35a628870a6d30fa9a63918405b6f99a.ppt
- Количество слайдов: 35

NANOMATERIALS: Risks and Regulatory Gaps SEJ 2012, Lubbock TX Jennifer Sass, Ph. D. SASS 1

Definition: Nanotechnology is the understanding and control of matter at dimensions between approximately 1 and 100 nanometers (nm), where unique phenomena enable novel applications. Encompassing nanoscale science, engineering, and technology, nanotechnology involves imaging, measuring, modeling, and manipulating matter at this length scale. SASS 2

NANO-ENABLED PRODUCTS 800+ consumer products using nanotechnologies, according to manufacturer Estimates predict 15% of goods globally, worth $2. 6 trillion by 2014 will be nano-enabled Every sector, and every Fortune 500 company is invested SASS 3

THE PROMISES Clothing covered in nano-zinc oxide wires could power devices. (Nature, Feb 2008) Iron nanoparticles can decontaminate solvent-soaked soil up to 1, 000 times faster than a conventional iron mixture. Improved hydrogen-fuel cells, lithium-ion batteries, and solar cell semiconductors SASS 4

THE PRODUCTS Stain-resistant clothing (nano-perfluorinated compounds) Clear sunscreen (Ti. O 2) The nanosolar utility panel carries 5 -10 times more current than typical panels Nanosilver antibiotic clothing, food packaging, and teddy bears Carbon nanotube lighter stronger building materials SASS 5

NANOTOXICOLOGY: basic assumptions Small size facilitates easier access to the lungs, passage through cell membranes, and possibly skin penetrance. Once inside the body, they seem to have access to all tissues and organs, including the brain and fetal circulation. Animal studies suggest that some nanomaterials cause inflammation, damage brain cells and cause pre-cancerous lesions. Ultrafine (nano) air pollution, is associated with size-dependent reduced lung function and increased likelihood of asthma, respiratory disease, and deaths from lung and heart disease. 6
It is clear that inhaled nanomaterials can pass into the blood stream, and from the blood through the blood-brain-barrier, and the placental barrier. Nano metal oxides in sunscreens may penetrate skin, though most tests on intact skin have reported only limited penetration. Not much is known about whether ingested nanomaterials can pass from the gut into the blood stream. SASS 7

NANO TITANIUM DIOXIDE When Ti. O 2 nanoparticles were fed to mice in drinking water (300 -3, 000 µg/day for five days), they showed DNA damage. (Trouiller et al, 2009) When pregnant mice were injected under the skin with Ti. O 2 (0. 1 mg at 3, 7, 10, and 14 days postcoitum) the nanoparticles were found in the offspring and caused reduced sperm production and brain cell death in the male offspring. (Takeda et al, 2008). 8

NANO TITANIUM DIOXIDE …Z-Cote (transparent zinc oxide) and T-Cote (transparent titanium dioxide), that do not deposit this chalky residue “YOUR BOOBS HAVE A MIND OF THEIR OWN. BUT WE KNOW WHAT THEY’RE THINKING” …antibacterial and odorless through the application of silver dioxide fiber technology, preserving garment freshness. Finally, integrated titanium oxide fiber technology protects against ultraviolet rays providing UPF 50+. http: //cw-x. com/Gear. Technology. aspx 9
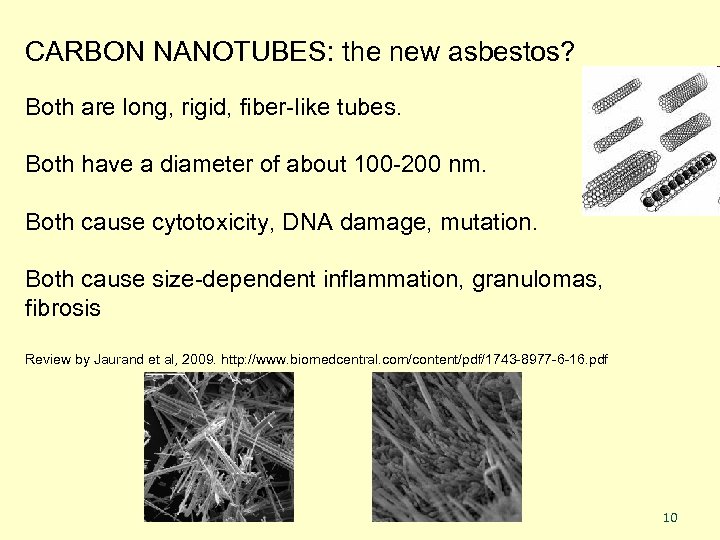
CARBON NANOTUBES: the new asbestos? Both are long, rigid, fiber-like tubes. Both have a diameter of about 100 -200 nm. Both cause cytotoxicity, DNA damage, mutation. Both cause size-dependent inflammation, granulomas, fibrosis Review by Jaurand et al, 2009. http: //www. biomedcentral. com/content/pdf/1743 -8977 -6 -16. pdf 10

