705e87f21864aeaab1b0383580911b67.ppt
- Количество слайдов: 24
 Nanoparticle Technology: Leveraging Rapid Dissolution to Improve Performance of Poorly Water-Soluble Drugs Advisory Committee for Pharmaceutical Science and Clinical Pharmacology Rockville, MD 22 July 2008 Stephen B. Ruddy, Ph. D. Elan Corporation, plc
Nanoparticle Technology: Leveraging Rapid Dissolution to Improve Performance of Poorly Water-Soluble Drugs Advisory Committee for Pharmaceutical Science and Clinical Pharmacology Rockville, MD 22 July 2008 Stephen B. Ruddy, Ph. D. Elan Corporation, plc
 What are Engineered Nanoparticles? Nanoscale particles of API: • characterized by an extremely high surface-area to mass ratio and stabilized against agglomeration using surface modifiers • not naturally occurring; prepared by: - molecular deposition/complexation (“bottom up”) - attrition of larger non-nanoscale materials (“top down”) • range in size from ca. 80 to 1000 nm for many pharmaceutical applications FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 2 –
What are Engineered Nanoparticles? Nanoscale particles of API: • characterized by an extremely high surface-area to mass ratio and stabilized against agglomeration using surface modifiers • not naturally occurring; prepared by: - molecular deposition/complexation (“bottom up”) - attrition of larger non-nanoscale materials (“top down”) • range in size from ca. 80 to 1000 nm for many pharmaceutical applications FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 2 –
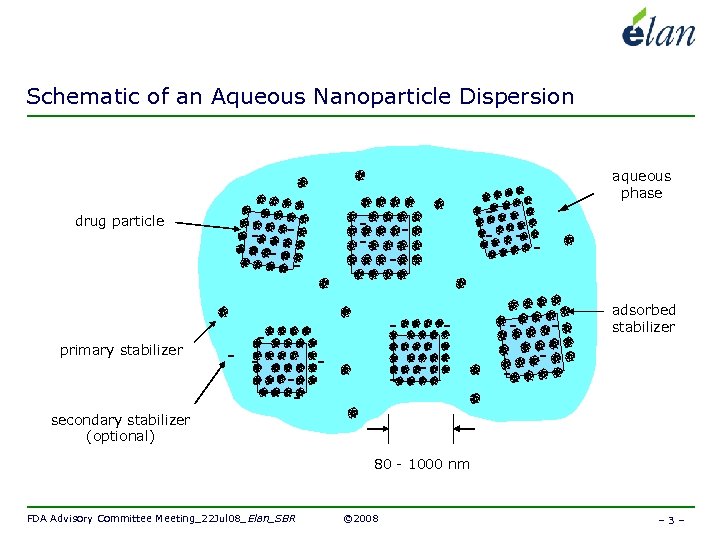 Schematic of an Aqueous Nanoparticle Dispersion aqueous phase drug particle adsorbed stabilizer primary stabilizer secondary stabilizer (optional) 80 - 1000 nm FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 3 –
Schematic of an Aqueous Nanoparticle Dispersion aqueous phase drug particle adsorbed stabilizer primary stabilizer secondary stabilizer (optional) 80 - 1000 nm FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 3 –
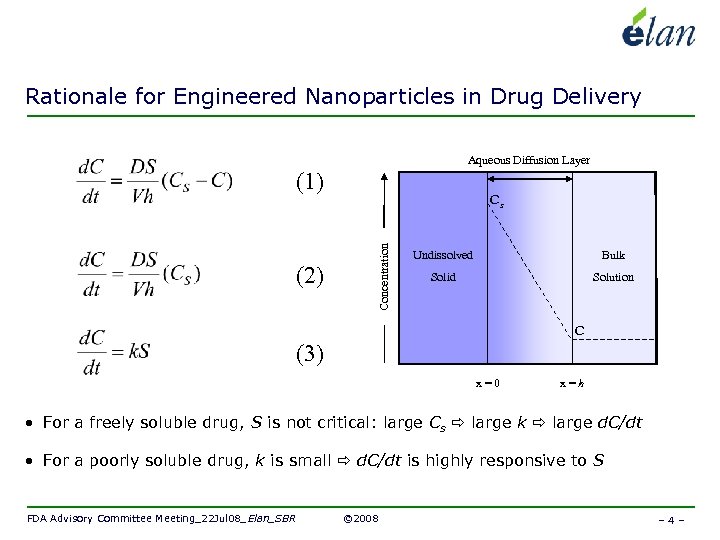 Rationale for Engineered Nanoparticles in Drug Delivery Aqueous Diffusion Layer (1) Concentration (2) Cs Undissolved Bulk Solid Solution C (3) x=0 x=h • For a freely soluble drug, S is not critical: large Cs large k large d. C/dt • For a poorly soluble drug, k is small d. C/dt is highly responsive to S FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 4 –
