cee40134c045de34b48c6c47ff8a83f9.ppt
- Количество слайдов: 40

Nanotecnologia e ambiente A. A. 2011 -2012

The Janus-faced nanotech The impact of new nanotechnology products with the environment are two-faced matter. Several nanotech are environmentally friend and provide good means for soil remediation, water purification and energy storage. On the other side, nanotechnology products can be considered a chemical hazard with unknown potential, poorly predictable from the knowledge about the corresponding bulk material.

Chemical hazard. Nanomaterials represent a chemical hazard unpredictable from known properties of corresponding bulk material Other variable tio be considered are: 1. Poorly controlled increment of production 2. Poorly known dynamics in the environmental matrices 3. Poorly known environmental toxicity

Need for standardization Standardization is recommended for: • Production • Manipulation • Transport • Waste dispersion • Nanometrology • Toxicology studies in environmental matrices

Agencies for nanotech monitoring: supernational. Organization for Economic Co-operation and Development (OECD: WPN): http: //www. oecd. org/ International Organization for Standardization (ISO): http: //www. iso. org/ International Electrotechnical Commission (IEC): http: //www. iec. ch/

Agencies for nanotech monitoring: USA Environmental Protection Agency (EPA): http: //www. epa. gov/ National Nanotechnology Initiative (NNI): http: //www. nano. gov/ Food and Drug Administration (FDA): http: //www. fda. gov/

Agencies for nanotech monitoring: EU Community Research and Development Information Service (CORDIS): http: //cordis. europa. eu/home_it. html Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR): http: //ec. europa. eu/health/scientific_committ ees/emerging/index_en. htm

The following list of needed methods for the International Standardization for Nanotechnologies Have been discussed by Dr Peter Hatto (Director of Research, Ion. Bond Ltd, Chairman ISO TC 229 and UK NTI/1 Nanotechnologies Standardization committees) at the meeting organized at the Michigan State University (East Lansing, Michigan, USA, 7 th February 2007).

Needs of standard methods for Nanoparticles 1. Concentrations in air and water 2. Stability, dissolution, aggregation and diffusion rates in the environment 3. Toxicological screening, physical and chemical hazard 4. Risk Assessments on exposure and use 5. Environmental Toxicity Assessment for Nanomaterials 6. Assessment of Product Degradation and Release of Nanomaterials from Consumer Products 7. Nanomaterial Product Labelling

Needs of standard methods for Nanotubes 1. Eco-toxicology testing 2. Exposure determination (environment and food contamination) 3. Inhalation testing 4. Toxicology testing
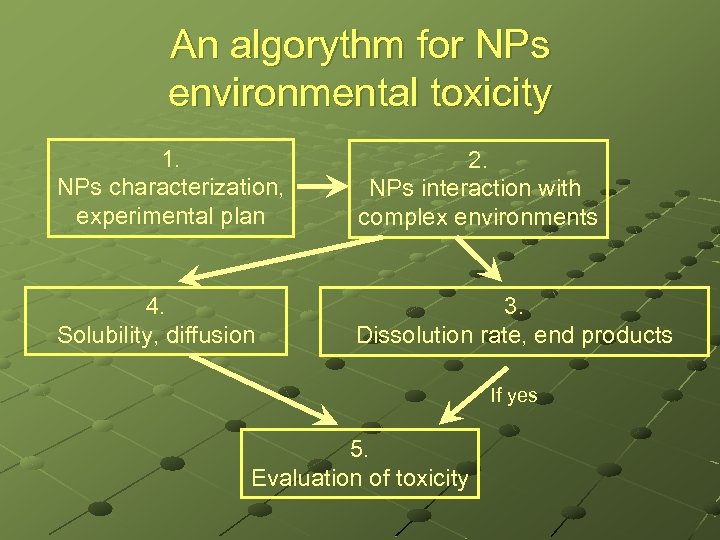
An algorythm for NPs environmental toxicity 1. NPs characterization, experimental plan 2. NPs interaction with complex environments 4. Solubility, diffusion 3. Dissolution rate, end products If yes 5. Evaluation of toxicity
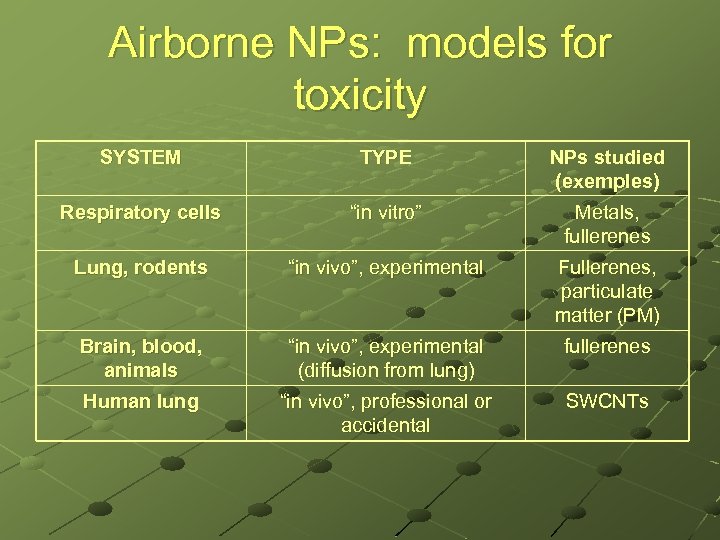
Airborne NPs: models for toxicity SYSTEM TYPE NPs studied (exemples) Respiratory cells “in vitro” Metals, fullerenes Lung, rodents “in vivo”, experimental Fullerenes, particulate matter (PM) Brain, blood, animals “in vivo”, experimental (diffusion from lung) fullerenes Human lung “in vivo”, professional or accidental SWCNTs