FEDERAL INSECTICIDE, FUNGICIDE, AND RODENTICIDE ACT (FIFRA) SASS 11

NANOSILVER: ANTIMICROBIAL Silver is a priority pollutant whose discharge is regulated by EPA under the Clean Water Act 100’s of consumer products claim to use nanosilver; Nanosilver, like silver, kills both harmful and beneficial microbes. The nanoscale version is more toxic than regular silver, and releases free ions. In cultured mouse sperm stem cells, a 48 hr treatment of nanosilver (15 nm diameter) was 45 -fold more toxic than silver carbonate (EC 50 of 8. 75 v 408 ug/ml). SASS 12

Silver is regulated as a contaminant under the Clean Water Act SASS 13

Calls to regulate nanosilver as a pesticide from the National Association of Clean Water Agencies (NACWA) and Tri. TAC (Calif water treatment agencies) sent multiple letters to EPA raising concern about nanosilver and nanomaterials impacts on water treatment facilities (2011, 2010, 2008, 2006) SASS 14

EPA REGULATES THE WASHING MACHINE … "ion generators that incorporate a substance (e. g. , silver or copper). . . for the purpose of preventing, destroying, repelling, or mitigating a pest (e. g. , bacteria or algae). . . are considered pesticides for purposes of FIFRA, and must be registered prior to sale or distribution. " (FR Notice, Sept 07) All products must have applied to EPA for registration by March 21, 2008, to continue to sell after that date. SASS 15
In March, 2008 US EPA fined a nanotech company, ATEN Technology $208, 000 because its subsidiary, IOGEAR, was selling nanosilver as an unregistered pesticide. SASS 16
SASS 17
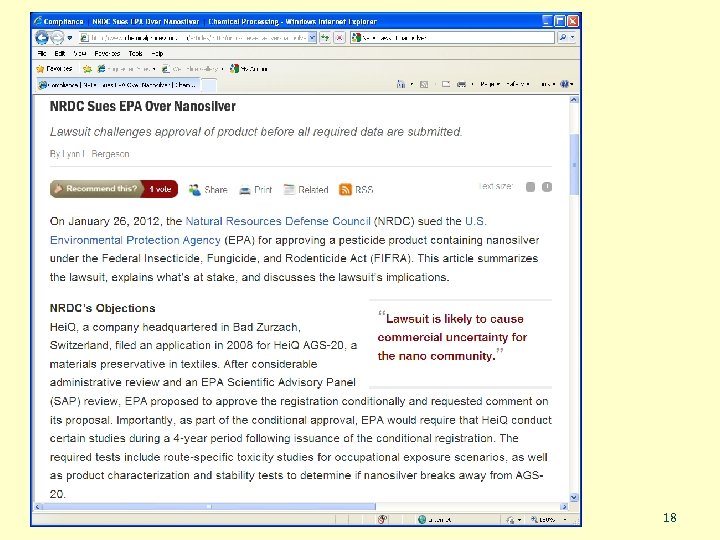
SASS 18

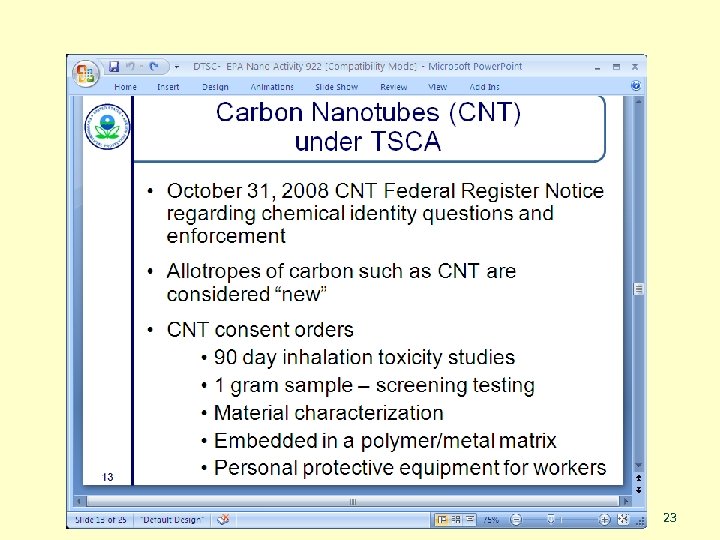
TOXICS SUBSTANCES CONTROL ACT (TSCA) SASS 19