Rationale for Engineered Nanoparticles in Drug Delivery Aqueous Diffusion Layer (1) Concentration (2) Cs Undissolved Bulk Solid Solution C (3) x=0 x=h • For a freely soluble drug, S is not critical: large Cs large k large d. C/dt • For a poorly soluble drug, k is small d. C/dt is highly responsive to S FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 4 –
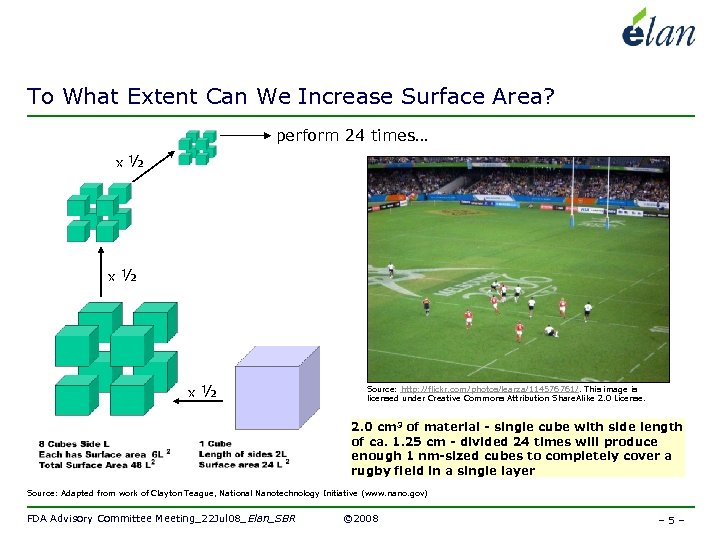 To What Extent Can We Increase Surface Area? perform 24 times… x ½ x ½ Source: http: //flickr. com/photos/learza/114576761/. This image is licensed under Creative Commons Attribution Share. Alike 2. 0 License. 2. 0 cm 3 of material - single cube with side length of ca. 1. 25 cm - divided 24 times will produce enough 1 nm-sized cubes to completely cover a rugby field in a single layer Source: Adapted from work of Clayton Teague, National Nanotechnology Initiative (www. nano. gov) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 5 –
To What Extent Can We Increase Surface Area? perform 24 times… x ½ x ½ Source: http: //flickr. com/photos/learza/114576761/. This image is licensed under Creative Commons Attribution Share. Alike 2. 0 License. 2. 0 cm 3 of material - single cube with side length of ca. 1. 25 cm - divided 24 times will produce enough 1 nm-sized cubes to completely cover a rugby field in a single layer Source: Adapted from work of Clayton Teague, National Nanotechnology Initiative (www. nano. gov) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 5 –
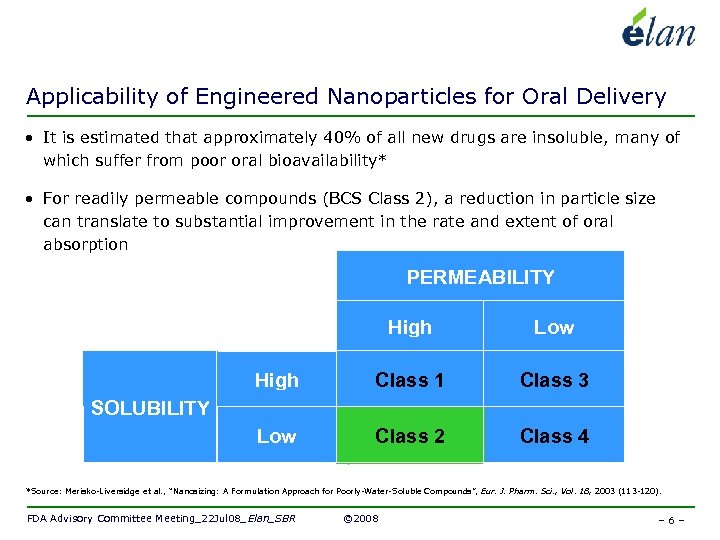 Applicability of Engineered Nanoparticles for Oral Delivery • It is estimated that approximately 40% of all new drugs are insoluble, many of which suffer from poor oral bioavailability* • For readily permeable compounds (BCS Class 2), a reduction in particle size can translate to substantial improvement in the rate and extent of oral absorption PERMEABILITY High Low High Class 1 Class 3 Low Class 2 Class 4 SOLUBILITY *Source: Merisko-Liversidge et al. , “Nanosizing: A Formulation Approach for Poorly-Water-Soluble Compounds”, Eur. J. Pharm. Sci. , Vol. 18, 2003 (113 -120). FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 6 –
Applicability of Engineered Nanoparticles for Oral Delivery • It is estimated that approximately 40% of all new drugs are insoluble, many of which suffer from poor oral bioavailability* • For readily permeable compounds (BCS Class 2), a reduction in particle size can translate to substantial improvement in the rate and extent of oral absorption PERMEABILITY High Low High Class 1 Class 3 Low Class 2 Class 4 SOLUBILITY *Source: Merisko-Liversidge et al. , “Nanosizing: A Formulation Approach for Poorly-Water-Soluble Compounds”, Eur. J. Pharm. Sci. , Vol. 18, 2003 (113 -120). FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 6 –
