e18a8f2cdfce7f0e88795484b84b57d9.ppt
- Количество слайдов: 21
 Myopathy in SSc SCTC-EUSTAR study
Myopathy in SSc SCTC-EUSTAR study
 EUSTAR-online EUSTAR database ≈ 10, 000 SSc patients included in database > 120 centres involved (mostly European but also centres in Israel, Egypt, South Africa, USA, Canada, Brazil, Argentina, Dominican Republic, New Zealand, China) Ø yearly follow-up data desirable The myopathy substudy is integrated into EUSTAR-online, hence you’ll need to become…
EUSTAR-online EUSTAR database ≈ 10, 000 SSc patients included in database > 120 centres involved (mostly European but also centres in Israel, Egypt, South Africa, USA, Canada, Brazil, Argentina, Dominican Republic, New Zealand, China) Ø yearly follow-up data desirable The myopathy substudy is integrated into EUSTAR-online, hence you’ll need to become…
 How to take part? FIRST- become a EUSTAR centre!
How to take part? FIRST- become a EUSTAR centre!
 How to take part? To become a EUSTAR-online centre you need… 1. Access to the internet 2. Positive statement of local ethics committee 3. Email the documents to the EUSTAR secretariat (eustar@unifi. it) 4. → you will receive your Unique Centre Number and a User Name and Password (each data entry clerk will get an individual log in, no shared log ins) …then You can enter patients into the database (signed consent of each patient needed).
How to take part? To become a EUSTAR-online centre you need… 1. Access to the internet 2. Positive statement of local ethics committee 3. Email the documents to the EUSTAR secretariat (eustar@unifi. it) 4. → you will receive your Unique Centre Number and a User Name and Password (each data entry clerk will get an individual log in, no shared log ins) …then You can enter patients into the database (signed consent of each patient needed).
 https: //www. eustar-online. org
https: //www. eustar-online. org
 https: //www. eustar-online. org • System was developed and validated by the standards set by FDA CFR 21 Part 11 for Good Clinical Practice and ISO 9001 for Quality Control. • 128 bit SSL encrypted data transmission (https: //) • Passwords are stored in encrypted form • Patient randomisation (patient ID’s) • Pseudonymisation (ONLY the patient’s physician can match a specific dataset with his patient(s)) • User-Role authentication enabling and restricting views and data entry • Audit trail (when/ how/ by whom data were entered first time/ were changed) • Secure Server Net (Firewall) • Complete and incremental data back-ups
https: //www. eustar-online. org • System was developed and validated by the standards set by FDA CFR 21 Part 11 for Good Clinical Practice and ISO 9001 for Quality Control. • 128 bit SSL encrypted data transmission (https: //) • Passwords are stored in encrypted form • Patient randomisation (patient ID’s) • Pseudonymisation (ONLY the patient’s physician can match a specific dataset with his patient(s)) • User-Role authentication enabling and restricting views and data entry • Audit trail (when/ how/ by whom data were entered first time/ were changed) • Secure Server Net (Firewall) • Complete and incremental data back-ups
 How to take part? SECOND- become a myopathy substudy participating centre!
How to take part? SECOND- become a myopathy substudy participating centre!
 Participate myopathy study
Participate myopathy study
 Participate myopathy study Dear EUSTAR member, You are asked whether you want to participate in the myopathy sub-study. Myopathy is a common and disabling, but often unaddressed problem of patients with SSc. This study aims to characterise primary muscle involvement in SSc with regard to patient’s history, physical examination including assessment of muscle strength and endurance, laboratory features, and autoantibody status. Additionally, MRI and/or ENMG and/or muscle biopsy should be performed. Thus, besides the characterisation of SSc-associated myopathy, this study aims also to identify appropriate diagnostic tools to assess myopathy in SSc patients.
Participate myopathy study Dear EUSTAR member, You are asked whether you want to participate in the myopathy sub-study. Myopathy is a common and disabling, but often unaddressed problem of patients with SSc. This study aims to characterise primary muscle involvement in SSc with regard to patient’s history, physical examination including assessment of muscle strength and endurance, laboratory features, and autoantibody status. Additionally, MRI and/or ENMG and/or muscle biopsy should be performed. Thus, besides the characterisation of SSc-associated myopathy, this study aims also to identify appropriate diagnostic tools to assess myopathy in SSc patients.