Environmental pollution: airborne Nanometals permanently aggregate in air (right)…. and enter the cells (left)
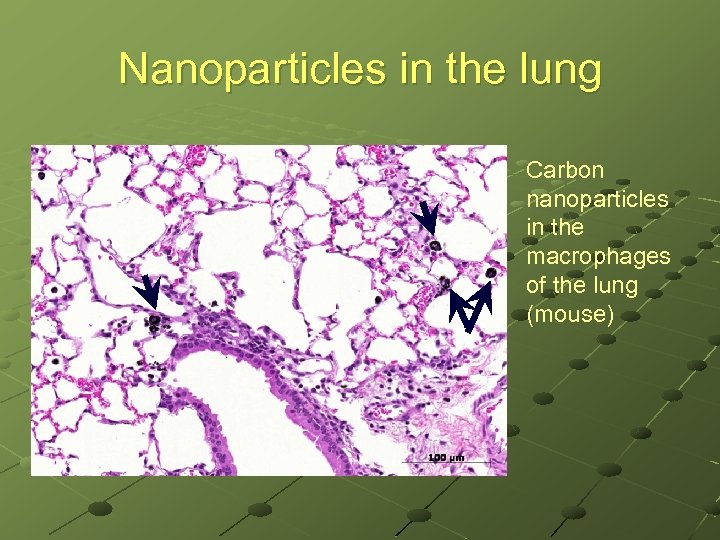
Nanoparticles in the lung Carbon nanoparticles in the macrophages of the lung (mouse)
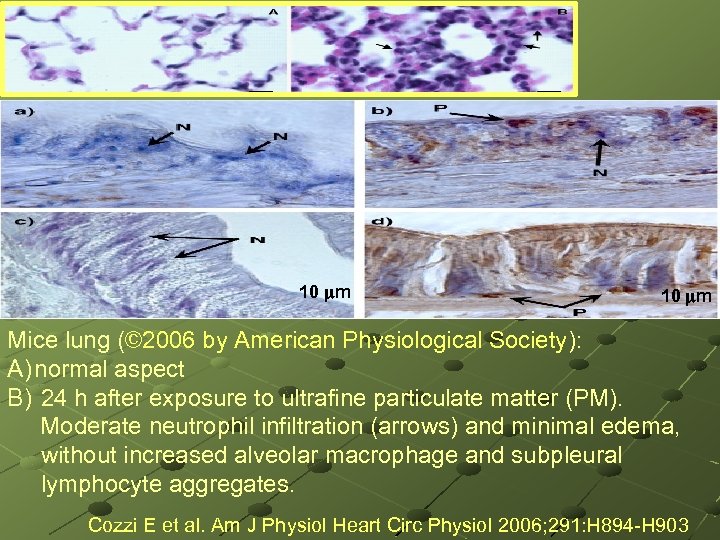
. 10 m Mice lung (© 2006 by American Physiological Society): A) normal aspect B) 24 h after exposure to ultrafine particulate matter (PM). Moderate neutrophil infiltration (arrows) and minimal edema, without increased alveolar macrophage and subpleural lymphocyte aggregates. Cozzi E et al. Am J Physiol Heart Circ Physiol 2006; 291: H 894 -H 903
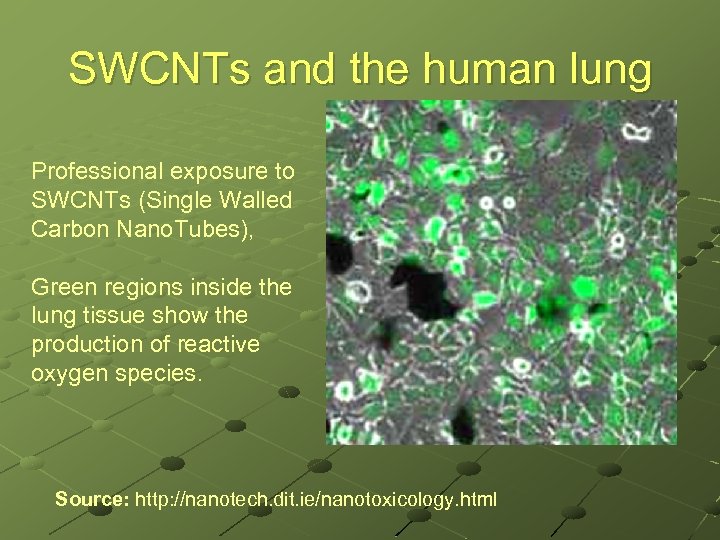
SWCNTs and the human lung Professional exposure to SWCNTs (Single Walled Carbon Nano. Tubes), Green regions inside the lung tissue show the production of reactive oxygen species. Source: http: //nanotech. dit. ie/nanotoxicology. html