 Benefits of Engineered Nanoparticles in Oral Drug Delivery • Increased bioavailability • Increased rate of absorption • Reduced fed/fasted variable absorption • Improved dose proportionality • Avoidance of uncontrolled precipitation after dosing FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 7 –
Benefits of Engineered Nanoparticles in Oral Drug Delivery • Increased bioavailability • Increased rate of absorption • Reduced fed/fasted variable absorption • Improved dose proportionality • Avoidance of uncontrolled precipitation after dosing FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 7 –
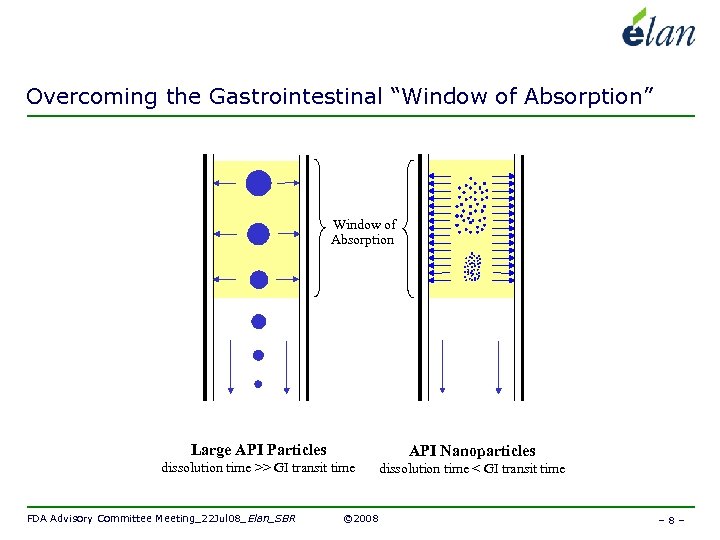 Overcoming the Gastrointestinal “Window of Absorption” Window of Absorption Large API Particles API Nanoparticles dissolution time >> GI transit time dissolution time < GI transit time FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 –
Overcoming the Gastrointestinal “Window of Absorption” Window of Absorption Large API Particles API Nanoparticles dissolution time >> GI transit time dissolution time < GI transit time FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 –
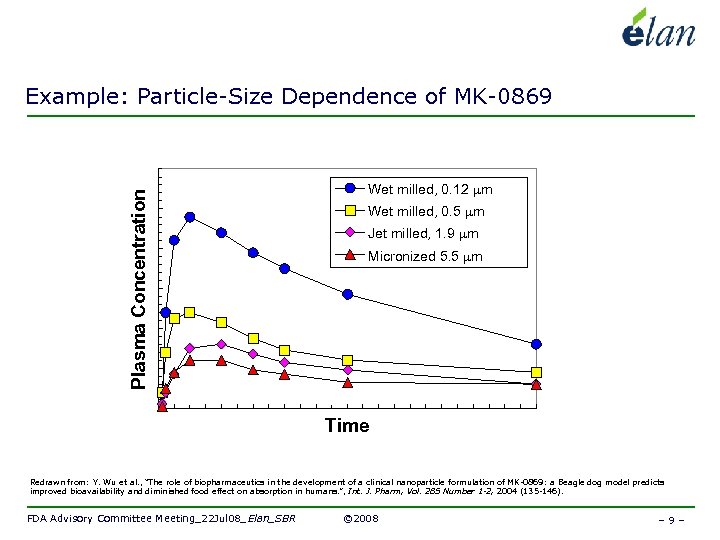 Plasma Concentration Example: Particle-Size Dependence of MK-0869 Wet milled, 0. 12 mm Wet milled, 0. 5 mm Jet milled, 1. 9 mm Micronized 5. 5 mm Time Redrawn from: Y. Wu et al. , “The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in humans. ”, Int. J. Pharm, Vol. 285 Number 1 -2, 2004 (135 -146). FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 9 –
Plasma Concentration Example: Particle-Size Dependence of MK-0869 Wet milled, 0. 12 mm Wet milled, 0. 5 mm Jet milled, 1. 9 mm Micronized 5. 5 mm Time Redrawn from: Y. Wu et al. , “The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in humans. ”, Int. J. Pharm, Vol. 285 Number 1 -2, 2004 (135 -146). FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 9 –
 Benefits of Engineered Nanoparticles in Parenteral Delivery • High drug loading in aqueous formulations (up to 45% w/w) • Avoidance of harsh vehicles (e. g. , cosolvents, solubilizers, p. H extremes) • Readily syringable formulations facilitate use of traditional small-bore needles • Safety established for IV*, IM and SC routes of administration See, for example: