7589293d6a0508974c71aa1d27461a10.ppt
- Количество слайдов: 50
 Mussel Aquaculture in Prince Edward Island Canada: Managing Seed Quality and Aquatic Invasive Species. T. Landry, A. Smith, N. Mc. Nair and J. Davidson Aquatic Health Division Fisheries and Oceans Canada Gulf Fisheries Centre, Moncton NB Oceans and Science Branch
Mussel Aquaculture in Prince Edward Island Canada: Managing Seed Quality and Aquatic Invasive Species. T. Landry, A. Smith, N. Mc. Nair and J. Davidson Aquatic Health Division Fisheries and Oceans Canada Gulf Fisheries Centre, Moncton NB Oceans and Science Branch
 Gulf of St. Lawrence (southern)
Gulf of St. Lawrence (southern)
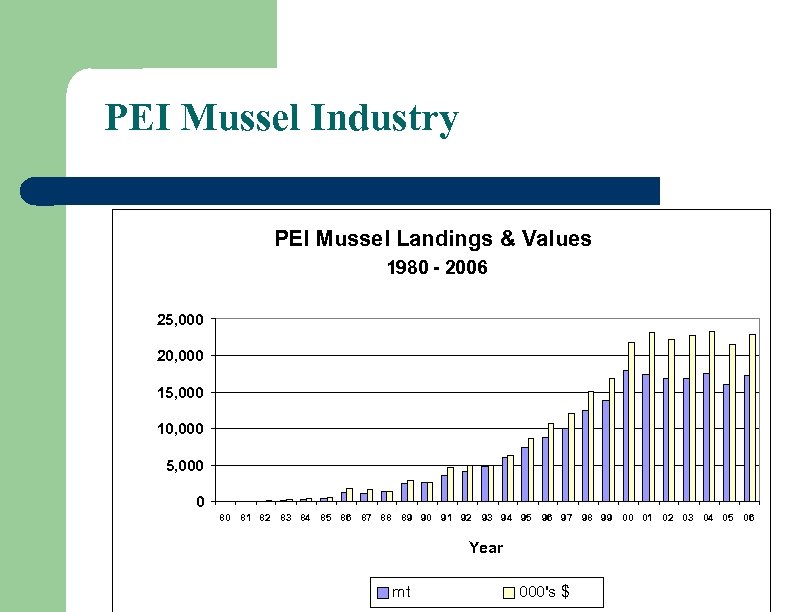 PEI Mussel Industry PEI Mussel Landings & Values 1980 - 2006 25, 000 20, 000 15, 000 10, 000 5, 000 0 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 00 01 02 03 04 05 06 Year mt 000's $
PEI Mussel Industry PEI Mussel Landings & Values 1980 - 2006 25, 000 20, 000 15, 000 10, 000 5, 000 0 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 00 01 02 03 04 05 06 Year mt 000's $
 PEI Mussel Industry ▪ Relatively new industry, beginnings in mid 1980 ▪ PEI ; 80% of the mussel market N. America ▪ 2006: ▸ 127 growers ▸ 295 leases ▸ 10, 000 acres ▸ Production 38 million pounds ($22. 8 million) ▸ 7 Processing plants (4 > 2, 000 mt) ▸ 1, 500 employed
PEI Mussel Industry ▪ Relatively new industry, beginnings in mid 1980 ▪ PEI ; 80% of the mussel market N. America ▪ 2006: ▸ 127 growers ▸ 295 leases ▸ 10, 000 acres ▸ Production 38 million pounds ($22. 8 million) ▸ 7 Processing plants (4 > 2, 000 mt) ▸ 1, 500 employed
 PEI Mussel Industry: Seed
PEI Mussel Industry: Seed
 PEI Mussel Industry: Grow out
PEI Mussel Industry: Grow out
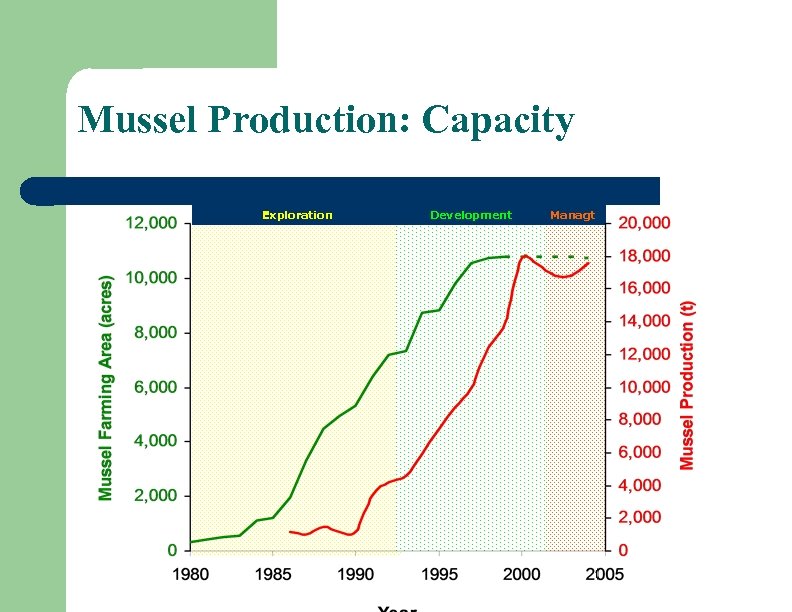 Mussel Production: Capacity Exploration Development Managt
Mussel Production: Capacity Exploration Development Managt
 R&D for the Mussel Aquaculture Industry in PEI. Exploration Development Management Environmental Interaction l Site l Yield l Capacity (Farm) l HAB’s l Productivity (Bay) l Impact Technique l Anchoring l Socking l Harvesting l Efficiency l Processing l Stocks (seed) l Prediction l Collection Site l Supply l Source l AIS Quality l Health
R&D for the Mussel Aquaculture Industry in PEI. Exploration Development Management Environmental Interaction l Site l Yield l Capacity (Farm) l HAB’s l Productivity (Bay) l Impact Technique l Anchoring l Socking l Harvesting l Efficiency l Processing l Stocks (seed) l Prediction l Collection Site l Supply l Source l AIS Quality l Health
 Seed Quality M. edulis vs. M. trossulus Cultured vs. Wild
Seed Quality M. edulis vs. M. trossulus Cultured vs. Wild
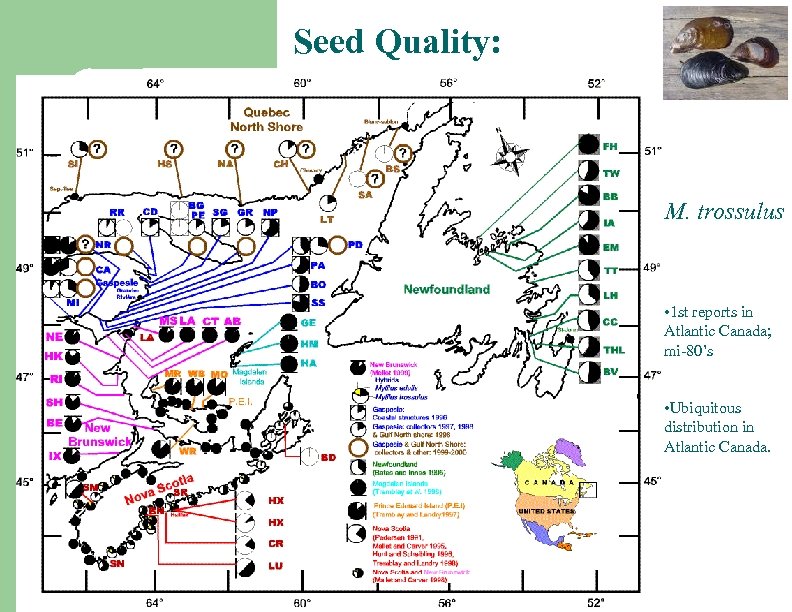 Seed Quality: M. trossulus • 1 st reports in Atlantic Canada; mi-80’s • Ubiquitous distribution in Atlantic Canada.
Seed Quality: M. trossulus • 1 st reports in Atlantic Canada; mi-80’s • Ubiquitous distribution in Atlantic Canada.