TSCA provides broad authority to: • Gather information on new and existing chemical substances and mixtures • Require testing of chemicals • Screen and control unreasonable risks of new and existing chemicals • Coordinate with other Federal agencies SASS 20

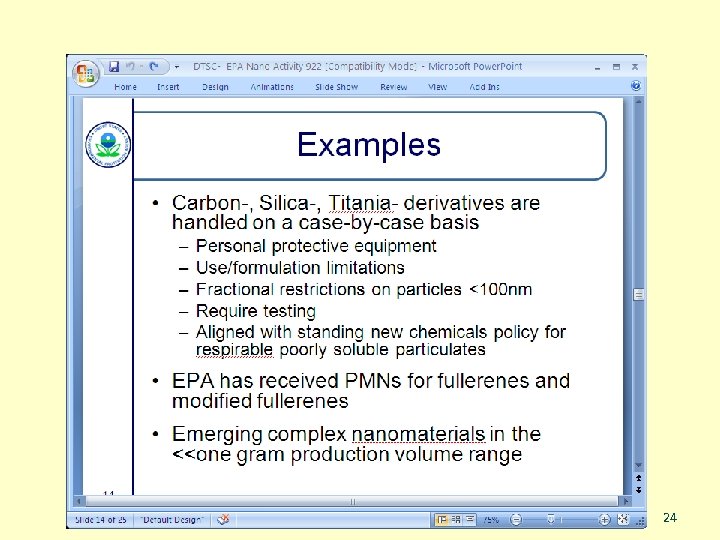
n New Chemicals Program n n n Pre-manufacture Notices (PMNs) Low Release/Exposure Exemption (Lo. REX) Significant New Use Rules (SNURs) Consent Orders Existing-chemical based nanomaterials n SNURs for Existing NMs n Section 4 test rule n Section 8(a) – report existing data n Section 8(e) – notices of substantial risk SASS 21

From a presentation by EPA’s Jim Allwood, Sept 22, 2010 SASS 22
SASS 23
SASS 24

EPA Publishes Proposed SNURs for 17 Chemicals, Including CNTs and Fullerenes – December 28, 2011 Under TSCA Section 5(e) - required to notify EPA at least 90 days before commencing manufacturing, producing, importing. EPA determined that nano SNURs may present and unreasonable risk of injury to human health. EPA requiring PPE – gloves, chemical protective clothing, respirators - and some workplace monitoring. SASS 25
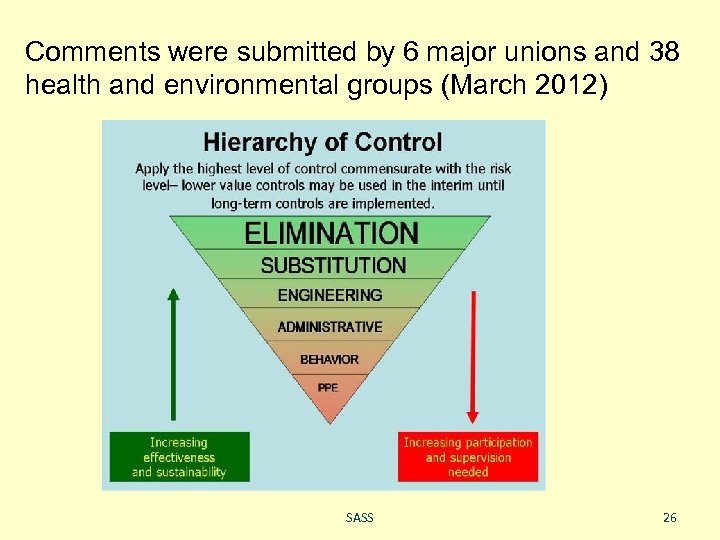
Comments were submitted by 6 major unions and 38 health and environmental groups (March 2012) SASS 26

FOOD AND DRUG ADMINISTRATION (FDA) • FDA does not have authority to require cosmetic companies to submit safety data • FDA does not have authority to obtain post-market health and safety data for any products SASS 27