J. P. Donnelly, J. W. Mouton, N. M. A. Blijlevens, A. Smiets, P. E. Verweij, B. E. De. Pauw, Dept of Hematology, Dept of Med Microbiology, Univ. Med. Ctr Nijmegen, Canisius Wilhelmina Ziekenhuis, Nijmegen, The Netherlands, “Pharmacokinetics of a 14 day course of itraconazole Nano. Crystal®s given intravenously to allogeneic haematopoietic stem cell transplant recipients” Paper presented at the Interscience Conference on Anti-microbials and Chemotherapy, 2001 FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 10 –
Benefits of Engineered Nanoparticles in Parenteral Delivery • High drug loading in aqueous formulations (up to 45% w/w) • Avoidance of harsh vehicles (e. g. , cosolvents, solubilizers, p. H extremes) • Readily syringable formulations facilitate use of traditional small-bore needles • Safety established for IV*, IM and SC routes of administration See, for example: J. P. Donnelly, J. W. Mouton, N. M. A. Blijlevens, A. Smiets, P. E. Verweij, B. E. De. Pauw, Dept of Hematology, Dept of Med Microbiology, Univ. Med. Ctr Nijmegen, Canisius Wilhelmina Ziekenhuis, Nijmegen, The Netherlands, “Pharmacokinetics of a 14 day course of itraconazole Nano. Crystal®s given intravenously to allogeneic haematopoietic stem cell transplant recipients” Paper presented at the Interscience Conference on Anti-microbials and Chemotherapy, 2001 FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 10 –
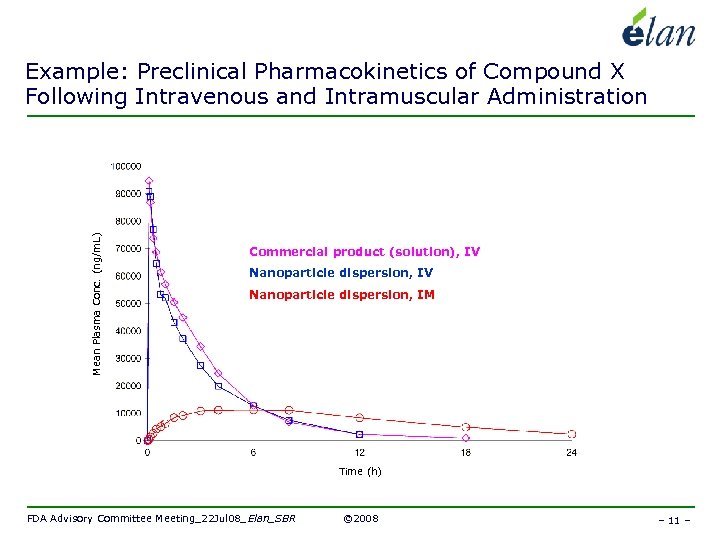 Mean Plasma Conc. (ng/m. L) Example: Preclinical Pharmacokinetics of Compound X Following Intravenous and Intramuscular Administration Commercial product (solution), IV Nanoparticle dispersion, IM Time (h) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 11 –
Mean Plasma Conc. (ng/m. L) Example: Preclinical Pharmacokinetics of Compound X Following Intravenous and Intramuscular Administration Commercial product (solution), IV Nanoparticle dispersion, IM Time (h) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 11 –
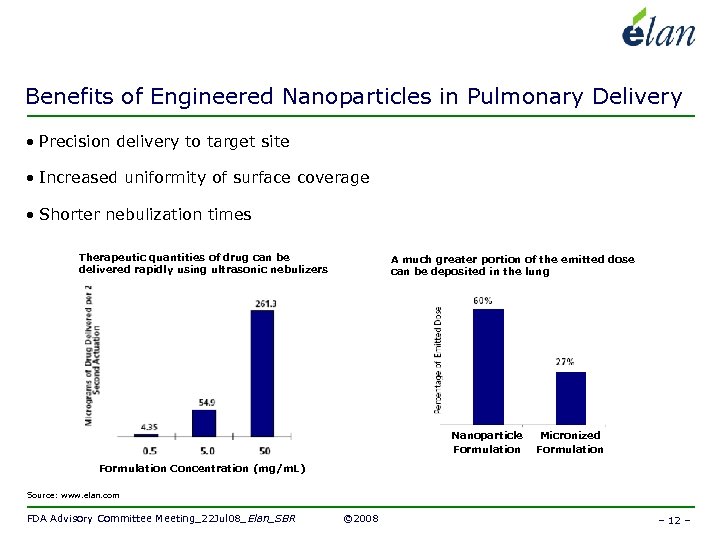 Benefits of Engineered Nanoparticles in Pulmonary Delivery • Precision delivery to target site • Increased uniformity of surface coverage • Shorter nebulization times Therapeutic quantities of drug can be delivered rapidly using ultrasonic nebulizers A much greater portion of the emitted dose can be deposited in the lung Nanoparticle Formulation Micronized Formulation Concentration (mg/m. L) Source: www. elan. com FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 12 –
