4. Nanomaterials Synthesis.ppt
- Количество слайдов: 29

Nanomaterials Synthesis Lecture-presentation on “Basics of Nanochemistry and Nanotechnology” by L. K. Tastanova

Objectives of lecture –presentation: Study of objects of Nanochemistry and their properties

Plan of lecture –presentation: 1. Liquid phase methods 2. Solid phase methods

1. Liquid Phase Methods Ø Molecular self-assembly Ø Supramolecular chemistry Ø Sol-gel processes Ø Single-crystal growth Ø Electrodeposition / electroplating Ø Anodizing Ø Molten salt solution electrolysis Ø Liquid template synthesis Ø Super-critical fluid expansion
1. Liquid Phase Methods Self-assembly - the ability of objects to assemble themselves into an orderly structure. Routinely seen in living cells, this is a property that nanotechnology may extend to inanimate matter. Molecular self-assembly is spontaneous organization of molecules into stable, structurally well-defined aggregates (nanometer length scale).
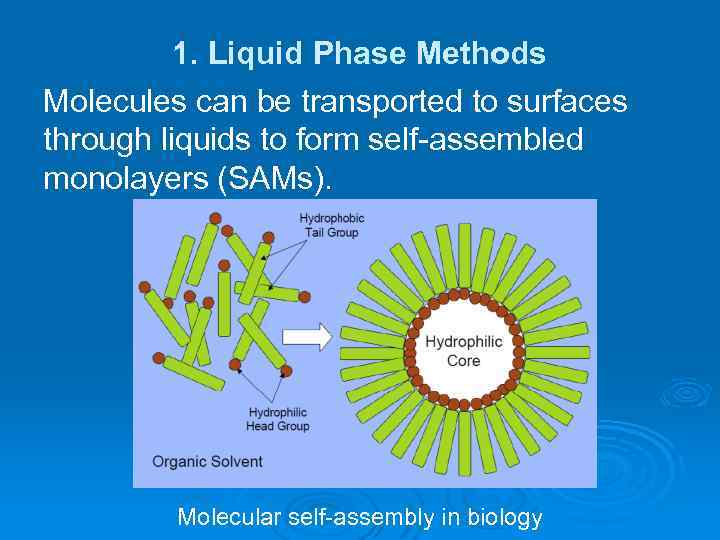
1. Liquid Phase Methods Molecules can be transported to surfaces through liquids to form self-assembled monolayers (SAMs). Molecular self-assembly in biology

1. Liquid Phase Methods Precipitating nanoparticles from a solution of chemical compounds can be classified into five major categories: (1) colloidal methods; (2) sol –gel processing; (3) water –oil microemulsions method; (4) hydrothermal synthesis; and (5) polyolmethod.
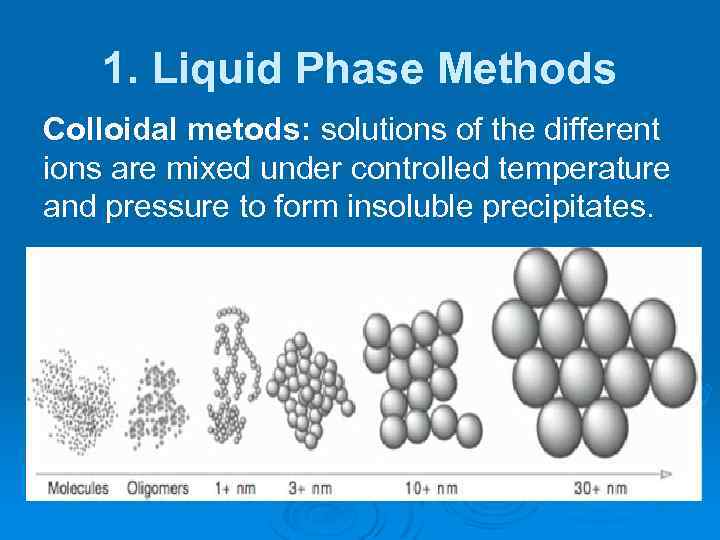
1. Liquid Phase Methods Colloidal metods: solutions of the different ions are mixed under controlled temperature and pressure to form insoluble precipitates.