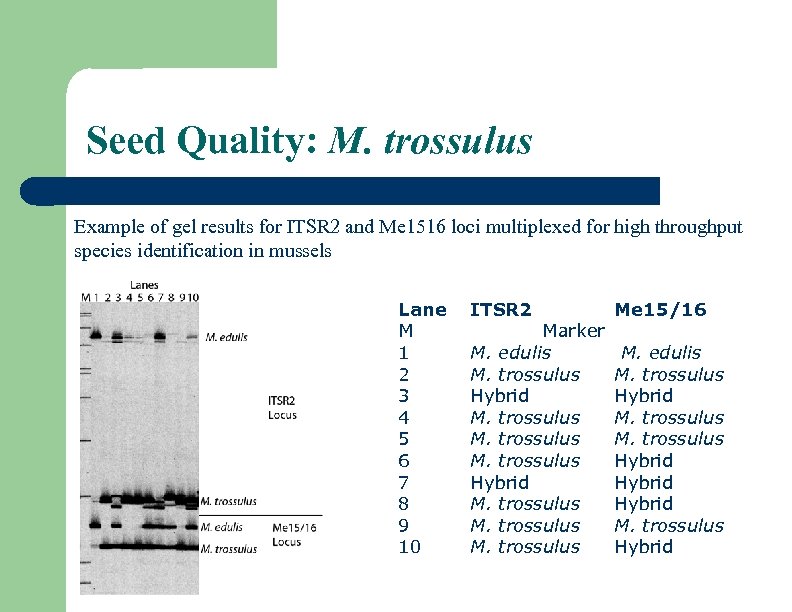 Seed Quality: M. trossulus Example of gel results for ITSR 2 and Me 1516 loci multiplexed for high throughput species identification in mussels Lane M 1 2 3 4 5 6 7 8 9 10 ITSR 2 Marker M. edulis M. trossulus Hybrid M. trossulus Me 15/16 M. edulis M. trossulus Hybrid Hybrid M. trossulus Hybrid
Seed Quality: M. trossulus Example of gel results for ITSR 2 and Me 1516 loci multiplexed for high throughput species identification in mussels Lane M 1 2 3 4 5 6 7 8 9 10 ITSR 2 Marker M. edulis M. trossulus Hybrid M. trossulus Me 15/16 M. edulis M. trossulus Hybrid Hybrid M. trossulus Hybrid
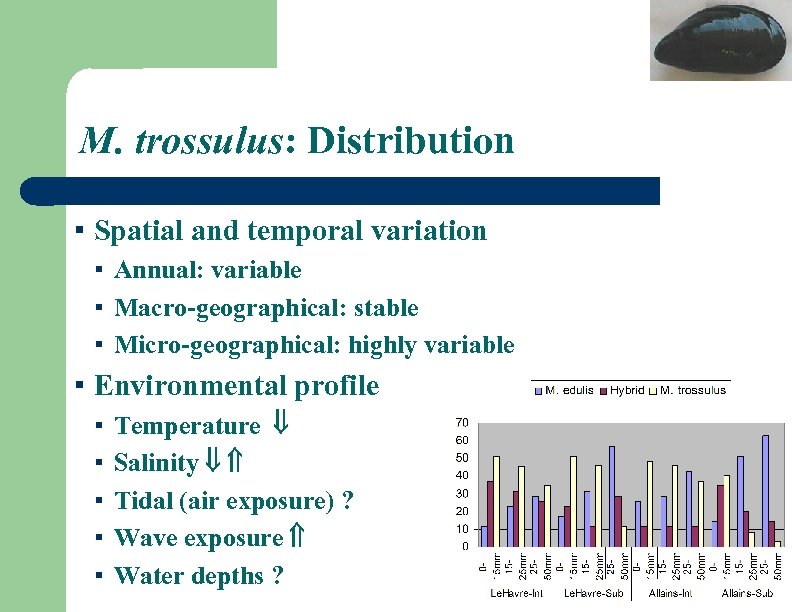 M. trossulus: Distribution ▪ Spatial and temporal variation ▪ Annual: variable ▪ Macro-geographical: stable ▪ Micro-geographical: highly variable ▪ Environmental profile ▪ ▪ ▪ Temperature Salinity Tidal (air exposure) ? Wave exposure Water depths ?
M. trossulus: Distribution ▪ Spatial and temporal variation ▪ Annual: variable ▪ Macro-geographical: stable ▪ Micro-geographical: highly variable ▪ Environmental profile ▪ ▪ ▪ Temperature Salinity Tidal (air exposure) ? Wave exposure Water depths ?
 M. trossulus: Aquaculture ▪ Shell ▪ ▪ Thickness Shape Colour Breakage > 10% ▪ Meat yield ▪ Lower ▪ Long post-spawning recovery ▪ Count per weight unit
M. trossulus: Aquaculture ▪ Shell ▪ ▪ Thickness Shape Colour Breakage > 10% ▪ Meat yield ▪ Lower ▪ Long post-spawning recovery ▪ Count per weight unit
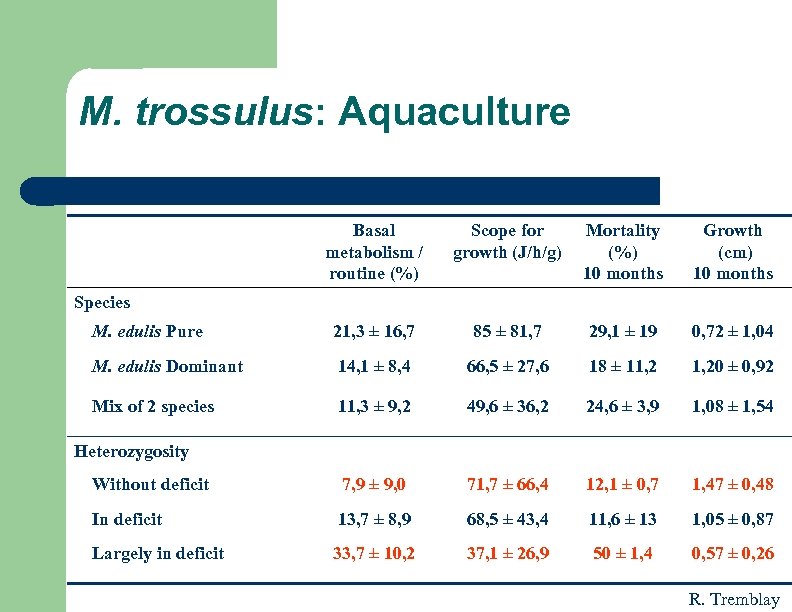 M. trossulus: Aquaculture Basal metabolism / routine (%) Scope for growth (J/h/g) Mortality (%) 10 months Growth (cm) 10 months M. edulis Pure 21, 3 ± 16, 7 85 ± 81, 7 29, 1 ± 19 0, 72 ± 1, 04 M. edulis Dominant 14, 1 ± 8, 4 66, 5 ± 27, 6 18 ± 11, 20 ± 0, 92 Mix of 2 species 11, 3 ± 9, 2 49, 6 ± 36, 2 24, 6 ± 3, 9 1, 08 ± 1, 54 Without deficit 7, 9 ± 9, 0 71, 7 ± 66, 4 12, 1 ± 0, 7 1, 47 ± 0, 48 In deficit 13, 7 ± 8, 9 68, 5 ± 43, 4 11, 6 ± 13 1, 05 ± 0, 87 Largely in deficit 33, 7 ± 10, 2 37, 1 ± 26, 9 50 ± 1, 4 0, 57 ± 0, 26 Species Heterozygosity R. Tremblay
M. trossulus: Aquaculture Basal metabolism / routine (%) Scope for growth (J/h/g) Mortality (%) 10 months Growth (cm) 10 months M. edulis Pure 21, 3 ± 16, 7 85 ± 81, 7 29, 1 ± 19 0, 72 ± 1, 04 M. edulis Dominant 14, 1 ± 8, 4 66, 5 ± 27, 6 18 ± 11, 20 ± 0, 92 Mix of 2 species 11, 3 ± 9, 2 49, 6 ± 36, 2 24, 6 ± 3, 9 1, 08 ± 1, 54 Without deficit 7, 9 ± 9, 0 71, 7 ± 66, 4 12, 1 ± 0, 7 1, 47 ± 0, 48 In deficit 13, 7 ± 8, 9 68, 5 ± 43, 4 11, 6 ± 13 1, 05 ± 0, 87 Largely in deficit 33, 7 ± 10, 2 37, 1 ± 26, 9 50 ± 1, 4 0, 57 ± 0, 26 Species Heterozygosity R. Tremblay
