d4aeb08e314a40604b72899e284ef4d1.ppt
- Количество слайдов: 32
 NAFDAC Regulation & Registration Procedure for Packaged Water by Ozigis AA Deputy Director NAFDAC FCT, Abuja. abdulsalamozigi@yahoo. com. SS At the “NIFST Workshop holding at Owerri, Imo state, Nigeria” 2015
NAFDAC Regulation & Registration Procedure for Packaged Water by Ozigis AA Deputy Director NAFDAC FCT, Abuja. abdulsalamozigi@yahoo. com. SS At the “NIFST Workshop holding at Owerri, Imo state, Nigeria” 2015
 Introduction Water is important in sustaining life however, provision of safe drinking water is a challenge all over the world due to: • The presence of microorganisms (biological contaminants) in drinking water which could give rise to waterborne infections such as typhoid fever, diarrhoea, hepatitis E, cholera etc, which affect hundreds of thousands of people. • The presence of chemicals (chemical contaminants) that could arise from water treatment & cleaning of equipment, packaging materials, source of water supply etc. This could lead to organ disease in consumers. 2
Introduction Water is important in sustaining life however, provision of safe drinking water is a challenge all over the world due to: • The presence of microorganisms (biological contaminants) in drinking water which could give rise to waterborne infections such as typhoid fever, diarrhoea, hepatitis E, cholera etc, which affect hundreds of thousands of people. • The presence of chemicals (chemical contaminants) that could arise from water treatment & cleaning of equipment, packaging materials, source of water supply etc. This could lead to organ disease in consumers. 2
 Introduction Contd. Trade in packaged water has increased globally due to water shortages and the recognition that traditional drinking water suppliers such as public and private waterworks may not always be able to guarantee the microbiological, chemical and physical safety of water to the extent previously thought possible. 3
Introduction Contd. Trade in packaged water has increased globally due to water shortages and the recognition that traditional drinking water suppliers such as public and private waterworks may not always be able to guarantee the microbiological, chemical and physical safety of water to the extent previously thought possible. 3
 Water Production-What is the situation? • A 2001 market research in the US showed that bottled water sales grew from roughly 6% to more than 13% per year over five years 1, 2. • Nigeria has also recorded astronomical growth of packaged water production facilities & sales but unfortunately this has not been matched by the quality of the product! 4
Water Production-What is the situation? • A 2001 market research in the US showed that bottled water sales grew from roughly 6% to more than 13% per year over five years 1, 2. • Nigeria has also recorded astronomical growth of packaged water production facilities & sales but unfortunately this has not been matched by the quality of the product! 4
 Water Production-What is the situation Contd. ? NAFDAC has observed a lot of noncompliant water products with poor labels that do not have date markings, batch numbers, NAFDAC Registration numbers, clear prints or graphics, nature & origin of water, net content, name & address of manufacturer etc & also fail laboratory analysis hence the need for this training workshop. 5
Water Production-What is the situation Contd. ? NAFDAC has observed a lot of noncompliant water products with poor labels that do not have date markings, batch numbers, NAFDAC Registration numbers, clear prints or graphics, nature & origin of water, net content, name & address of manufacturer etc & also fail laboratory analysis hence the need for this training workshop. 5
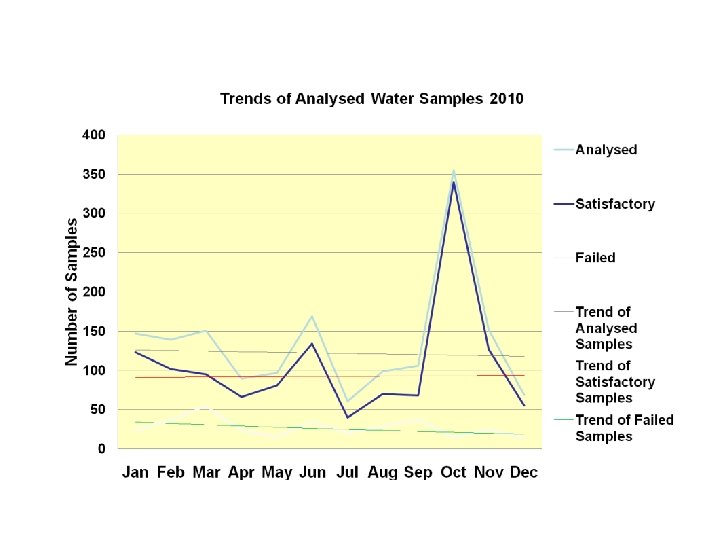
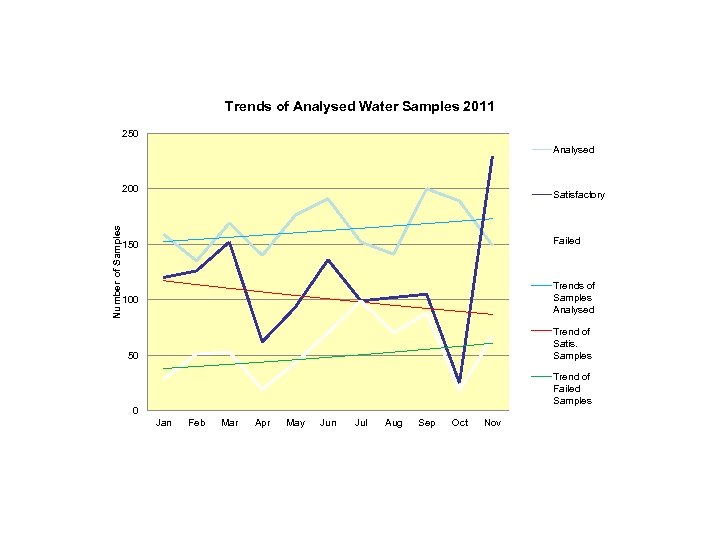
 Analysis of Laboratory Results Analysis of the cumulated laboratory monthly reports trends for the years 2010 & 2011 seen in the graph slides below showed that : • Failure of water samples increased in 2011 compared to 2010. This shows that GMP was getting poorer and there may be need for appropriate staffing and adequate training. • Failure of water samples tended to increase during the rainy season. Water production facilities should therefore ensure that water treatment is effective at this period. 6
Analysis of Laboratory Results Analysis of the cumulated laboratory monthly reports trends for the years 2010 & 2011 seen in the graph slides below showed that : • Failure of water samples increased in 2011 compared to 2010. This shows that GMP was getting poorer and there may be need for appropriate staffing and adequate training. • Failure of water samples tended to increase during the rainy season. Water production facilities should therefore ensure that water treatment is effective at this period. 6