Carbon nanospheres and cytotoxicity Carbon nanospheres CNs, functionalized with fluorescent dye at the surface. Vital staining, fluorescent dye. Carbon nanospheres in the cells (B) and in the nucleus (C; time- dependent migration). Dissolution occurs, with delivery of the toxic functionalizing dye (a semiconductor or a QD). Dissolution is time-dependent and happens in the nucleus. Selvi et al. Nano Letters 2008, 8(10): 3182– 3188 DOI: 10. 1021/nl 801503 m
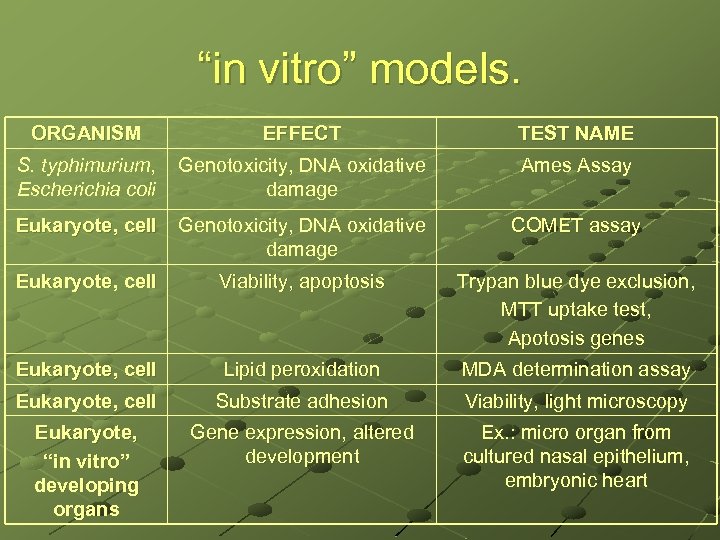
“in vitro” models. ORGANISM EFFECT S. typhimurium, Genotoxicity, DNA oxidative Escherichia coli damage TEST NAME Ames Assay Eukaryote, cell Genotoxicity, DNA oxidative damage COMET assay Eukaryote, cell Viability, apoptosis Trypan blue dye exclusion, MTT uptake test, Apotosis genes Eukaryote, cell Lipid peroxidation MDA determination assay Eukaryote, cell Substrate adhesion Viability, light microscopy Eukaryote, “in vitro” developing organs Gene expression, altered development Ex. : micro organ from cultured nasal epithelium, embryonic heart
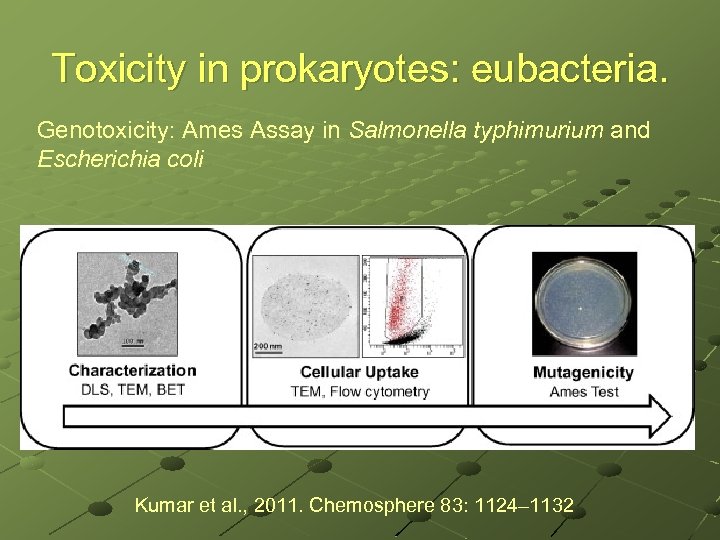
Toxicity in prokaryotes: eubacteria. Genotoxicity: Ames Assay in Salmonella typhimurium and Escherichia coli Kumar et al. , 2011. Chemosphere 83: 1124– 1132
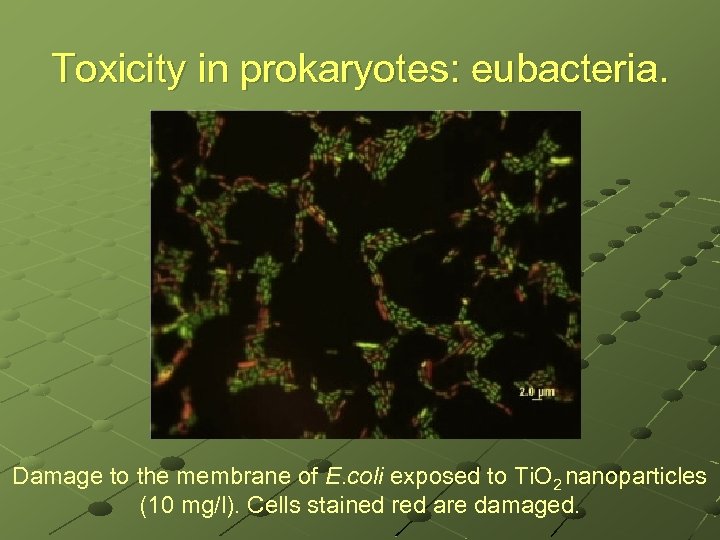
Toxicity in prokaryotes: eubacteria. Damage to the membrane of E. coli exposed to Ti. O 2 nanoparticles (10 mg/l). Cells stained red are damaged.
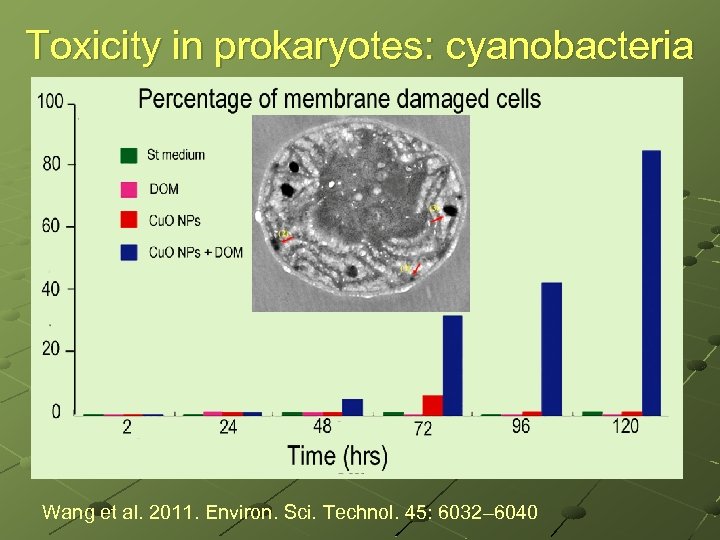
Toxicity in prokaryotes: cyanobacteria Wang et al. 2011. Environ. Sci. Technol. 45: 6032– 6040
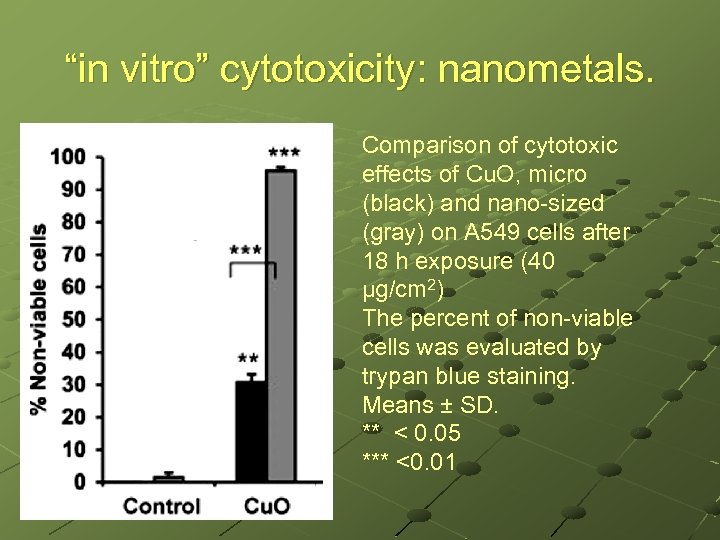
“in vitro” cytotoxicity: nanometals. Comparison of cytotoxic effects of Cu. O, micro (black) and nano-sized (gray) on A 549 cells after 18 h exposure (40 μg/cm 2) The percent of non-viable cells was evaluated by trypan blue staining. Means ± SD. ** < 0. 05 *** <0. 01