Benefits of Engineered Nanoparticles in Pulmonary Delivery • Precision delivery to target site • Increased uniformity of surface coverage • Shorter nebulization times Therapeutic quantities of drug can be delivered rapidly using ultrasonic nebulizers A much greater portion of the emitted dose can be deposited in the lung Nanoparticle Formulation Micronized Formulation Concentration (mg/m. L) Source: www. elan. com FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 12 –
 How are Engineered Nanoparticles Produced? 1. Spray freezing into liquid (SFL) 2. Emulsification 3. Precipitation with a compressed fluid antisolvent (PCA) 4. Rapid expansion from a liquefied-gas solution (RESS) 5. Evaporative precipitation into aqueous solution (EPAS) 6. High-pressure homogenization 7. Microfluidization 8. High-energy wet milling Source: V. Kharb, “Nanoparticle Technology for the Delivery of Poorly Water-Soluble Drugs”, Pharmaceutical Technology, Vol. 30 Number 2, February, 2006. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 13 –
How are Engineered Nanoparticles Produced? 1. Spray freezing into liquid (SFL) 2. Emulsification 3. Precipitation with a compressed fluid antisolvent (PCA) 4. Rapid expansion from a liquefied-gas solution (RESS) 5. Evaporative precipitation into aqueous solution (EPAS) 6. High-pressure homogenization 7. Microfluidization 8. High-energy wet milling Source: V. Kharb, “Nanoparticle Technology for the Delivery of Poorly Water-Soluble Drugs”, Pharmaceutical Technology, Vol. 30 Number 2, February, 2006. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 13 –
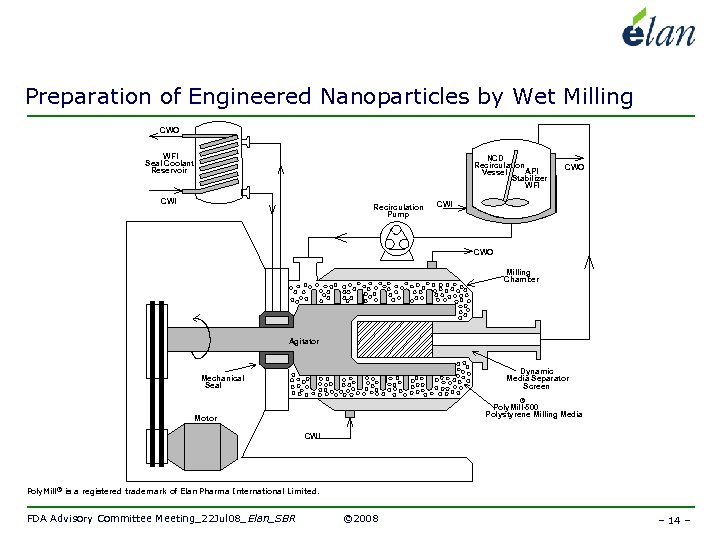 Preparation of Engineered Nanoparticles by Wet Milling CWO WFI Seal Coolant Reservoir NCD Recirculation API Vessel Stabilizer WFI CWI Recirculation Pump CWO CWI CWO Milling Chamber Agitator Dynamic Media Separator Screen Mechanical Seal ® Poly. Mill-500 Polystyrene Milling Media Motor CWI Poly. Mill ® is a registered trademark of Elan Pharma International Limited. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 14 –
Preparation of Engineered Nanoparticles by Wet Milling CWO WFI Seal Coolant Reservoir NCD Recirculation API Vessel Stabilizer WFI CWI Recirculation Pump CWO CWI CWO Milling Chamber Agitator Dynamic Media Separator Screen Mechanical Seal ® Poly. Mill-500 Polystyrene Milling Media Motor CWI Poly. Mill ® is a registered trademark of Elan Pharma International Limited. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 14 –