1. Liquid Phase Methods A colloid is a type of chemical mixture where one substance is dispersed evenly throughout another one but the particles of the dispersed substance are suspended in the mixture, they are not completely dissolved in it. Sol-gel synthesis is performed in liquid phase. It is a useful self-assembly process for fabricating nanoparticles as well as nanostructured surfaces and three-dimensional nanostructured materials such as aerogels.

1. Liquid Phase Methods ØA “sol” is a type of colloid in which a dispersed solid phase is mixed in a homogeneous liquid medium. Ø The gel can be considered as a solid macromolecule immersed in a solvent. Ø Sol-gel process consists in the chemical transformation of a liquid (the sol) into a gel state and with subsequent posttreatment and transition into solid oxide material.
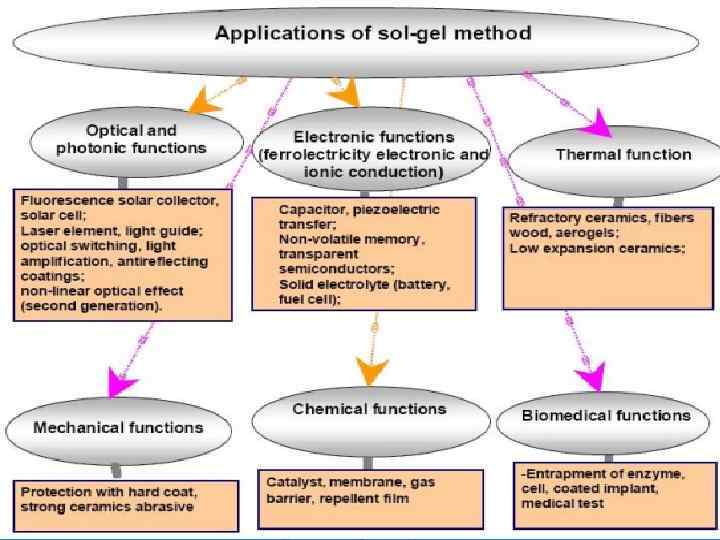
3. Liquid Phase Methods

1. Liquid Phase Methods Ø The Sol-Gel process allows to synthesize ceramic materials of high purity and homogeneity by means of preparation techniques different from the traditional process of fusion of oxides.