The COMET assay H 2 O 2 Co NPs Seriously (H 2 O 2, 300 M) and moderately (n. Co, 1 M) damaged DNA. Hartung & Sabbioni. 2011. Nanomedicine and Nanobiotechnology, DOI: 10. 1002/wnan. 153
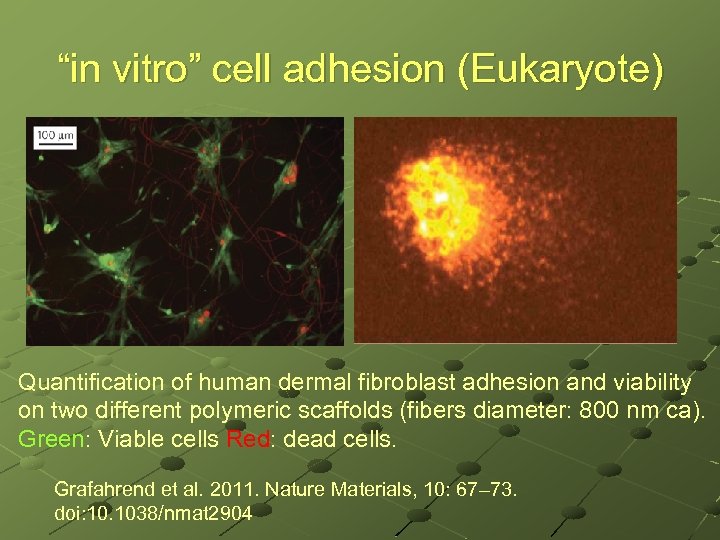
“in vitro” cell adhesion (Eukaryote) Quantification of human dermal fibroblast adhesion and viability on two different polymeric scaffolds (fibers diameter: 800 nm ca). Green: Viable cells Red: dead cells. Grafahrend et al. 2011. Nature Materials, 10: 67– 73. doi: 10. 1038/nmat 2904