NANOCHEMICALS IN MEDICINE Emend ® (Merck, USA) approved by FDA in 2003 as an antinausea drug for chemotherapy patients. Nanocrystals. Doxil® (ALZA Corp, USA) approved by FDA in 2005 to treat ovarian cancer and Kaposi’s sarcoma. Lipid nanoparticles. Estrasorb™ (Novavax, Inc, USA) approved by FDA in 2003 as topical estradiol lotion to treat menopause. Micellar nanoparticles. Rapamune ® (Wyeth, USA) approved by FDA in 2000 as an immunesuppresant for renal transplant patients. Nanocrystal form. Zirconium Oxide ® (Altair Nanotechnologies, Inc, USA) commercially available since 2003 for dental fillings 28

NANOCHEMICALS IN FOOD AND BEVERAGES Nanoceuticals ™ Slim Shake Chocolate (RBC Life Sciences, USA). Pure cocoa is added to a nano-cluster Canola Active Oil (Shemen Industries, Israel). Uses Nanosized self assembled structured lipids, NSSL, to deliver insoluble vitamins through the cellular membrane Nanotea (Shenzhen Become Industry&Trade Co. , China) 29

NANOCHEMICALS IN FOOD CONTACT MATERIALS Kitchen cutting board (S Korea) nanosilver Home and garden spray (ABL, USA) nanosilver Aluminum foil (Melitta, Germany). With non-stick coating. “Put simply, is that the black coating material to carbon, in a glass matrix is embedded. The black area reached up to 100 degrees Celsius higher surface temperatures when cooking … the food is prepared quickly. ” 30

CONSUMER PRODUCT SAFETY COMMISSION • CPSAct prohibits the agency from imposing mandatory safety standards if the industry agrees to write its own standards • CPSAct prohibits the agency from informing the public about a product without pre-approval of the manufacturer • CPSC has no authority to require pre-market testing; only has authority to implement post-market product recalls SASS 31
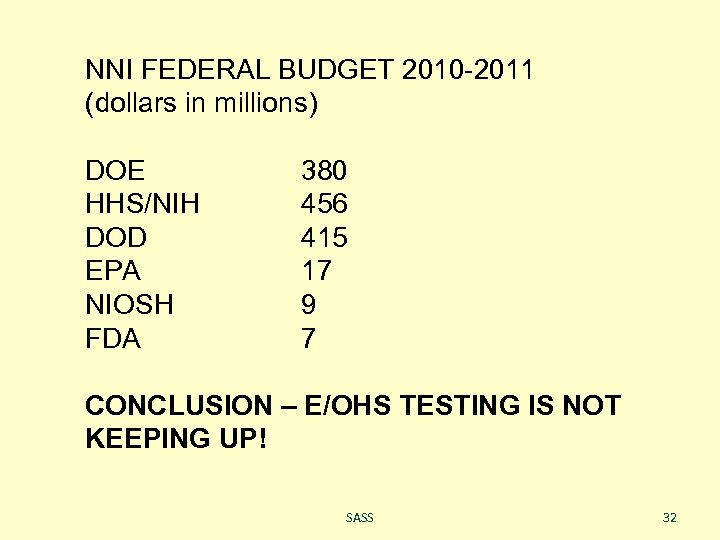
NNI FEDERAL BUDGET 2010 -2011 (dollars in millions) DOE HHS/NIH DOD EPA NIOSH FDA 380 456 415 17 9 7 CONCLUSION – E/OHS TESTING IS NOT KEEPING UP! SASS 32

Allocation of EPA/ORD EHS budget, $20 M: 50% Sources, Fate, Transport, and Exposure 30% Human Health and Ecological Effects 10% Risk Assessment Methods and Case Studies 10% Preventing and Mitigating Risks SASS 33
SASS 34

The Solution: We need reform of our nation’s chemical regulatory act, TSCA. Support Senator Lautenberg’s SAFE CHEMICALS ACT, which would require all new chemicals, including nanomaterials, to be tox tested before they are commercialized. The Safe Chemicals Act is supported by a coalition of over 11 million individuals, including parents, health professionals, advocates for people with learning and developmental disabilities, reproductive health advocates, environmentalists and businesses from across the nation. http: //www. saferchemicals. org/safe-chemicals-act/ SASS 35
35a628870a6d30fa9a63918405b6f99a.ppt