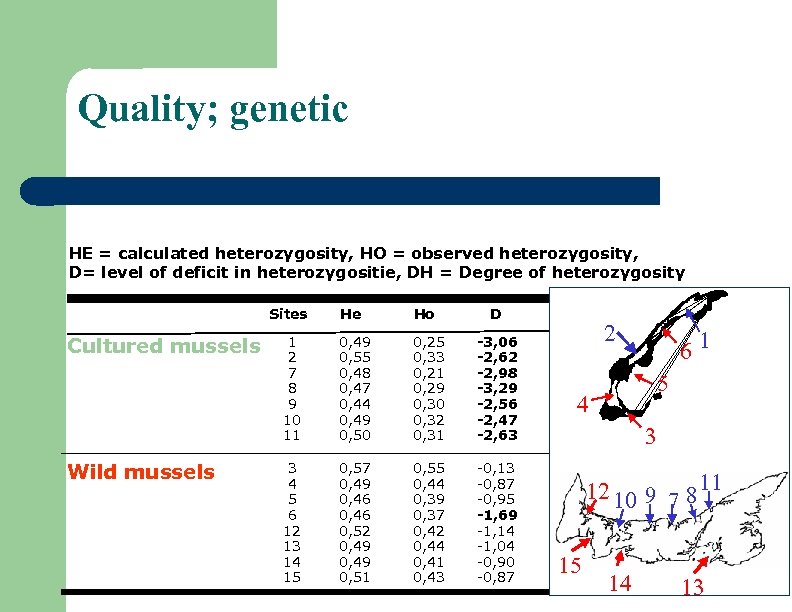 Quality; genetic HE = calculated heterozygosity, HO = observed heterozygosity, D= level of deficit in heterozygositie, DH = Degree of heterozygosity Sites Cultured mussels Wild mussels He Ho D 1 2 7 8 9 10 11 0, 49 0, 55 0, 48 0, 47 0, 44 0, 49 0, 50 0, 25 0, 33 0, 21 0, 29 0, 30 0, 32 0, 31 -3, 06 -2, 62 -2, 98 -3, 29 -2, 56 -2, 47 -2, 63 3 4 5 6 12 13 14 15 0, 57 0, 49 0, 46 0, 52 0, 49 0, 51 0, 55 0, 44 0, 39 0, 37 0, 42 0, 44 0, 41 0, 43 -0, 13 -0, 87 -0, 95 -1, 69 -1, 14 -1, 04 -0, 90 -0, 87 2 61 5 4 3 12 10 9 7 8 11 15 14 13
Quality; genetic HE = calculated heterozygosity, HO = observed heterozygosity, D= level of deficit in heterozygositie, DH = Degree of heterozygosity Sites Cultured mussels Wild mussels He Ho D 1 2 7 8 9 10 11 0, 49 0, 55 0, 48 0, 47 0, 44 0, 49 0, 50 0, 25 0, 33 0, 21 0, 29 0, 30 0, 32 0, 31 -3, 06 -2, 62 -2, 98 -3, 29 -2, 56 -2, 47 -2, 63 3 4 5 6 12 13 14 15 0, 57 0, 49 0, 46 0, 52 0, 49 0, 51 0, 55 0, 44 0, 39 0, 37 0, 42 0, 44 0, 41 0, 43 -0, 13 -0, 87 -0, 95 -1, 69 -1, 14 -1, 04 -0, 90 -0, 87 2 61 5 4 3 12 10 9 7 8 11 15 14 13
 Heterozygosity? l l Heterozygosity has been shown to be an indicator for the performance of a stock Link between heterozygosity and physiological fitness well documented in several molluscan species including the blue mussel
Heterozygosity? l l Heterozygosity has been shown to be an indicator for the performance of a stock Link between heterozygosity and physiological fitness well documented in several molluscan species including the blue mussel
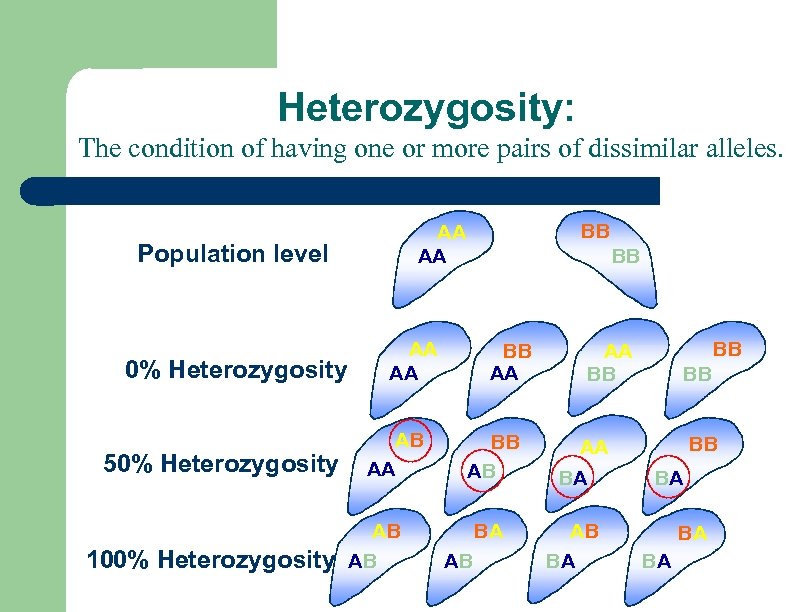 Heterozygosity: The condition of having one or more pairs of dissimilar alleles. Population level 0% Heterozygosity 50% Heterozygosity 100% Heterozygosity BB AA AA AB AB BB AA BB AB BA AB BB AA BB BB BB AA BA AB BA BA
Heterozygosity: The condition of having one or more pairs of dissimilar alleles. Population level 0% Heterozygosity 50% Heterozygosity 100% Heterozygosity BB AA AA AB AB BB AA BB AB BA AB BB AA BB BB BB AA BA AB BA BA
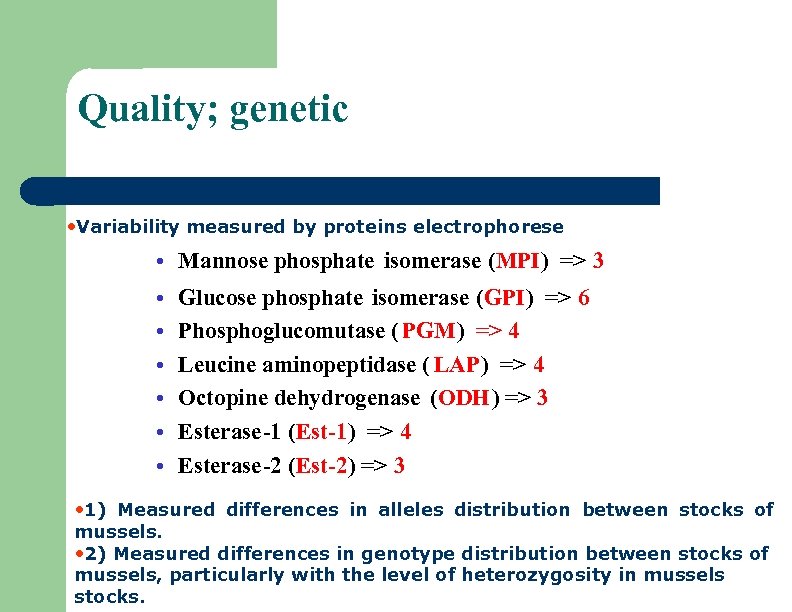 Quality; genetic • Variability measured by proteins electrophorese • Mannose phosphate isomerase (MPI) => 3 • • • Glucose phosphate isomerase (GPI) => 6 Phosphoglucomutase ( PGM) => 4 Leucine aminopeptidase ( LAP) => 4 Octopine dehydrogenase (ODH) => 3 Esterase-1 (Est-1) => 4 Esterase-2 (Est-2) => 3 • 1) Measured differences in alleles distribution between stocks of mussels. • 2) Measured differences in genotype distribution between stocks of mussels, particularly with the level of heterozygosity in mussels stocks.
Quality; genetic • Variability measured by proteins electrophorese • Mannose phosphate isomerase (MPI) => 3 • • • Glucose phosphate isomerase (GPI) => 6 Phosphoglucomutase ( PGM) => 4 Leucine aminopeptidase ( LAP) => 4 Octopine dehydrogenase (ODH) => 3 Esterase-1 (Est-1) => 4 Esterase-2 (Est-2) => 3 • 1) Measured differences in alleles distribution between stocks of mussels. • 2) Measured differences in genotype distribution between stocks of mussels, particularly with the level of heterozygosity in mussels stocks.