Models for aquatic toxicity (fresh and salt water), invertebrates ORGANISM Test approved by ASTM/EPA/OECD/EU/ISO Daphnids: D. magna, D. pulex, C. dubia + Chydorus sphaericus - Thamnocephalus platyurus - Brachionus calyciflorus + Hydra attenuata + Elliptio complanata - Crustaceans: harpacticoida copepods + Mytilus edulis +

Fluorescently labelled polymeric nanoparticles in the gut of Daphnia magna following ingestion after aqueous exposure. (Image taken by Dag Altin, Bio. Trix) Nanometals and fullerens intake by D. magna. (Chen et al. , 2008 Progress Report: Methodology Development for Manufactured Nanomaterial Bioaccumulation Test EPA Grant Number: R 833327)
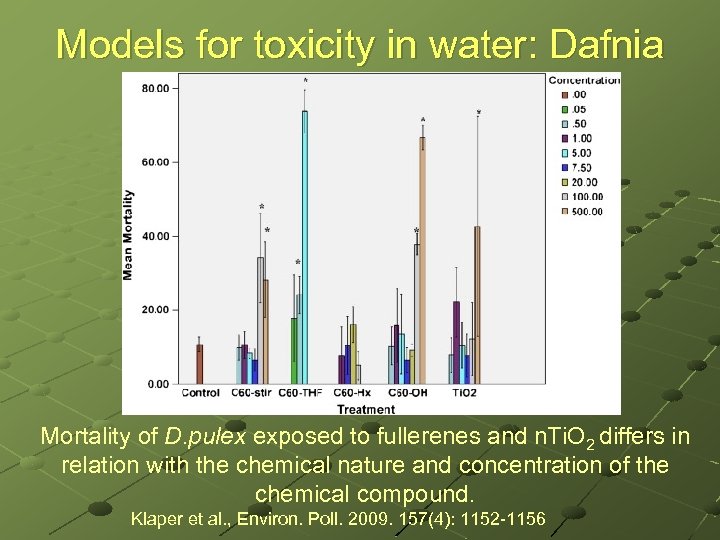
Models for toxicity in water: Dafnia Mortality of D. pulex exposed to fullerenes and n. Ti. O 2 differs in relation with the chemical nature and concentration of the chemical compound. Klaper et al. , Environ. Poll. 2009. 157(4): 1152 -1156
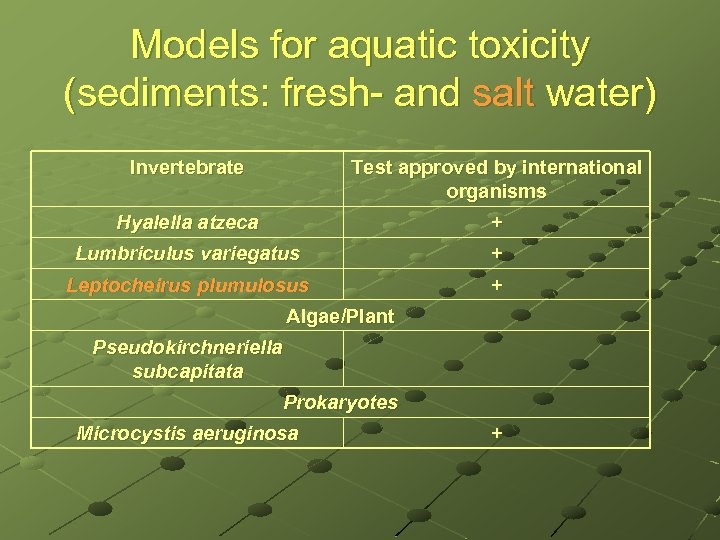
Models for aquatic toxicity (sediments: fresh- and salt water) Invertebrate Test approved by international organisms Hyalella atzeca + Lumbriculus variegatus + Leptocheirus plumulosus + Algae/Plant Pseudokirchneriella subcapitata Prokaryotes Microcystis aeruginosa +
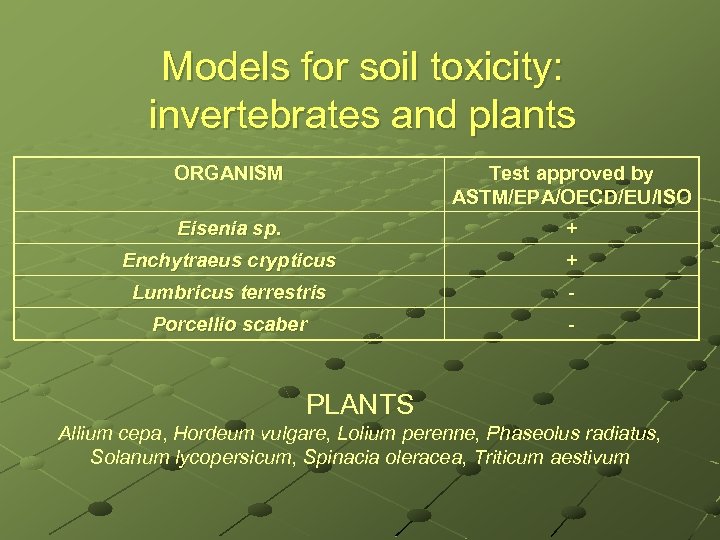