 Trends of Analysed Water Samples 2011 250 Analysed Number of Samples 200 Satisfactory 150 Failed 100 Trends of Samples Analysed Trend of Satis. Samples 50 Trend of Failed Samples 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov
Trends of Analysed Water Samples 2011 250 Analysed Number of Samples 200 Satisfactory 150 Failed 100 Trends of Samples Analysed Trend of Satis. Samples 50 Trend of Failed Samples 0 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov
 What Should You Do? Be able to perform simple tests on your water e. g. using the PDA petri dish film at the point before packaging the water product as follows: • Pipette 1 ml of the water sample into the petri dish at the point before packaging • Observe and count the colonies seen after 24 hrs • Not more than 100 colony forming units (cfu) should be seen • If the CFUs are more than 100, repeat the water treatment procedure and make sure you have less than 100 cfu before bottling/packaging. 9
What Should You Do? Be able to perform simple tests on your water e. g. using the PDA petri dish film at the point before packaging the water product as follows: • Pipette 1 ml of the water sample into the petri dish at the point before packaging • Observe and count the colonies seen after 24 hrs • Not more than 100 colony forming units (cfu) should be seen • If the CFUs are more than 100, repeat the water treatment procedure and make sure you have less than 100 cfu before bottling/packaging. 9
 Food Grade Packaging Materials Only seven types of plastic are considered "food grade" by the U. S. Food & Drug Administration 8. • 1 -PET (or PETE): Polyethylene Terephthalate (PET) used most often in bottles carrying carbonated drinks because it has better gascontaining properties than other plastics. • • 2 -HDPE: High-density polyethylene (HDPE) is the plastic of choice for milk and juice 3 -Vinyl (PVC): Polyvinyl chloride (PVC) is used in all manner of food wraps 10
Food Grade Packaging Materials Only seven types of plastic are considered "food grade" by the U. S. Food & Drug Administration 8. • 1 -PET (or PETE): Polyethylene Terephthalate (PET) used most often in bottles carrying carbonated drinks because it has better gascontaining properties than other plastics. • • 2 -HDPE: High-density polyethylene (HDPE) is the plastic of choice for milk and juice 3 -Vinyl (PVC): Polyvinyl chloride (PVC) is used in all manner of food wraps 10
 Food Grade Packaging materials Contd. • • 4 -LDPE: Low-density polyethylene (LDPE) is similar to HDPE, but is more flexible and is used to make squeeze bottles and tubing 5 -PP : Polypropylene (PP) is perfect for containing products that are bottled while hot and allowed to cool before shipment 6 -PS: Polystyrene (PP) is an excellent insulator and is often injected with air to make foam packing products such as egg cartons, coffee cups and meat trays 7 -Other : All other types of plastics are denoted with the "7 Other" designation, and cover a wide range of materials including acrylic, fiberglass, nylon and polycarbonate 11
Food Grade Packaging materials Contd. • • 4 -LDPE: Low-density polyethylene (LDPE) is similar to HDPE, but is more flexible and is used to make squeeze bottles and tubing 5 -PP : Polypropylene (PP) is perfect for containing products that are bottled while hot and allowed to cool before shipment 6 -PS: Polystyrene (PP) is an excellent insulator and is often injected with air to make foam packing products such as egg cartons, coffee cups and meat trays 7 -Other : All other types of plastics are denoted with the "7 Other" designation, and cover a wide range of materials including acrylic, fiberglass, nylon and polycarbonate 11
 What NAFDAC Regulations/Guidelines do You Need? 1. 2. 3. 4. 5. 6. 7. Bottled Water (Labelling) Regulations 1995 Bottled Water (Advertisement) Regulations 1995 Pre-packaged Food Labelling Regulations 2005 Draft Food Safety Regulations 2009 Guidelines for Production and Registration of Packaged Water Guidelines for Registration of Food And Water Manufactured in Nigeria, NAFDAC/RR 005/00 Guidelines for Establishment/Re-certification of Packaged Water Plants in Nigeria 12
What NAFDAC Regulations/Guidelines do You Need? 1. 2. 3. 4. 5. 6. 7. Bottled Water (Labelling) Regulations 1995 Bottled Water (Advertisement) Regulations 1995 Pre-packaged Food Labelling Regulations 2005 Draft Food Safety Regulations 2009 Guidelines for Production and Registration of Packaged Water Guidelines for Registration of Food And Water Manufactured in Nigeria, NAFDAC/RR 005/00 Guidelines for Establishment/Re-certification of Packaged Water Plants in Nigeria 12