 Quality; genetic St. Peters Bay Tracadie Bay Mill River Morell River Winter Bay Enmore Bay Bedeque Bay West River
Quality; genetic St. Peters Bay Tracadie Bay Mill River Morell River Winter Bay Enmore Bay Bedeque Bay West River
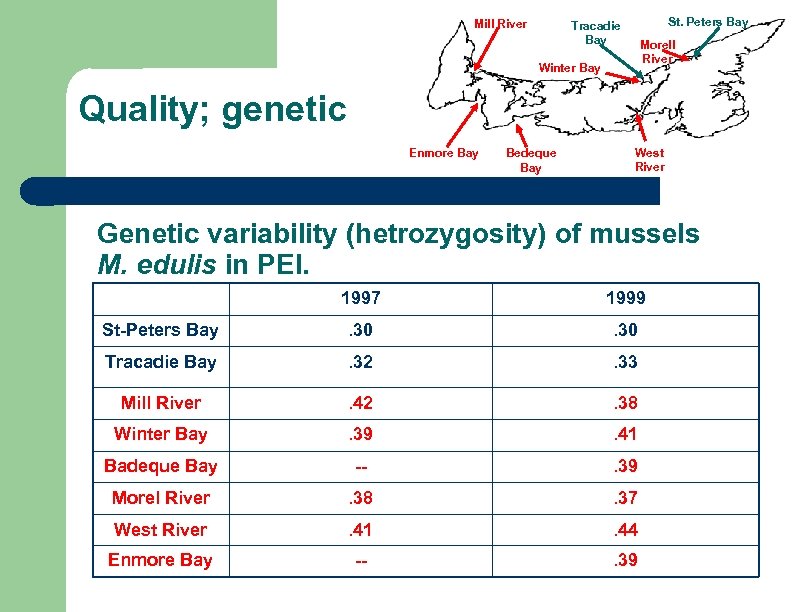 Mill River St. Peters Bay Tracadie Bay Morell River Winter Bay Quality; genetic Enmore Bay Bedeque Bay West River Genetic variability (hetrozygosity) of mussels M. edulis in PEI. 1997 1999 St-Peters Bay . 30 Tracadie Bay . 32 . 33 Mill River . 42 . 38 Winter Bay . 39 . 41 Badeque Bay -- . 39 Morel River . 38 . 37 West River . 41 . 44 Enmore Bay -- . 39
Mill River St. Peters Bay Tracadie Bay Morell River Winter Bay Quality; genetic Enmore Bay Bedeque Bay West River Genetic variability (hetrozygosity) of mussels M. edulis in PEI. 1997 1999 St-Peters Bay . 30 Tracadie Bay . 32 . 33 Mill River . 42 . 38 Winter Bay . 39 . 41 Badeque Bay -- . 39 Morel River . 38 . 37 West River . 41 . 44 Enmore Bay -- . 39
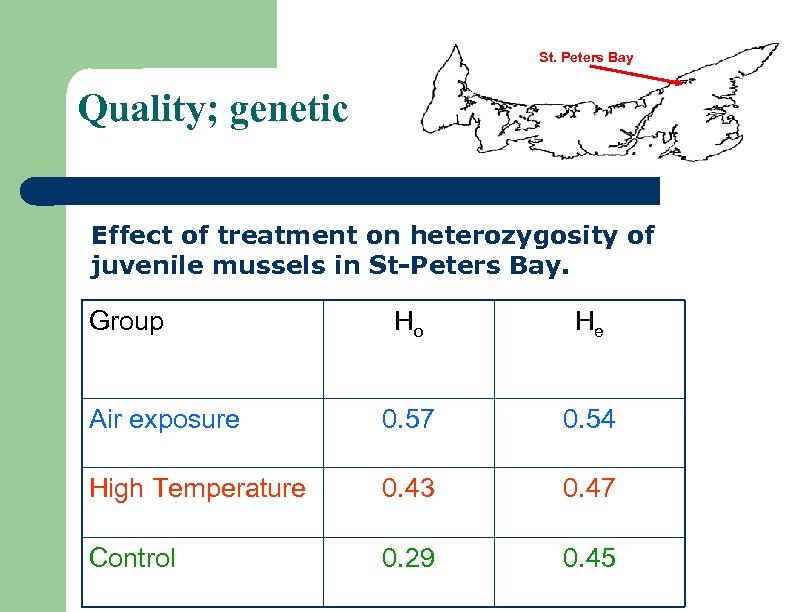 St. Peters Bay Quality; genetic Effect of treatment on heterozygosity of juvenile mussels in St-Peters Bay. Group Ho He Air exposure 0. 57 0. 54 High Temperature 0. 43 0. 47 Control 0. 29 0. 45
St. Peters Bay Quality; genetic Effect of treatment on heterozygosity of juvenile mussels in St-Peters Bay. Group Ho He Air exposure 0. 57 0. 54 High Temperature 0. 43 0. 47 Control 0. 29 0. 45
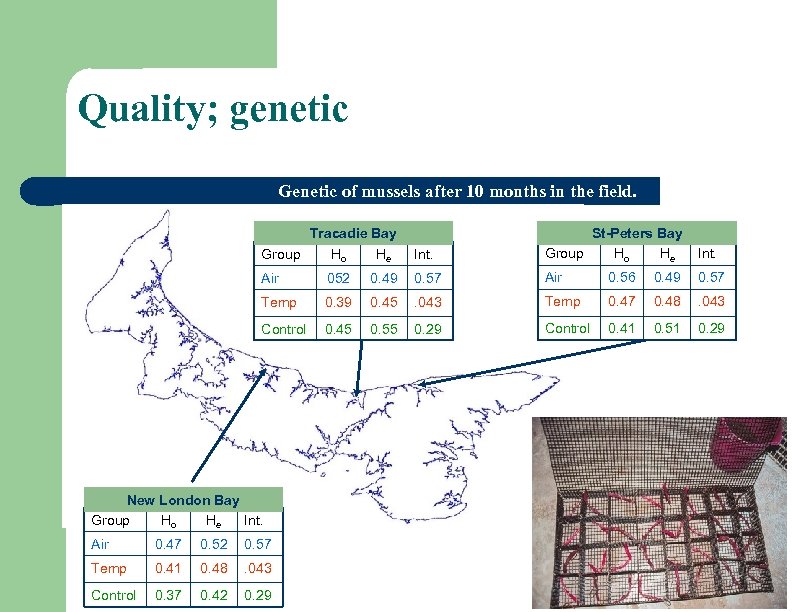 Quality; genetic Genetic of mussels after 10 months in the field. Int. St-Peters Bay Group Ho He Int. 0. 49 0. 57 Air 0. 56 0. 49 0. 57 0. 39 0. 45 . 043 Temp 0. 47 0. 48 . 043 0. 45 0. 55 0. 29 Control 0. 41 0. 51 0. 29 Tracadie Bay Group Ho He Air 052 Temp Control New London Bay Group Ho He Int. Air 0. 47 0. 52 0. 57 Temp 0. 41 0. 48 . 043 Control 0. 37 0. 42 0. 29