Models for soil toxicity: invertebrates and plants ORGANISM Test approved by ASTM/EPA/OECD/EU/ISO Eisenia sp. + Enchytraeus crypticus + Lumbricus terrestris - Porcellio scaber - PLANTS Allium cepa, Hordeum vulgare, Lolium perenne, Phaseolus radiatus, Solanum lycopersicum, Spinacia oleracea, Triticum aestivum
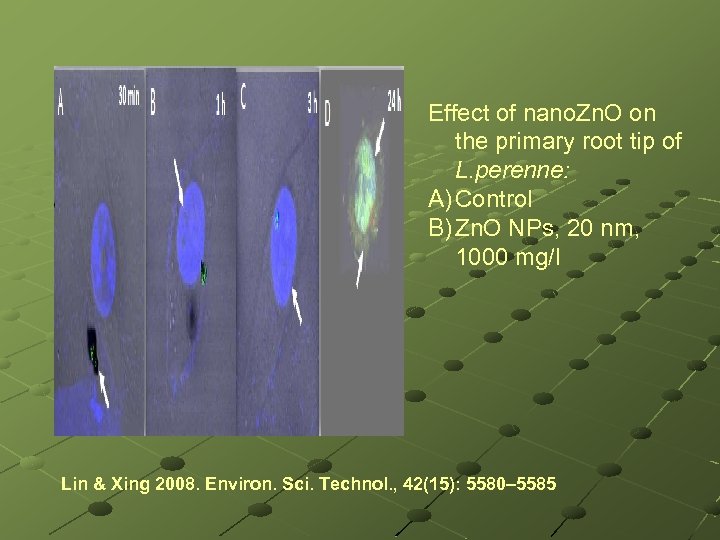
Effect of nano. Zn. O on the primary root tip of L. perenne: A) Control B) Zn. O NPs, 20 nm, 1000 mg/l Lin & Xing 2008. Environ. Sci. Technol. , 42(15): 5580– 5585
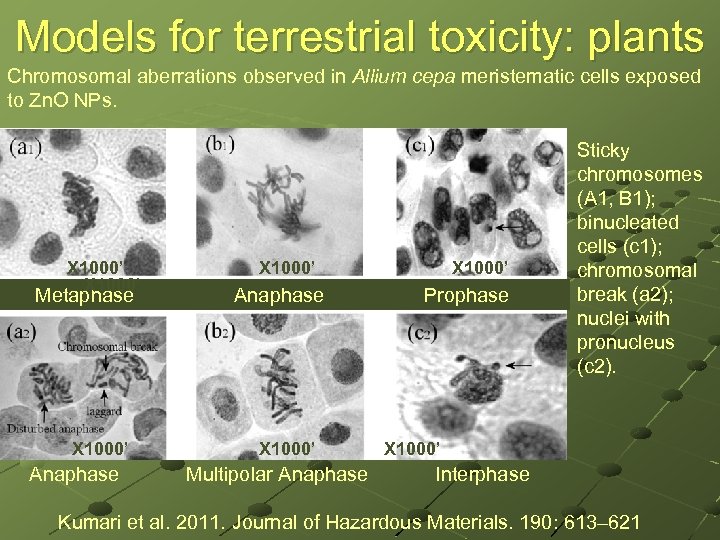
Models for terrestrial toxicity: plants Chromosomal aberrations observed in Allium cepa meristematic cells exposed to Zn. O NPs. X 1000’ X 1000’ Metaphase Anaphase Prophase Sticky chromosomes (A 1, B 1); binucleated cells (c 1); chromosomal break (a 2); nuclei with pronucleus (c 2). X 1000’ Anaphase Multipolar Anaphase Interphase Kumari et al. 2011. Journal of Hazardous Materials. 190: 613– 621
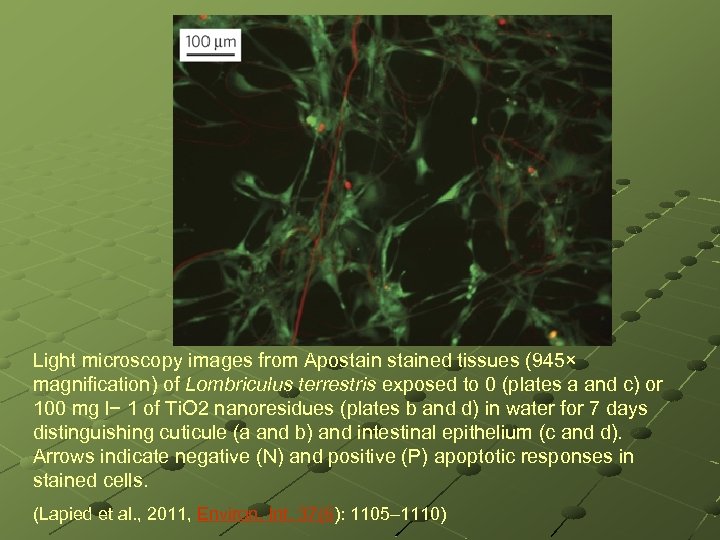
Light microscopy images from Apostained tissues (945× magnification) of Lombriculus terrestris exposed to 0 (plates a and c) or 100 mg l− 1 of Ti. O 2 nanoresidues (plates b and d) in water for 7 days distinguishing cuticule (a and b) and intestinal epithelium (c and d). Arrows indicate negative (N) and positive (P) apoptotic responses in stained cells. (Lapied et al. , 2011, Environ. Int. 37(6): 1105– 1110)