 How to take part? THIRD- Enter patients’ data!
How to take part? THIRD- Enter patients’ data!
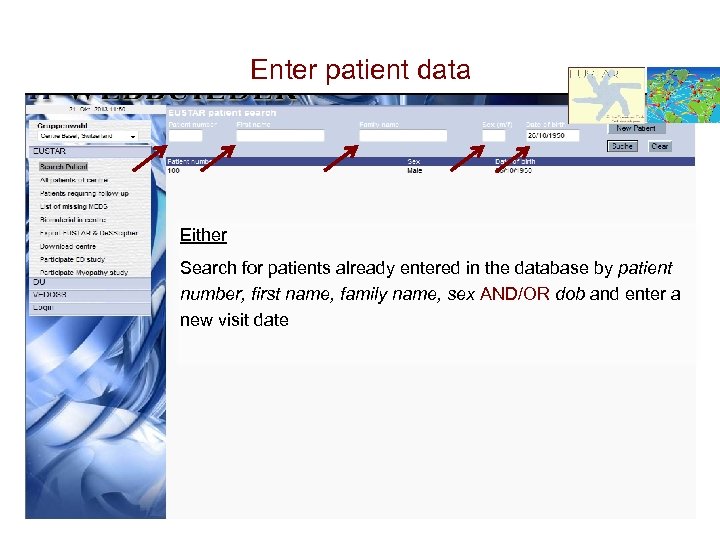 Enter patient data Either Search for patients already entered in the database by patient number, first name, family name, sex AND/OR dob and enter a new visit date
Enter patient data Either Search for patients already entered in the database by patient number, first name, family name, sex AND/OR dob and enter a new visit date
 Enter patient data
Enter patient data
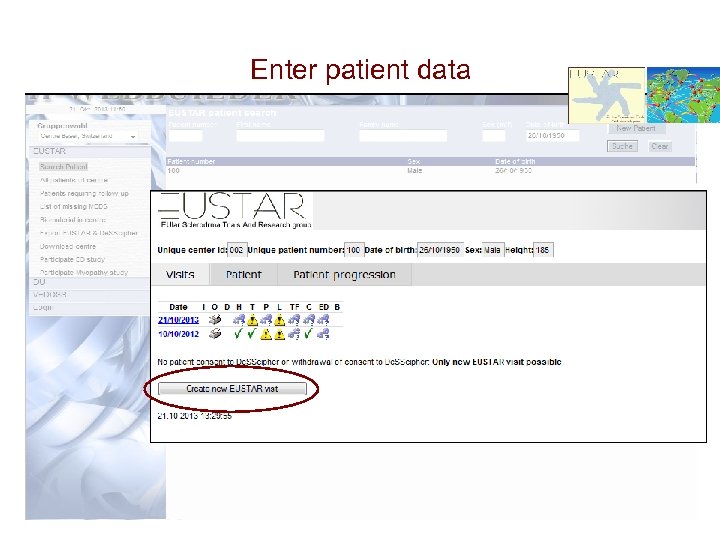
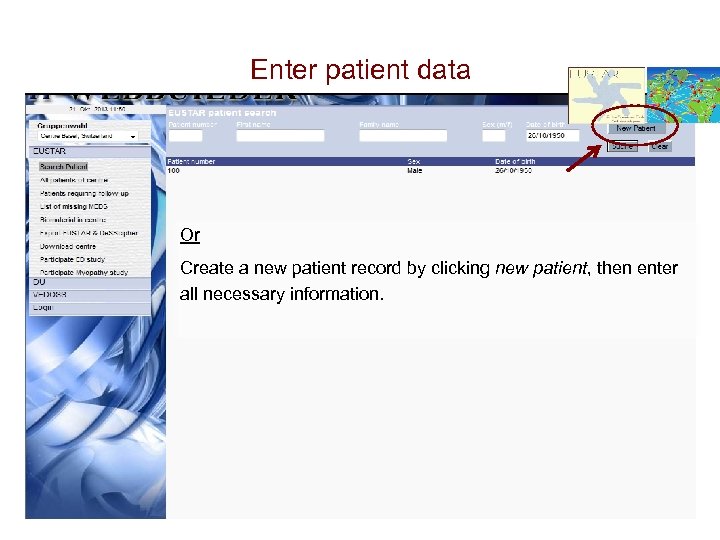 Enter patient data Or Create a new patient record by clicking new patient, then enter all necessary information.