Quality; genetic Genetic of mussels after 10 months in the field. Int. St-Peters Bay Group Ho He Int. 0. 49 0. 57 Air 0. 56 0. 49 0. 57 0. 39 0. 45 . 043 Temp 0. 47 0. 48 . 043 0. 45 0. 55 0. 29 Control 0. 41 0. 51 0. 29 Tracadie Bay Group Ho He Air 052 Temp Control New London Bay Group Ho He Int. Air 0. 47 0. 52 0. 57 Temp 0. 41 0. 48 . 043 Control 0. 37 0. 42 0. 29
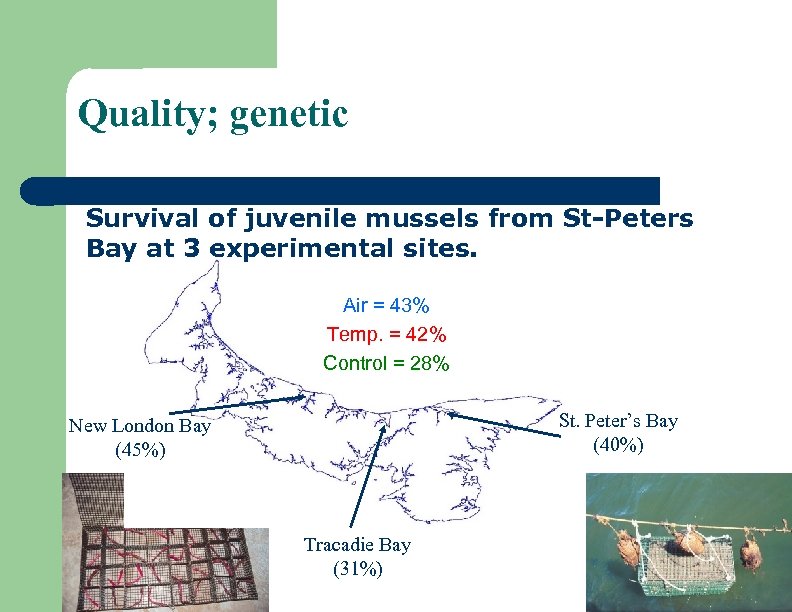 Quality; genetic Survival of juvenile mussels from St-Peters Bay at 3 experimental sites. Air = 43% Temp. = 42% Control = 28% St. Peter’s Bay (40%) New London Bay (45%) Tracadie Bay (31%)
Quality; genetic Survival of juvenile mussels from St-Peters Bay at 3 experimental sites. Air = 43% Temp. = 42% Control = 28% St. Peter’s Bay (40%) New London Bay (45%) Tracadie Bay (31%)
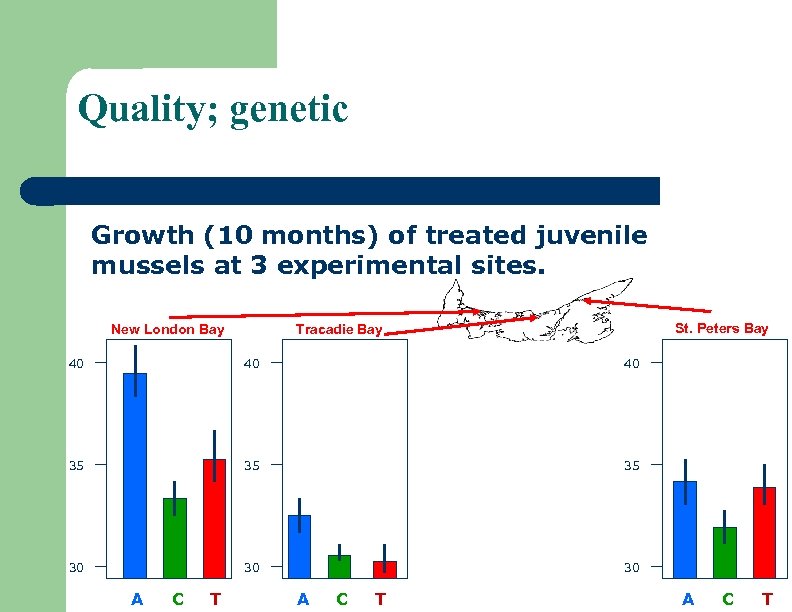 Quality; genetic Growth (10 months) of treated juvenile mussels at 3 experimental sites. New London Bay St. Peters Bay Tracadie Bay 40 40 40 35 35 35 30 30 30 A C T
Quality; genetic Growth (10 months) of treated juvenile mussels at 3 experimental sites. New London Bay St. Peters Bay Tracadie Bay 40 40 40 35 35 35 30 30 30 A C T
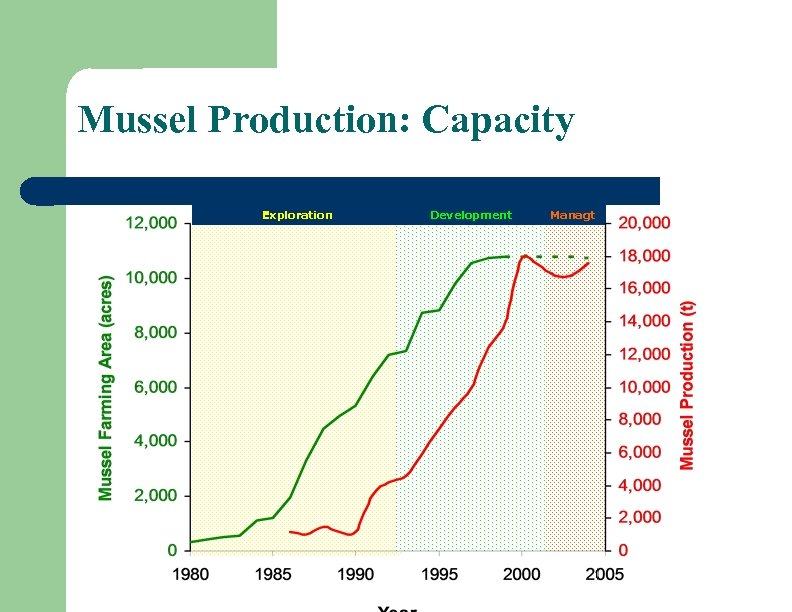 Mussel Production: Capacity Exploration Development Managt
Mussel Production: Capacity Exploration Development Managt
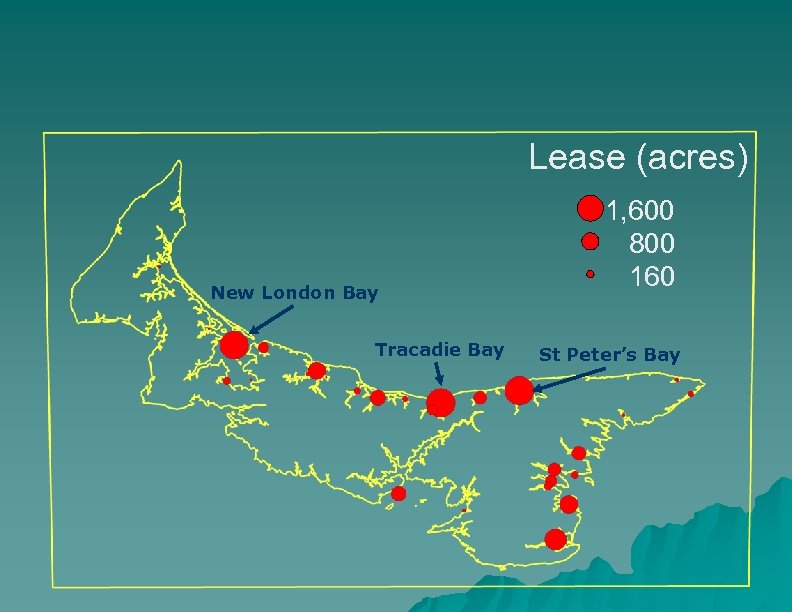 Lease (acres) New London Bay Tracadie Bay 1, 600 800 160 St Peter’s Bay
Lease (acres) New London Bay Tracadie Bay 1, 600 800 160 St Peter’s Bay
 1990 DFO Leasing
1990 DFO Leasing
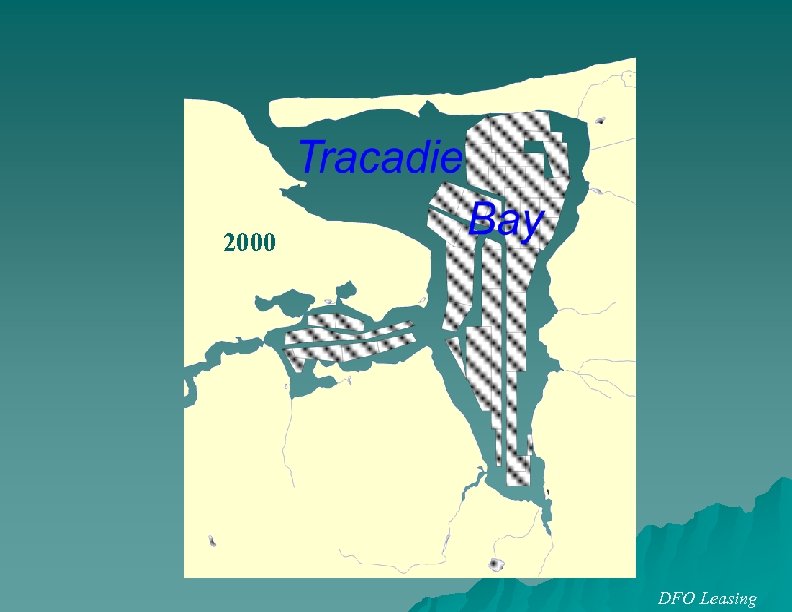 2000 DFO Leasing
2000 DFO Leasing
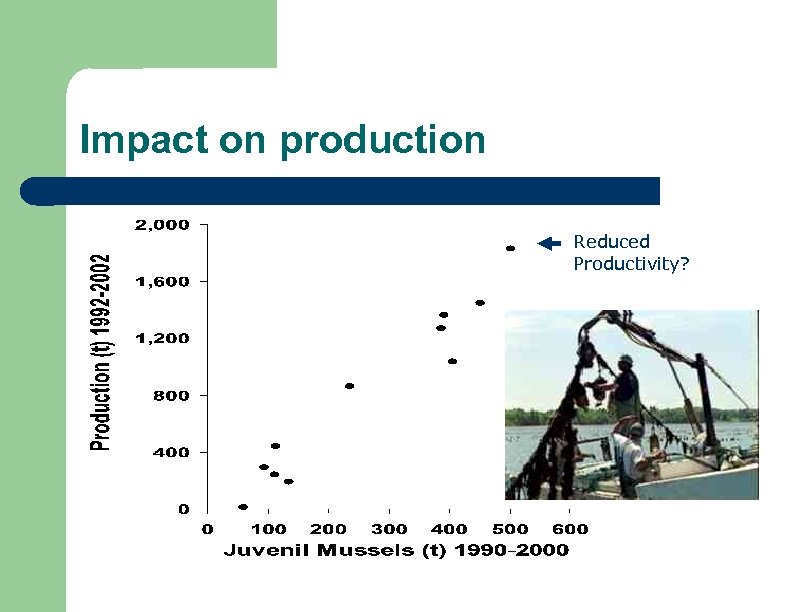 Impact on production Reduced Productivity?
Impact on production Reduced Productivity?