Models for aquatic toxicity: vertebrate The fishes O. mykiss, O. latipes and D. rerio have been studied during exposure to NPs, in natural or experimental conditions. The developmental effects on the model organism X. laevis, a clawed frog, have been also studied in laboratory conditions.
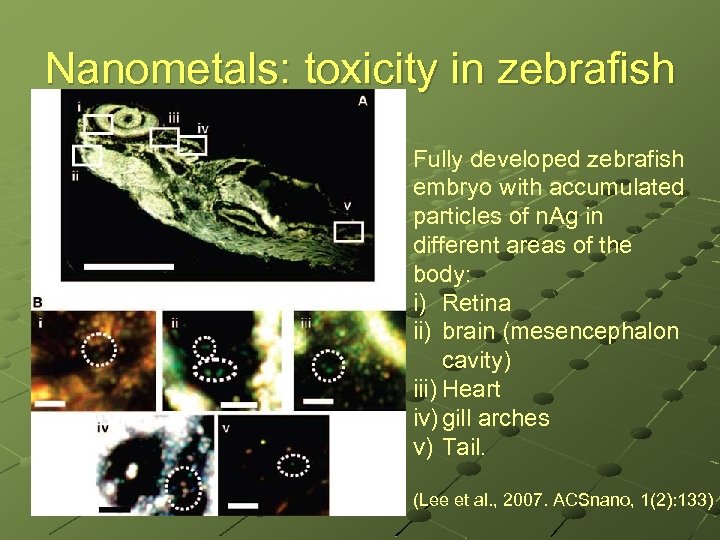
Nanometals: toxicity in zebrafish Fully developed zebrafish embryo with accumulated particles of n. Ag in different areas of the body: i) Retina ii) brain (mesencephalon cavity) iii) Heart iv) gill arches v) Tail. (Lee et al. , 2007. ACSnano, 1(2): 133)
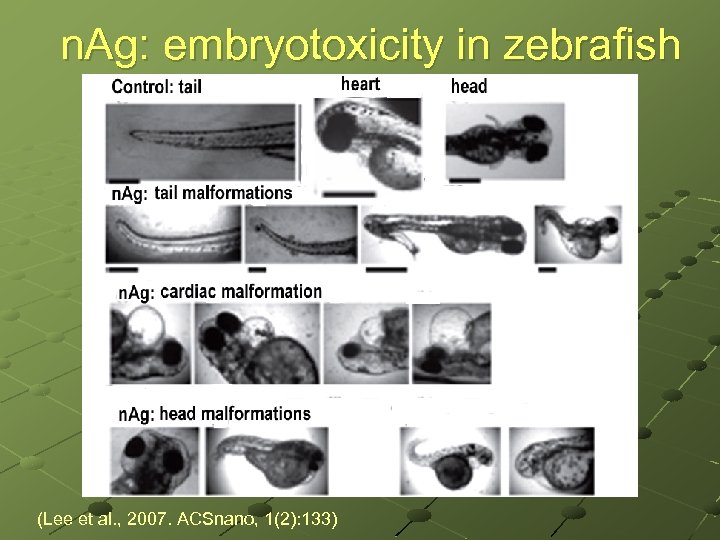
n. Ag: embryotoxicity in zebrafish (Lee et al. , 2007. ACSnano, 1(2): 133)