 Labeling Requirements for Water The Bottled water (Labelling) Regulations states that “No person shall sell packaged water unless a label has been affixed to it”. 1. Labeling shall be informative, clear and accurate. 2 Minimum requirements on the package label in accordance with food labeling regulations shall be: (a)Name of food (brand name) (where applicable). (b)Name and location address of the manufacturer, distributor, packer or advertiser. 13
Labeling Requirements for Water The Bottled water (Labelling) Regulations states that “No person shall sell packaged water unless a label has been affixed to it”. 1. Labeling shall be informative, clear and accurate. 2 Minimum requirements on the package label in accordance with food labeling regulations shall be: (a)Name of food (brand name) (where applicable). (b)Name and location address of the manufacturer, distributor, packer or advertiser. 13
 Labeling Requirements for Water contd. (c) Provision for NAFDAC Registration Number on product label or listing number. (d) Batch No. , Manufacturing date and Expiry date. (e) Net content. 3. Where a brand name is used, the type of product should also be stated 14
Labeling Requirements for Water contd. (c) Provision for NAFDAC Registration Number on product label or listing number. (d) Batch No. , Manufacturing date and Expiry date. (e) Net content. 3. Where a brand name is used, the type of product should also be stated 14
 Labeling Requirements for Water contd. 4. Any food/water product whose name or package label bears close resemblance to an already registered product or is likely to be mistaken for such registered product, shall not be considered for registration. 5. Any food/water product which is labeled in a foreign language shall not be considered for registration unless an English translation is included on the label. 15
Labeling Requirements for Water contd. 4. Any food/water product whose name or package label bears close resemblance to an already registered product or is likely to be mistaken for such registered product, shall not be considered for registration. 5. Any food/water product which is labeled in a foreign language shall not be considered for registration unless an English translation is included on the label. 15
 Registration of Water NAFDAC Guidelines for Registration of Food & Water in Nigeria stipulates that: A manufacturer who intends to register a food or related product in Nigeria shall first have the factory inspected by the FSAN Directorate of NAFDAC and be assigned a Certificate of Recognition as a manufacturer before an application to register the product can be made. 16
Registration of Water NAFDAC Guidelines for Registration of Food & Water in Nigeria stipulates that: A manufacturer who intends to register a food or related product in Nigeria shall first have the factory inspected by the FSAN Directorate of NAFDAC and be assigned a Certificate of Recognition as a manufacturer before an application to register the product can be made. 16
 Inspection &Sampling • • NAFDAC officers from the FSAN are responsible for inspection of water production facilities to ensure that GMP/GHP are observed. Three vetting samples shall be submitted upon satisfactory Pre- Registration inspection. 17
Inspection &Sampling • • NAFDAC officers from the FSAN are responsible for inspection of water production facilities to ensure that GMP/GHP are observed. Three vetting samples shall be submitted upon satisfactory Pre- Registration inspection. 17
 Registration Process The registration process involves: 1. Documentation processes, 2. Establishment inspections • Good Manufacturing Practices (GMP)/ Good Hygienic Practices (GHP) Inspections & • Sampling, 3. Laboratory analysis of product, 18
Registration Process The registration process involves: 1. Documentation processes, 2. Establishment inspections • Good Manufacturing Practices (GMP)/ Good Hygienic Practices (GHP) Inspections & • Sampling, 3. Laboratory analysis of product, 18
 Registration Process Contd. 4. Vetting of product labels and advertisement, 5. A three-level product approval committee meeting for consideration of the products and 6. Finally, issuance of NAFDAC Reg. Nos. and certificates for products that meet the registration requirements. 19