1. Liquid Phase Methods This process occurs in liquid solution of organometallic precursors (TMOS, TEOS, Zr(IV) - Propoxide, Ti(IV) Butoxide, etc. ), which, by means of hydrolysis and condensation reactions, lead to the formation of a new phase (SOL). M-O-R + H 2 O → M-OH + R-OH (hydrolysis) M-OH + HO-M → M-O-M + H 2 O (water condensation) M-O-R + HO-M → M-O-M + R-OH (alcohol condensation) (M = Si, Zr, Ti)
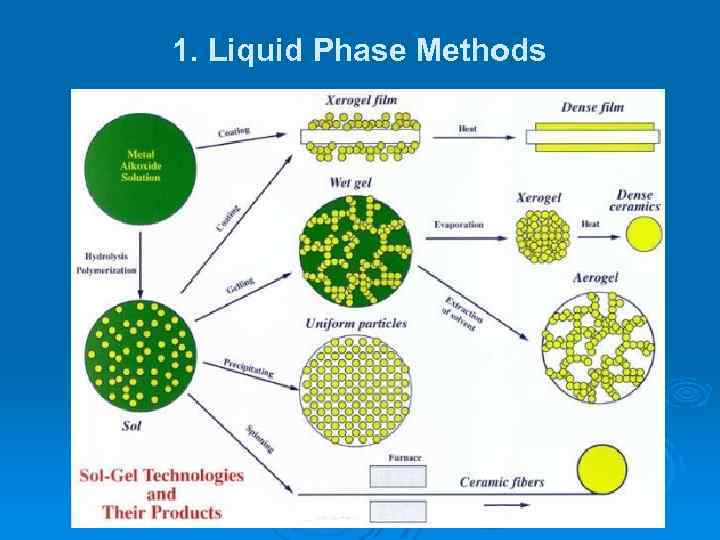
1. Liquid Phase Methods
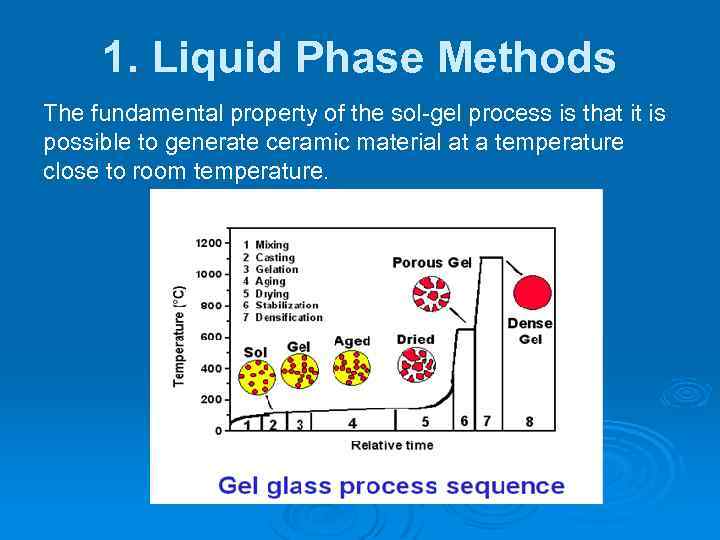
1. Liquid Phase Methods The fundamental property of the sol-gel process is that it is possible to generate ceramic material at a temperature close to room temperature.
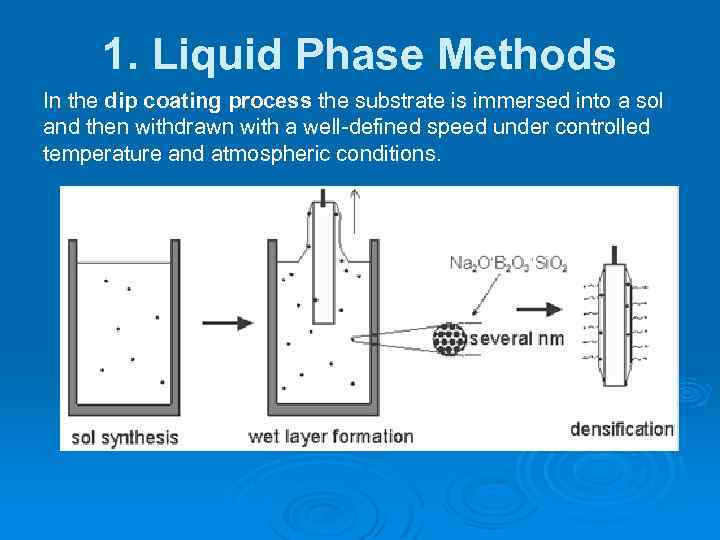
1. Liquid Phase Methods In the dip coating process the substrate is immersed into a sol and then withdrawn with a well-defined speed under controlled temperature and atmospheric conditions.