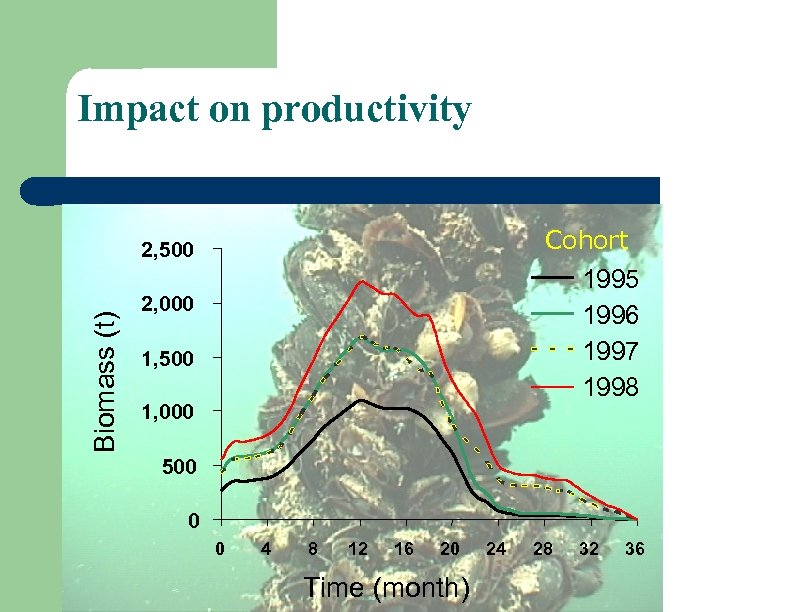 Impact on productivity Cohort Biomass (t) 2, 500 1995 1996 1997 1998 2, 000 1, 500 1, 000 500 0 0 4 8 12 16 20 Time (month) 24 28 32 36
Impact on productivity Cohort Biomass (t) 2, 500 1995 1996 1997 1998 2, 000 1, 500 1, 000 500 0 0 4 8 12 16 20 Time (month) 24 28 32 36
 Standardized Monitoring Objectives – Monitor shellfish aquaculture production – Monitor spatial and temporal variation in mollusc productivity
Standardized Monitoring Objectives – Monitor shellfish aquaculture production – Monitor spatial and temporal variation in mollusc productivity
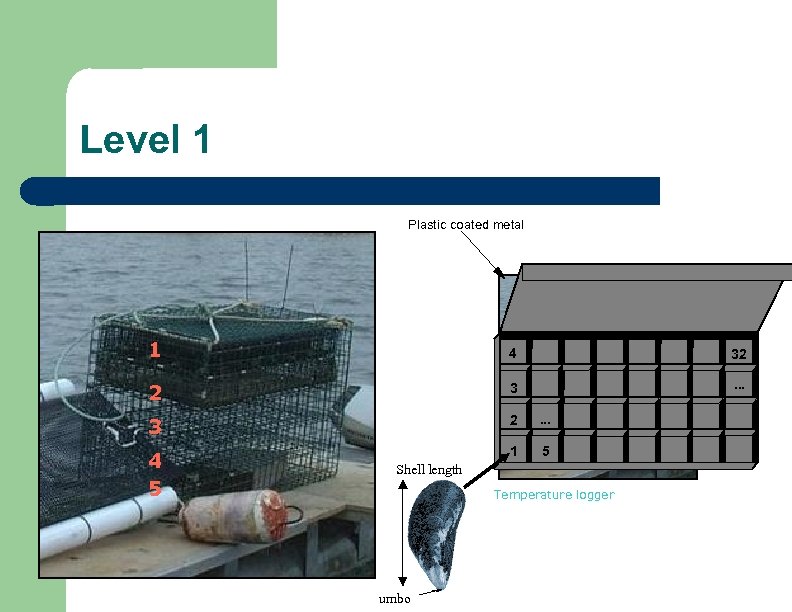 Level 1 Plastic coated metal 1 4 2 3 3 2 . . . 4 5 1 2 3 4 5 Shell length Temperature logger umbo 32. . .
Level 1 Plastic coated metal 1 4 2 3 3 2 . . . 4 5 1 2 3 4 5 Shell length Temperature logger umbo 32. . .
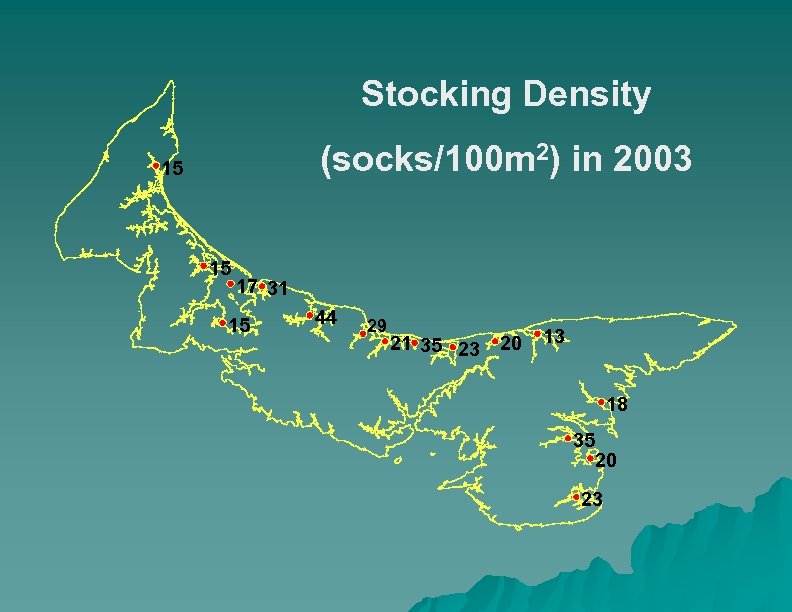 Stocking Density (socks/100 m 2) in 2003 15 15 17 31 15 44 29 21 35 23 20 13 18 35 20 23
Stocking Density (socks/100 m 2) in 2003 15 15 17 31 15 44 29 21 35 23 20 13 18 35 20 23
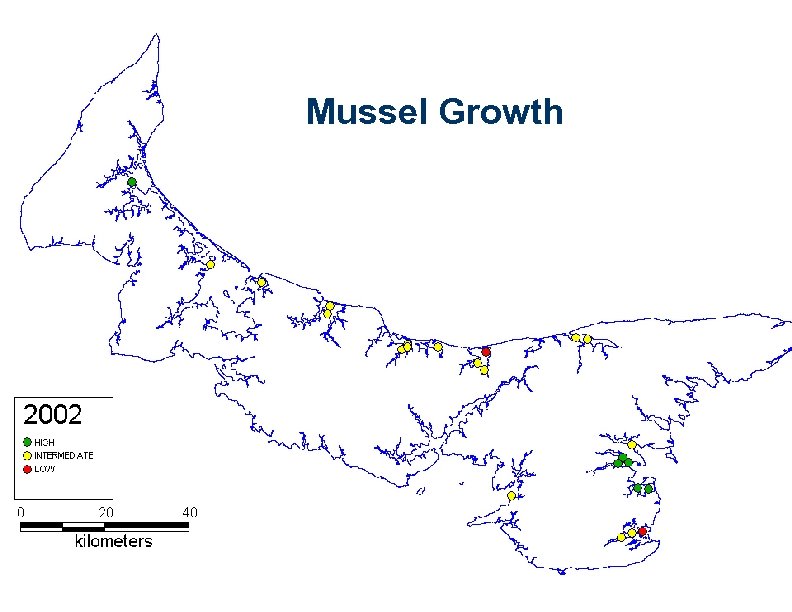 Mussel Growth
Mussel Growth
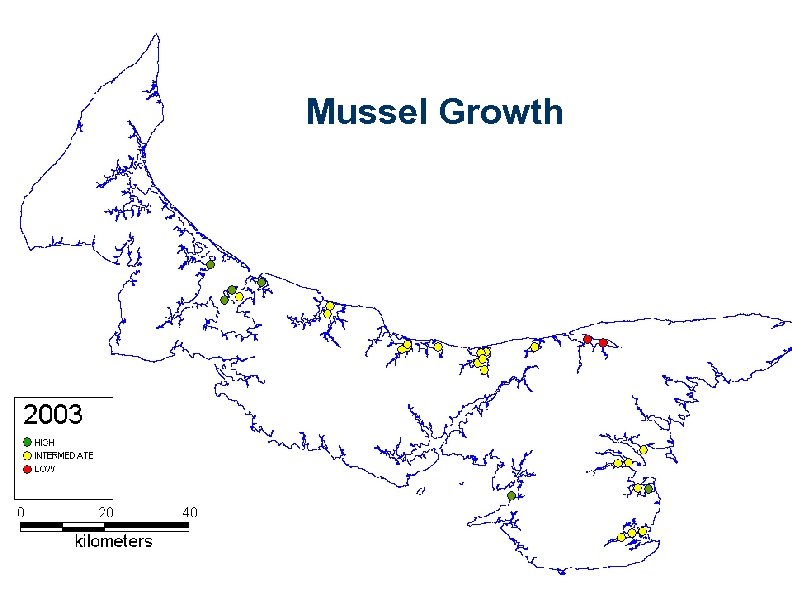 Mussel Growth
Mussel Growth
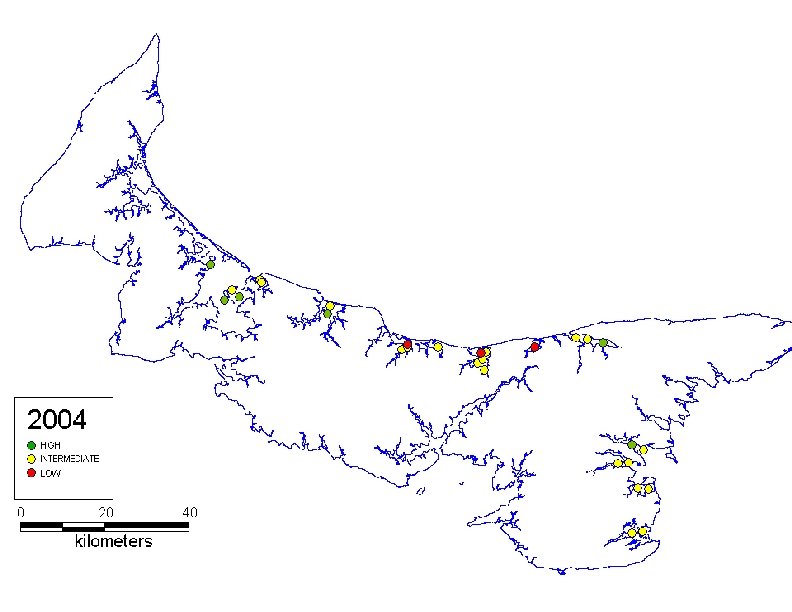
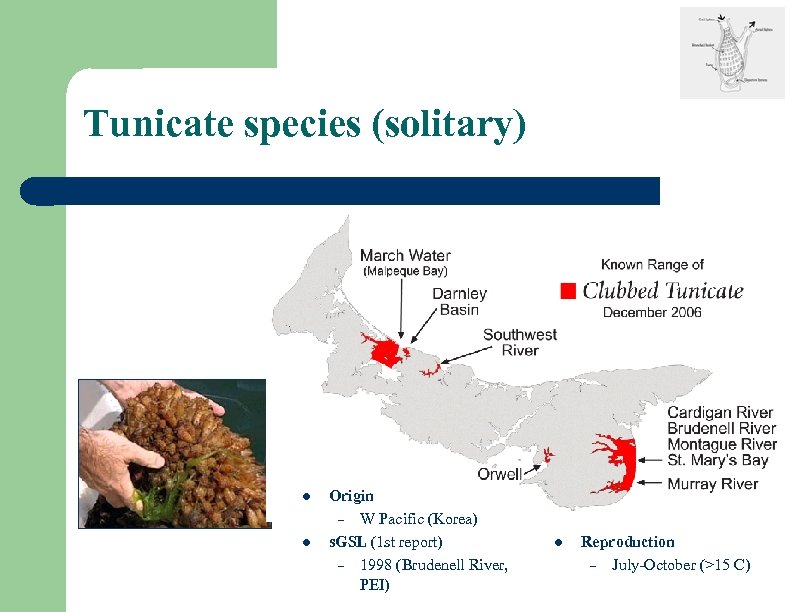 Tunicate species (solitary) l l Origin – W Pacific (Korea) s. GSL (1 st report) – 1998 (Brudenell River, PEI) l Reproduction – July-October (>15 C)
Tunicate species (solitary) l l Origin – W Pacific (Korea) s. GSL (1 st report) – 1998 (Brudenell River, PEI) l Reproduction – July-October (>15 C)
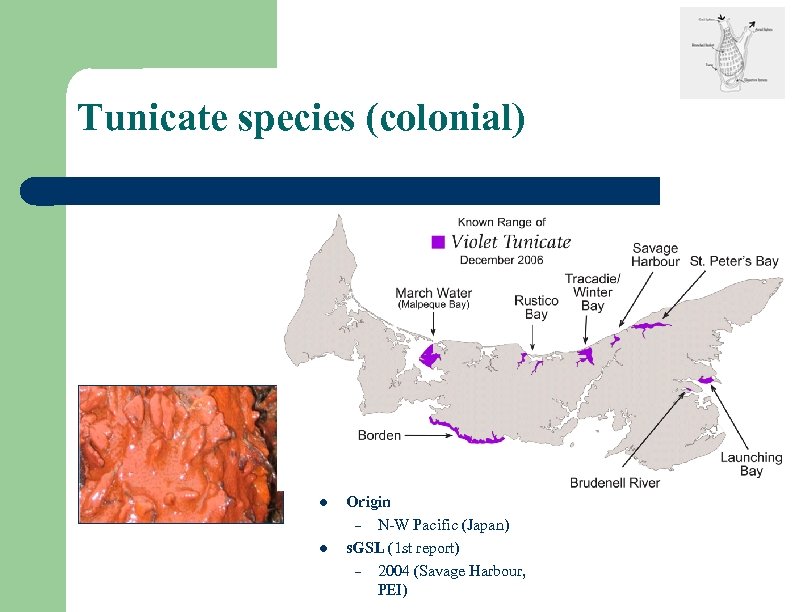 Tunicate species (colonial) l l Origin – N-W Pacific (Japan) s. GSL (1 st report) – 2004 (Savage Harbour, PEI)
Tunicate species (colonial) l l Origin – N-W Pacific (Japan) s. GSL (1 st report) – 2004 (Savage Harbour, PEI)
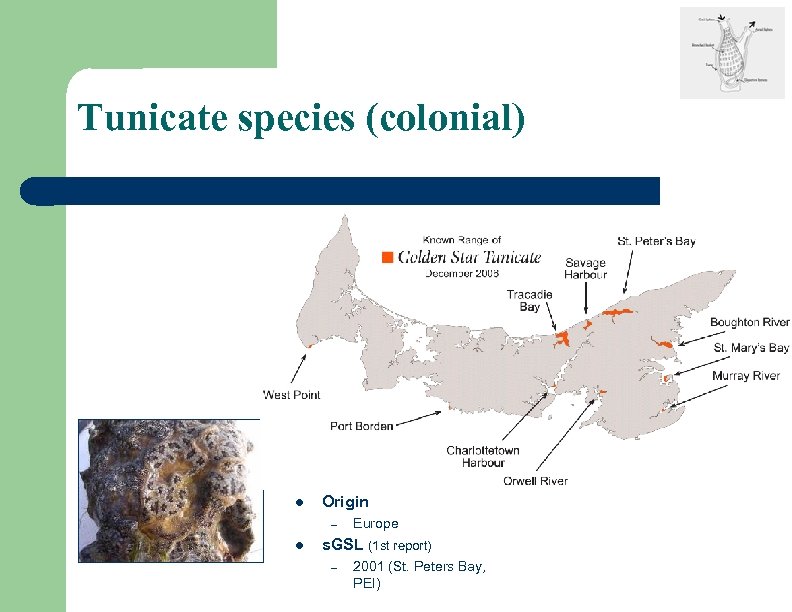 Tunicate species (colonial) l Origin – l Europe s. GSL (1 st report) – 2001 (St. Peters Bay, PEI)
Tunicate species (colonial) l Origin – l Europe s. GSL (1 st report) – 2001 (St. Peters Bay, PEI)
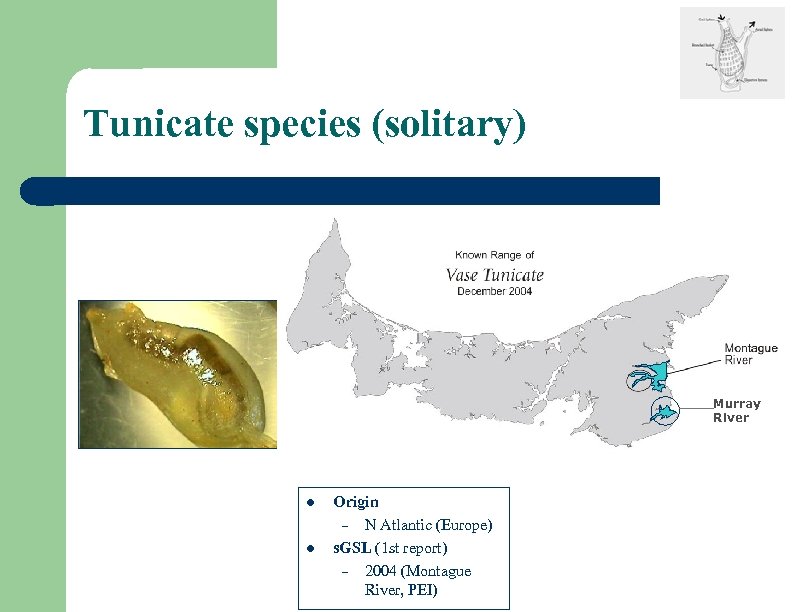 Tunicate species (solitary) Murray River l l Origin – N Atlantic (Europe) s. GSL (1 st report) – 2004 (Montague River, PEI)
Tunicate species (solitary) Murray River l l Origin – N Atlantic (Europe) s. GSL (1 st report) – 2004 (Montague River, PEI)
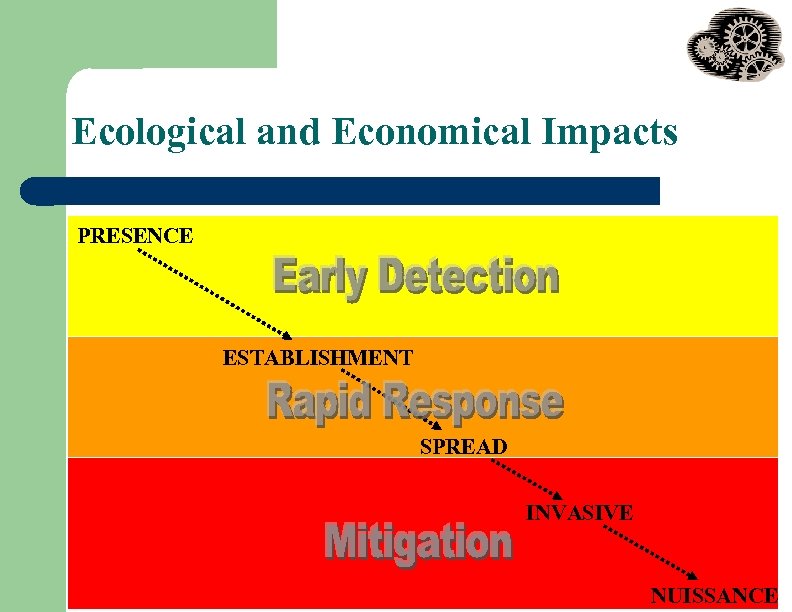 Ecological and Economical Impacts PRESENCE ESTABLISHMENT SPREAD INVASIVE NUISSANCE
Ecological and Economical Impacts PRESENCE ESTABLISHMENT SPREAD INVASIVE NUISSANCE