Registration Process Contd. 4. Vetting of product labels and advertisement, 5. A three-level product approval committee meeting for consideration of the products and 6. Finally, issuance of NAFDAC Reg. Nos. and certificates for products that meet the registration requirements. 19
 INITIATION OF REGISTRATION OF WATER Submission of application letter: • • An application for Registration of the water product addressed to the Director General, NAFDAC, Abuja submit at the location concerned is submitted. A copy of the letter is sent to the Director Registration and Regulatory Affairs (R&R) Directorate, NAFDAC, Lagos 20
INITIATION OF REGISTRATION OF WATER Submission of application letter: • • An application for Registration of the water product addressed to the Director General, NAFDAC, Abuja submit at the location concerned is submitted. A copy of the letter is sent to the Director Registration and Regulatory Affairs (R&R) Directorate, NAFDAC, Lagos 20
 INITIATION OF REGISTRATION OF WATER The application letter submitted should be on company letterhead and bear the name of the manufacturer, full location address, contact phone number of the company, the generic name of product, & the brand name (where applicable). 21
INITIATION OF REGISTRATION OF WATER The application letter submitted should be on company letterhead and bear the name of the manufacturer, full location address, contact phone number of the company, the generic name of product, & the brand name (where applicable). 21
 Registration Application Form • • The applicant shall purchase the registration application form at N 250/copy from the NAFDAC Registration & Regulatory Affairs (R&R) Directorate Lagos, NAFDAC State offices or the NAFDAC EID office in Abuja and submit the duly completed registration forms at the same offices. A separate application form shall be submitted for each food/water product. 22
Registration Application Form • • The applicant shall purchase the registration application form at N 250/copy from the NAFDAC Registration & Regulatory Affairs (R&R) Directorate Lagos, NAFDAC State offices or the NAFDAC EID office in Abuja and submit the duly completed registration forms at the same offices. A separate application form shall be submitted for each food/water product. 22
 What About the Tariffs? All payments to the Agency should be in Bank Draft payable to National Agency for Food and Drug Administration and Control (NAFDAC) 1 Food - Fifty thousand naira (N 50, 000: 00) + 5% VAT. 2 Water in sachet -Thirty thousand naira (N 30, 000: 00) + 5% VAT. 3 Water in bottle- Fifty thousand naira (N 50, 000: 00) + 5% VAT. 23
What About the Tariffs? All payments to the Agency should be in Bank Draft payable to National Agency for Food and Drug Administration and Control (NAFDAC) 1 Food - Fifty thousand naira (N 50, 000: 00) + 5% VAT. 2 Water in sachet -Thirty thousand naira (N 30, 000: 00) + 5% VAT. 3 Water in bottle- Fifty thousand naira (N 50, 000: 00) + 5% VAT. 23
 Documentation The following documents shall accompany the application letter for registration/ re certification written on company’s letterhead: 1 Evidence of payment 2 Evidence of current membership of Association of Table Water Producers (ATWAP)/recommendation letter 24
Documentation The following documents shall accompany the application letter for registration/ re certification written on company’s letterhead: 1 Evidence of payment 2 Evidence of current membership of Association of Table Water Producers (ATWAP)/recommendation letter 24
 Documentation Contd. 3. Evidence of Trademark Registration in the Relevant Class. 4. Evidence of pre-production inspection/ Certificate of Recognition issued by NAFDAC. 5. Certificate of Incorporation of the company issued by the Corporate Affairs Commission. 25
Documentation Contd. 3. Evidence of Trademark Registration in the Relevant Class. 4. Evidence of pre-production inspection/ Certificate of Recognition issued by NAFDAC. 5. Certificate of Incorporation of the company issued by the Corporate Affairs Commission. 25
 Documentation 6. Certificate of analysis for raw water and treated water 7. Food Handlers’ Test Certificates or Medical Certificates of fitness 8. Current Certificate of Fumigation 9. Organizational structure showing key personnel 10. Letters of employment & acceptance of offer of key personnel etc 26
Documentation 6. Certificate of analysis for raw water and treated water 7. Food Handlers’ Test Certificates or Medical Certificates of fitness 8. Current Certificate of Fumigation 9. Organizational structure showing key personnel 10. Letters of employment & acceptance of offer of key personnel etc 26