1. Liquid Phase Methods In an angle-dependent dip coating process the coating thickness is dependent also on the angle between the substrate and the liquid surface, so different layer thickness can be obtained on the top and bottom side of the substrate.
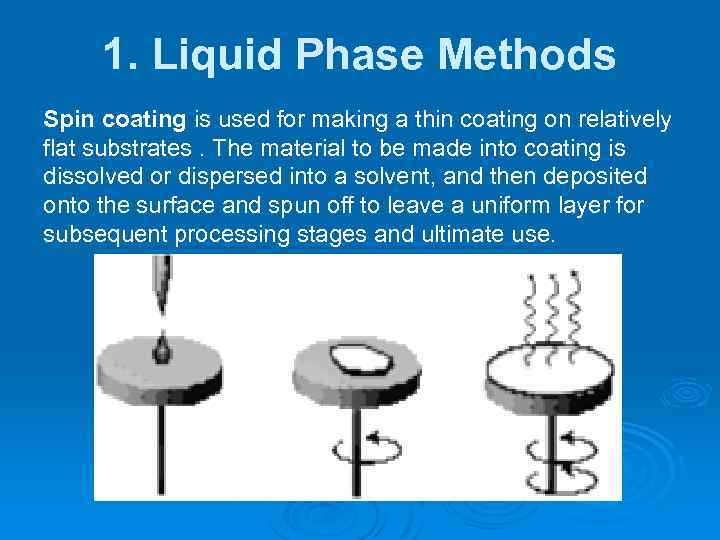
1. Liquid Phase Methods Spin coating is used for making a thin coating on relatively flat substrates. The material to be made into coating is dissolved or dispersed into a solvent, and then deposited onto the surface and spun off to leave a uniform layer for subsequent processing stages and ultimate use.
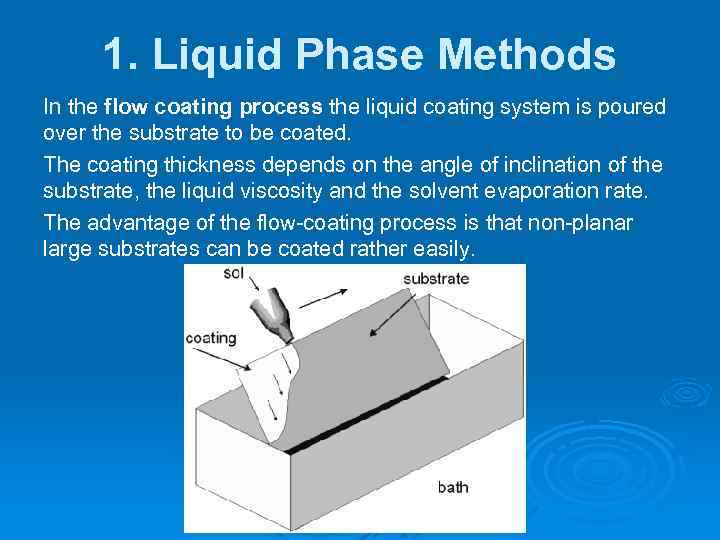
1. Liquid Phase Methods In the flow coating process the liquid coating system is poured over the substrate to be coated. The coating thickness depends on the angle of inclination of the substrate, the liquid viscosity and the solvent evaporation rate. The advantage of the flow-coating process is that non-planar large substrates can be coated rather easily.
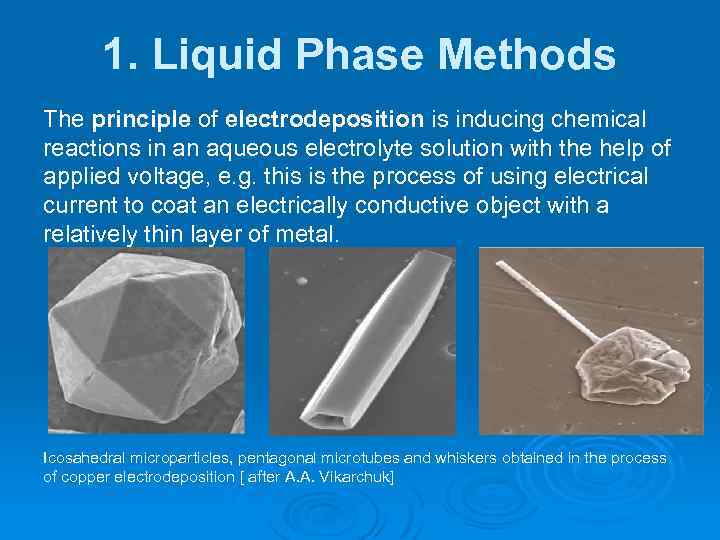
1. Liquid Phase Methods The principle of electrodeposition is inducing chemical reactions in an aqueous electrolyte solution with the help of applied voltage, e. g. this is the process of using electrical current to coat an electrically conductive object with a relatively thin layer of metal. Icosahedral microparticles, pentagonal microtubes and whiskers obtained in the process of copper electrodeposition [ after A. A. Vikarchuk]

