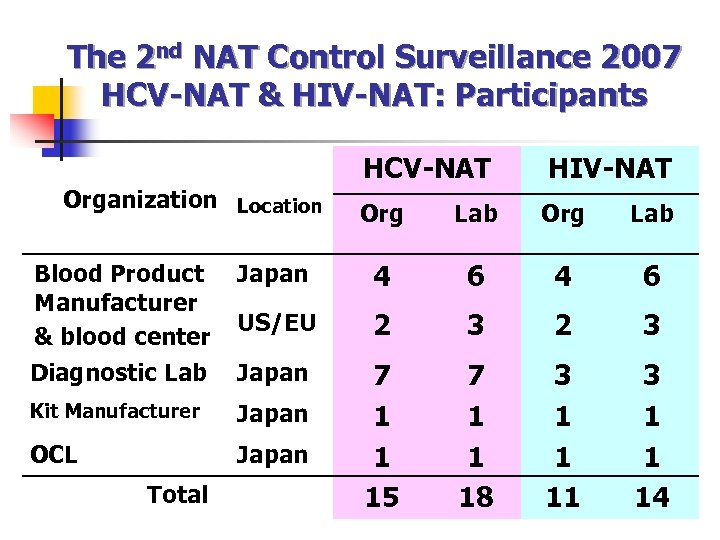 The 2 nd NAT Control Surveillance 2007 HCV-NAT & HIV-NAT: Participants Organization Location HCV-NAT HIV-NAT Org Lab Blood Product Manufacturer & blood center Japan 4 6 US/EU 2 3 Diagnostic Lab Japan Kit Manufacturer Japan OCL Japan 7 1 1 15 7 1 1 18 3 1 1 11 3 1 1 14 Total
The 2 nd NAT Control Surveillance 2007 HCV-NAT & HIV-NAT: Participants Organization Location HCV-NAT HIV-NAT Org Lab Blood Product Manufacturer & blood center Japan 4 6 US/EU 2 3 Diagnostic Lab Japan Kit Manufacturer Japan OCL Japan 7 1 1 15 7 1 1 18 3 1 1 11 3 1 1 14 Total
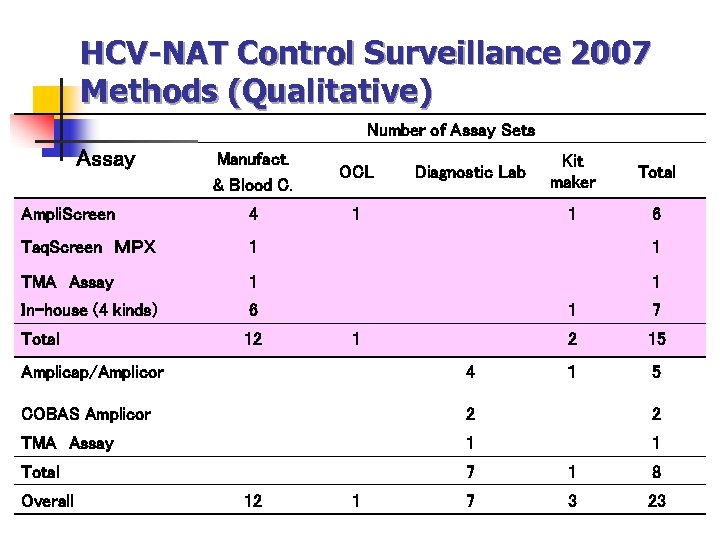 HCV-NAT Control Surveillance 2007 Methods (Qualitative) Number of Assay Sets Assay Manufact. & Blood C. OCL Ampli. Screen 4 1 Taq. Screen MPX 1 1 TMA Assay 1 1 In-house (4 kinds) 6 Total 12 Kit maker Total 1 Diagnostic Lab 6 1 2 1 7 15 1 5 Amplicap/Amplicor 4 COBAS Amplicor 2 2 TMA Assay 1 1 Total 7 1 8 7 3 23 Overall 12 1
HCV-NAT Control Surveillance 2007 Methods (Qualitative) Number of Assay Sets Assay Manufact. & Blood C. OCL Ampli. Screen 4 1 Taq. Screen MPX 1 1 TMA Assay 1 1 In-house (4 kinds) 6 Total 12 Kit maker Total 1 Diagnostic Lab 6 1 2 1 7 15 1 5 Amplicap/Amplicor 4 COBAS Amplicor 2 2 TMA Assay 1 1 Total 7 1 8 7 3 23 Overall 12 1
 HCV-NAT Control Surveillance 2007 45/45 44/45
HCV-NAT Control Surveillance 2007 45/45 44/45
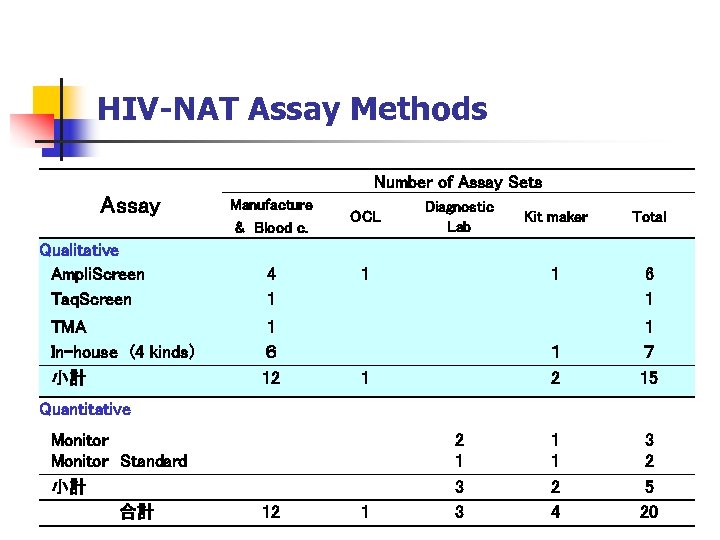 HIV-NAT Assay Methods Number of Assay Sets Assay Manufacture & Blood c. Qualitative Ampli. Screen Taq. Screen 4 1 TMA In-house (4 kinds) 小計 1 6 12 OCL Diagnostic Lab Total 1 1 Kit maker 6 1 1 2 1 1 7 15 1 1 2 4 3 2 5 20 Quantitative Monitor Standard 小計 合計 12 1 3 3