 Product Advertisement • Registration of a product does not automatically confer advertising permit. A separate approval by the Agency shall be required if the product is to be advertised. • NAFDAC may withdraw the certificate of Registration in the event that the product is advertised without express approval from the Agency. 27
Product Advertisement • Registration of a product does not automatically confer advertising permit. A separate approval by the Agency shall be required if the product is to be advertised. • NAFDAC may withdraw the certificate of Registration in the event that the product is advertised without express approval from the Agency. 27
 What Should You Expect? • A successful application will attract a Certificate of Registration with a validity period of 5 (five) years. • Products with Listing status will attract a validity of 2 (two) years. • Failure to comply with registration requirements may result in the disqualification of the application or lead to considerable delays in the registration processing time. . 28
What Should You Expect? • A successful application will attract a Certificate of Registration with a validity period of 5 (five) years. • Products with Listing status will attract a validity of 2 (two) years. • Failure to comply with registration requirements may result in the disqualification of the application or lead to considerable delays in the registration processing time. . 28
 Important Things to Note! • NAFDAC reserves the right to revoke, suspend or vary the certificate during its validity period. • Filling an application form or paying for an application form does not confer registration status. • Failure to respond promptly (within 30 work days) to queries or enquiries raised by NAFDAC on the application, will automatically lead to suspension of further processing of the application. 29
Important Things to Note! • NAFDAC reserves the right to revoke, suspend or vary the certificate during its validity period. • Filling an application form or paying for an application form does not confer registration status. • Failure to respond promptly (within 30 work days) to queries or enquiries raised by NAFDAC on the application, will automatically lead to suspension of further processing of the application. 29
 Conclusion The importance of safe drinking water cannot be overemphasized because of the serious health implications and the effect on morbidity and mortality of any nation. Water producers must therefore improve on their Good Manufacturing Practices (GMP) so that their products would be compliant all the time. Water producers need to join hands with NAFDAC to safeguard the health of Nigerians as their products gain consumer confidence which will lead 30
Conclusion The importance of safe drinking water cannot be overemphasized because of the serious health implications and the effect on morbidity and mortality of any nation. Water producers must therefore improve on their Good Manufacturing Practices (GMP) so that their products would be compliant all the time. Water producers need to join hands with NAFDAC to safeguard the health of Nigerians as their products gain consumer confidence which will lead 30
 References 1 Anon. Bottled water thirst reaches 5 billion gal. Contractor, 48(12), pp. 7, 26. December 2001. 2 IBWA. www. bottledwater. org/public/BWFacts. Home_main. htm 3 Bottled Water (labeling) Regulations, 1995 4 February/March 2002 Ask the Regulators -- Bottled Water Regulation and the FDA http: //www. fda. gov/foodsafety/productspecificinformation/bottledwater carbonatedsoftdrinks/ucm 077079. htm#authors 5 Standard for Potable Water, NIS 306: 2008 6 Guidelines for Production and Registration of Packaged Water 7 Bottled Water (labeling) Regulations, 1995 8 www. ehow. com/way_5819448_do-tell-food-grade-plastic_. ht 9 Guidelines For Establishment/Re-Certification of Packaged Water Plants in Nigeria 31
References 1 Anon. Bottled water thirst reaches 5 billion gal. Contractor, 48(12), pp. 7, 26. December 2001. 2 IBWA. www. bottledwater. org/public/BWFacts. Home_main. htm 3 Bottled Water (labeling) Regulations, 1995 4 February/March 2002 Ask the Regulators -- Bottled Water Regulation and the FDA http: //www. fda. gov/foodsafety/productspecificinformation/bottledwater carbonatedsoftdrinks/ucm 077079. htm#authors 5 Standard for Potable Water, NIS 306: 2008 6 Guidelines for Production and Registration of Packaged Water 7 Bottled Water (labeling) Regulations, 1995 8 www. ehow. com/way_5819448_do-tell-food-grade-plastic_. ht 9 Guidelines For Establishment/Re-Certification of Packaged Water Plants in Nigeria 31
 Thank You For Your Attention !!! 32
Thank You For Your Attention !!! 32