 Nano. Mill®– 2 Manufacturing Platform Nano. Mill® is a registered trademark of Elan Pharma International Limited. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 15 –
Nano. Mill®– 2 Manufacturing Platform Nano. Mill® is a registered trademark of Elan Pharma International Limited. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 15 –
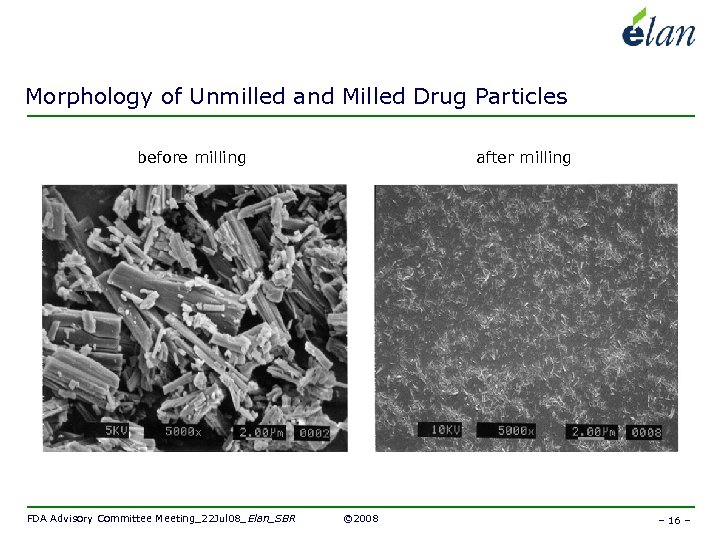 Morphology of Unmilled and Milled Drug Particles before milling FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR after milling © 2008 – 16 –
Morphology of Unmilled and Milled Drug Particles before milling FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR after milling © 2008 – 16 –
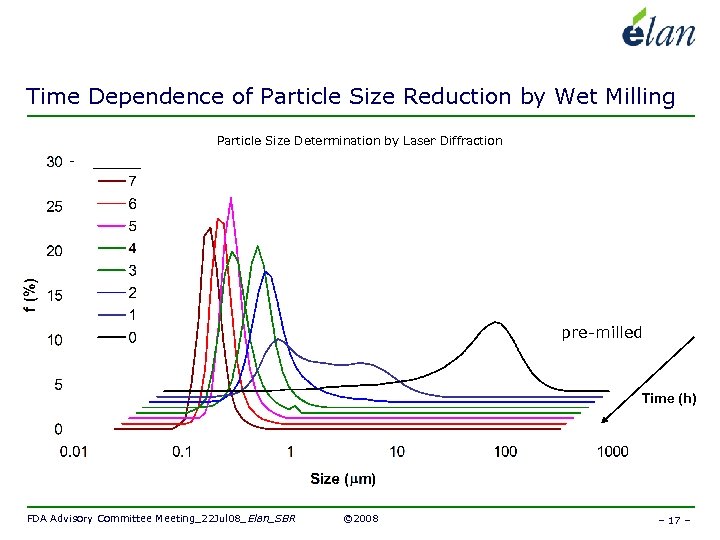 Time Dependence of Particle Size Reduction by Wet Milling Particle Size Determination by Laser Diffraction Broad Range Required in Our Application pre-milled Time (h) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 17 –
Time Dependence of Particle Size Reduction by Wet Milling Particle Size Determination by Laser Diffraction Broad Range Required in Our Application pre-milled Time (h) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 17 –
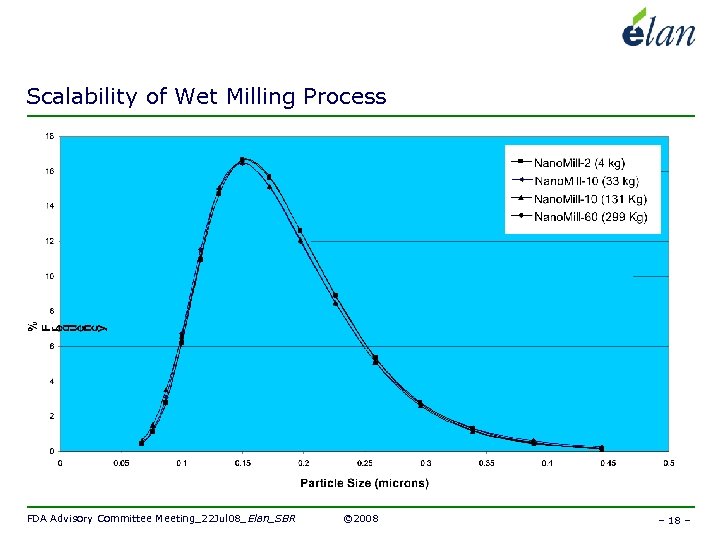 Scalability of Wet Milling Process FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 18 –
Scalability of Wet Milling Process FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 18 –
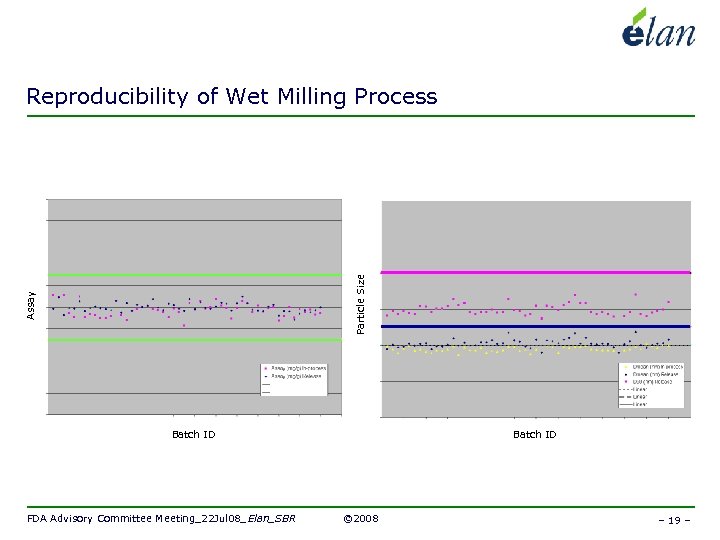 Assay Particle Size Reproducibility of Wet Milling Process Batch ID FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR Batch ID © 2008 – 19 –
Assay Particle Size Reproducibility of Wet Milling Process Batch ID FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR Batch ID © 2008 – 19 –
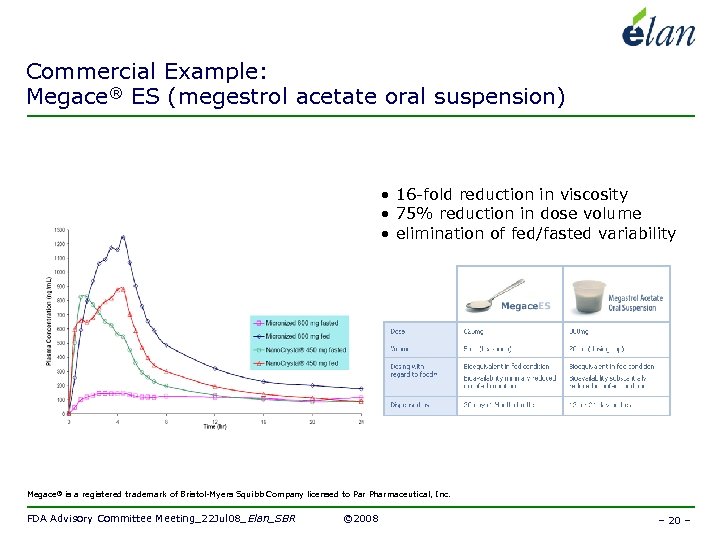 Commercial Example: Megace® ES (megestrol acetate oral suspension) • 16 -fold reduction in viscosity • 75% reduction in dose volume • elimination of fed/fasted variability Megace® is a registered trademark of Bristol-Myers Squibb Company licensed to Par Pharmaceutical, Inc. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 20 –
Commercial Example: Megace® ES (megestrol acetate oral suspension) • 16 -fold reduction in viscosity • 75% reduction in dose volume • elimination of fed/fasted variability Megace® is a registered trademark of Bristol-Myers Squibb Company licensed to Par Pharmaceutical, Inc. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 20 –