HIV-NAT Assay Methods Number of Assay Sets Assay Manufacture & Blood c. Qualitative Ampli. Screen Taq. Screen 4 1 TMA In-house (4 kinds) 小計 1 6 12 OCL Diagnostic Lab Total 1 1 Kit maker 6 1 1 2 1 1 7 15 1 1 2 4 3 2 5 20 Quantitative Monitor Standard 小計 合計 12 1 3 3
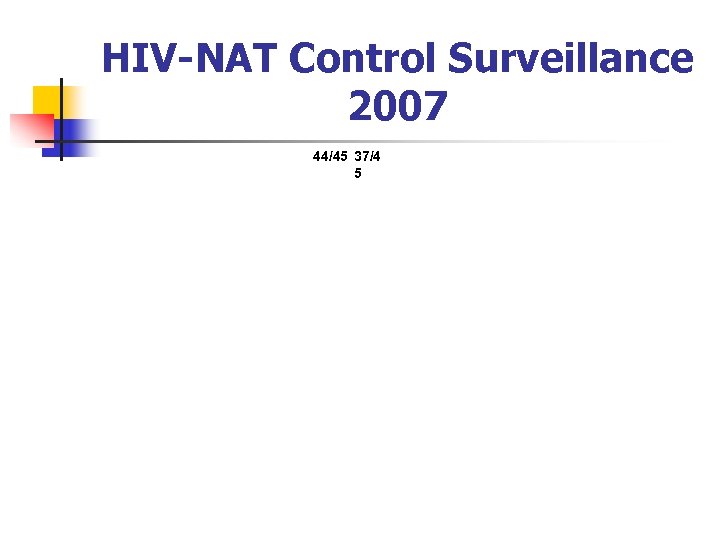 HIV-NAT Control Surveillance 2007 44/45 37/4 5
HIV-NAT Control Surveillance 2007 44/45 37/4 5
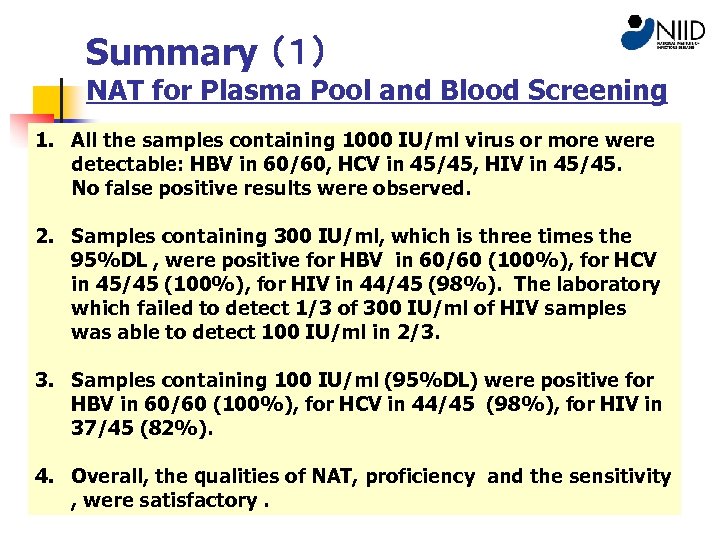 Summary (1) NAT for Plasma Pool and Blood Screening 1. All the samples containing 1000 IU/ml virus or more were detectable: HBV in 60/60, HCV in 45/45, HIV in 45/45. No false positive results were observed. 2. Samples containing 300 IU/ml, which is three times the 95%DL , were positive for HBV in 60/60 (100%), for HCV in 45/45 (100%), for HIV in 44/45 (98%). The laboratory which failed to detect 1/3 of 300 IU/ml of HIV samples was able to detect 100 IU/ml in 2/3. 3. Samples containing 100 IU/ml (95%DL) were positive for HBV in 60/60 (100%), for HCV in 44/45 (98%), for HIV in 37/45 (82%). 4. Overall, the qualities of NAT, proficiency and the sensitivity , were satisfactory.
Summary (1) NAT for Plasma Pool and Blood Screening 1. All the samples containing 1000 IU/ml virus or more were detectable: HBV in 60/60, HCV in 45/45, HIV in 45/45. No false positive results were observed. 2. Samples containing 300 IU/ml, which is three times the 95%DL , were positive for HBV in 60/60 (100%), for HCV in 45/45 (100%), for HIV in 44/45 (98%). The laboratory which failed to detect 1/3 of 300 IU/ml of HIV samples was able to detect 100 IU/ml in 2/3. 3. Samples containing 100 IU/ml (95%DL) were positive for HBV in 60/60 (100%), for HCV in 44/45 (98%), for HIV in 37/45 (82%). 4. Overall, the qualities of NAT, proficiency and the sensitivity , were satisfactory.