Enter patient data Or Create a new patient record by clicking new patient, then enter all necessary information.
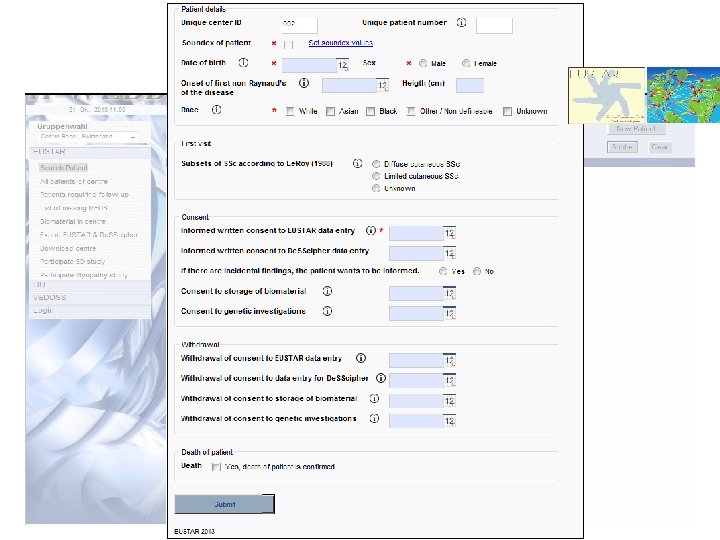 Enter data
Enter data
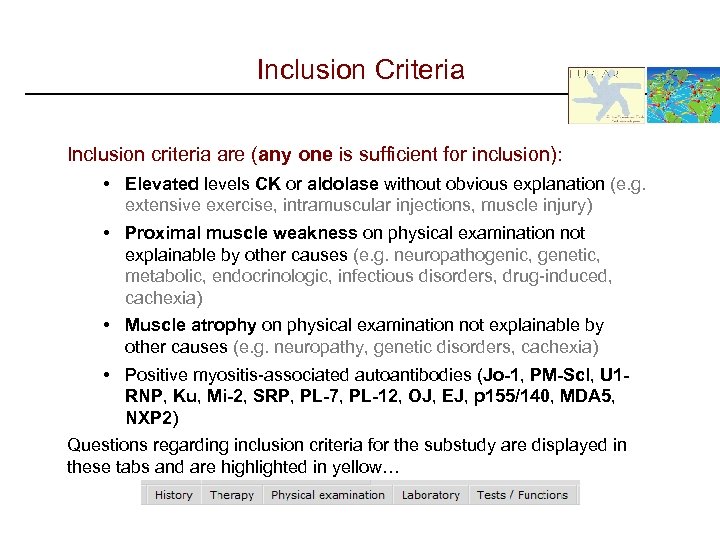 Inclusion Criteria Inclusion criteria are (any one is sufficient for inclusion): • Elevated levels CK or aldolase without obvious explanation (e. g. extensive exercise, intramuscular injections, muscle injury) • Proximal muscle weakness on physical examination not explainable by other causes (e. g. neuropathogenic, genetic, metabolic, endocrinologic, infectious disorders, drug-induced, cachexia) • Muscle atrophy on physical examination not explainable by other causes (e. g. neuropathy, genetic disorders, cachexia) • Positive myositis-associated autoantibodies (Jo-1, PM-Scl, U 1 RNP, Ku, Mi-2, SRP, PL-7, PL-12, OJ, EJ, p 155/140, MDA 5, NXP 2) Questions regarding inclusion criteria for the substudy are displayed in these tabs and are highlighted in yellow…
Inclusion Criteria Inclusion criteria are (any one is sufficient for inclusion): • Elevated levels CK or aldolase without obvious explanation (e. g. extensive exercise, intramuscular injections, muscle injury) • Proximal muscle weakness on physical examination not explainable by other causes (e. g. neuropathogenic, genetic, metabolic, endocrinologic, infectious disorders, drug-induced, cachexia) • Muscle atrophy on physical examination not explainable by other causes (e. g. neuropathy, genetic disorders, cachexia) • Positive myositis-associated autoantibodies (Jo-1, PM-Scl, U 1 RNP, Ku, Mi-2, SRP, PL-7, PL-12, OJ, EJ, p 155/140, MDA 5, NXP 2) Questions regarding inclusion criteria for the substudy are displayed in these tabs and are highlighted in yellow…