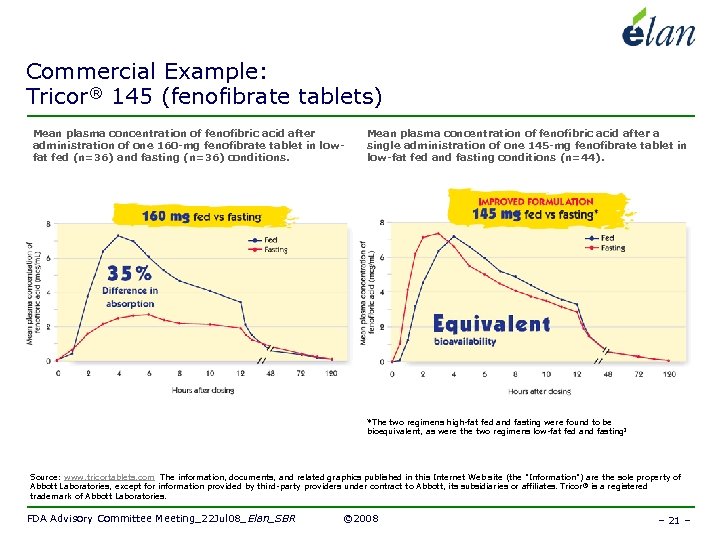 Commercial Example: Tricor® 145 (fenofibrate tablets) Mean plasma concentration of fenofibric acid after administration of one 160 -mg fenofibrate tablet in lowfat fed (n=36) and fasting (n=36) conditions. Mean plasma concentration of fenofibric acid after a single administration of one 145 -mg fenofibrate tablet in low-fat fed and fasting conditions (n=44). *The two regimens high-fat fed and fasting were found to be bioequivalent, as were the two regimens low-fat fed and fasting 2 Source: www. tricortablets. com The information, documents, and related graphics published in this Internet Web site (the "Information") are the sole property of Abbott Laboratories, except for information provided by third-party providers under contract to Abbott, its subsidiaries or affiliates. Tricor® is a registered trademark of Abbott Laboratories. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 21 –
Commercial Example: Tricor® 145 (fenofibrate tablets) Mean plasma concentration of fenofibric acid after administration of one 160 -mg fenofibrate tablet in lowfat fed (n=36) and fasting (n=36) conditions. Mean plasma concentration of fenofibric acid after a single administration of one 145 -mg fenofibrate tablet in low-fat fed and fasting conditions (n=44). *The two regimens high-fat fed and fasting were found to be bioequivalent, as were the two regimens low-fat fed and fasting 2 Source: www. tricortablets. com The information, documents, and related graphics published in this Internet Web site (the "Information") are the sole property of Abbott Laboratories, except for information provided by third-party providers under contract to Abbott, its subsidiaries or affiliates. Tricor® is a registered trademark of Abbott Laboratories. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 21 –
 Potential Challenges in Developing Nanoparticle Products 1. Particle agglomeration 2. Particle size growth (Ostwald ripening) 3. Changes in particle morphology 4. Changes in polymorphic form 5. Process-related impurities (e. g. , residual solvents, media attrition) 6. Process scalability and reproducibility 7. Lack of a universal particle sizing method FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 22 –
Potential Challenges in Developing Nanoparticle Products 1. Particle agglomeration 2. Particle size growth (Ostwald ripening) 3. Changes in particle morphology 4. Changes in polymorphic form 5. Process-related impurities (e. g. , residual solvents, media attrition) 6. Process scalability and reproducibility 7. Lack of a universal particle sizing method FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 22 –