 Summary (2) NAT for Look-back Study 1. Two quantitative HBV-DNA kits were available (Kit A and B). No false positive results were observed in both kit A and kit B. 2. Kit A was able to measure all the positive samples (10 – 10000 IU/ml) while kit B was able to measure samples containing 3000 IU/ml or more.
Summary (2) NAT for Look-back Study 1. Two quantitative HBV-DNA kits were available (Kit A and B). No false positive results were observed in both kit A and kit B. 2. Kit A was able to measure all the positive samples (10 – 10000 IU/ml) while kit B was able to measure samples containing 3000 IU/ml or more.
 Summary (3) NAT control surveillance should be continued: n. To reflect the results on regulations. n. To improve the performance of participants n. To confirm that routine NAT is able to detect all the genotypes/subtypes. n. To assess performance of newly developed assay systems.
Summary (3) NAT control surveillance should be continued: n. To reflect the results on regulations. n. To improve the performance of participants n. To confirm that routine NAT is able to detect all the genotypes/subtypes. n. To assess performance of newly developed assay systems.
 Collaboration with: Department of Bacteriology II, NIID Yoshinobu Horiuchi Department of Blood and Safety Research, NIID Toshiaki Mizuochi Kazunari Yamaguchi Working Group on NAT Control Surveillance Chaired by Dr. Teruhide Yamaguchi National Institute of Health Sciences
Collaboration with: Department of Bacteriology II, NIID Yoshinobu Horiuchi Department of Blood and Safety Research, NIID Toshiaki Mizuochi Kazunari Yamaguchi Working Group on NAT Control Surveillance Chaired by Dr. Teruhide Yamaguchi National Institute of Health Sciences