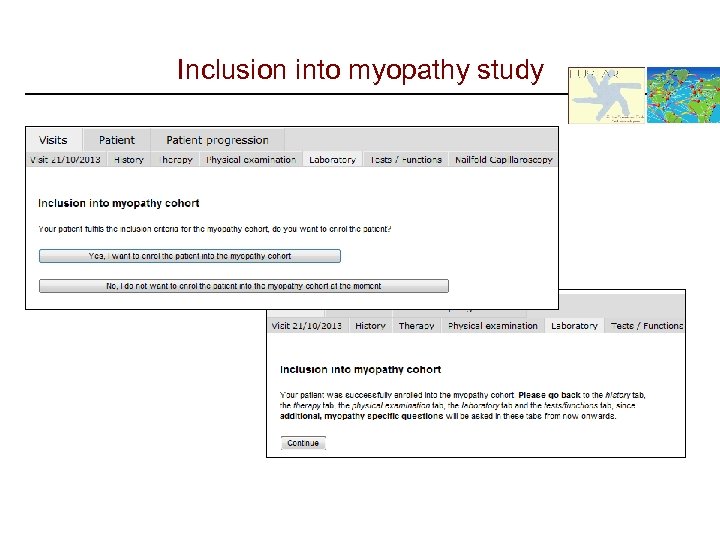 Inclusion into myopathy study
Inclusion into myopathy study
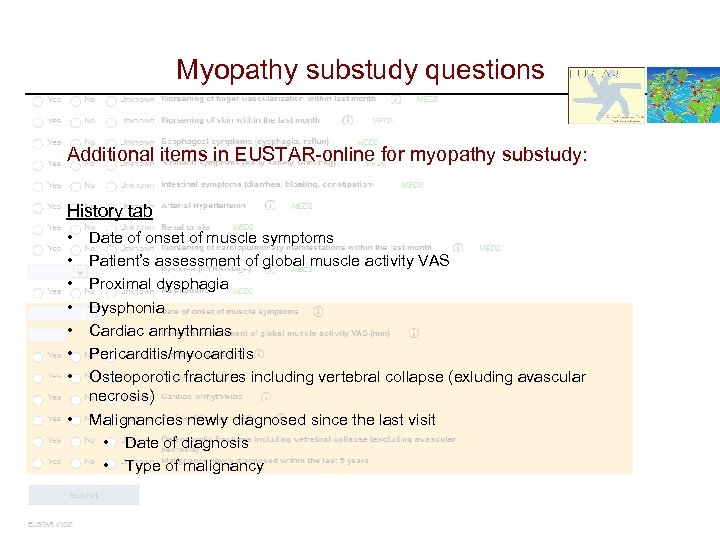 Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: History tab • • Date of onset of muscle symptoms Patient’s assessment of global muscle activity VAS Proximal dysphagia Dysphonia Cardiac arrhythmias Pericarditis/myocarditis Osteoporotic fractures including vertebral collapse (exluding avascular necrosis) Malignancies newly diagnosed since the last visit • Date of diagnosis • Type of malignancy
Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: History tab • • Date of onset of muscle symptoms Patient’s assessment of global muscle activity VAS Proximal dysphagia Dysphonia Cardiac arrhythmias Pericarditis/myocarditis Osteoporotic fractures including vertebral collapse (exluding avascular necrosis) Malignancies newly diagnosed since the last visit • Date of diagnosis • Type of malignancy
 Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Therapy tab • • • Tacrolimus IVIG Physiotherapy
Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Therapy tab • • • Tacrolimus IVIG Physiotherapy
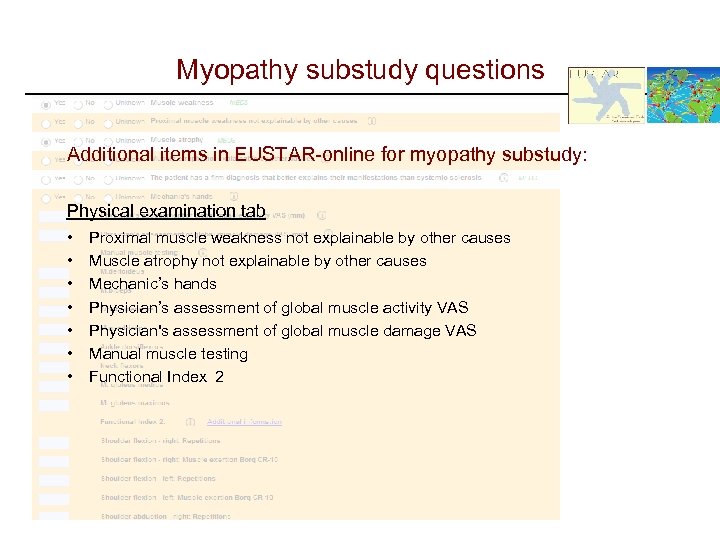 Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Physical examination tab • • Proximal muscle weakness not explainable by other causes Muscle atrophy not explainable by other causes Mechanic’s hands Physician’s assessment of global muscle activity VAS Physician's assessment of global muscle damage VAS Manual muscle testing Functional Index 2
Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Physical examination tab • • Proximal muscle weakness not explainable by other causes Muscle atrophy not explainable by other causes Mechanic’s hands Physician’s assessment of global muscle activity VAS Physician's assessment of global muscle damage VAS Manual muscle testing Functional Index 2