 Key Characterization Needs for Nanoparticle Applications 1. Particle size distribution 2. Solid-state properties 3. Dissolution behavior 4. Microbial limits testing (for aqueous products or product intermediates) 5. Application specific methods (e. g. , route of administration) 6. Technology specific methods (i. e. , novel, unique to formulation/process) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 23 –
Key Characterization Needs for Nanoparticle Applications 1. Particle size distribution 2. Solid-state properties 3. Dissolution behavior 4. Microbial limits testing (for aqueous products or product intermediates) 5. Application specific methods (e. g. , route of administration) 6. Technology specific methods (i. e. , novel, unique to formulation/process) FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 23 –
 Concluding Remarks • Nanoparticle engineering offers significant potential to improve the delivery performance of poorly water-soluble drugs, and hence the treatment outcomes of patients who will benefit from these novel drug products. • A number of commercial drug products employing nanoparticle technology have already been approved by FDA. • FDA’s current requirements for assessing drug product safety, efficacy and quality appear adequate for evaluation of nanoparticle-based drug products. • Future evolution of more complex nanotechnologies (e. g. , drug targeting, intracellular delivery, etc. ) will likely drive the need for periodic evaluation of FDA policy and procedures for regulating nanotechnology based drug products. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 24 –
Concluding Remarks • Nanoparticle engineering offers significant potential to improve the delivery performance of poorly water-soluble drugs, and hence the treatment outcomes of patients who will benefit from these novel drug products. • A number of commercial drug products employing nanoparticle technology have already been approved by FDA. • FDA’s current requirements for assessing drug product safety, efficacy and quality appear adequate for evaluation of nanoparticle-based drug products. • Future evolution of more complex nanotechnologies (e. g. , drug targeting, intracellular delivery, etc. ) will likely drive the need for periodic evaluation of FDA policy and procedures for regulating nanotechnology based drug products. FDA Advisory Committee Meeting_22 Jul 08_Elan_SBR © 2008 – 24 –