 AIS Monitoring : Directed (plates) Objectives • Early detection • Vectors • Effect of mitigation • Evolution of spread
AIS Monitoring : Directed (plates) Objectives • Early detection • Vectors • Effect of mitigation • Evolution of spread
 AIS Monitoring: Stewardship Objectives • Early detection • Frequent observation • Education • Buy-in COORDINATION • Who do you call? • Data gathering • Education • Communication CLEARING HOUSE • Identification • Specimen collection • Validation • Training
AIS Monitoring: Stewardship Objectives • Early detection • Frequent observation • Education • Buy-in COORDINATION • Who do you call? • Data gathering • Education • Communication CLEARING HOUSE • Identification • Specimen collection • Validation • Training
 Invasive tunicate in Atlantic Canada Impacts ØCompete for food and space ØAdd weight and increase drop-off ØIncrease production cost ØIncrease cost of processing ØHard on employees
Invasive tunicate in Atlantic Canada Impacts ØCompete for food and space ØAdd weight and increase drop-off ØIncrease production cost ØIncrease cost of processing ØHard on employees
 Mitigation measures (in part funded by the Development Fund) Chemical Physical (lime dipping and spraying) (high pressure spray)
Mitigation measures (in part funded by the Development Fund) Chemical Physical (lime dipping and spraying) (high pressure spray)
 Mitigation measures (in part funded by the Development Fund) Husbandry (socking density and re-socking) Biological (predation)
Mitigation measures (in part funded by the Development Fund) Husbandry (socking density and re-socking) Biological (predation)
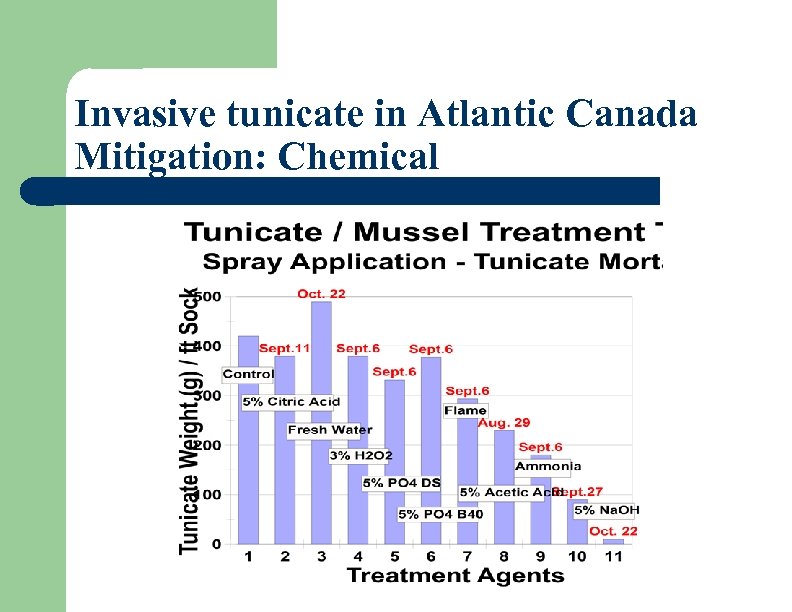 Invasive tunicate in Atlantic Canada Mitigation: Chemical
Invasive tunicate in Atlantic Canada Mitigation: Chemical
 Invasive tunicate in Atlantic Canada Mitigation: Physical
Invasive tunicate in Atlantic Canada Mitigation: Physical
 Invasive tunicate in Atlantic Canada Mitigation: Physical
Invasive tunicate in Atlantic Canada Mitigation: Physical
 Methods and Materials: Marker development: • Seven nuclear loci (18 S (Kenchington et al. , 1995), Glu 5’ (Heath et al. , 1995), ITS (Heath et al. , 1995), MAL-I (Rawson et al. , 1996), PLIIa (Heath et al. , 1995), mac-1 (Bierne et al. , 2003 a; Bierne et al. , 2003 b), EFbis (Bierne et al. , 2003 a; Bierne et al. , 2003 b) and seven microsatellite loci Mgu 1, Mgu 2, Mgu 3, Mgu 4, Mgu 5, Mgu 6 and Mgu 7 (Presa et al. , 2002) were evaluated for their use in species identification mussels from Atlantic Canada • Species identification markers were tested on pedigreed mussel crosses (developed by E. Kenchinton’s group DFO, BIO (Kenchington et al. , 2002)). o X 102 K (M. edulis ♀) x J 17 F (M. edulis ♂) o 04 WF 01 T (M. trossulus ♀) x J 17 F (M. edulis ♂) o X 102 K (M. edulis ♀) x 01 WM 05 T (hybrid ♂) o 02 WF 01 T (M. trossulus ♀) x 01 WM 05 T (hybrid ♂). • ITS optimization: o Cloned and sequenced PCR products from M. edulis, M. trossulus and hybrid individuals o Relocated PCR primer to eliminate small bands that interfere with interpretation (Figure 1) • Glu 5’ optimization: o Tested other primers for the polyphenolic adhesive protein (Me 15 (Inoue et al. , 1995), Me 16 (Inoue et al. , 1995) and Me 17 (Bierne et al. , 2003) Conclusions: • ITSR 2 and Me 15/16 provide a higher throughput method for species identification in mussels. o The methods used were suitable for small mussels (<5 mm) • This modified method was suitable for high throughput species identification o We were able to process 659 individual mussels from 11 locations at two loci (ITSR 2 and Me 15/16)
Methods and Materials: Marker development: • Seven nuclear loci (18 S (Kenchington et al. , 1995), Glu 5’ (Heath et al. , 1995), ITS (Heath et al. , 1995), MAL-I (Rawson et al. , 1996), PLIIa (Heath et al. , 1995), mac-1 (Bierne et al. , 2003 a; Bierne et al. , 2003 b), EFbis (Bierne et al. , 2003 a; Bierne et al. , 2003 b) and seven microsatellite loci Mgu 1, Mgu 2, Mgu 3, Mgu 4, Mgu 5, Mgu 6 and Mgu 7 (Presa et al. , 2002) were evaluated for their use in species identification mussels from Atlantic Canada • Species identification markers were tested on pedigreed mussel crosses (developed by E. Kenchinton’s group DFO, BIO (Kenchington et al. , 2002)). o X 102 K (M. edulis ♀) x J 17 F (M. edulis ♂) o 04 WF 01 T (M. trossulus ♀) x J 17 F (M. edulis ♂) o X 102 K (M. edulis ♀) x 01 WM 05 T (hybrid ♂) o 02 WF 01 T (M. trossulus ♀) x 01 WM 05 T (hybrid ♂). • ITS optimization: o Cloned and sequenced PCR products from M. edulis, M. trossulus and hybrid individuals o Relocated PCR primer to eliminate small bands that interfere with interpretation (Figure 1) • Glu 5’ optimization: o Tested other primers for the polyphenolic adhesive protein (Me 15 (Inoue et al. , 1995), Me 16 (Inoue et al. , 1995) and Me 17 (Bierne et al. , 2003) Conclusions: • ITSR 2 and Me 15/16 provide a higher throughput method for species identification in mussels. o The methods used were suitable for small mussels (<5 mm) • This modified method was suitable for high throughput species identification o We were able to process 659 individual mussels from 11 locations at two loci (ITSR 2 and Me 15/16)