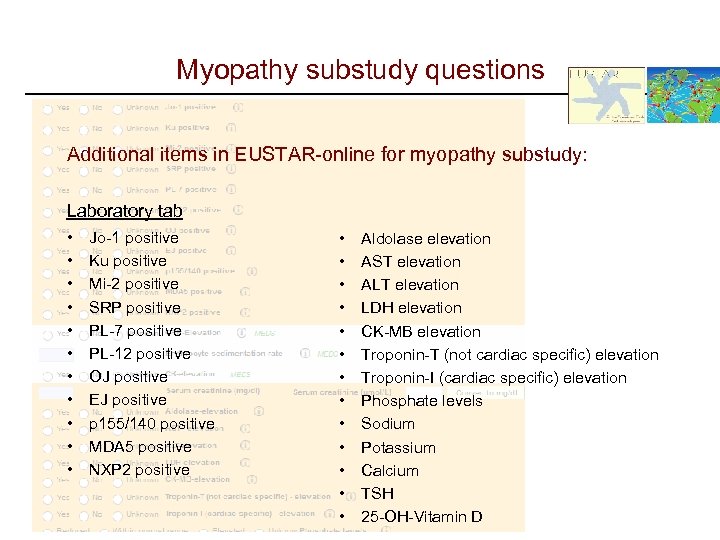 Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Laboratory tab • • • Jo-1 positive Ku positive Mi-2 positive SRP positive PL-7 positive PL-12 positive OJ positive EJ positive p 155/140 positive MDA 5 positive NXP 2 positive • • • • Aldolase elevation AST elevation ALT elevation LDH elevation CK-MB elevation Troponin-T (not cardiac specific) elevation Troponin-I (cardiac specific) elevation Phosphate levels Sodium Potassium Calcium TSH 25 -OH-Vitamin D
Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Laboratory tab • • • Jo-1 positive Ku positive Mi-2 positive SRP positive PL-7 positive PL-12 positive OJ positive EJ positive p 155/140 positive MDA 5 positive NXP 2 positive • • • • Aldolase elevation AST elevation ALT elevation LDH elevation CK-MB elevation Troponin-T (not cardiac specific) elevation Troponin-I (cardiac specific) elevation Phosphate levels Sodium Potassium Calcium TSH 25 -OH-Vitamin D
 Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Tests/functions tab • EMG - • Date of EMG, neuropathy, myopathy, non-specific MRI Date of MRI, muscle inflammation (contrast enhancement or T 2 weighted + fat suppressed or STI), muscle atrophy, fasciitis • Muscle biopsy - Date of muscle biopsy, histology, immunohistochemistry, electron microscopy
Myopathy substudy questions Additional items in EUSTAR-online for myopathy substudy: Tests/functions tab • EMG - • Date of EMG, neuropathy, myopathy, non-specific MRI Date of MRI, muscle inflammation (contrast enhancement or T 2 weighted + fat suppressed or STI), muscle atrophy, fasciitis • Muscle biopsy - Date of muscle biopsy, histology, immunohistochemistry, electron microscopy