1. Liquid Phase Methods Electrodeposition has three main attributes that make it so well suited for nano-, bio-and microtechnologies. • It can be used to grow functional material through complex 3 D masks. • It can be performed near room temperature from water-based electrolytes. • It can be scaled down to the deposition of a few atoms or up to large dimensions.

1. Liquid Phase Methods Miniature copper mask from the site of Loma Negra on the far north coast of Peru, ca. 200 C. E. Removal of the green copper corrosion products reveals a bright gold surface. The extremely thin layer of gold was applied to the sheet copper by electrochemical replacement plating. [Heather Lechtman, Sci. Amer. , 250(6), 56 (1984). ]

2. Solid Phase Methods Ø Method of compact nanocrystalline materials preparation is widely known and widely spread. Ø This technology uses the method of vaporization and condensation to obtain nanocrystalline particles which are deposited on cold surface of rotating cylinder; vaporization and condensation is carried out in rarefied inert gas atmosphere.

2. Solid Phase Methods Ø Deposited condensate is collected from the surface of cylinder and then, after pumping out the inert gas, nanocrystalline particles are pressed. Ø Pressing is held under the vacuum, i. e. without contact of material with the environment, therefore prepared nanopowder is clean.

2. Solid Phase Methods Nanoceramics prepared by powder compacting is highly porous. Ø Decrease of degree of dispersion of powder is followed with remarkable decrease of their packing ability while pressing at constant pressure. Ø In order to prepare compact nanocrystalline materials, especially ceramic materials, it is perspective to press with following caking of nanopowder at high temperature. Ø

2. Solid Phase Methods Ø Topochemistry studies solid phase reactions which take place locally in curtain areas of solid matter.
2. Solid Phase Methods Topochemical reactions consist of the following stages: 1) Formation of molecules or elementary cells of reaction product on the surface of initial substance. 2) Formation of cores of reaction product new phase. 3) Gross of cores until they are connected to each other, formation of solid layer of reaction product. 4) Gross of solid layer of reaction product due to decrease of volume and surface of initial reactant. Ø

Check Yourself 1. 2. 3. 4. 5. 6. What is self-assembly? Give examples of selfassembly. How is precipitation of nanoparticles from a solution classified? What is called colloid? How does sol-gel synthesis go? What are the benefits of sol-gel processing? Describe coating processes. How does electrodeposition work?

Literature: 1. Roco M. C. J. Nanoparticle Res. , 2001, v. 3, № 5– 6, 2001, p. 353– 360. 2. NSTC, National Nanotechnology Initiative and Its Implementation Plan, Washington, D. C. , 2000. 3. Societal Implications of Nanoscience and Nanotechnology. Eds. M. C. Roco, W. S. Bainbridge Dordrecht: Kluver Acad. Publ. , 2001. 4. NSTC, National Nanotechnology Initiative and Its Implementation Plan, Washington, D. C. , 2002. 5. Gleiter H. Nanostructured materials – State-oftheart and perspectives. // Z/ Metallkunde. , 1995. V. 86. P. 78 -83. 6. Charitidis C. , Logothetidis S. Nanomechanical and nanotribological properties of carbon based films // Thin Solid Films, 2005. V. 482. P. 120– 125.
4. Nanomaterials Synthesis.ppt