And now, the good news.

Nanotechnologies for soil and groundwater remediation NPs amphiphilic polyurethane (APU) Nanoscale or emulsified zero-valent iron (NZVI, EZVI) Environment Ref. Groundwater and soils DOI: 10. 1021/es 0348997 from polynuclear aromatic hydrocarbons. PAHs EPA 542 -F-08 -009, 2008 bi-metallic nanoscale particles (BNPs) Au. Soils from DOI: 10. 1002/cs polymeric oil, nanofoam volatile sc. 201000 haromatic 410 http: //www. epa. gov/tio/do wnload/remed/542 -f-08009. pdf

Nanotechnology for wastewater purification NPs Dendrimers Metal nanoparticles Zeolite nanoparticles Pollutants toxic metal ions, radionuclide and inorganic anions, delivery vehicles for antimicrobial Antimicrobial action, remove As (Zn. O NPs) Remove Cr(III), Ni(II), Zn(II), Cu(II) and Cd(II). Reverse osmosis for desalination. Carbon-based nanomaterials Wide-range applications Boron nanotubes Desalination Tiwari et al. , 2008 World Applied Sciences Journal 3 (3): 417 -433,

Nanotechnology for solar power. Photoactive materials: Semiconductors with appropriate band gap and high absorption coefficient. 1. Inorganic Semiconductors: Si, Ge, Cd. S, Ga. As, Cd. Te, Ti. O 2, etc. 2. Organic Semiconductors: 3. A. Small molecules: phthalocyanines, porphyrins, etc. 4. B. Conducting polymers: Polythiophenes, polypyrrole, polyaniline, etc. 5. Solid-state polymers have lesser problems than liquid ones. 6. Coupling solid-state polymers with C 60 improves the performance. 7. Teketel Y. , Solid-State Photoelectrochemical Solar Energy Conversion Based 8. On Conducting Polymers. ICPC Nano. Net 3 rd Annual Workshop, May 2011,

Nanotechnology for solar power. Photoactive materials: Semiconductors with appropriate band gap and high absorption coefficient. 1. Inorganic Semiconductors: Si, Ge, Cd. S, Ga. As, Cd. Te, Ti. O 2, 2. etc. 3. Organic Semiconductors: 4. A. Small molecules: phthalocyanines, porphyrins, etc. 5. B. Conducting polymers: Polythiophenes, polypyrrole, 6. polyaniline, etc.
cee40134c045de34b48c6c47ff8a83f9.ppt